Abstract
In developing chordates, retinoic acid (RA) signaling patterns the rostrocaudal body axis globally and affects gene expression locally in some differentiating cell populations. Here we focus on development of epidermal sensory neurons in an invertebrate chordate (amphioxus) to determine how RA signaling influences their rostrocaudal distribution and gene expression (for AmphiCoe, a neural precursor gene; for amphioxus islet and AmphiERR, two neural differentiation genes; and for AmphiHox1, -3, -4, and -6). Treatments with RA or an RA antagonist (BMS009) shift the distribution of developing epidermal neurons anteriorly or posteriorly, respectively. These treatments also affect gene expression patterns in the epidermal neurons, suggesting that RA levels may influence specification of neuronal subtypes. Although colinear expression of Hox genes is well known for the amphioxus central nervous system, we find an unexpected comparable colinearity for AmphiHox1, -3, -4, and -6 in the developing epidermis; moreover, RA levels affect the anteroposterior extent of these Hox expression domains, suggesting that RA signaling controls a colinear Hox code for anteroposterior patterning of the amphioxus epidermis. Thus, in amphioxus, the developing peripheral nervous system appears to be structured by mechanisms parallel to those that structure the central nervous system. One can speculate that, during evolution, an ancestral deuterostome that structured its panepidermal nervous system with an RA-influenced Hox code gave rise to chordates in which this patterning mechanism persisted within the epidermal elements of the peripheral nervous system and was transferred to the neuroectoderm as the central nervous system condensed dorsally.
Chordates (tunicates, amphioxus, and vertebrates) are the only animals known to use endogenous retinoic acid (RA) signaling through heterodimers of RA receptors and retinoid X receptors for developmental patterning (1–4). In vertebrates, RA plays a relatively global role in establishing broad regional identities along the anteroposterior axis as well as more spatially limited roles in patterning specific tissues (see Discussion). The involvement of RA signaling in global patterning is best known for the neural ectoderm (3, 5, 6), endoderm (3, 7, 8), and mesoderm (9) but has been less studied for the epidermal ectoderm (so designated to emphasize its distinction from the neural ectoderm). To date, the only structures known to be patterned by RA signaling within the vertebrate epidermal ectoderm are some of the placodes (10–12).
We have taken advantage of the relatively simple but vertebrate-like genome (13) and body plan (14) of amphioxus to examine effects of RA signaling on the sensory neurons of the epidermal ectoderm. Each of these sensory neurons is a primary neuron (i.e., its cell body is located in the epidermis and sends an axon centripetally to the central nervous system). An evolutionary relationship between these epidermal sensory neurons of amphioxus and some vertebrate neurogenic placodes (especially the olfactory placode) has been tentatively suggested (14, 15). In amphioxus, the epidermal sensory neurons are scattered individually along the anteroposterior axis on either side of the body (16), where they can first be detected morphologically at the late neurula stage (17). Initially there are ≈24 such cells per embryo, but their numbers steadily increase to several hundred during subsequent development (15). Previous work on gene expression patterns in the developing epidermal ectoderm of amphioxus suggests an origin of these sensory neurons from general epidermal cells: starting at the neurula stage, scattered epidermal cells express AmphiHu, a homolog of a vertebrate pan-neuronal marker (18), and AmphiCoe, a homolog of vertebrate markers for neural precursors and neurons differentiating from them (17).
To investigate RA involvement in patterning the amphioxus epidermal ectoderm, we focused on the epidermal sensory neurons and determined how RA or an RA antagonist (BMS009) influenced their anteroposterior distribution as well as aspects of their gene expression. We studied the effects of RA levels on the expression of seven amphioxus genes. One (AmphiCoe) is a marker of neural precursor cells, two (amphioxus islet and AmphiERR) are markers of differentiating neurons, and four (AmphiHox1, -3, -4, and -6) evidently have roles in global regionalization of the anteroposterior body axis and may also be involved in neuron differentiation.
We show that RA levels affect the anteroposterior distribution and possibly also the subtype identity of sensory neurons in the epidermal ectoderm. Our results reveal parallels in nervous system patterning and differentiation between amphioxus and vertebrates. Because amphioxus is the closest living invertebrate relative of the vertebrates, it is the best available proxy for the protochordate ancestor of the vertebrates (19). Thus, we suggest that this protochordate ancestor used an RA-influenced Hox code to pattern both its epidermal ectoderm and its neural ectoderm and that this developmental mechanisms might, in turn, have been derived from a similar one that helped pattern the panepidermal nervous system of a more basal deuterostome lacking a clear distinction between the central and peripheral nervous systems (20, 21).
Materials and Methods
Embryonic Culture and Treatment with RA or the RA Antagonist BMS009. Sexually mature males and females of the Florida amphioxus (Branchiostoma floridae) were collected in Tampa Bay, Florida, during the summer breeding season. The animals were stimulated to spawn electrically (22), and the embryos were raised in seawater at 25°C. At late blastula (3 h of development), cultures were treated with a 1:1,000 dilution of DMSO, all-trans RA in DMSO (23), or BMS009 in DMSO (3) to final concentrations of 10–6 M, determined as the minimal dose giving a maximal effect (3, 23). Virtually all of the embryos raised in seawater and in the DMSO control cultures underwent normal morphological development. At the early neurula stage (10 h of development), all of the experimental and control animals were transferred to clean seawater and allowed to develop further. During the next 2 days, developmental stages (controls, RA-treated, or BMS009-treated) were fixed at frequent intervals in 0.1 M Mops/0.5 M NaCl/2mM MgSO4/1 mM EGTA (pH 7.4) for scanning electron microscopy (SEM) or for in situ hybridization (24).
SEM. Paraformaldehyde-fixed embryos were briefly washed in distilled water, dehydrated in ethanol, and critical point-dried from CO2. Each sample of dried embryos was then sprinkled onto the upper adhesive surface of a double-coated carbon conductive pad (Ted Pella, Redding, CA) mounted on an aluminum stub. The adhesive of a second stub-mounted pad was then gently touched to the embryos and pulled away to leave both stubs with some embryos from which the epidermis was partially stripped away from the underlying tissues. The mounted embryos were then sputter-coated with a gold/palladium mixture and observed in a Cambridge S360 SEM (Zeiss). The distribution of sensory neurons was quantified from SEM prints of the control, RA-treated, and BMS009-treated embryos. The anteroposterior axis along one side of each embryo was divided into five equally spaced regions, and the number of sensory neurons in each region was counted.
In Situ Hybridization and Optical Microscopy. We obtained clones of AmphiCoe (provided by Sebastian M. Shimeld, University of Reading, Reading, U.K.), amphioxus islet (provided by William R. Jackman, University of Colorado, Boulder), AmphiERR (provided by Jean-Marc Vanacker, Ecole Normale Supérieure, Lyon, France), and AmphiHox1, -3, -4, and -6 (provided by Jordi Garcia-Fernàndez, University of Barcelona, Barcelona, and Peter W. H. Holland, Cambridge University, Cambridge, U.K.). Synthesis of antisense riboprobes and in situ hybridization were as described in ref. 24. For each gene tested, in situ hybridization was performed with amphioxus embryos from control, RA-treated, and BMS009-treated cultures. In situ preparations were first photographed as whole mounts and then counterstained in Ponceau S, embedded in Spurr's resin, and prepared as 4-μm serial sections for optical microscopy (24).
Results
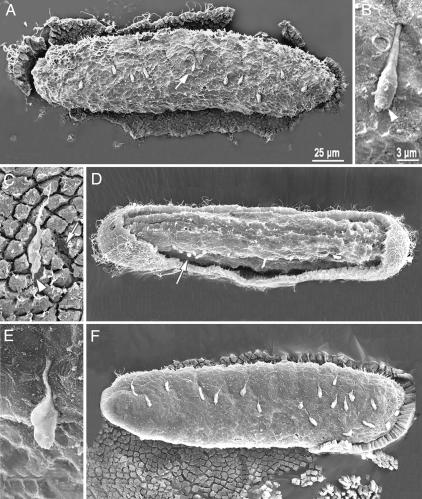
Distribution of Epidermal Sensory Neurons in Control Neurulae. In embryos of the Florida amphioxus (B. floridae), epidermal sensory neurons first become detectable by SEM at the late neurula stage, at ≈18 h (Fig. 1). In controls, ≈12 such cells appear relatively evenly distributed along each flank of the embryo (Figs. 1 A and 2). The cell body of each sensory neuron produces a basal axon that soon penetrates the subepidermal basal lamina and enters the deeper tissues (Fig. 1B). When the epidermis has been removed, these neurons almost always remain attached to the deeper tissues. Where each neuron has been pulled away from the inner surface of the stripped-off epidermis, a dent remains (Fig. 1C, arrow). Such neurons only rarely remain attached to the epidermis (one of these rare exceptions is indicated by the arrowhead in Fig. 1C). At this early stage, there is some morphological variation from one neuron to the next; the cell apex may bear a circle of nascent microvilli (Fig. 1B, arrowhead) or may be pulled out into a thin apical strand (Fig. 1C, arrowhead).
Fig. 1.
SEM of late neurula (18-h) embryos of the Florida amphioxus with the anterior toward the left. The scale bar in A applies to A, D, and F; the scale bar in B applies to B, C, and E. The general epidermis of each embryo has been removed, exposing the underlying tissue surface, on which differentiating sensory neurons are conspicuous. (A) Control neurula in side view (dorsal up) with sensory neurons scattered along its flank. (B) Enlargement of the neuron indicated by an arrow in A. The apical end of the cell body (arrowhead) bears a ring of nascent microvilli, and the basal end gives rise to an axon that continues into the deeper tissues (toward top right). (C) Inner surface of the epidermis of a control neurula. A sensory neuron (with its apical end indicated by an arrowhead) remains attached to the inner surface of the epidermis, and the depression (arrow) among the general epidermal cells indicates where another sensory neuron has been pulled away. (D) Neurula that was exposed to 1 × 10–6 M RA from late blastula through late gastrula. In this dorsolateral view, the distribution of sensory neurons (arrow) has been shifted anteriorly. (E) Enlargement of a differentiating sensory neuron in a neurula embryo after RA treatment; the axon extends toward the top center. (F) Side view of a neurula (dorsal up) that was exposed to 1 × 10–6 M BMS009, an antagonist of RA, from late blastula through late gastrula. The distribution of sensory neurons has been shifted posteriorly.
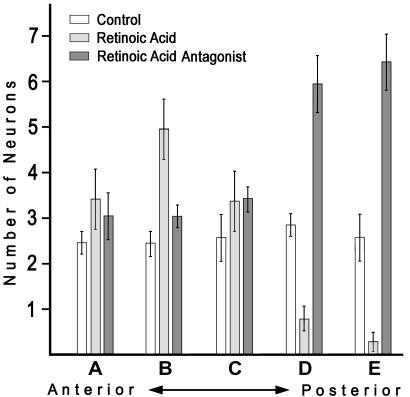
Fig. 2.
Quantification of sensory neuron distributions along the anteroposterior axis of late neurula embryos of amphioxus. Five sectors were demarcated on the body of each embryo (from A, the most anterior, through E, the most posterior), and the number of neurons in each sector was counted. For each sector, the bars show the mean number of neurons for 14 control animals, 15 animals treated with RA, and 16 animals treated with the RA antagonist BMS009 (error bars are ±1 standard error). Sensory neurons, which are distributed relatively uniformly along the sides of control neurulae, become concentrated anteriorly or posteriorly, respectively, by treatment with RA or the RA antagonist BMS009.
Altered Distribution of Epidermal Sensory Neurons in Embryos Treated with RA or the RA Antagonist BMS009. Treatment with RA shifts the distribution of epidermal neurons anteriorly (Figs. 1D and 2), and treatment with the RA antagonist BMS009 shifts their distribution posteriorly (Figs. 1F and 2). Altered levels of RA signaling do not substantially change neuronal morphology (Fig. 1, compare E with controls in B and C).
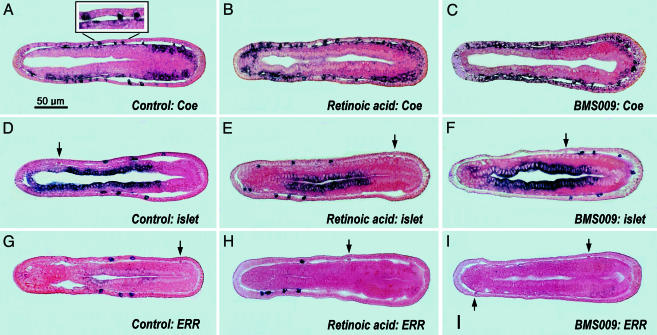
RA and BMS009 Effects on Gene Expression in the Developing Amphioxus Epidermis. The normal developmental transcription of AmphiCoe has been reported for amphioxus (17). This gene is homologous to vertebrate markers for neural cell precursors and differentiating neurons (25). In control neurulae (Fig. 3A), AmphiCoe is weakly to strongly expressed by some cells of the epidermis. The strongly expressing ones (Fig. 3A Inset) are likely precursors of nerve cells and differentiating sensory neurons (the cell bodies of the latter are partly recessed beneath the epidermis). RA or BMS009 treatment shifts the distribution of the epidermal sensory neurons anteriorly (Fig. 3B) or posteriorly (Fig. 3C), respectively. Despite these shifts, the neurons all express AmphiCoe strongly, as would be expected for a marker of nerve cell precursors.
Fig. 3.
In situ preparations of neural precursor and neural differentiation gene expression in late neurulae of amphioxus (frontal sections with anterior toward left). The scale bar in A applies to all panels. Arrows indicate unlabeled epidermal sensory neurons. Some sections show mesodermal (A–C) or endodermal (D–F) expression domains, which are beyond the scope of the present study. (A–C) Expression of AmphiCoe in control (A), RA-treated (B), and BMS009-treated (C) neurulae. (Inset) An enlargement of part of the epidermis showing strong AmphiCoe expression in a general epidermal cell (at left, probably destined to become an epidermal sensory neuron) and in two differentiating epidermal sensory neurons (toward the right). (D–F) Expression of amphioxus islet in control (D), RA-treated (E), and BMS009-treated (F) embryos. (G–I) Expression of AmphiERR in control (G), RA-treated (H), and BMS009-treated (I) neurulae.
Amphioxus islet expression in control neurulae is limited to a few, but not all, of the differentiating epidermal sensory neurons (Fig. 3D) and was overlooked in the original study of this gene (26). In amphioxus embryos exposed to RA (Fig. 3E) or BMS009 (Fig. 3F), amphioxus islet expression is shifted anteriorly (Fig. 3E) or posteriorly (Fig. 3F), respectively. As in the controls, amphioxus islet is expressed in only a subset of the sensory neurons in the RA- or BMS009-treated embryos.
AmphiERR resembles islet in being strongly expressed in only a subset of the differentiating epidermal sensory neurons in the controls (Fig. 3G). In addition, RA treatment shifts the expression of AmphiERR anteriorly (Fig. 3H), still in only some of the epidermal sensory neurons, whereas BMS009 treatment suppresses transcription of AmphiERR throughout the epidermis (Fig. 3I). The transcription of amphioxus islet and AmphiERR in only some (and sometimes in none) of the epidermal sensory cells in the control and experimental embryos indicates that these genes may be differentially expressed to help control cell fate of individual neurons.
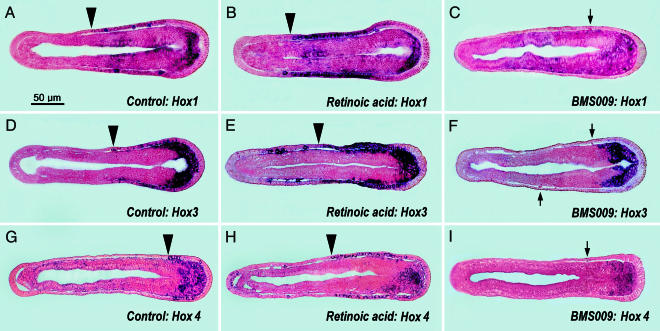
Of the four amphioxus Hox genes studied here, AmphiHox1 is the only one that has been reported to be transcribed in the normally developing epidermis (27, 28). The epidermal expression domains of AmphiHox3, -4, and -6 have been overlooked (28, 29), presumably because these genes are much more weakly expressed in the epidermal cells than in deeper tissues. In 18-h control neurulae, AmphiHox1, -3, and -4 are expressed in some general epidermal cells and differentiating epidermal neurons (Fig. 4 A, D, and G). The contiguous epidermal expression domains of AmphiHox1, -3, and -4 (the anterior limits of which are indicated by arrowheads in Fig. 4 A, D, and G) extend, respectively, three-fifths, two-fifths, and one-fifth of the distance from the posterior end of the embryo.
Fig. 4.
In situ preparations of Hox gene expression in late neurulae of amphioxus (frontal sections with anterior toward left). The scale bar in A applies to all panels. Arrows indicate unlabeled epidermal sensory neurons. The mesodermal and/or endodermal expression domains are beyond the scope of the present study. The arrowheads indicate the anterior boundaries of relatively continuous domains of epidermal Hox expression (not including some isolated labeled cells located more anteriorly in the epidermis of RA-treated embryos). (A–C) Expression of AmphiHox1 in control (A), RA-treated (B), and BMS009-treated (C) neurulae. (D–F) Expression of amphioxus AmphiHox3 in control (D), RA-treated (E), and BMS009-treated (F) embryos. (G–I) Expression of AmphiHox4 in control (G), RA-treated (H), and BMS009-treated (I) neurulae.
During amphioxus embryology, RA treatments affect epidermal Hox genes in two ways: gene expression is both up-regulated and extended anteriorly along the anteroposterior axis of the body. Anterior to the region of contiguously expressing epidermal cells (with anterior boundary marked by arrowheads in Fig. 4 B, E, and H), there are also a few additional cells scattered in the epidermis that express these Hox genes relatively strongly. In comparison with RA treatments, BMS009 (Fig. 4 C, F, and I) has the converse effect of suppressing epidermal Hox expression; however, this suppression is so strong that possible shifts in the expression domains (even if they occur) cannot be visualized by in situ hybridization.
Amphioxus embryos also express AmphiHox6 in the epidermis, but transcription does not begin until 24 h of development (data not shown). In the normal 24-h embryo, the zone of epidermal cells expressing AmphiHox6 is limited to the posterior tenth of the body. Vertebrate homologs of this amphioxus gene are expressed posterior to the developing head (30, 31), and, because the earlier embryonic stages of amphioxus are thought to constitute mainly a head region (32), expression of AmphiHox6 seems to be delayed until postneurula stages when the trunk/tail region begins to elongate posteriorly. AmphiHox6 also responds to RA or BMS009 treatments like the other amphioxus Hox genes studied here. RA treatment up-regulates transcription of the gene and expands its expression domain anteriorly, whereas BMS009 treatment down-regulates its expression to undetectable levels (data not shown).
Discussion
Numerous studies of vertebrate embryos implicate a combinatorial Hox code in global-scale, anteroposterior patterning of developing tissues (33), including the epidermis (34, 35). For vertebrates, much is also known about how RA signaling relates to Hox code patterning, but chiefly for the central nervous system (6) and skeleton (36). In contrast, for the developing vertebrate epidermis, the influence of RA on Hox expression has so far been considered for only one Hox gene at a time (37–39). Such studies are thus not comprehensive enough to establish that global-scale patterning of the vertebrate epidermis is influenced by an RA-dependent Hox code. The effect of exogenous RA on epidermal expression has also been studied for only a single Hox gene (Hox1) of ascidian tunicates (40). This tunicate study showed that increased RA levels up-regulate the gene and shift its anterior expression boundary anteriorly.
Despite the limited data available for vertebrate embryos, there are two reasons for suspecting that RA levels may control a Hox code in the developing epidermis (at least in the cranial portion of the body). First, RA response elements are present in the regulatory regions of Hox genes generally (41). Moreover, RA signaling can influence the anteroposterior positioning and/or cytodifferentiation of some placodes in the developing vertebrate epidermis (10–12), although Hox genes were not considered in these studies.
We find that amphioxus Hox genes are expressed in the developing epidermis in a colinear, nested manner and that their expression domains are influenced by exogenous RA or RA antagonist. This raises the possibility that RA signaling in amphioxus might act through Hox genes to regionalize the epidermal ectoderm and to influence the distribution of epidermal sensory neurons along the anteroposterior axis of the embryo. The notion that the developing amphioxus epidermis may have its own intrinsic Hox code that is influenced by RA signaling is especially interesting because of similarities between the patterning of epidermal sensory neurons of amphioxus and the central nervous systems of amphioxus (23) and vertebrates (6, 42). The anterior limits of the expression domains of AmphiHox1, -3, and -4 in the epidermis are roughly in register with those of the same genes in the amphioxus neural tube (27, 28) and resemble the anterior limits of the expression domains of their corresponding homologs in the vertebrate hindbrain.
On a second, more limited spatial scale, RA signaling and Hox genes may play a role in the cytodifferentiative steps that subdivide populations of nerve cell precursors into neuronal subtypes. The role of RA in specifying subtypes of cells has been discussed for the vertebrate central nervous system (42, 43), retina (44), limb bud mesenchyme (45), heart (46), kidney (47), lung (48), and skin (49, 50). Similarly, for amphioxus, the present results suggest that RA (signaling through Hox genes) may also be involved in the differentiation of neurons into subtypes within the epidermal ectoderm. This more restricted role for Hox would be a specific instance of the general evolutionary phenomenon that chordate Hox genes have tended to be recruited from their original global patterning role to take on additional functions in the differentiation of particular tissue types (49). Hox expression in this category tends to begin somewhat later in development and may be spatially unrestricted along the anteroposterior axis of the embryo (50).
In amphioxus, the subtypes of epidermal neurons presumably differ among themselves in axonal trajectories and possibly also in sensory modalities. Recent work on the developing vertebrate hindbrain and spinal cord have demonstrated a role for Hox genes in subdividing larger neuronal populations into neuronal subtypes (51–54). Our present results show that four amphioxus Hox genes and two neuronal differentiation genes (amphioxus islet and AmphiERR) are expressed in nonoverlapping patterns in different epidermal sensory neurons. This finding suggests that such genes may be part of a combinatorial code that subdivides the epidermal sensory cell population as a whole into diverse neuronal subtypes.
The relationship between the epidermal sensory neurons of amphioxus and the chordate central nervous system is also interesting from a functional standpoint. For the proper functioning of the chordate nervous system as a whole, the developing sensory neurons must project accurately to specific targets in the central nervous system (55). For developing amphioxus, axons from the most anterior of these sensory neurons have been reconstructed from three-dimensional transmission electron microscopy and found to synapse with motoneurons in the nerve cord and with notochord cells (56). In contrast, nothing definite is yet known about the sensory modalities of the epidermal sensory cells of amphioxus, although it has been speculated that they function in mechanoreception and/or chemoreception (57). Recent work on vertebrates has indicated that sensory neurons do not have their fates determined late by target interactions, as once thought, but are subdivided into subtypes very soon after they begin to differentiate (58). This model is in general agreement with our proposal that amphioxus sensory neurons are subdivided into subtypes soon after they begin to differentiate within the epidermal ectoderm.
In sum, both amphioxus and vertebrates evidently pattern their developing epidermis (at least in the cephalic region) with a Hox code. In amphioxus this epidermal Hox code appears to be RA-dependent. The presence of a similar RA-dependent Hox code in the developing vertebrate epidermis remains a definite possibility. However, for vertebrates, the degree of autonomy of epidermal development needs further study because the positioning of epidermal neurons may be influenced strongly by factors from the nearby central nervous system and/or mesoderm (10–12). If epidermal development turns out to be more autonomous in amphioxus than in vertebrates, it could reflect either an independent loss or a gain of autonomy, respectively, in the vertebrate or amphioxus lines of descent.
Additional evolutionary insights may be gained from comparing development of the amphioxus epidermis with development of the amphioxus central nervous system. The similarities in the early developmental mechanisms structuring these two amphioxus tissues may have their evolutionary origins in a more basal deuterostome resembling a modern acorn worm (enteropneust hemichordate). Acorn worms lack a clear distinction between a peripheral and central nervous system, and they evidently pattern their entire epidermis (their “skin brain”) with mechanisms that include colinear nested Hox expression (20, 21). It would be very interesting to test the effects of exogenous RA and RA antagonist on morphology and gene expression of developing acorn worms.
In the course of deuterostome evolution, there appears to have been a progressive condensation of the pervasive epidermal nervous system into a central nervous system on the dorsal side. In amphioxus (and by extension in the invertebrate ancestor of vertebrates), the molecular mechanisms patterning the general epidermal plexus have tended to be transferred to the relatively condensed central nervous system; this finding could account for the similar roles for RA signaling and Hox genes during neural development in these two tissues. In the developing vertebrates, it is possible that an RA-influenced Hox code helps pattern elements of the peripheral nervous system in the epidermis. However, further work is needed to determine whether the developing vertebrate epidermis is patterned relatively autonomously or more indirectly, under the influence of molecular patterning mechanisms that have come to reside in the central nervous system.
Acknowledgments
We thank John M. Lawrence for providing laboratory facilities at the University of South Florida in Tampa, Hinrich Gronemeyer (Institut de Génétique et de Biologie Moleculaire et Cellulaire, Strasbourg, France) for supplying BMS009, Evelyn York for helping with SEM, and Jr-Kai Yu for critical reading of the manuscript. We are also grateful to Jordi Garcia-Fernàndez, Peter W. H. Holland, William R. Jackman, Sebastian M. Shimeld, and Jean-Marc Vanacker for providing clones of amphioxus genes. This work was supported by a European Community Marie Curie Fellowship (to M.S.), National Science Foundation Research Grant IBN-00-78599 (to N.D.H. and L.Z.H.), and grants from the Ministère de l'Éducation Nationale de la Recherche et de la Technologie, Centre National de la Recherche Scientifique, and the Association pour la Recherche sur le Cancer Région Rhône-Alpes (to V.L.).
Abbreviations: RA, retinoic acid; SEM, scanning electron microscopy/microscope.
References
- 1.Shimeld, S. M. (1996) BioEssays 18, 613–616. [Google Scholar]
- 2.Balmer, J. E. & Blomhoff, R. (2002) J. Lipid Res. 43, 1773–1808. [DOI] [PubMed] [Google Scholar]
- 3.Escriva, H., Holland, N. D., Gronemeyer, H., Laudet, V. & Holland, L. Z. (2002) Development (Cambridge, U.K.) 129, 2905–2916. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara, S. & Kawamura, K. (2003) Zool. Sci. 20, 809–818. [DOI] [PubMed] [Google Scholar]
- 5.Kelsh, R. N. & Raible, D. W. (2002) Results Probl. Cell Differ. 40, 216–236. [DOI] [PubMed] [Google Scholar]
- 6.Maden, M. (2002) Nat. Rev. Neurosci. 3, 843–853. [DOI] [PubMed] [Google Scholar]
- 7.Yelon, D. & Stainier, D. Y. R. (2002) Curr. Biol. 12, R707–R709. [DOI] [PubMed] [Google Scholar]
- 8.Matt, N., Ghyselinck, N. B., Wendling, O., Chambon, P. & Mark, M. (2003) Development (Cambridge, U.K.) 130, 2083–2093. [DOI] [PubMed] [Google Scholar]
- 9.Hochgreb, T., Linhares, V. L., Menezes, D. C., Sampaio, A. C., Yan, C. Y., Cardoso, W. V., Rossenthal, N. & Xavier-Neto, J. (2003) Development (Cambridge, U.K.) 130, 5363–5374. [DOI] [PubMed] [Google Scholar]
- 10.White, J. C., Highland, M., Kaiser, M. & Clagett-Dame, M. (2000) Dev. Biol. 220, 263–284. [DOI] [PubMed] [Google Scholar]
- 11.Phillips, B. T., Bolding, K. & Riley, B. B. (2001) Dev. Biol. 235, 351–365. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs, M. A. & Northcutt, R. G. (2004) Int. J. Dev. Biol. 48, 63–66. [DOI] [PubMed] [Google Scholar]
- 13.Panopoulou, G., Hennig, S., Groth, D., Krause, A., Poutska, A. J., Herwig, R., Vingron, M. & Lehrach, H. (2003) Genome Res. 13, 1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holland, L. Z. & Holland, N. D. (2001) J. Anat. 199, 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland, N. D. & Yu, J. K. (2002) Acta Zool. (Stockholm) 83, 309–319. [Google Scholar]
- 16.Stokes, M. D. & Holland, N. D. (1995) Acta Zool. (Stockholm) 76, 105–120. [Google Scholar]
- 17.Mazet, F., Masood, S., Luke, G. N., Holland, N. D. & Shimeld, S. M. (2004) Genesis 38, 58–65. [DOI] [PubMed] [Google Scholar]
- 18.Satoh, G., Wang, Y., Zhang, P. J. & Satoh, N. (2001) J. Exp. Zool. 291, 354–364. [DOI] [PubMed] [Google Scholar]
- 19.Holland, L. Z. & Gibson-Brown, J. J. (2003) BioEssays 25, 529–532. [DOI] [PubMed] [Google Scholar]
- 20.Lowe, C. J., Wu, M., Salic, A., Evans, L., Lander, E., Stange-Thomann, N., Gruber, C. E., Gerhart, J. & Kirschner, M. (2003) Cell 113, 853–865. [DOI] [PubMed] [Google Scholar]
- 21.Holland, N. D. (2003) Nat. Rev. Neurosci. 4, 617–627. [DOI] [PubMed] [Google Scholar]
- 22.Holland, N. D. & Holland, L. Z. (1993) in Essential Developmental Biology: A Practical Approach, eds. Stern, C. D. & Holland, P. W. H. (IRL, Oxford), pp. 21–32.
- 23.Holland, L. Z. & Holland, N. D. (1996) Development (Cambridge, U.K.) 122, 1829–1838. [DOI] [PubMed] [Google Scholar]
- 24.Holland, L. Z., Holland, P. W. H. & Holland, N. D. (1996) in Molecular Zoology: Advances, Strategies and Protocols, eds. Ferraris, J. D. & Palumbi, S. R. (Wiley, New York), pp. 267–282, 473–483.
- 25.Dubois, L. & Vincent, A. (2001) Mech. Dev. 108, 3–12. [DOI] [PubMed] [Google Scholar]
- 26.Jackman, W. R., Langeland, J. A. & Kimmel, C. B. (2000) Dev. Biol. 220, 16–26. [DOI] [PubMed] [Google Scholar]
- 27.Holland, P. W. H. & Garcia-Fernàndez, J. (1996) Dev. Biol. 173, 382–395. [DOI] [PubMed] [Google Scholar]
- 28.Wada, H., Garcia-Fernàndez, J. & Holland, P. W. H. (1999) Dev. Biol. 213, 131–141. [DOI] [PubMed] [Google Scholar]
- 29.Cohn, M. J. (2002) Nature 416, 386–387. [DOI] [PubMed] [Google Scholar]
- 30.Sharpe, P. T., Miller, J. R., Evans, E. P., Burtenshaw, M. D. & Gaunt, S. J. (1988) Development (Cambridge, U.K.) 102, 397–407. [DOI] [PubMed] [Google Scholar]
- 31.Burke, A. C., Nelson, C. E., Morgan, B. A. & Tabin, C. (1995) Development (Cambridge, U.K.) 121, 333–346. [DOI] [PubMed] [Google Scholar]
- 32.Gilland, E. & Baker, R. (1993) Acta Anat. 148, 110–123. [DOI] [PubMed] [Google Scholar]
- 33.Kmita, M. & Duboule, D. (2003) Science 301, 331–333. [DOI] [PubMed] [Google Scholar]
- 34.McGinnis, W. & Krumlauf, R. (1992) Cell 68, 283–302. [DOI] [PubMed] [Google Scholar]
- 35.Couly, G., Grapin-Botton, A., Coltey, P., Ruhin, B. & Le Douarin, N. M. (1998) Development (Cambridge, U.K.) 125, 3445–3459. [DOI] [PubMed] [Google Scholar]
- 36.Kessel, W. & Gruss, P. (1991) Cell 67, 88–104. [DOI] [PubMed] [Google Scholar]
- 37.Sive, H. L. & Cheng, P. F. (1991) Genes Dev. 5, 1321–1332. [DOI] [PubMed] [Google Scholar]
- 38.Sundin, I. O. & Eichele, G. (1992) Development (Cambridge, U.K.) 114, 841–852. [DOI] [PubMed] [Google Scholar]
- 39.Kolm, P. J. & Sive, H. L. (1995) Dev. Biol. 167, 34–49. [DOI] [PubMed] [Google Scholar]
- 40.Katsuyama, Y., Wada, S., Yasugi, S. & Saiga, H. (1995) Development (Cambridge, U.K.) 121, 3197–3205. [DOI] [PubMed] [Google Scholar]
- 41.Manzanares, M., Wada, H., Itasaki, N., Trainor, P. A., Krumlauf, R. & Holland, P. W. H. (2000) Nature 408, 854–857. [DOI] [PubMed] [Google Scholar]
- 42.Guidato, S., Barrett, C. & Guthrie, S. (2003) Mol. Cell. Neurosci. 23, 81–95. [DOI] [PubMed] [Google Scholar]
- 43.Sockanathan, S., Perlmann, T. & Jessell, T. M. (2003) Neuron 40, 97–111. [DOI] [PubMed] [Google Scholar]
- 44.Eagleson, G. W., Johnson-Meeter, L. J. & Frideres, J. (2001) Dev. Dyn. 221, 350–364. [DOI] [PubMed] [Google Scholar]
- 45.Weston, A. D., Hoffman, L. M. & Underhill, T. M. (2003) Birth Defects Res. C Embryo Today 69, 156–173. [DOI] [PubMed] [Google Scholar]
- 46.Xavier-Neto, J., Rosenthal, N., Silva, F. A., Freitas Matos, T. G, Hochgreb, T. & Ferreira Linhares, V. L. (2001) Genesis 31, 97–104. [DOI] [PubMed] [Google Scholar]
- 47.Batourina, E., Gim, S., Bello, N., Shy, N., Clagett-Dame, M., Srinivas, S., Costantini, F. & Mendelsohn, C. (2001) Nat. Genet. 27, 74–84. [DOI] [PubMed] [Google Scholar]
- 48.Chazaud, C., Dolle, P., Rossant, J. & Mollard, R. (2003) Mech. Dev. 120, 691–700. [DOI] [PubMed] [Google Scholar]
- 49.Duboule, D. (1997) Genes Dev. 12, 1–4. [DOI] [PubMed] [Google Scholar]
- 50.Reid, A. I. & Gaunt, S. J. (2002) Int. J. Dev. Biol. 46, 209–215. [DOI] [PubMed] [Google Scholar]
- 51.Cooper, K. L., Leisenring, W. M. & Moens, C. B. (2003) Dev. Biol. 253, 200–213. [DOI] [PubMed] [Google Scholar]
- 52.Dasen, J. S., Liu, J. P. & Jessell, T. M. (2003) Nature 425, 926–933. [DOI] [PubMed] [Google Scholar]
- 53.Gaufo, G. O., Thomas, K. R. & Capecchi, M. R. (2003) Development (Cambridge, U.K.) 130, 5191–5201. [DOI] [PubMed] [Google Scholar]
- 54.Pattyn, A., Vallstedt, A., Dias, J. M., Samad, O. A., Krumlauf, R., Rijli, F. M., Burnet, J. F. & Ericson, J. (2003) Genes Dev. 17, 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson, D. J. (1999) Curr. Opin. Neurobiol. 9, 517–524. [DOI] [PubMed] [Google Scholar]
- 56.Lacalli, T. C. (2002) Acta Zool. (Stockholm) 83, 149–166. [Google Scholar]
- 57.Baatrup, E. (1981) Acta Zool. (Stockholm) 62, 147–157. [Google Scholar]
- 58.Guan, W., Puthenveedu, M. A. & Condic, M., L. (2003) J. Neurosci. 23, 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]