Abstract
Optical coherence tomography (OCT), a noninvasive imaging modality that uses low-coherence light to obtain a high-resolution cross-section of biological structures, has evolved dramatically over the years. The Swept-source OCT (SS-OCT) makes use of a single detector with a rapidly tunable laser as a light source. The Casia SS-1000 OCT is a Fourier-domain, SS-OCT designed specifically for imaging the anterior segment. This system achieves high resolution imaging of 10΅m (Axial) and 30΅m (Transverse) and high speed scanning of 30,000 A-scans per second. With a substantial improvement in scan speed, the anterior chamber angles can be imaged 360 degrees in 128 cross sections (each with 512 A-scans) in about 2.4 seconds. We summarize the clinical applications of anterior segment SS-OCT in Glaucoma. Literature search: We searched PubMed and included Medline using the phrases anterior segment optical coherence tomography in ophthalmology, swept-source OCT, use of AS-OCT in glaucoma, use of swept-source AS-OCT in glaucoma, quantitative assessment of angle, filtering bleb in AS-OCT, comparison of AS-OCT with gonioscopy and comparison of AS-OCT with UBM. Search was made for articles dating 1990 to August 2015.
Keywords: Fourier-domain optical coherence tomography, frequency-domain optical coherence tomography, optical frequency-domain imaging, swept-source optical coherence tomography, time-domain optical coherence tomography
Introduction
Optical coherence tomography (OCT), a noninvasive imaging modality that uses low-coherence light to obtain a high-resolution cross-section of biological structures, is changing the field of ophthalmology. The technology has evolved dramatically since its first description by Huang et al. in 1991.[1] The first-generation OCT system was based on a time-domain OCT (TD-OCT) imaging method that relies on a moving reference mirror to scan each depth position in the image pixel. This mechanical scanning process limits the rate at which images can be acquired. The most significant leap forward occurred when the moving reference mirror used during the collection of TD-OCT data was abandoned in favor of Fourier analysis of collected data. These systems are known as frequency-domain OCT (FD-OCT), Fourier-domain OCT, swept-source OCT (SS-OCT), or optical frequency-domain imaging.[2,3] In FD-OCT imaging methods, interferometric data are measured as a function of optical wavelength and time, rather than as a function of time alone, based on the introduction of a fixed reference mirror and a tunable laser light source with a sweep range of 1250–1370 nm, instead of the broadband light source used in TD-OCT imaging systems.
The SS-OCT makes use of a single detector with a rapidly tunable laser as a light source. The CASIA SS-1000 OCT (Tomey, Nagoya, Japan) is a Fourier-domain, SS-OCT designed, specifically for imaging the anterior segment. This system achieves high-resolution imaging of 10 μm (axial) and 30 μm (transverse) and high-speed scanning of 30,000 A-scans per second. With a substantial improvement in scan speed, the anterior chamber angles can be imaged 360° in 128 cross-sections (each with 512 A-scans) in about 2.4 s.
Indications
The CASIA is indicated for cross-sectional imaging of the anterior segment components of the human eye such as cornea, anterior chamber, and bleb segment of the sclera, and also for dimension measurements of these such as curvature, length, area, and volume by computed analysis [Figure 1].
Figure 1.
Photograph of a CASIA SS-1000 OCT unit
Technique
The image acquisition in anterior segment OCT (AS-OCT) is similar to traditional OCT using modes such as corneal, anterior segment, pachymetry, high-resolution corneal, high-resolution anterior segment and enhanced anterior segment imaging. The examination is carried out with the patient in a sitting position. As it is a noncontact procedure, topical anesthesia is not required. Wide palpebral apertures are required to get a scan unobstructed by the eyelids. This is particularly important for vertical scans, and it may sometimes be necessary to lift the eyelid during the scan. The default mode provides an internal fixation. The patient is asked to look into the imaging aperture and focus on the center of fixation target. The patient's distance refractive correction is entered; the eye is aligned, and the desired scan is taken. The images are subsequently analyzed.
Types of scans
Anterior segment: Measure the cornea as high-resolution
Angle analysis: Measure the angle and the lens as high-resolution
Angle HD: Measure the angle with peripheral fixation light
Bleb: Measure the bleb
Corneal map: Measure the cornea like a topographer.
The AS-OCT image is either a grayscale or a false-color two-dimensional representation of backscattered light intensity in a cross-sectional plane or a colored rainbow scheme. The default grayscale scheme is recommended as it relates directly to the tissue reflectivity. Other color schemes may aid in the delineation of certain structures, depending on the application and interpretation by the user; care should be taken; however, so that these alternate color schemes do not artificially create tissue boundaries. High-resolution images of the cornea and angle region including iris root, angle recess, anterior ciliary body, scleral spur (SS), and, in some eyes, canal of Schlemm are possible.
Normal Anterior Segment Optical Coherence Tomography
Normal ocular structures
AS-OCT imaging modality allows visualization of the entire anterior segment structures in a single image and also to perform precise quantitative measurements of these structures. The clinical applications of AS-OCT imaging depend on the ability to visualize and assess the anatomic relationships of the angle and anterior segment structures including the cornea and to perform quantitative estimations of their measurements.
Generally, for the quantitative assessment of the different anterior segment parameters, prior identification of a landmark is necessary. The SS is one such anatomical landmark that is customarily used. It also serves as a landmark for the trabecular meshwork, which cannot be easily distinguished on AS-OCT images. The trabecular meshwork is located approximately 250–500 μm anterior to SS along the angle wall.[4] In AS-OCT images, SS is identified as the point where there is a change in curvature of the inner surface of the angle wall, often appearing as an inward protrusion of the sclera [Figure 2].[5] Sometimes, it appears as a highly reflective region.
Figure 2.
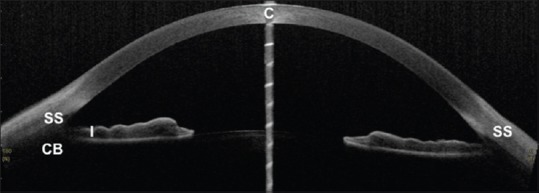
Anterior segment optical coherence tomography image showing scleral spur (SS), Iris (I), and ciliary body (CB) area
Quantitative parameters
The quantitative parameters [Figures 3-7] that can be determined from an AS-OCT image include angle, anterior chamber, iris, lens, and corneal parameters.
Figure 3.
SS-OCT showing measurement of Angle opening distance at 500 μm (AOD500) and 750 μm (AOD750) respectively
Figure 7.
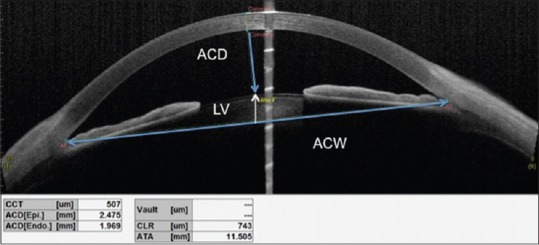
CASIA SS-OCT anterior segment analysis showing the measurements of CCT: Central corneal thickness, ACD: Anterior chamber depth; ACw: Anterior chamber width and LV: Lens vault
Angle parameters
Angle opening distance at 500 μm from the SS (AOD500)[6] is defined as the perpendicular distance between the trabecular meshwork and the iris at 500 μm anterior to the SS. AOD250 and AOD750 are at 250 μm and 750 μm from the spurs, respectively [Figure 3].
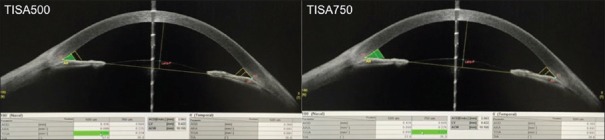
Trabecular iris space area at 500 μm from the SS (TISA500)[7] is defined as the trapezoidal area with the following boundaries: Anterior, the AOD500; posterior, a line drawn from the SS perpendicular to the plane of the inner scleral wall to the opposing iris; superior, the inner corneoscleral wall; and inferior, the iris surface. TISA750 is at 750 μm from the SS [Figure 4].
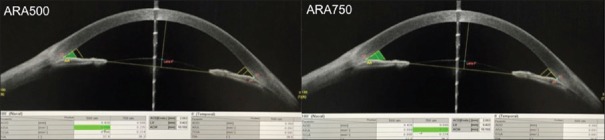
Angle recess area at 500 μm from the SS (ARA500)[8] is defined as the triangular area bounded by AOD500 (the base), angle recess (the apex), iris surface, and inner corneoscleral wall (sides of triangle) [Figure 5].
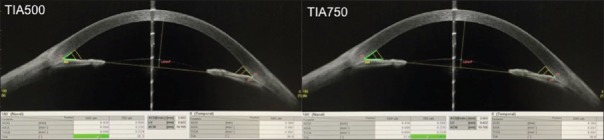
Trabecular Iris Angle at at 500 μm from the scleral spur (TIA500) is defined as an angle measured with the apex in the iris recess and the arms of the angle passing through a point on the trabecular meshwork 500 μm from the scleral spur and the point on the iris perpendicularly [Figure 6].
Figure 4.
SS-OCT showing measurement of Trabecular iris space area at 500 μm (TISA500) and 750 μm (TISA750) respectively
Figure 5.
SS-OCT showing measurement of Angle recess area at 500 μm (ARA500) and 750 μm (ARA750) respectively
Figure 6.
SS-OCT showing measurement of Trabecular iris angle at 500 μm (TIA500) and 750 μm (TIA750) respectively
TISA is considered clinically superior to the AOD and ARA. As AOD is simply a perpendicular line from the trabecular meshwork to a point on the iris, its magnitude is therefore likely to be affected by the iris contour. TISA, on the other hand, overcomes this possible source of error as it takes into account the entire iris contour. In addition, ARA incorporates the area posterior to the SS that represents the nonfiltering region of the angle, whereas TISA is a modification of the ARA and excludes the area posterior to the SS. It is presumed to better represent the filtering area of the angles. Furthermore, TISA is better correlated with gonioscopy compared to AOD or ARA.[9]
Anterior chamber parameters
Anterior chamber width[9] is defined as the spurs to spur distance
Anterior chamber depth (ACD) is defined as the distance from the corneal endothelium to the anterior surface of the lens [Figure 7]
Anterior chamber area[10] is defined as the cross-sectional area of anterior segment bounded by endothelium, anterior surface of iris, and anterior surface of lens (within the pupil).
Iris parameters
Iris thickness 750 and 2000[11] are defined as the iris thickness measured at 750 and 2000 μm from the SS, respectively
Iris curvature (ICurv):[11] To calculate ICurv, the software draws a line from the most peripheral to the most central points of iris pigment epithelium, and then a perpendicular line is extended from this line to the iris pigment epithelium at the point of greatest convexity. The perpendicular line is a measure of the ICurv
Iris area:[11] It is calculated as the cross-sectional area of the iris.
Lens
Lens vault[12] is defined as the perpendicular distance between the anterior pole of the crystalline lens and the horizontal line joining the two SSs [Figure 7].
Corneal parameters
Corneal thickness measurements can be obtained from cross-sectional AS-OCT images. Analysis of flap thickness and residual stromal bed in post-laser-assisted in situ keratomileusis (LASIK) patients can be performed on the high-resolution corneal scans.
Qualitative Analysis
Iridotrabecular contact
In AS-OCT, iridotrabecular contact (ITC) is defined as any contact between the iris and angle wall anterior to the SS. An anterior chamber angle quadrant is considered closed on the AS-OCT in the presence of ITC, while on gonioscopy, a quadrant is considered closed if the posterior pigmented trabecular meshwork is not visualized in the primary position without indentation.[13] It has been found that the AS-OCT detected more closed angles than gonioscopy. The differences in definition of angle closure and description of landmarks may explain the disparate findings between AS-OCT and gonioscopy.[13]
Iridotrabecular contact index (ITC index)
The CASIA SS-OCT (Tomey Corporation, Nagoya, Japan) allows image acquisition of the entire circumference of the anterior chamber angle. The inbuilt semiautomated software of the CASIA can be used to measure the ITC index which is a quantitative measure of the extent of angle closure across 360° of the angles expressed as a percentage. The ITC analysis uses the anterior segment cross-sectional meridional images (which are not corrected for index of refraction) to determine the extent of contact between the iris and angle wall.
Evaluation of iridotrabecular contact index
To determine the ITC index, the SS and the ITC endpoint (EP) are manually marked for both quadrants of the anterior image frames. The SS is identified as the point where there is a change in curvature of the corneoscleral interface. The EP is identified as the most anterior point of contact of the iris to the angle wall [Figure 8a]. Upon marking all the frames, the ITC index can be calculated by clicking on the “ITC” button. The minimum number of frames that needs to be marked for evaluation of the ITC index is 8. The other inbuilt options for frame selection are 16, 32, 64, and 128. The software automatically selects the image frames to be analyzed depending on the number of frames chosen by the examiner, such that the images chosen are placed evenly apart.
Figure 8.
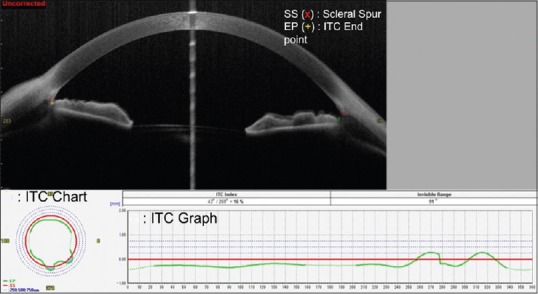
Illustration of the evaluation of Iridotrabecular contact (ITC) on CASIA. (a) Single frame cross-sectional image with the scleral spur (SS) and iris-trabecular contact end-point (EP) marked by an observer grading the image. (b) The ITC chart with the blue area illustrating the amount and distribution of ITC. (c) The ITC graph with the green line above the red line (representing the SS) depicting the amount of angle closure, measured as the ITC index
Output and interpretation
The results of the ITC analysis are depicted in the ITC chart [Figure 8b] and the ITC graph [Figure 8c]. The ITC chart is analogous to a goniogram and allows easier interpretation of the extent of angle closure. The red, circular line represents the SS, and the circumferential dotted lines represent 0.25, 0.50, and 0, respectively, of the distance anterior to the SS along the angle wall. The blue area indicates the extent and distribution of the ITC. The ITC graph also illustrates the circumferential extent of the ITC, with the Y-axis representing the ITC (in arbitrary units) and the X-axis representing the degree of the angle. Angle closure or positive ITC is shown above the red line while open angles or negative ITC is shown below the line.
Comparison with gonioscopy
The ITC index was found to have moderate to good agreement with gonioscopic angle closure at 0.69 and 0.71 for ITC index cutoffs of >35% and ≥50%, respectively.[14] The ITC index also identifies more closed angles than gonioscopy; with possible reasons being the inadvertent compression of the globe and excessive exposure to visible light during gonioscopy resulting in spurious opening of the angles.[14]
Three-dimensional imaging/gonioscopic view
The image can be displayed as three-dimensional (3D) image. Gonioscopic view can display the image similar to gonio lens image [Figure 9].
Figure 9.
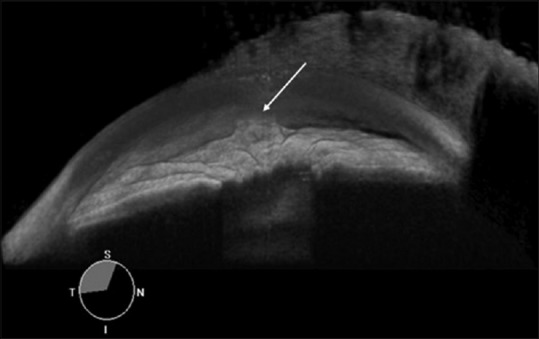
Three-dimensional gonioscopic view of a case of primary angle closure glaucoma with peripheral anterior synechiae in situ (arrow)
Corneal map
Corneal shape is analyzed like a topographer. Corneal map has several mode similar to dual map, different map, and so on (measurement range: 10.2 mm, 16 lines, and 32 directions) [Figure 10].
Figure 10.
CASIA image illustrating the Corneal Map
Map type:
Axial (keratometric/anterior/posterior/real)
Refractive power pachymetry
Instantaneous (keratometric/anterior/posterior/real)
Elevation (anterior/posterior).
Bleb analysis
SS-OCT is used to visualize not only bleb morphology but also bleb area and volume [Figure 11].
Figure 11.
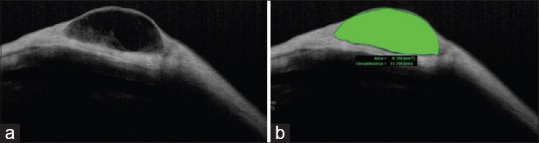
CASIA SS-OCT image of a filtering bleb (a), also illustrating bleb area and volume (b)
Case Records
Primary angle closure glaucoma
AS-OCT can identify and document occludable angles and, in eyes with angle closure, can identify the presence and extent of peripheral anterior synechiae (PAS). Anterior chamber is defined as closed on AS-OCT by the presence of contact between the iris and angle wall anterior to the SS. The diagnostic performance of AS-OCT for detecting eyes with angle closure varies according to the adapted scanning protocol
Primary angle closure glaucoma - showing closed angles [Figure 12]
Post-laser peripheral iridotomy (LPI) - changes in the angle morphology can be imaged and objectively quantified. Pre- and post-LPI AS-OCT showing opening up of narrow angles after the procedure [Figure 13]. Laser iridotomy leads to reduction of iris convexity and widening of angles.
Figure 12.
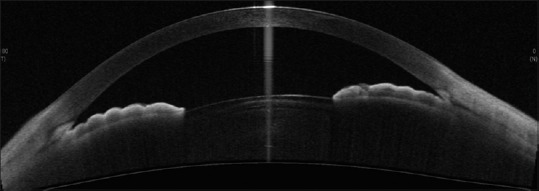
CASIA SS-OCT showing closed angles in a case of PACG
Figure 13.
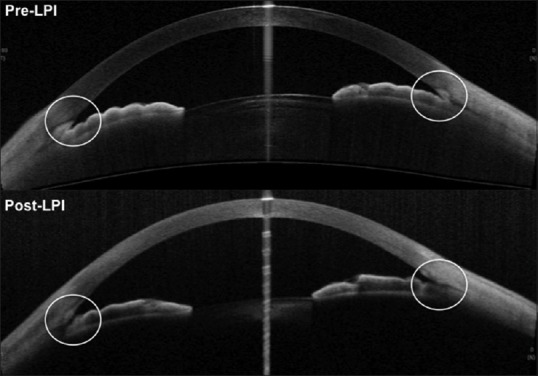
Pre and post LPI (laser peripheral iridotomy) SS-OCT showing opening up of narrow angles after the procedure
Primary open angle glaucoma
Primary open angle glaucoma (POAG) with a clear view of the anterior chamber angle on ASOCT. An open anterior chamber angle recess and scleral spur ar clearly visible [Figure 14].
Figure 14.
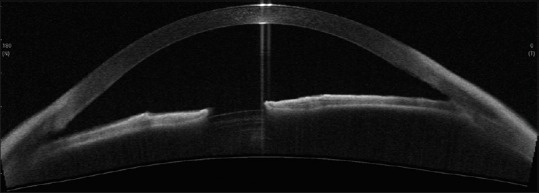
CASIA SS-OCT showing open angles in a case of POAG
Pigment dispersion syndrome
Pigment dispersion syndrome is associated with wide open angle and a typical posterior bowing of the peripheral iris causing reverse pupillary block [Figure 15].
Figure 15.
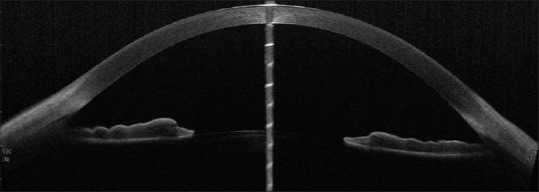
CASIA SS-OCT showing open angles in a case of PDS with posterior bowing of the peripheral iris
Bleb
Bleb morphology can indicate the functional outcome of the surgery. AS-OCT is used to visualize the bleb wall, cavity, flap, ostium status, and characterization of the bleb, flap location, cysts within the bleb, wall thickness, and bleb dimensions [Figures 17-19] and can identify failing blebs and the cause of failure.
Figure 17.

CASIA SS-OCT showing a failed bleb associated with ostium occlusion, apposition of conjunctiva-episclera to sclera, and apposition of scleral flap to its bed with absence of bleb wall thickening and cystic spaces
Figure 19.
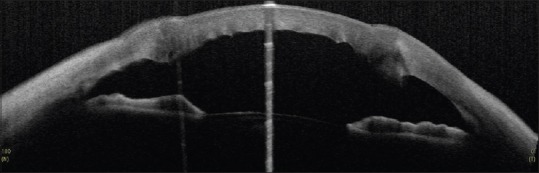
Anterior segment optical coherence tomography of a case of operated penetrating keratoplasty showing abnormal apposition at the graft-host junction
Functioning - successful blebs to be associated with bleb wall thickening [Figure 16]
Failed - failed blebs had ostium occlusion, apposition of conjunctiva-episclera to sclera, or apposition of sclera flap to its bed with absence of bleb wall thickening, features that would be difficult to see with the slit-lamp alone [Figure 17].
Figure 16.

CASIA SS-OCT showing a filtering bleb associated with bleb wall thickening and cystic spaces
Corneal opacities
Slit-lamp evaluation of the anterior chamber is limited by corneal opacity. AS-OCT allows visualization of anterior segment structures behind opaque corneas [Figure 18]. Memarzadeh et al.[15] evaluated secondary glaucoma with corneal opacity after penetrating keratoplasty and found the causes to be iridolenticular adhesion, PAS leading to angle closure, and blockage of the tip of aqueous drainage tube by PAS. They found AS-OCT to be as useful as ultrasonographic biomicroscopy (UBM) with the advantage of being a noncontact procedure. Dada et al.[16] used AS-OCT in opaque grafts with secondary glaucoma following tectonic penetrating keratoplasty for perforated corneal ulcers.
Figure 18.

Anterior segment optical coherence tomography of a case of adherent leukoma areas of iridocorneal touch with peripheral anterior synechiae
Post penetrating keratoplasty (PK)
In glaucoma following Penetrating Keratoplasty, ASOCT imaging can be used to evaluate the graft host junction, corneal thickness and can pick up irido-corneal adhesions [Figure 19]. It can also be used to guide placement of glaucoma drainage devices away from areas of peripheral anterior synechiae.
Implantable collamer lens
AS-OCT can be used in preoperative workup of phakic intraocular lenses (IOLs) and to follow these patients and guide management in case of complications [Figure 20].
Figure 20.
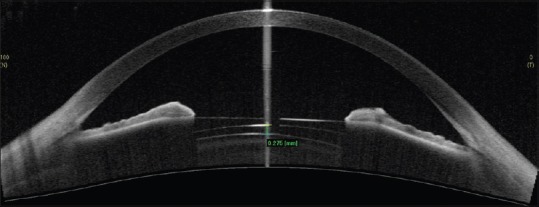
Anterior segment optical coherence tomography showing glaucoma drainage device tube in the ciliary sulcus, below the iris
Glaucoma drainage devices
AS-OCT provides high-resolution imaging for glaucoma drainage devices, which helps in knowing the position, patency, state of drainage, and intraluminal suture and can also show any tube-corneal or tube-iris touch [Figure 21].
Figure 21.
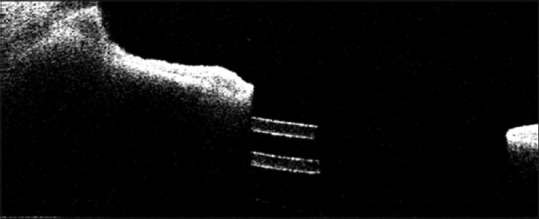
Anterior segment optical coherence tomography of a case of implantable collamer lens in situ with a normal vaulting
Effect of cataract surgery
Cataract surgery and IOL implantation lead to significant increases in ACD and anterior chamber angle [Figure 22].
Figure 22.
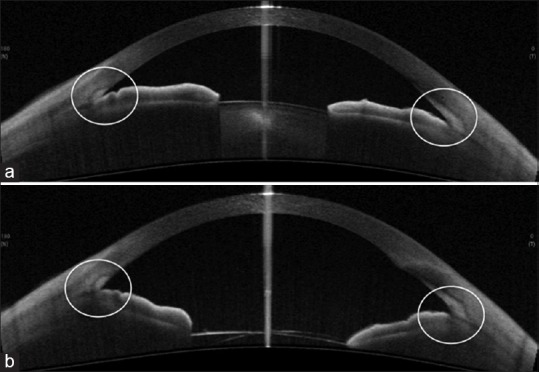
(a) Preoperative anterior segment optical coherence tomography of a case of primary angle closure glaucoma with cataract, showing narrow angles. (b) Postoperative anterior segment optical coherence tomography of the same patient following phacoemulsification showing wide open angles
Comparison with Gonioscopy
Gonioscopy remains the gold standard for evaluation of anterior segment and iridocorneal angle; it is a subjective, contact method, however potentially causing angle distortion. AS-OCT, on the other hand, is a noncontact procedure, has less inter-operator variability, requires less expertise, is easy to learn, and views the angle in its natural state because of the use of infrared light. The criteria for diagnosis of closed angle in gonioscopy and AS-OCT are different. Gonioscopy uses nonvisibility of posterior pigmented trabecular meshwork as the criteria for closed angle. AS-OCT defines closed angles as any contact between peripheral iris and angle wall anterior to the SS.[17] This has resulted in varied results in different studies comparing gonioscopy with AS-OCT for detection of closed angles. Sakata et al. have found a fair agreement (k = 0.40, 95% confidence interval, 0.35-0.45) between gonioscopy and AS-OCT for detecting angle closure.[13] They said that variations in the iris profile and the level of irido angle contact also may explain some of the differences seen between gonioscopy and AS-OCT. Park et al. found good agreement between gonioscopy and the Van Herick technique for assessment of narrow-angle, but both methods showed poor agreement with AS-OCT.[18] Wirbelauer et al. compared gonioscopy and AS-OCT and found a significant correlation with gonioscopy and AS-OCT findings.[19] Pekmezci found a nonlinear relationship between AS-OCT parameters and gonioscopy, the ARA having the highest correlation.[20] Nolan found that AS-OCT identified about 87% of occludable angle quadrants as occludable or closed.[21] More patients were found to have closed angle on AS-OCT than with gonioscopy.[22]
Ultrasonographic biomicroscopy
UBM and AS-OCT allow anterior ocular structure imaging,[23,24] but both have limitations. AS-OCT uses infra-red light and hence does not require a coupling fluid. As a noncontact procedure, AS-OCT can be used in the pediatric population and cases of suspected ocular injuries. UBM requires a coupling medium and is a contact procedure. Landmarks such as SS are more distinct on AS-OCT compared with UBM. AS-OCT is done in the sitting position and hence depicts a more physiological picture. UBM requires a supine position and hence causes falling back of the iris diaphragm, leading to a false opening of angle. The use of the UBM eyecup can also influence angle configuration.[25] The lateral resolution obtained with AS-OCT with the standard software is 30 μm and axial resolution is 10 μm, compared with 50 μm and 25 μm with the UBM. The posterior lens capsule can be examined using AS-OCT but not UBM. UBM allows imaging of a single quadrant at a time, but AS-OCT allows imaging of all the four quadrants at once. With AS-OCT, the fixation angle is the angle between the instrument's optical axis and the eye's line of sight. Keeping the fixation angle at 0, the exact location of the measured angle in degrees can be found with AS-OCT. This exact localization is not possible with UBM due to lack of reference point. AS-OCT is easier to perform and allows more rapid imaging in comparison to UBM.
Limitations
Limitations of AS-OCT include the inability to see the ciliary body and to see through opaque media. Therefore, it cannot be used for diagnosing some situations that require clear ciliary body imaging, such as plateau iris. Upper and lower eyelids obstruct the imaging of superior and inferior angles, making it difficult in the case of AS-OCT. UBM is advantageous in such cases. Third, in 3D gonioscopic views – the determination of synechial or appositional angle closure would not be possible as indentation/manipulation cannot be performed on AS-OCT.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlis P, Schmitt JM. Current and future developments in intracoronary optical coherence tomography imaging. EuroIntervention. 2009;4:529–33. doi: 10.4244/eijv4i4a89. [DOI] [PubMed] [Google Scholar]
- 3.Takarada S, Imanishi T, Liu Y, Ikejima H, Tsujioka H, Kuroi A, et al. Advantage of next-generation frequency-domain optical coherence tomography compared with conventional time-domain system in the assessment of coronary lesion. Catheter Cardiovasc Interv. 2010;75:202–6. doi: 10.1002/ccd.22273. [DOI] [PubMed] [Google Scholar]
- 4.Pavlin CJ, Harasiewicz K, Sherar MD, Foster FS. Ultrasound Biomicroscopy of the Eye. New York: Springer; 1995. [Google Scholar]
- 5.Sakata LM, Lavanya R, Friedman DS, Aung HT, Seah SK, Foster PJ, et al. Assessment of the scleral spur in anterior segment optical coherence tomography images. Arch Ophthalmol. 2008;126:181–5. doi: 10.1001/archophthalmol.2007.46. [DOI] [PubMed] [Google Scholar]
- 6.Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992;113:381–9. doi: 10.1016/s0002-9394(14)76159-8. [DOI] [PubMed] [Google Scholar]
- 7.Radhakrishnan S, Huang D, Smith SD. Optical coherence tomography imaging of the anterior chamber angle. Ophthalmol Clin North Am. 2005;18:375–81, vi. doi: 10.1016/j.ohc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa H, Esaki K, Liebmann JM, Uji Y, Ritch R. Ultrasound biomicroscopy dark room provocative testing: A quantitative method for estimating anterior chamber angle width. Jpn J Ophthalmol. 1999;43:526–34. doi: 10.1016/s0021-5155(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 9.Radhakrishnan S, Goldsmith J, Huang D, Westphal V, Dueker DK, Rollins AM, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005;123:1053–9. doi: 10.1001/archopht.123.8.1053. [DOI] [PubMed] [Google Scholar]
- 10.Nongpiur ME, Sakata LM, Friedman DS, He M, Chan YH, Lavanya R, et al. Novel association of smaller anterior chamber width with angle closure in Singaporeans. Ophthalmology. 2010;117:1967–73. doi: 10.1016/j.ophtha.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Wu RY, Nongpiur ME, He MG, Sakata LM, Friedman DS, Chan YH, et al. Association of narrow angles with anterior chamber area and volume measured with anterior-segment optical coherence tomography. Arch Ophthalmol. 2011;129:569–74. doi: 10.1001/archophthalmol.2011.68. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Sakata LM, Friedman DS, Chan YH, He M, Lavanya R, et al. Quantitative iris parameters and association with narrow angles. Ophthalmology. 2010;117:11–7. doi: 10.1016/j.ophtha.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Sakata LM, Lavanya R, Friedman DS, Aung HT, Gao H, Kumar RS, et al. Comparison of gonioscopy and anterior segment ocular coherence tomography in detecting angle closure in different quadrants of the anterior chamber angle. Ophthalmology. 2008;115:769–74. doi: 10.1016/j.ophtha.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Baskaran M, Ho SW, Tun TA, How AC, Perera SA, Friedman DS, et al. Assessment of circumferential angle-closure by the iris-trabecular contact index with swept-source optical coherence tomography. Ophthalmology. 2013;120:2226–31. doi: 10.1016/j.ophtha.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Memarzadeh F, Li Y, Francis BA, Smith RE, Gutmark J, Huang D. Optical coherence tomography of the anterior segment in secondary glaucoma with corneal opacity after penetrating keratoplasty. Br J Ophthalmol. 2007;91:189–92. doi: 10.1136/bjo.2006.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dada T, Shah BM, Bali SJ, Bansal N, Panda A, Vanathi M. Anterior segment OCT imaging in opaque grafts with secondary glaucoma following tectonic penetrating keratoplasty for perforated corneal ulcers. Eye (Lond) 2011;25:1522–4. doi: 10.1038/eye.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asrani S, Sarunic M, Santiago C, Izatt J. Detailed visualization of the anterior segment using fourier-domain optical coherence tomography. Arch Ophthalmol. 2008;126:765–71. doi: 10.1001/archopht.126.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SB, Sung KR, Kang SY, Jo JW, Lee KS, Kook MS. Assessment of narrow angles by gonioscopy, Van Herick method and anterior segment optical coherence tomography. Jpn J Ophthalmol. 2011;55:343–50. doi: 10.1007/s10384-011-0036-0. [DOI] [PubMed] [Google Scholar]
- 19.Wirbelauer C, Karandish A, Häberle H, Pham DT. Noncontact goniometry with optical coherence tomography. Arch Ophthalmol. 2005;123:179–85. doi: 10.1001/archopht.123.2.179. [DOI] [PubMed] [Google Scholar]
- 20.Pekmezci M, Porco TC, Lin SC. Anterior segment optical coherence tomography as a screening tool for the assessment of the anterior segment angle. Ophthalmic Surg Lasers Imaging. 2009;40:389–98. doi: 10.3928/15428877-20096030-07. [DOI] [PubMed] [Google Scholar]
- 21.Nolan W. Anterior segment imaging: Identifying the landmarks. Br J Ophthalmol. 2008;92:1575–6. doi: 10.1136/bjo.2008.146175. [DOI] [PubMed] [Google Scholar]
- 22.Nolan WP, See JL, Chew PT, Friedman DS, Smith SD, Radhakrishnan S, et al. Detection of primary angle closure using anterior segment optical coherence tomography in Asian eyes. Ophthalmology. 2007;114:33–9. doi: 10.1016/j.ophtha.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 23.Garcia JP, Jr, Rosen RB. Anterior segment imaging: Optical coherence tomography versus ultrasound biomicroscopy. Ophthalmic Surg Lasers Imaging. 2008;39:476–84. doi: 10.3928/15428877-20081101-02. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa H. Anterior segment imaging for glaucoma: OCT or UBM? Br J Ophthalmol. 2007;91:1420–1. doi: 10.1136/bjo.2007.121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa H, Inazumi K, Liebmann JM, Ritch R. Inadvertent corneal indentation can cause artifactitious widening of the iridocorneal angle on ultrasound biomicroscopy. Ophthalmic Surg Lasers. 2000;31:342–5. [PubMed] [Google Scholar]