Abstract
Thyroid hormones are involved in the regulation of many physiological processes and regulate gene transcription by binding to their nuclear receptors TRα and TRβ. In the absence of triiodothyronine (T3), the unliganded receptors (aporeceptors) do bind DNA and repress the transcription of target genes. The role of thyroid hormone aporeceptors as repressors was observed in hypothyroid adult mice, but its physiological relevance in nonpathological hypothyroid conditions remained to be determined. Here we show that, in the normal mouse fetus, TRα aporeceptors repress heart rate as well as the expression of TRβ and several genes encoding ion channels involved in cardiac contractile activity. Right after birth, when T3 concentration sharply increases, liganded TRα (holoreceptors) turn on the expression of some of these same genes concomitantly with heart rate increase. These data describe a physiological situation under which conversion of TRα from apo-receptors into holo-receptors, upon changes in T3 availability, plays a determinant role in a developmental process.
Thyroid hormones (THs) are involved in the regulation of many physiological and developmental processes. At the molecular level, THs regulate gene transcription mostly through the triiodothyronine (T3) form, which binds to nuclear receptors, encoded by the TRα and TRβ genes (1). TH receptors (TRs) are transcription factors with ligand-regulated activity. In the absence of T3, the unliganded receptors (aporeceptors) recruit corepressors and repress the transcription of target genes. Upon hormone binding, the receptors (holoreceptors) exchange corepressors for coactivators and activate transcription (2). This repressive effect of aporeceptors has been clearly documented in hypothyroid mice for TRα in brain, bone, intestine, spleen, and heart (3, 4). These observations suggested that the conversion of TR aporeceptors into holoreceptors as a result of a change in T3 concentration in vivo might act as a molecular switch under physiological conditions. One situation in which T3 level naturally changes during life is during the transition from fetal to neonatal life. In mice, fetal circulating levels of T3 are quite low (5–7) and highly increase at birth, reaching a maximum during the first postnatal weeks (almost 2,000-fold increase) and decreasing to the adult level by the end of the second week (6, 7). Therefore, the early postnatal period appears as a natural hyperthyroid situation after a fetal period of relative hypothyroidism. This transition may allow for rapid and simultaneous triggering of different gene expression programs involved in the major developmental and maturation events of this postnatal period. It is already well known that T3 plays a critical role during this period, regulating the maturation of different organs such as bone, brain, and intestine. All these events are severely impaired in mice devoid of TRs (8–12) and in hypothyroid mice (3, 4). Heart function is critically controlled by T3 in the adult where hypothyroidism and hyperthyroidism respectively induce bradycardia and tachycardia (1). In this study, cardiac activity was chosen as the model to address the physiological relevance of the T3 switch during the neonatal ontogenetic process.
The respective role of each receptor in the regulation of cardiac function was previously established in adult mice by different studies using either genetically modified mice harboring mutation of TRα and/or TRβ (13–20) or treatment with specific TRβ agonist (13). TRα, which is the predominant isoform in the heart, plays a major role in determining heart rate (HR) under baseline conditions, whereas TRβ, even though expressed at a lower level, is involved in the response to acute stimulation by T3 (14–16).
Several genes encoding ion channels involved in cardiac contractile activity have been shown to be positively or negatively regulated by THs in the adult mouse heart (14, 17). Among these genes, HCN2, which encodes a component of the pacemaker, is of particular interest. Its expression has been shown to be positively regulated exclusively through the TRα pathway and to be severely repressed in hypothyroid mice (14). This repression seems to depend on TRα aporeceptors, because HCN2 expression was restored by knocking out the TRα gene in the hypothyroid background (4). In adult heart, HCN2 can thus be considered as a bona fide TRα target gene, repressed by the aporeceptor and activated by the holoreceptor. Some other genes such as the ones encoding KCBN1 and KCNA5, two voltage-gated K+ channel α-subunits contributing to the main repolarizing K+ currents in the adult mouse ventricle (14, 17), are also positively regulated by T3. In contrast, the KCNQ1 and KCNE1 (minK) genes that encode K+ channel subunits, which generate the slow component of delayed cardiac rectification, have been shown to be down-regulated by T3 in hyperthyroid adult mice and up-regulated in hypothyroid mice. This regulation seems to be mediated mostly by TRα (14, 17).
The aim of the present work was to determine whether TRα aporeceptors play a role during fetal and postnatal development. This question was addressed by using the heart as a model.
This study shows that in the fetus TRα aporeceptors repress HR as well as the expression of several genes like HCN2, KCNE1, KCBN1, KCNA5, KCNQ1, and TRβ. In contrast, after birth, when T3 becomes available, TRα holoreceptors lose this repressing effect and even activate the expression of some of these genes concomitantly with stimulation of HR. These data provide therefore evidence for a role of TRα aporeceptors under physiological conditions. They further establish that the TRα receptor is a molecular switch controlling heart function near birth time.
Methods
Animal Conditioning. Animal care procedures were conducted in accordance with the guidelines set by the European Community Council Directives.
Mice devoid of all known isoforms produced by the TRα locus (TRα0/0) are described in ref. 18. Fifteen TRα0/0 female mice were mated with TRα0/0 male mice to obtain 100% TRα0/0 littermates. Twenty-four WT female mice were mated with WT males to provide WT control animals. To produce 100% TRα0/+, heterozygous littermates carried by TRα0/0 homozygous mothers, TRα0/0 females were mated with WT males. The TRα0/0 and WT genitors used in this study were both derived from parallel littermates obtained by intercrossing TRα0/+ heterozygous genitors to provide control and mutant animals with the same genetic background.
Embryonic day 0.5 postcoitum (pc) was defined as noon of the day a vaginal plug was detected after overnight mating. Mice were studied between days 9.5 and 19.5 pc in the WT and TRα0/0 populations and at days 18.5 and 19.5 pc for heterozygous littermates. Day 9.5 pc corresponds to the onset of spontaneous cardiac activity.
Studies were performed with mice under general anesthesia (1–1.5% isoflurane) by using specific equipment and a dedicated control system of body temperature (Minerve, Esternay, France). The rectal temperature of pregnant mice was maintained at 37°C ± 1°C throughout the experiments.
Hair on the abdominal wall was clipped prior to examination, and acoustic gel was applied to provide good coupling between the transducer and the skin.
Ultrasound Acquisition. A commercially available ultrasound machine was used (HDI 5000, Philips Medical Systems, Andover, MA) with a per-operative linear transducer (Entos CL15-7). A midline or parasagittal transabdominal approach was used to examine the uterine horns. The abdominal cavity was virtually divided in four quadrants: left and right cranial segments, and left and right caudal segments. When more than four fetuses were present in the abdomen, measurements were performed on only one fetus per abdominal quadrant to ensure that no fetus would be studied twice during the experiment.
The ultrasonic probe was operated at the highest frequency range (“resolution mode,” 15 MHz; axial resolution measured at –20 dB, 600 microns; lateral resolution measured at –20 dB, 700 microns). Fetuses were located by using gray-scale B mode images and were selected so anatomical landmarks were present nearby (kidneys and bladder), allowing for a consistent identification of the fetus being studied at each measurement step.
A color-Doppler volume was superimposed on the gray-scale image to locate blood flow within the heart, the dorsal aorta, and the umbilical cord (Fig. 1A). After identification of the vessels of interest, pulsed-wave Doppler interrogations were performed, placing the sample volume successively on the dorsal aorta and the umbilical artery. The Doppler settings were as follows: sample volume size, 0.5 mm; wall filter, medium level; gain level, 65–80%; angle of insonation, <60°C. The pulse repetition frequency was set to record the maximum velocities without aliasing (3,500–4,000 Hz). From these time-velocity curves, the fetal HR was determined. The maternal HR also was recorded at the beginning and at the end of the procedure from Doppler interrogation of the abdominal aorta.
Fig. 1.
Heart rate in WT and mutant mice. (A) Color-Doppler image of a fetus showing the signal from the umbilical artery (in red) and vein (in blue). This color image was used to place a sample volume accurately on the umbilical cord to obtain the spectral-Doppler time velocity curve in the top left corner, showing the continuous signal from the vein (at the top) and the pulsatile, systolic, signal from the artery (at the bottom). The HR was derived from the measurement of the cardiac cycle duration (CC) defined as the time separating to arterial systolic signals. (B) Mean HR in WT and TRα0/0-mutant fetuses and in WT and mutant pregnant mice. For the fetuses, average values and a 95% confidence interval are presented. For P18 and A11 of each genotype, individual mean HR values are presented. (C) Comparison of HR among WT and homozygous and heterozygous mutants at days 18.5 and 19.5 pc. Results are presented as mean HR with 95% confidence intervals. bpm, Beats per minute; Indiv., individual.
Data were acquired from embryos and fetuses between days 9.5 and 19.5 pc. The total number of data sets was 138, corresponding to 46 fetuses for the TRα0/0 group, and 186, corresponding to 75 fetuses for the WT group. To record HRs from TRα0/+ heterozygous fetuses, 10 measurements were obtained, corresponding to six fetuses from two different littermates. Data were collected at days 18.5 and 19.5 pc.
Alteration of TH Status in Pregnant Mice. To obtain a hyperthyroid status, WT and TRα0/0 pregnant mice were treated by daily i.p. injections of T3 (200 μg/kg) and T4 (2 mg/kg) hormones in PBS during 5 days from day 9.5 pc to day 14.5 pc. Control animals received PBS i.p. injections.
RNA Extraction and cDNA Synthesis. Hearts from control and treated animals were dissected and frozen in liquid nitrogen. Isolation of total RNA was performed by RNeasy extraction kit procedure (Qiagen, Valencia, CA), according to the manufacturer's protocol. Reverse transcription was carried out with the Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega). Total RNA (1 μg) was reverse transcribed into cDNA in a 20-μl reaction including 500 ng of random hexamers, 4 μl of 5× RT buffer (2,540 mM Tris·HCl, pH 8.3/375 mM KCl/15 mM MgCl2/50 mM DTT) 1 μl of 20 mM dNTPs, 0.5 μlof 40 units/μl RNasin (Promega), 3.5 μl of diethyl pyrocarbonate-treated water, and 200 units of M-MLV reverse transcriptase. The mixture was heated at 37°C for 60 min and then at 70°C for 5 min to inactivate the M-MLV reverse transcriptase.
Quantitative PCR. Two kinds of quantitative PCR systems were used: the SYBR green DNA-binding dye and the Assays-On-Demand TaqMan probe (Applied Biosystems). Amplifications were performed with a DNA Engine Opticon 2 system (MJ Research, Cambridge, MA). For data analysis, the relative standard curve method was used to determine relative quantification of genes of interest and compare the amounts of mRNA in control and treated groups of animals, normalized to the Arbp reference gene (UniGene no. Mm.5286), a gene encoding a ribosomal protein used as an internal standard.
The following seven genes were studied (all listed in the UniGene Database, www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=Search&DB=unigene): HCN2 (no. Mm.12956), KCNE1 (no. Mm.4955), TRβ (no. Mm.317589), KCNB1 (no. Mm.188175), KCNA5 (no. Mm.222831), KCNQ1 (no. Mm.5177), and TRα1.
Quantification of KCNE1, TRβ, KCNB1, KCNA5, KCNQ1, TRα1, and Arbp mRNAs. Diluted cDNA (2 μl) was added to 15 μl of quantitative PCR reactions containing 7.5 μl of 2× Quantitect SYBR Green Master Mix (Qiagen) and 300 nmol of gene-specific primers or Arbp primers used as the reference gene. The PCR started with the HotStarTaq Polymerase enzyme activation step of 15 min at 95°C, followed by 45 cycles each consisting of 15 sec at 94°C, 20 sec at 55°C, and 20 sec at 72°C. The forward and reverse primers were as follows: KCNE1-forward, 5′-ACTCAGTGGTGCCCCTACAATAAAG-3′; KCNE1-reverse, 5′-GAACTGAAGCCATTGTCGTGAACCC-3′; TRβ-forward, 5′-CTCTTCTCACGGTTCTCCTC-3′; TRβ-reverse, 5′-AACCAGTGCCAGGAATGTCG-3′; KCNB1-forward, 5′-GCACTTGCTGTGGTGTAGATGG-3′; KCNB1-reverse, 5′-TCTTAGGAACAGAGGAGGCG-3′; KCNA5-forward, 5′-CAGAGTCTCCAAGCAGAAGG-3′; KCNA5-reverse, 5′-CCAGGTGTGGCTTATCTTCG-3′; KCNQ1-forward, 5′-ACCGTCTTCCTCATTGTTCTGG-3′; KCNQ1-reverse, 5′-GACAATCTCCATCCAGAAGAGG-3′; Arbp-forward, 5′-ACCTCCTTCTTCCAGGCTTT-3′; Arbp-reverse, 5′-CCCACCTTGTCTCCAGTCTTT-3′; TRα1-forward, 5′-TTCTCTCCTTCCTCCCATCCTT-3′; and TRα1-reverse, 5′-GGCTGGAGGGTCTGAGGG-3′. Except for the TRα1 gene for which the primers were obtained from Sadow et al. (19), all other primers were designed specifically for this work.
Quantification of HCN2 mRNA. For HCN2 gene expression, quantification the following protocol was used. Diluted cDNA (2 μl) was added to 20 μl of quantitative PCR reactions containing 10 μl of 2× Universal PCR Master mix (Applied Biosystems) and 1 μlof HCN2-specific TaqMan probe (Assays-on-Demand). The PCR started with the AmpliTaq Gold polymerase enzyme activation step of 10 min at 95°C, followed by 40 cycles each consisting of 15 sec at 95°C and 1 min at 72°C. Amounts of mRNA were expressed relatively to that of Arbp mRNA.
Results
HR Measurements. We first measured the HR of pregnant females and their fetuses from either WT or TRα0/0 genotype (7) during course of pregnancy by using Doppler ultrasound. In this first experiment, mothers and fetuses were from the same genotype.
As shown in Fig. 1B, the HR of pregnant mice of either genotype did not change significantly over the whole gestation period and was slightly higher than HR of nonpregnant 11-wk-old adult mice (A11). At any time point, adult TRα0/0 females displayed bradycardia as compared with their WT counterparts.
Heartbeats of WT fetuses were slow (100 beats per minute) at day 9.5 pc, increased slowly until day 17.5 pc, and then became faster to the end of gestation. Adult rate was not yet reached in 18-day-old pups (P18). Quite surprisingly, the HR of the TRα0/0 fetuses was always higher than the one recorded for WT. In contrast, 18 days after birth, both strains of mice showed very similar HR.
To check whether the higher HR recorded for TRα0/0 mutant fetuses was a consequence of the TRα0/0 mothers' environment, we measured the HR of heterozygous fetuses (TRα0/+) carried by TRα0/0 mothers. As shown in Fig. 1C, measurements at days 18.5 and 19.5 pc clearly showed that HRs of the heterozygotes were intermediate between those of WT and homozygous TRα0/0 fetuses, suggesting an autonomous fetus phenotype.
Gene Expression Analysis in Euthyroid Mice. To further understand the molecular basis of this effect on the developing heart, we investigated the expression of cardiac genes known to be regulated by T3 or relevant to the observed phenotype. Measurements were done at three different developmental stages: 15.5-day-old fetuses (E15.5 fetuses), P18, and A11.
As shown in Fig. 2 A–F, mRNA levels of HCN2, TRβ, KCNE1, KCNB1, and KCNA5 strongly increased in WT animals during the first 18 postnatal days. Except for KCNA5, their expression then decreased to different levels in the adult. Expression of KCNQ1 decreased constantly from fetus to adult stages. The level of expression of the TRα1 mRNA in the WT animals was the same at all of the developmental stages (data not shown).
Fig. 2.
Changes in expression of genes in the hearts of WT and mutant mice. (A–F) Quantification of transcripts in the heart of WT (plain bars) and TRα0/0-mutant (hatched bars) mice. The amounts of HCN2 mRNA (A), TRβ mRNA (B), KCNE1 mRNA (C), KCNB1 mRNA (D), KCNA5 mRNA (E), and KCNQ1 mRNA (F) were estimated by quantitative RT-PCR, as described in Methods, and normalized to the corresponding Arbp mRNA levels. Each value represents the mean ± SD obtained from several independent animals (two for E15.5 WT fetuses, seven for E15.5 mutants, three for P18 WT, three for P18 mutants, two for A11 WT, and two for A11 mutants). (G) Changes in the levels of expression of genes in the heart as a result of TRα knockout. Bars represent the percentages of variation in TRα0/0 mutants vs. WT and were calculated from the values presented in A–F. Data are presented as positive when values for TRα0/0 were superior to WT and negative when inferior to WT.
In the TRα0/0 fetuses, all six genes showed a much higher level of expression than in the WT fetuses (Fig. 2 A–F, hatched bars). In mutant pups the level of expression of all six genes was lower than that observed in WT pups. In the adult mutants, expression of HCN2 and KCNA5 was inferior to that observed in WT adults (Fig. 2 A and E), whereas expression of TRβ, KCNB1, and KCNQ1 was not different between mutant and WT animals (Fig. 2 B, D, and F), and the expression of KCNE1 was superior in mutant as compared with WT animals (Fig. 2C).
An overview of the change in the expression of these genes as a result of TRα knockout, at the three different stages analyzed, is presented in Fig. 2G. It appears clear that the situation is significantly different for fetal and postnatal stages. In the fetus, all six genes appeared activated in the mutants. In contrast, in the pups, the levels of expression of all these genes were decreased to different levels in mutant animals as compared with WT. In the adult, the situation is similar to that in P18 for all genes except for KCNE1. In the mutant adult heart, this last gene showed an “activation” phenotype similar to what is described in ref. 14.
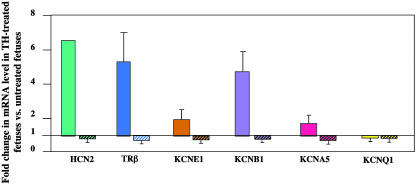
Effect of Stimulation by THs on Gene Expression. The previous results strongly suggested that these genes were actively repressed by the TRα aporeceptor in WT fetuses. To further investigate this hypothesis, we checked whether this repression could be alleviated by chronic TH treatment of the mother. As shown in Fig. 3, all genes except KCNQ1 showed a strong enhancement of their level of expression in the heart of E15.5 WT fetuses carried by T3-treated mothers, reinforcing our hypothesis. The level of expression of the TRα1 mRNA in the WT fetuses was not affected by treatment of the mothers with THs (data not shown).
Fig. 3.
Change in the amounts of mRNAs of different genes in hearts of E15.5 fetuses induced by treatment of mothers with THs. For each gene, the graph presents the ratios between mRNA levels in TH-injected mice and levels in untreated mice. Bars without hatching correspond to ratios for WT fetuses carried by WT mothers. Hatched bars correspond to ratios for TRα0/0 mutant fetuses carried by TRα0/0 mothers. Mean values are given with 95% confidence intervals.
Furthermore, to exclude any role of TRβ receptors in the enhanced level of expression of the tested genes in the TRα0/0 fetuses, we checked whether these genes could be activated by T3 in TRα0/0 fetuses (Fig. 3, hatched bars). We did not see any significant change in the expression of any of the tested genes in homozygous E15.5 mutants carried by TRα0/0 mothers under chronic treatment with T3. All of the genes even showed a slight decrease in expression for unexplained reasons.
Discussion
Our data strongly suggest that in the WT fetus, TRα receptors repress cardiac function. Previous data have shown that dominant negative TRα mutant receptors working as constitutive transcriptional repressors strongly reduce the HR in adult mice (20, 21). Therefore, we might assume that in the fetal heart, TRα behave as aporeceptors repressing HR.
These data also confirmed that the adult mutant mice devoid of TRα are bradycardic (14, 15, 22–24). They further show that the basal HR of both WT and mutant pregnant mice is slightly elevated as compared with nonpregnant animals with the respective genotype. Such a difference was already described for WT mice (25).
Our observation of an intermediate HR in heterozygous fetuses as compared with WT and mutant homozygous fetuses shows that the increase in HR in the TRα0/0 fetuses is genetically controlled at the level of the TRα gene and that the degree of fetal tachycardia depends on an autonomous gene dosage effect.
From a molecular standpoint, we observed an increased expression of the cardiac genes studied in TRα0/0 fetuses as compared with WT fetuses. It was shown previously that HCN2 is a target for repression by TRα aporeceptors in hypothyroid adult mice (4). We might then assume that, during normal fetal life when T3 circulating level is low, TRα aporeceptors actually work as repressors on some cardiac genes that are up-regulated by T3 later in life. This hypothesis was confirmed by the observation that all those genes could actually be up-regulated in the heart of WT fetuses if T3 is provided by chronic T3 treatment of the mother.
Interestingly, it should be noted that the differences between treated and untreated groups observed for HCN2, TRβ, KCNE1, and KCNB1 were within the same range as those seen between TRα0/0 mutants and WT in euthyroid mothers (compare Figs. 3 and 2G). Taken together, these observations strongly suggest that in the WT fetus, the HCN2 gene and presumably all other tested genes are repressed by the TRα aporeceptors and that the availability of T3 releases this blockage. We could exclude the hypothesis that remaining TRβ receptors in TRα0/0 mutants could be responsible for activating expression of the tested genes because in these mutant fetuses the treatment with T3 was totally insufficient to induce gene expression.
In the adult mouse, the KCNE1 gene has been shown to be repressed by T3 (14, 17). This could explain why the expression of the gene is enhanced in TRα0/0 adult mutants (Fig. 2G, A11). It is therefore interesting to see that this gene undergoes opposite regulations in response to T3 in fetus vs. adult. KCNQ1 is also down-regulated in hyperthyroid adult mice (14, 17), whereas it is insensitive to T3 in the fetus.
Conclusion
This work shows that the expression of all tested genes in the heart is controlled by TRα in opposite ways before and after birth. Because the HCN2 gene is repressed by TRα aporeceptors in hypothyroid adult mice (4), we propose that the same mechanism accounts for repression of the gene in normal fetal heart, and by extension for repression of the other tested genes. At the end of the fetal development and mainly during the early postnatal period, the availability of T3 mostly produced by the fetus itself releases this blockage. It should be noted that in P18, the differences in expression of all of the tested genes between WT and TRα0/0 do not strictly correlate with differences in HR, suggesting that these genes are not the only genes controlling heart activity at this period of development.
Activation of the expression of the TRβ gene by TRα holo-receptors has not been previously documented in the mouse, although it has been described in the metamorphosing tadpole (26). Together with the fact that the expression of the TRβ gene is increased in TRα0/0 mutants, it strongly suggests that this expression in the fetal heart is directly controlled by the TRα receptor. Because such an activation was not observed in the hyperthyroid adult heart (17), we might conclude that this kind of regulation of the TRβ gene by TRα in the heart is specific to the fetal organ. Similarly, differences in regulation by T3 of KCNE1 and KCNQ1 between adults and fetuses suggest that TRα receptors work differently in adult and fetal heart environments.
These data clearly demonstrate that during normal fetal development the TRα aporeceptors created by low levels of T3 exert a physiologic role by repressing heart function. Therefore, the TRα receptor might be considered a molecular switch controlling heart function near birth time.
So far, no mutation has been identified in the TRα gene in humans, whereas mutations in the TRβ gene result in the syndrome of resistance to THs (27, 28). Presumably, if mutations of TRα do exist in humans, their symptoms might be quite different from those of resistance to THs. Although the ontogeny of heart function is quite different between mice and humans, the data presented here for mice suggest that deregulation of heart function in the human fetus and newborn might constitute relevant symptoms for TRα mutations.
The present observations made on the fetal heart might be instructive for investigating similar effects of TRα aporeceptors on the development and/or function of other fetal organs. Indeed, it has been shown that TRα aporeceptors block the maturation of several tissues in experimentally induced hypothyroid mice (4), and these same tissues might be appropriate to extend this model apo-TRα to holo-TRα-switching effect during normal development.
Acknowledgments
We thank Karine Gauthier for comments and advice on the manuscript and Nadine Aguilera for technical assistance in preparing the animals. This work was supported by grants from the Ligue Nationale contre le Cancer (to J.S.) and the Consortium National de la Recherche en Génomique, by European Union Grant Eumorphia QLG2-CT-2002-00930 (to J.S. and M.F.J.), and by the Région Rhône-Alpes Program Thématique Biotechnologie (M.F.J.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TH, thyroid hormone; T3, triiodothyronine; TR, TH receptor; HR, heart rate; pc, postcoitum; P18, 18-day-old pups; A11, 11-wk-old adult mice; E15.5 fetuses, 15.5-day-old fetuses.
References
- 1.Yen, P. M. (2001) Physiol. Rev. 81, 1097–1142. [DOI] [PubMed] [Google Scholar]
- 2.Hu, X. & Lazar, M. A. (2000) Trends Endocrinol. Metab. 11, 6–10. [DOI] [PubMed] [Google Scholar]
- 3.Morte, B., Manzano, J., Scanlan, T., Vennstrom, B. & Bernal, J. (2002) Proc. Natl. Acad. Sci. USA 99, 3985–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flamant, F., Poguet, A. L., Plateroti, M., Chassande, O., Gauthier, K., Streichenberger, N., Mansouri, A. & Samarut, J. (2002) Mol. Endocrinol. 16, 24–32. [DOI] [PubMed] [Google Scholar]
- 5.Morreale de Escobar, G., Calvo, R., Escobar del Rey, F. & Obregon, M. J. (1994) Endocrinology 134, 2410–2415. [DOI] [PubMed] [Google Scholar]
- 6.Hadj-Sahraoui, N., Seugnet, I., Ghorbel, M. T. & Demeneix, B. (2000) Neurosci. Lett. 280, 79–82. [DOI] [PubMed] [Google Scholar]
- 7.Gauthier, K., Chassande, O., Plateroti, M., Roux, J., Legrand, C., Pain, B., Rousset, B., Weiss, R., Trouillas, J. & Samarut, J. (1999) EMBO J. 18, 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forrest, D., Hallbook, F., Persson, H. & Vennstrom, B. (1991) EMBO J. 10, 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campos-Barros, A., Amma, L. L., Faris, J. S., Shailam, R., Kelley, M. W. & Forrest, D. (2000) Proc. Natl. Acad. Sci. USA 97, 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plateroti, M., Gauthier, K., Domon-Dell, C., Freund, J., Samarut, J. & Chassande, O. (2001) Mol. Cell. Biol. 21, 4761–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraichard, A., Chassande, O., Plateroti, M., Roux, J., Trouillas, J., Dehay, C., Legrand, C., Gauthier, K., Kedinger, M., Malaval, L., et al. (1997) EMBO J. 16, 4412–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plateroti, M., Chassande, O., Fraichard, A., Gauthier, K., Freund, J., Samarut, J. & Kedinger, M. (1999) Gastroenterology 116, 1367–1378. [DOI] [PubMed] [Google Scholar]
- 13.Trost, S. U., Swanson, E., Gloss, B., Wang-Iverson, D. B., Zhang, H., Volodarsky, T., Grover, G. J., Baxter, J. D., Chiellini, G., Scanlan, T. S., et al. (2000) Endocrinology 141, 3057–3064. [DOI] [PubMed] [Google Scholar]
- 14.Gloss, B., Trost, S., Bluhm, W., Swanson, E., Clark, R., Winkfein, R., Janzen, K., Giles, W., Chassande, O., Samarut, J. & Dillmann, W. (2001) Endocrinology 142, 544–550. [DOI] [PubMed] [Google Scholar]
- 15.Johansson, C., Gothe, S., Forrest, D., Vennström, B. & Thoren, P. (1999) Am. J. Physiol. 276, 2006–2012. [DOI] [PubMed] [Google Scholar]
- 16.Weiss, R. E., Korcarz, C., Chassande, O., Cua, K., Sadow, P. M., Koo, E., Samarut, J. & Lang, R. (2002) Am. J. Physiol. 283, E428–E435. [DOI] [PubMed] [Google Scholar]
- 17.Le Bouter, S., Demolombe, S., Chambellan, A., Bellocq, C., Aimond, F., Toumaniantz, G., Lande, G., Siavoshian, S., Baro, I., Pond, A. L., et al. (2003) Circ. Res. 92, 234–242. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier, K., Plateroti, M., Harvey, C., Williams, G., Weiss, R., Refetoff, S., Willot, J., Sundin, V., Roux, J., Malaval, L., Hara, M., Samarut, J. & Chassande, O. (2001) Mol. Cell. Biol. 21, 4748–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadow, P. M., Chassande, O., Koo, E. K., Gauthier, K., Samarut, J., Xu, J., O'Malley, B. W. & Weiss, R. E. (2003) Mol. Cell. Endocrinol. 203, 65–75. [DOI] [PubMed] [Google Scholar]
- 20.Liu, Y. Y., Schultz, J. J. & Brent, G. A. (2003) J. Biol. Chem. 278, 38913–38920. [DOI] [PubMed] [Google Scholar]
- 21.Tinnikov, A., Nordstrom, K., Thoren, P., Kindblom, J. M., Malin, S., Rozell, B., Adams, M., Rajanayagam, O., Pettersson, S., Ohlsson, C., et al. (2002) EMBO J. 21, 5079–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson, C., Lannergren, J., Lunde, P. K., Vennstrom, B., Thoren, P. & Westerblad, H. (2000) Am. J. Physiol. 278, R598–R603. [DOI] [PubMed] [Google Scholar]
- 23.Pazos-Moura, C., Abel, E. D., Boers, M. E., Moura, E., Hampton, T. G., Wang, J., Morgan, J. P. & Wondisford, F. E. (2000) Circ. Res. 86, 700–706. [DOI] [PubMed] [Google Scholar]
- 24.Weiss, R. E., Murata, Y., Cua, K., Hayashi, Y., Seo, H. & Refetoff, S. (1998) Endocrinology 139, 4945–4952. [DOI] [PubMed] [Google Scholar]
- 25.Wong, A. Y., Kulandavelu, S., Whiteley, K. J., Qu, D., Langille, B. L. & Adamson, S. L. (2002) Am. J. Physiol. 282, H918–H925. [DOI] [PubMed] [Google Scholar]
- 26.Machuca, I., Esslemont, G., Fairclough, L. & Tata, J. R. (1995) Mol. Endocrinol. 9, 96–107. [DOI] [PubMed] [Google Scholar]
- 27.Usala, S. J., Bale, A. E., Gesundheit, N., Weinberger, C., Lash, R. W., Wondisford, F. E., McBride, O. W. & Weintraub, B. D. (1988) Mol. Endocrinol. 2, 1217–1220. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai, A., Takeda, K., Ain, K., Ceccarelli, P., Nakai, A., Seino, S., Bell, G. I., Refetoff, S. & DeGroot, L. J. (1989) Proc. Natl. Acad. Sci. USA 86, 8977–8981. [DOI] [PMC free article] [PubMed] [Google Scholar]