Abstract
Background:
Retinal vein occlusion (RVO) is the most common occlusive retinal vascular disorder and results in varying degrees of visual loss.
Aim:
To determine the pattern of presentation, risk factors, and treatment outcomes in a group of patients with RVO seen in a tertiary hospital in Nigeria.
Materials and Methods:
Medical records of patients who presented to the University of Benin Teaching Hospital, Benin City, Nigeria in whom a diagnosis of RVO was made over a 5 years period were reviewed. Data obtained were analyzed with the GraphPad Instat Software, Inc. version V2.05a program, San Diego, Califonia and a P < 0.05 considered significant.
Results:
There were 20 patients made of 14 (70.0%) males and 6 (30.0%) females with a mean age of 62.7 ± 10.4 years. There were 15 (68.2%) eyes with central RVO, 3 (13.6%) eyes with branch RVO, and 4 (18.2%) eyes with hemi RVO. Bilateral involvement occurred in 2 (10.0%) patients. Risk factors included hypertension 14 (70.0%), diabetes mellitus 9 (45.0%), and glaucoma 5 (22.7%). Multiple risk factors were present in 14 (70.0%) patients. Complications included macula edema 15 (68.2%), retinal neovascularization 5 (22.7%), neovascular glaucoma 3 (13.6%), and vitreous hemorrhage 2 (9.1%). Eyes which had definitive treatment with intravitreal antivascular endothelial growth factors and laser photocoagulation for macula edema and retinal neovascularization, respectively, had better visual acuity compared to eyes which did not receive these treatment, P = 0.002.
Conclusion:
The incidence and visual loss that occurs from RVO can be reduced by modifying known risk factors and early institution of appropriate therapy for complications that occur.
KEYWORDS: Intravitreal antivascular endothelial growth factors, laser photocoagulation, retinal vein occlusion
INTRODUCTION
Retinal vein occlusion (RVO) is the second most common retinal vascular disease after diabetic retinopathy.[1,2] It is the most common occlusive retinal vascular disorder occurring more commonly in the older age group and results in varying degrees of visual loss.[1,2,3] RVO has a prevalence that varies from of 0.7% to 2% in persons older than 40 years of age.[4,5,6] The 10-year incidence of RVO in a population based cohort was 1.6%.[7] It is broadly classified based on the site of obstruction into central RVO (CRVO) when the site of obstruction is at or proximal to the lamina cribrosa, branch RVO (BRVO) when the occlusion is typically at an arteriovenous intersection, and hemi RVO (HRVO) when the obstruction is of one of two trunks of the central retinal vein within the optic nerve.[8,9] Its pathogenesis is multifactorial and thought to follow the principles of Virchow's triad for thrombogenesis, involving vessel damage, stasis, and hypercoagulability, which could occur in conditions such as atherosclerosis or inflammation.[8,9,10] Ocular and systemic factors which predispose to RVO have been described. The systemic risk factors include cardiovascular disease, hypertension, diabetes mellitus, hyperlipidemia, and hypercoagulable states. Ocular risk factors implicated include glaucoma with elevated intraocular pressure and hypermetropia.[10,11,12,13,14,15,16]
The aim of the study is to determine the pattern of presentation, risk factors, and treatment outcomes in a group of patients with RVO seen in a tertiary hospital in Nigeria.
MATERIALS AND METHODS
The case files of all new patients who presented to the University of Benin Teaching Hospital, Benin City, Nigeria in whom a diagnosis of RVO was made from January 1, 2010, to December 31, 2014 were retrieved and reviewed. Data obtained were analyzed using the GraphPad Instat Software, Inc. version V2.05a program, San Diego, Califonia. For statistical comparison, a P < 0.05 was considered significant. Demographic and clinical data extracted include age, gender, visual acuity, clinical diagnosis, type of vein occlusion, ocular and systemic risk factors, treatment offered, outcomes, and ocular complications. During the study period, there was a change in the standard operating procedure with regards to the management of RVO in the department following subspecialty training in vitreous and retina. In the first 2 years of the study period prior to subspecialization, management was primarily conservative with prescription of topical and/or oral medications for ocular complications such as macula edema and neovascular glaucoma with carbonic anhydrase inhibitors such as oral acetazolamide, nonsteroidal anti-inflammatory drugs such as indomethacin tablets and gutt diclofenac, topical intraocular pressure lowering medications such as timolol, and treatment of known predisposing risk factors. The last 3 years of the study period now included laboratory investigations to identify predisposing risk factors and definitive treatment with use of intravitreal pharmacologic agents (anti vascular endothelial growth factors [anti-VEGF] either 0.05 mg in 0.05 ml of ranibizumab or 1.25 mg in 0.05 ml of bevacizumab) in complications such as macula edema and initial treatment of neovascular glaucoma and laser (panretinal photocoagulation in ischemic CRVO with retinal neovascularization or sector retinal photocoagulation in BRVO or HRVO with retinal neovascularization).
A diagnosis of CRVO was made in the presence of generalized, scattered hemorrhages consisting of dot, blot, or flame shaped hemorrhages located in the superficial or deep layers of the retina, retinal edema, venous dilatation, and areas of occluded veins. BRVO or HRVO was characterized by retinal hemorrhages within the sector of the retina supplied by the occluded vein. The presence of severe relative afferent pupillary defect and neovascularization was used to differentiate patients with ischemic and nonischemic RVO. A diagnosis of retinal thickening, macula edema, or retinal neovascularization was made following clinical biomicroscopic examination with +78D stereoscopic noncontact lens. Further confirmatory investigations were then advised consisting of fundus fluorescein angiography and/or optical coherence tomography. Tailored laboratory investigations requested include full blood count, fasting plasma glucose, glycosylated hemoglobin, fasting serum lipid profile, urinalysis, and renal function tests (serum electrolyte, urea, and creatinine). The guidelines concerning the ethical use of subjects were followed in this research.
RESULTS
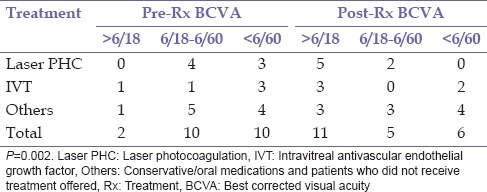
A diagnosis of RVO was documented in 20 patients consisting of 14 (70.0%) males and 6 (30.0%) females, during the study period. Bilateral involvement was found in 2 (10.0%) patients giving a total of 22 eyes. The mean age at the presentation was 62.7 ± 10.4 years (range 43–87 years). This is presented in Table 1. There were 15 (68.2%) eyes with CRVO, 3 (13.6%) eyes with BRVO, and 4 (18.2%) eyes with HRVO. Bilateral involvement occurred in 2 (10.0%) patients; this occurred in 1 patient each with CRVO and HRVO while ischemic RVO was present in 6 (27.2) eyes, of which 3 (13.6%) eyes had ischemic CRVO. The duration of symptoms prior to presentation ranged from 1 week to 10 years with a mean of 84.6 ± 171.2 weeks. Systemic risk factors for RVO found were hypertension 14 (70.0%), diabetes mellitus 9 (45.0%), and dyslipidemia 2 (10.0%). Ocular risk factors were glaucoma 5 (22.7%) and hypermetropia 1 (5.0%). Multiple risk factors were present in 14 (70.0%) patients. No identifiable risk factor was found in 1 (4.5%) aside increasing age present in all patients. Complications of RVO found in this study were macula edema 15 (68.2%) eyes, retinal neovascularization 5 (22.7%) of which 1 (20.0%) occurred in BRVO, 2 (40.0%) in HRVO, and 2 (40.0%) in CRVO, vitreous hemorrhage 2 (9.1%), localized tractional retinal detachment 1 (4.5%) which was in BRVO with fibrovascular proliferation in the affected region, rubeosis iridis 3 (13.6%), and neovascular glaucoma 3 (13.6%). Table 2 shows the initial and final best corrected visual acuity in the eyes. In eyes that were lost to follow-up, the last documented visual acuity was regarded as final BCVA. Table 3 shows the treatment modality used in the eyes. One (4.5%) eye with ischemic CRVO had both intravitreal bevacizumab and panretinal laser photocoagulation for macula edema and retinal neovascularization. Five (22.75) eyes consisting of 4 (18.2) CRVO and 1 (4.5%) HRVO did not receive intravitreal anti-VEGF which were advised. Table 4 shows that eyes which had definitive treatment with intravitreal anti-VEGF and laser photocoagulation for complications with macula edema and neovascularization had better outcomes in visual acuity compared to eyes which did not receive this treatment, P = 0.002. The mean duration of follow-up was 8.21 ± 11.04 months (range 0–44 months). Six (30.0%) patients did not attend any follow-up appointment following diagnosis for treatment while 7 (35%) patients had follow-up duration <1-year.
Table 1.
Age and sex distribution of patients
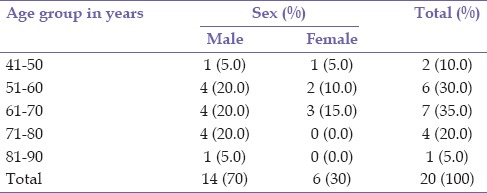
Table 2.
Visual acuity of eyes pre- and post- intervention
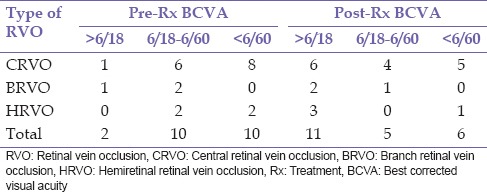
Table 3.
RVO and treatment provided
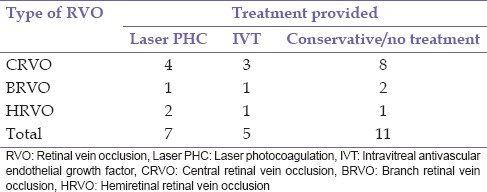
Table 4.
Treatment provided and visual acuity of eyes pre- and post-intervention
DISCUSSION
The low incidence of RVO in ophthalmic cases as reflected in this study with only 20 patients has been documented in similar studies in Nigeria.[10,17] Males were the majority constituting 70% of cases with RVO, in this study. This is contrary to previous studies in Nigeria in which a female preponderance of 60.5% and 74% was documented in Onitsha and Port Harcourt, respectively.[10,17] The female preponderance has been attributed to possible use of oral contraceptives and hormone replacement therapy, a risk factor for RVO in females.[10,17,18] However, population-based studies are needed to accurately determine the role of gender in RVO due to the drawbacks of hospital-based studies. Furthermore, while some studies have reported a male predominance, others find no difference between both sexes.[7,19,20] The mean age of the presentation in this study was 62.7 years. This is not surprising as increasing age is an established risk factor for RVO. However, the previous studies in Nigeria have reported a lower mean age being 58 years and 54.8 years in Onitsha and Port Harcourt, respectively.[10,17] Bilateral involvement occurred in 10.0%. This is very similar to the study in Onitsha with 11.6%,[10] but lower than 25.9% reported in Port Harcourt.[17] The mean duration of symptoms prior to presentation was 84.6 ± 171.2 weeks. Delayed presentation for treatment adversely affects outcomes in the management of RVO. Early intervention with intravitreal anti-VEGF for macula edema in RVO results in better visual outcomes.
A greater proportion of eyes with RVO had CRVO (68.2%) in comparison with BRVO or HRVO. This is similar to the previous studies in Nigeria[10,17] but is at variance with studies in the Western world.[6,7,20,21] Perhaps, the interplay of environmental factors, diet, and genetic/constitutional makeup may have a contributory role in the pattern of presentation of RVO. Indeed, Hayreh[8] has reported that the pathologic mechanisms for the occurrence of various types of retinal venous occlusion viz., CRVO, HRVO, and BRVO are very different. Hypertension was the major culprit identified as a risk factor of RVO in this study in 70.0% of the patients. In Onitsha, Eastern Nigeria, hypertension (58.1%) was the predominant risk factor in the cohort while diabetes mellitus (44.4%) was the most common risk factor identified in Port Harcourt, Southern Nigeria. Studies conducted in the Asian population also found hypertension and elevated blood pressures to be strong risk factors for RVO.[11,20] The rising incidence of noncommunicable diseases such as hypertension and diabetes mellitus in our environment may herald a concomitant increase in the incidence of RVO in our society. Thus, there is a need to create measures to raise awareness in a bid to ensure that these risk factors which are to a great extent modifiable with a healthy lifestyle and dietary habits are properly controlled. The presence of multiple risk factors in most of the patients (70%) may indicate a greater risk of occlusion. Ischemic RVO was present in 27.2% eyes. This is higher than the study in Port Harcourt (14.8%) but much lower than that documented in Onitsha (60.4%). Perhaps the higher prevalence in Onitsha may be due to the longer duration of study in Onitsha as RVO could convert from nonischemic to ischemic as time progresses.
The most common complication of RVO in this study was macular edema in 68.2%. This is in contrast to the study in Port Harcourt, which had vitreous hemorrhage as the most common complication. Vitreous hemorrhage occurs from either break through bleeding in the internal limiting membrane in RVO or from bleeding from the abnormal retinal neovascularization which could occur in ischemic RVO, the risk of which increases with large areas of capillary nonperfused areas. The only complication documented in the study in Onitsha was neovascular glaucoma.
There was a significant improvement in visual outcomes with the use of laser photocoagulation in cases of retinal neovascularization and pharmacotherapeutic agents mainly intravitreal anti-VEGF in cases with macula edema and adjuvant treatment in neovascular glaucoma compared to the conservative approach which was previously utilized, P = 0.002. This occurred despite the delayed duration of symptoms prior to presentation for subsequent prompt and effective treatment that affects outcomes and prognosis. This is attributed to improvement in service delivery with subspecialty training and manpower development, and provision of appropriate equipment. This may translate to a better quality of life in affected patients with better prognosis for restoration of useful vision or stabilization of visual function to limit a decline or deterioration with complications such as chronic macula edema, retinal neovascularization, and subsequent possible vitreous hemorrhage or proliferative retinopathy with tractional retinal detachment. Various studies have demonstrated ample evidence of improvement in visual function with intravitreal anti-VEGF in the management of macular edema following CRVO and BRVO.[22,23,24,25,26,27,28,29] Steroids such as intravitreal triamcinolone and dexamethasone implants were not used in the treatment of macula edema due to the high incidence of raised intraocular pressures and cataract in phakic patients when used.[30] The poor follow-up rate noted in this study is only slightly higher to that reported in Onitsha.[17]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994;117:429–41. doi: 10.1016/s0002-9394(14)70001-7. [DOI] [PubMed] [Google Scholar]
- 2.David R, Zangwill L, Badarna M, Yassur Y. Epidemiology of retinal vein occlusion and its association with glaucoma and increased intraocular pressure. Ophthalmologica. 1988;197:69–74. doi: 10.1159/000309923. [DOI] [PubMed] [Google Scholar]
- 3.Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol. 1997;115:486–91. doi: 10.1001/archopht.1997.01100150488006. [DOI] [PubMed] [Google Scholar]
- 4.Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–9. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243–7. doi: 10.1001/archopht.1996.01100140443012. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: The Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–41. [PMC free article] [PubMed] [Google Scholar]
- 7.Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: The Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726–32. doi: 10.1001/archopht.124.5.726. [DOI] [PubMed] [Google Scholar]
- 8.Hayreh SS. Retinal vein occlusion. Indian J Ophthalmol. 1994;42:109–32. [PubMed] [Google Scholar]
- 9.Wong TY, Scott IU. Clinical practice.Retinal-vein occlusion. N Engl J Med. 2010;363:2135–44. doi: 10.1056/NEJMcp1003934. [DOI] [PubMed] [Google Scholar]
- 10.Fiebai B, Ejimadu CS, Komolafe RD. Incidence and risk factors for retinal vein occlusion at the University of Port Harcourt Teaching Hospital, Port Harcourt, Nigeria. Niger J Clin Pract. 2014;17:462–6. doi: 10.4103/1119-3077.134040. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Yoon YH, Kim HK, Yoon HS, Kang SW, Kim JG, et al. Baseline characteristics and risk factors of retinal vein occlusion: A study by the Korean RVO Study Group. J Korean Med Sci. 2013;28:136–44. doi: 10.3346/jkms.2013.28.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong TY, Larsen EK, Klein R, Mitchell P, Couper DJ, Klein BE, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: The Atherosclerosis Risk in Communities and Cardiovascular Health studies. Ophthalmology. 2005;112:540–7. doi: 10.1016/j.ophtha.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Rath EZ, Frank RN, Shin DH, Kim C. Risk factors for retinal vein occlusions. A case-control study. Ophthalmology. 1992;99:509–14. doi: 10.1016/s0161-6420(92)31940-2. [DOI] [PubMed] [Google Scholar]
- 14.Hayreh SS, Zimmerman B, McCarthy MJ, Podhajsky P. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61–77. doi: 10.1016/s0002-9394(00)00709-1. [DOI] [PubMed] [Google Scholar]
- 15.Elman MJ, Bhatt AK, Quinlan PM, Enger C. The risk for systemic vascular diseases and mortality in patients with central retinal vein occlusion. Ophthalmology. 1990;97:1543–8. doi: 10.1016/s0161-6420(90)32379-5. [DOI] [PubMed] [Google Scholar]
- 16.Cheung N, Klein R, Wang JJ, Cotch MF, Islam AF, Klein BE, et al. Traditional and novel cardiovascular risk factors for retinal vein occlusion: The multiethnic study of atherosclerosis. Invest Ophthalmol Vis Sci. 2008;49:4297–302. doi: 10.1167/iovs.08-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nwosu SN. Pattern and risk factors for retinal vein occlusion in Onitsha, Nigeria. Niger J Ophthamol. 2008;16:30–2. [Google Scholar]
- 18.Stowe GC, 3rd, Zakov ZN, Albert DM. Central retinal vascular occlusion associated with oral contraceptives. Am J Ophthalmol. 1978;86:798–801. doi: 10.1016/0002-9394(78)90124-1. [DOI] [PubMed] [Google Scholar]
- 19.Lim LL, Cheung N, Wang JJ, Islam FM, Mitchell P, Saw SM, et al. Prevalence and risk factors of retinal vein occlusion in an Asian population. Br J Ophthalmol. 2008;92:1316–9. doi: 10.1136/bjo.2008.140640. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda M, Kiyohara Y, Arakawa S, Hata Y, Yonemoto K, Doi Y, et al. Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: The Hisayama study. Invest Ophthalmol Vis Sci. 2010;51:3205–9. doi: 10.1167/iovs.09-4453. [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: The Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:513–8. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 22.Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, et al. Ranibizumab for macular edema following branch retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1102–1112. doi: 10.1016/j.ophtha.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, et al. Ranibizumab for macular edema following central retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124–1133. doi: 10.1016/j.ophtha.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:1594–602. doi: 10.1016/j.ophtha.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: Twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041–9. doi: 10.1016/j.ophtha.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 26.Heier JS, Campochiaro PA, Yau L, Li Z, Saroj N, Rubio RG, et al. Ranibizumab for macular edema due to retinal vein occlusions: Long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119:802–9. doi: 10.1016/j.ophtha.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Prager F, Michels S, Kriechbaum K, Georgopoulos M, Funk M, Geitzenauer W, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009;93:452–6. doi: 10.1136/bjo.2008.141085. [DOI] [PubMed] [Google Scholar]
- 28.Demir M, Oba E, Gulkilik G, Odabasi M, Ozdal E. Intravitreal bevacizumab for macular edema due to branch retinal vein occlusion: 12-month results. Clin Ophthalmol. 2011;5:745–9. doi: 10.2147/OPTH.S19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heier JS, Clark WL, Boyer DS, Brown DM, Vitti R, Berliner AJ, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: Two-year results from the COPERNICUS study. Ophthalmology. 2014;121:1414–20. doi: 10.1016/j.ophtha.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 30.Channa R, Smith M, Campochiaro PA. Treatment of macular edema due to retinal vein occlusions. Clin Ophthalmol. 2011;5:705–13. doi: 10.2147/OPTH.S7632. [DOI] [PMC free article] [PubMed] [Google Scholar]


