Abstract
Background:
World Health Organization (WHO) recommends the use of Xpert MTB/RIF assay for rapid diagnosis of tuberculosis (TB) and detection of rifampicin resistance. This systematic review was done to know about the diagnostic accuracy and cost-effectiveness of the Xpert MTB/RIF assay.
Methods:
A systematic literature search was conducted in following databases: Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews, MEDLINE, PUBMED, Scopus, Science Direct and Google Scholar for relevant studies for studies published between 2010 and December 2014. Studies given in the systematic reviews were accessed separately and used for analysis. Selection of studies, data extraction and assessment of quality of included studies was performed independently by two reviewers. Studies evaluating the diagnostic accuracy of Xpert MTB/RIF assay among adult or predominantly adult patients (≥14 years), presumed to have pulmonary TB with or without HIV infection were included in the review. Also, studies that had assessed the diagnostic accuracy of Xpert MTB/RIF assay using sputum and other respiratory specimens were included.
Results:
The included studies had a low risk of any form of bias, showing that findings are of high scientific validity and credibility. Quantitative analysis of 37 included studies shows that Xpert MTB/RIF is an accurate diagnostic test for TB and detection of rifampicin resistance.
Conclusion:
Xpert MTB/RIF assay is a robust, sensitive and specific test for accurate diagnosis of tuberculosis as compared to conventional tests like culture and microscopic examination.
Keywords: Diagnosis, Tuberculosis, Xpert MTB/RIF assay
INTRODUCTION
World Health Organization (WHO) fact sheet of the year 2015 shows a mixed picture of global tuberculosis (TB); on one hand, there is a decline in the incidence and the mortality rate attributed to TB, while on the other hand, the decrease in rate is very slow and TB is still the greatest killer worldwide due to a single infectious agent.[1,2] According to WHO estimates of the year 2013, approximately, 9.0 million people developed TB and 1.5 million died from the disease.[1] Out of these, more than half (56%) of new TB cases occurred in the South-East Asia and Western Pacific Regions.[1] According to the Ministry of Health Affairs, India, every year, approximately, 100 million work days are lost due to illness. India incurs a huge cost due to TB, which is close to US$ 3 billion in indirect costs and US $ 300 million in indirect costs.[3]
As TB is a curable disease, the role of accurate diagnosis and treatment is vital for improving the global picture. Between the years 2000 and 2013, approximately, 37 million lives were saved through TB diagnosis and treatment.[1,2] These numbers are encouraging and an achievement for the epidemiologists, however, lots more needs to be achieved to control this disease. Use of highly sensitive and specific diagnostic assays for screening, monitoring, diagnosing co-infection with Human Immunodeficiency Virus (HIV), and pattern of drug resistance may help the national and international TB control programs to get close to the targets.
Of the 9.0 million (range: 8.7-9.4 million) incident cases in 2013, only 5.7 million were detected and notified, giving a case detection rate of 64%, thus creating a gap of approximately 3.3 million people with TB who were “missed” either because they were not diagnosed or because they were diagnosed, but not reported.[2] Apart from the poor detection rate, increase in the incidence of multidrug-resistant (MDR) TB and extensively drug resistant (XDR) TB is also critical. In the same year, 3.5% of new and 20.5% of previously treated TB cases were diagnosed to have MDR-TB and 9% of these had XDR-TB.[4] In 2013, there were 3,00,000 estimated cases of MDR-TB worldwide and out of these 1,36000 cases were actually detected, which is an improvement in the detection rates as compared to earlier years.[4] Another area of concern is co-infection with HIV as TB causes one-fourth of all HIV-related deaths.[1]
Now, major thrust is being laid on the accessibility of economically feasible accurate diagnostic assays.[5] In early 2011, WHO endorsed a novel, rapid, automated, molecular diagnostic test, the Xpert MTB/RIF assay that can simultaneously detect TB and rifampicin resistance.[6] Xpert MTB/RIF provides result within 2 h, thus the patients can be started with proper treatment even on the same day. Furthermore, it requires minimal hands-on technical time, training, and can be installed in nonconventional laboratories.[7,8] All these features give Xpert MTB/RIF assay an edge over the other available diagnostic techniques. Sample processing and polymerase chain reaction (PCR) amplification are integrated into a single self-enclosed test unit unlike conventional nucleic acid amplification tests (NAAT).[9] In addition, the assay's sample reagent, used to liquefy sputum, has potent tuberculocidal properties and so largely eliminates biosafety concerns during the test procedure.[10]
Other available tests include the direct examination of sputum smears with Ziehl–Neelsen (ZN) staining for acid-fast Bacilli, mycobacterial culture sensitivity, and other NAATs and immunological tests.[11,12] Microscopic examination is suitable for laboratories at peripheral and higher levels and it can be done safely under minimal biosafety conditions.[11] Mycobacterial culture is taken as the reference standard test for the diagnosis of TB, but this process is cumbersome and time-consuming.[11] NAATs are molecular systems that can detect small quantities of genetic material (DNA or RNA) from microorganisms such as Mycobacterium tuberculosis. PCR is the most common among the variety of NAATs. NAATs are available as commercial kits and in-house tests and are used routinely in high-income countries for TB detection.[13] In-house PCR tests are widely used in the developing countries because these tests are less expensive than commercial kits. The line probe assays (LPA) are suitable only for national or regional level laboratories because of its complexity and bio safety requirements.[13]
We tried to analyze the sensitivity and specificity of Xpert MTB/RIF assay in this review. Two earlier Cochrane reviews had assessed the diagnostic accuracy of Xpert MTB/RIF as an initial diagnostic test replacing microscopy and add-on test following negative smear microscopy result.[9,13] We tried to include more studies published after the publication of these two Cochrane reviews.
Objective
To determine the diagnostic accuracy of Xpert MTB/RIF for the diagnosis of pulmonary TB among adults as compared to other available diagnostic tests.
MATERIALS AND METHODS
Criteria for considering studies for this review
Types of studies
Studies that assessed the diagnostic accuracy of Xpert MTB/RIF assay were included in the analysis. Diagnostic accuracy studies are typically cross-sectional in design. Studies that reported data comparing Xpert MTB/RIF to an acceptable reference standard from which we could extract true positive (TP), true negative (TN), false positive (FP), and false negative (FN) values were included. Xpert MTB/RIF assay can be assessed alone or together with other tests.
Types of participants
Studies that had recruited adult or predominantly adult patients (≥14 years), presumed to have pulmonary TB with or without HIV infection were included in the review. In addition, studies that had assessed the diagnostic accuracy of Xpert MTB/RIF assay using sputum and other respiratory specimens (such as fluid obtained from bronchial alveolar lavage and tracheal aspiration) and studies from all the types of health facilities and all laboratory levels (peripheral, intermediate, and central) from all countries were included.
Types of interventions
Xpert MTB/RIF assay was the index test under evaluation. Comparison was made between Xpert MTB/RIF and smear microscopy, either ZN microscopy, fluorescence microscopy, or both microscopy methods, liquid or solid culture.
Search methods for identification of studies
Electronic searches
A systematic literature search was conducted in the following databases: Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews, MEDLINE, PubMed, Scopus, Science Direct, and Google Scholar for relevant studies. Studies given in the systematic reviews were accessed separately and used for analysis. Secondary referencing was done by manually reviewing the list of key articles and searching citations using PubMed and Google Scholar. Study search was done on from all the published literature from 1st January 2010 till December 2014. The key words used were “diagnosis” or “TB” or “developing countries” or “GeneXpert” or “diagnosis and TB and GeneXpert” or “Xpert MTB/RIF”.
Data collection and analysis
Selection of studies
Population: Adult population both males and females above 14 years of age suspected of having TB with or without HIV.
Diagnostic test: Xpert MTB/RIF assay.
Comparator: Smear microscopy.
Outcome: Diagnosis of TB.
Data extraction and management
Two review authors (RK and KK) independently performed the data extraction by reading every study. The data included characteristics of patients. Whenever possible, data were extracted for TP, FP, FN, and TN values based on one Xpert MTB/RIF result for one specimen provided by one patient. However, in some of the studies, the number of specimens exceeded the number of patients, suggesting that a single patient may have provided multiple specimens. Therefore, pooled sensitivity and specificity for TB detection in all the studies were compared with pooled sensitivity and specificity in the subset of studies that provided one Xpert MTB/RIF result based on one specimen provided by one patient.
Assessment of risk of bias in included studies
Two reviewers (RK and KK) independently assessed the quality of all the studies. With the help of Review Manager 5.3, we interpreted the bias among all the studies and explained that in terms of legible diagrams as a risk of bias and summary table.[14]
RESULTS
Results of the search
After deduplication, we were left with 123 studies, out of these, 78 studies were excluded as they were not relevant to the diagnosis of TB [Figure 1]. The remaining 45 full text articles were read out and eight of these were excluded mainly because the data to calculate TP, TN, FP, and FN were insufficient to assess the sensitivity and specificity of the Xpert MTB/RIF. The excluded studies were listed and the reasons for their exclusion were also mentioned. The remaining 37 studies were included in the quantitative synthesis of data. Studies were named according to the surname of the first author and year of publication. For multicenter studies, the study-naming scheme uniquely identified multiple study centers from within each study (e.g., Al-Ateah et al. [2012a]; Al-Ateah et al. [2012b]), each of which reported data separately for a distinct population at a given study site.[15] Hence, the number of study centers exceeds the number of studies.
Figure 1.
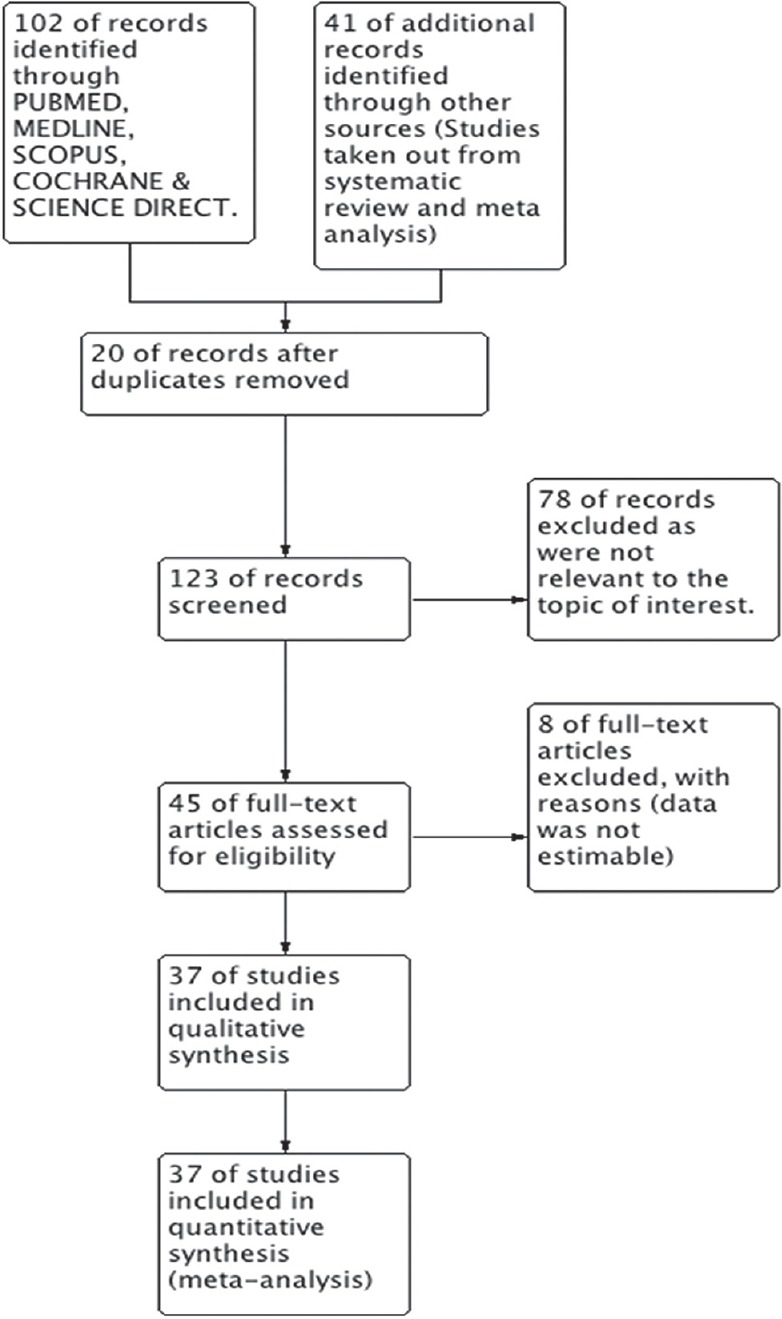
Study search diagram
Included studies
Thirty-seven different studies were identified. Out of these, Boehme et al. and Boehme et al. were international multicenter studies, carried out at five and six study centers, respectively.[16,17] These two studies had involved different patients.[16,17] Another study, Marlowe et al. had been conducted at three sites, reported accuracy data for the three sites combined; which was considered to be a single study and a single study center.[18] Thus, there were 37 studies representing 47 study centers.
Methodological quality of the included studies
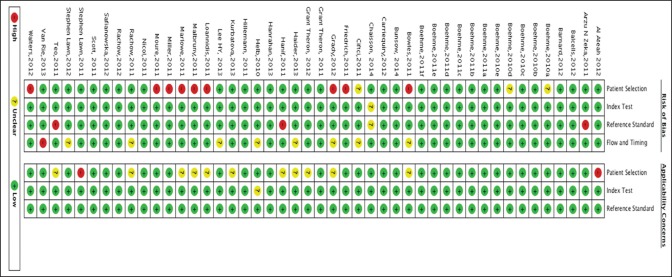
Risk of bias table and summary shows the overall risk of bias and applicability concerns for the 47 study centers [Figures 2 and 3]. Xpert MTB/RIF assay has been developed by only one manufacturer and, as a new test for which there has been considerable attention and scrutiny; it was believed that reporting bias was minimal. In the figure showing risk of bias summary, 10 presents the quality assessment results for the individual study centers. In the patient selection domain, 73% were at low risk of bias because the center enrolled participants consecutively and avoided inappropriate exclusions. The remaining study centers were at 23% high risk of bias because either the manner of patient selection was by convenience (Bowles et al. [2011]; Hanif et al. [2011]; Ioannidis et al. [2011]; Malbruny et al. [2011]; Marlowe et al.; and Miller et al. [2011]) or the study preselected smear positive patients (Friedrich et al. [2011]; Williamson et al. [2012]) or 4% (Ciftci et al. [2011]) at unclear risk of bias because the manner of patient selection was not stated. High concern was among 2% studies and 25% of studies had unclear concern. In the index test (Xpert MTB/RIF) domain, 99% were considered to be at low risk of bias and low concern regarding applicability and the remaining at unclear concerns and risk. In the reference standard domain, 95% were judged to be at low risk of bias because the reference standard results were interpreted without knowledge of the results of the Xpert MTB/RIF assay. Applicability was of low concern for all the studies in the reference standard domain. Furthermore, 77% of the studies were at low risk of bias in the flow and timing domain. Overall, the studies show a low risk of any form of bias, showing that findings are of high scientific validity and credibility.
Figure 2.
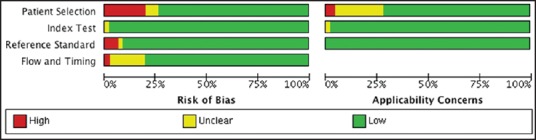
Risk of bias and applicability concerns graph for 47 included study centers (37 studies)
Figure 3.
Risk of bias and applicability concerns summary: Review authors’ judgments about each domain for each included study center
Findings
The results have been depicted in the form of forest plot, + likelihood ratio (LR), −LR and receiver operating curve (ROC). As per the choice of optimal test, +LR should be greater than 10 and −LR should be less than equal to 0.1, for the test to be considered as “robust” and 24 studies were showing +LR ≥10 and −LR ≤0.1. Therefore, Xpert MTB/RIF, diagnostic test for TB fulfilled the criteria for being a robust test [Figure 4].
Figure 4.
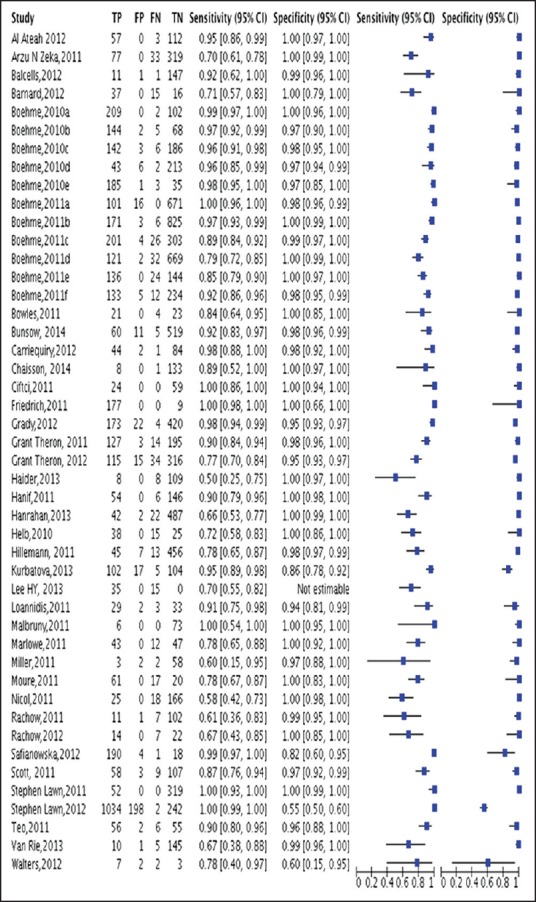
Forest plot showing sensitivity and specificity results of Xpert test
Figure 5 shows two ROC curves representing Xpert MTB/RIF versus other tests (Smear microscopy) plotted on the same graph. The accuracy of the test depends on how well the test separates the group being tested into those with and without the disease in question. Accuracy is measured by the area under the ROC curve. An area of “1” represents a perfect test and an area of 0.5 represents a worthless test. A rough guide for classifying the accuracy of a diagnostic test is the traditional academic point system shown below − 0.90-1 = Excellent (A); 0.80-0.90 = Good (B); 0.70-0.80 = Fair (C); 0.60-0.70 = Poor (D); and 0.50-0.60 = Fail (F) [Table 1].
Figure 5.
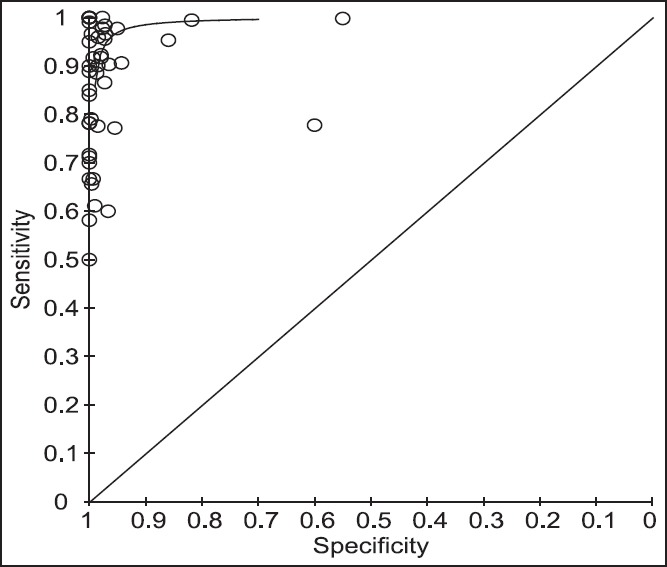
Receiver operating curve curves representing Xpert versus other tests (smear microscopy, culture, culture and smear, and amplicor MTB test)
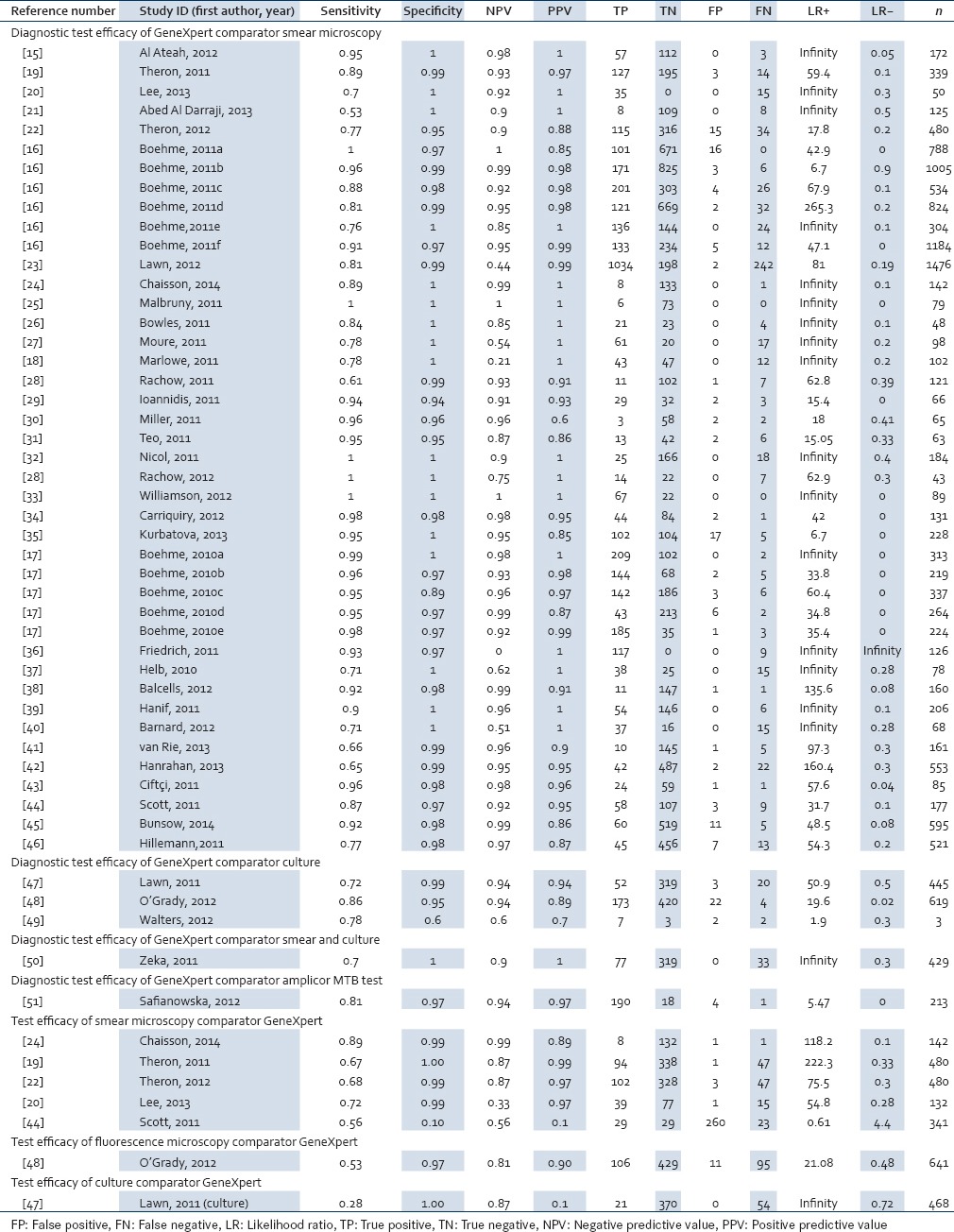
Table 1.
Data showing diagnostic test efficacy of Xpert
DISCUSSION
Our results show that Xpert MTB/RIF assay is a significantly more sensitive and specific test as compared to other diagnostic tests (microscopic and smear culture) for accurate diagnosis of TB. Diagnosis and appropriate treatment of TB can help in achieving the targets. TB is mainly curable, if it is detected and treated effectively, thus it is highly significant to have robust economically viable diagnostic tests.
According to the WHO data of 2011, only 48% of the MDR-TB patients detected were treated successfully, 16% were died and 12% were not cured regardless of treatments and the rest did not get their outcome reported. Worse, the treatment success rate was only 22% for XDR TB.[4] WHO has set high goals to end the global TB epidemic such that there should be a 95% reduction in TB deaths and a 90% decrease in TB incidence by year 2035 as compared to the 2015 targets.[2]
The priority areas to achieve these surmountable targets include administration of highly effective treatment to prevent MDR-TB and second, expansion of rapid diagnostic assays for MDR-TB.[7] However, diagnosis with the bacterial culture still remains the gold standard in many developing countries and the 125-year-old sputum microscopic examination is still the most widely used method for the detection of TB.[6,12] On the other hand, a single Xpert MTB/RIF run can provide both detection of TB and detection of rifampicin resistance within 2 h.[6] It is a quick assay with minimum hands-on time and negligible safety concerns for persons handling the samples. WHO recommends the use of Xpert MTB/RIF assay worldwide.[2,52] For pulmonary TB, the strong recommendation is to use Xpert MTB/RIF assay as the initial diagnostic assay in adults and children presumed to have MDR-TB or HIV-associated TB and the conditional recommendation is for initial diagnosis among adults and children presumed to have TB. It is also strongly recommended for the initial diagnostic test of cerebrospinal fluid of patients presumed to have TB meningitis.[6]
Xpert MTB/RIF assay can be used directly on the clinical specimens, either raw sputum samples or sputum sediments created after decontaminating the sample and concentrating it.[53] Even the unprocessed sputum samples and those from the extrapulmonary sites can be used for this assay.[8] Xpert MTB/RIF detects both live and dead bacteria.[54] The Xpert MTB/RIF test is being used both as an initial test replacing smear microscopy in a population unselected by smear status and as an add-on test following a negative smear microscopy result. Efforts are being made to increase the coverage area of use of Xpert MTB/RIF across the world. Subsidies are being provided by various organizations so that this assay is available in the developing and underdeveloped countries where health systems work with economic constraints. The inclusion of Xpert MTB/RIF assay in the national control programs by different countries will definitely shift the center of diagnosis of HIV-associated and resistant TB from specialized laboratories to decentralized health care centers.[7]
As the alternative conventional diagnostic tests are less sensitive, we require specialized training and biosafety set up and are not so quick, the use of Xpert MTB/RIF assay can be increased, especially in countries with higher prevalence of HIV with TB.[7,9,13] Sensitivity and specificity of Xpert MTB/RIF is more than smear microscopy, liquid and solid culture, and even is less prone to contamination and is more biocompatible as it has tuberculocidal properties and does not pose a biosafety concern to the human kind. Although the sensitivity of the alternative tests like microscopic examination can be improved, still a large fraction cannot be detected and there is no provision for detecting the drug resistance. The conventional methods of detection of drug resistance include bacterial culture and drug susceptibility testing (DST), which are slow and cumbersome. This delay can lead to the administration of inappropriate treatment to the patient, thus increasing the risk of spread and resistance.[7] Results of NAAT results can be highly inconsistent with in-house kits.[55] The main advantage of NAATs is that they can provide results several weeks earlier than culture.[56] Drawbacks are that these tests are often too expensive and complex for routine use by TB programs in resource-limited settings.[56] Advantages of LPAs are that they can provide a result for detection of TB in 1-2 days. In addition, these assays have high sensitivity (>90%) and specificity (>99%) on TB isolates and smear-positive sputum specimens.[57] However, LPAs are expensive and must be used in reference laboratories.[58]
Other WHO recommended diagnostic tests at the levels of laboratories include LED microscopy, which can be used as a replacement of conventional fluorochrome and light microscopy; commercial liquid culture, DST system; rapid speciation strip technology; commercial molecular; and LPAs. LPAs can help in the detection of rifampicin and/or isoniazid resistance in smear positive specimens or culture isolates.[11] Xpert MTB/RIF assay has not replaced the conventional methods (microscopy, culture, and DST) of diagnosis; those are required for monitoring and for detecting resistance to other drugs.[11]
Rapid and accurate case detection is critical for effective treatment, prevention of transmission of infection, treatment failures, relapse, and development of resistant cases. As we are lagging behind the targets of STOP TB, consistent research and funding is required for the development of propagation of the best monitoring centers.[5] Inflow of huge funds for specific projects related to accessibility of Xpert MTB/RIF assay will help in achieving the TB control targets. The escalation in consumption of cartridges by the developing countries in last 3 years has been very encouraging.[11]
CONCLUSION
Xpert MTB/RIF assay is a sensitive specific robust assay as compared to other conventional tests for accurate initial TB diagnosis and detection of rifampicin resistance. Xpert MTB/RIF assay may be used as an add-on test to microscopy for smear negative patients. This diagnostic test can be, especially beneficial for the diagnosis of TB among HIV patients in the developing countries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.World Health Organization. [Last accessed on 2015 Jun 25]. Available from: http://www.who.int/mediacentre/factsheets/fs104/en/
- 2.World Health Organization. [Last accessed on 2015 Jun 25]. Available from: http://www.who.int/tb/publications/global_report/gtbr14_executive_summary.pdf?ua=1 .
- 3.Tuberculosis Control, India. [Last accessed on 2015 Jun 25]. Available from: http://www.tbcindia.nic.in/rntcp.html .
- 4.World Health Organization. [Last accessed on 2015 Jun 26]. Available from: http://www.who.int/tb/challenges/mdr/mdr_tb_factsheet.pdf?ua=1 .
- 5.Lawn SD, Mwaba P, Bates M, Piatek A, Alexander H, Marais BJ, et al. Advances in tuberculosis diagnostics: The Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13:349–61. doi: 10.1016/S1473-3099(13)70008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. [Last accessed on 2015 Jun 26]. Available from: http://www.who.int/tb/publications/Xpert_factsheet.pdf .
- 7.Weyer K, Mirzayev F, Migliori GB, Van Gemert W, D’Ambrosio L, Zignol M, et al. Rapid molecular TB diagnosis: Evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J. 2013;42:252–71. doi: 10.1183/09031936.00157212. [DOI] [PubMed] [Google Scholar]
- 8.Lawn SD, Nicol MP. Xpert® MTB/RIF assay: Development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6:1067–82. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593. doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banada PP, Sivasubramani SK, Blakemore R, Boehme C, Perkins MD, Fennelly K, et al. Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings. J Clin Microbiol. 2010;48:3551–7. doi: 10.1128/JCM.01053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. [Last accessed on 2015 Jun 26]. Available from: http://www.who.int/tb/publications/tbDiagnostics_factsheet.pdf?ua=1 .
- 12.Iram S, Zeenat A, Hussain S, Wasim Yusuf N, Aslam M. Rapid diagnosis of tuberculosis using Xpert MTB/RIF assay—Report from a developing country. Pak J Med Sci. 2015;31:105–10. doi: 10.12669/pjms.311.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steingart KR, Sohn H, Schiller I, Kloda LA, Boehme CC, Pai M, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;1:CD009593. doi: 10.1002/14651858.CD009593.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Green S, editors. Guide to the contents of a Cochrane protocol and review. Cochrane Handbook for Systematic Reviews of Interventions. Ch. 4, Ver. 5.1.0. The Cochrane Collaboration. 2011. [Last updated on 2015 Sep 01]. Available from: http://www.cochrane-handbook.org .
- 15.Al-Ateah SM, Al-Dowaidi MM, El-Khizzi NA. Evaluation of direct detection of Mycobacterium tuberculosis complex in respiratory and non-respiratory clinical specimens using the Cepheid Gene Xpert® system. Saudi Med J. 2012;33:1100–5. [PubMed] [Google Scholar]
- 16.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: A multicentre implementation study. Lancet. 2011;377:1495–505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marlowe EM, Novak-Weekley SM, Cumpio J, Sharp SE, Momeny MA, Babst A, et al. Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol. 2011;49:1621–3. doi: 10.1128/JCM.02214-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, Murray S, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011;184:132–40. doi: 10.1164/rccm.201101-0056OC. [DOI] [PubMed] [Google Scholar]
- 20.Lee HY, Seong MW, Park SS, Hwang SS, Lee J, Park YS, et al. Diagnostic accuracy of Xpert® MTB/RIF on bronchoscopy specimens in patients with suspected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2013;17:917–21. doi: 10.5588/ijtld.12.0885. [DOI] [PubMed] [Google Scholar]
- 21.Abed Al-Darraji HA, Abd Razak H, Ng KP, Altice FL, Kamarulzaman A. The diagnostic performance of a single GeneXpert MTB/RIF assay in an intensified tuberculosis case finding survey among HIV-infected prisoners in Malaysia. PLoS One. 2013;8:e73717. doi: 10.1371/journal.pone.0073717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theron G, Peter J, Lenders L, van Zyl-Smit R, Meldau R, Govender U, et al. Correlation of Mycobacterium tuberculosis specific and non-specific quantitative Th1 T-cell responses with bacillary load in a high burden setting. PLoS One. 2012;7:e37436. doi: 10.1371/journal.pone.0037436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawn SD, Kerkhoff AD, Vogt M, Ghebrekristos Y, Whitelaw A, Wood R. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis. 2012;54:1071–9. doi: 10.1093/cid/cir1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaisson LH, Roemer M, Cantu D, Haller B, Millman AJ, Cattamanchi A, et al. Impact of GeneXpert MTB/RIF assay on triage of respiratory isolation rooms for inpatients with presumed tuberculosis: A hypothetical trial. Clin Infect Dis. 2014;59:1353–60. doi: 10.1093/cid/ciu620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malbruny B, Le Marrec G, Courageux K, Leclercq R, Cattoir V. Rapid and efficient detection of Mycobacterium tuberculosis in respiratory and non-respiratory samples. Int J Tuberc Lung Dis. 2011;15:553–5. doi: 10.5588/ijtld.10.0497. [DOI] [PubMed] [Google Scholar]
- 26.Bowles EC, Freyée B, van Ingen J, Mulder B, Boeree MJ, van Soolingen D. Xpert MTB/RIF®, a novel automated polymerase chain reaction-based tool for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2011;15:988–9. doi: 10.5588/ijtld.10.0574. [DOI] [PubMed] [Google Scholar]
- 27.Moure R, Muñoz L, Torres M, Santin M, Martín R, Alcaide F. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. J Clin Microbiol. 2011;49:1137–9. doi: 10.1128/JCM.01831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rachow A, Zumla A, Heinrich N, Rojas-Ponce G, Mtafya B, Reither K, et al. Rapid and accurate detection of Mycobacterium tuberculosis in sputum samples by Cepheid Xpert MTB/RIF assay—A clinical validation study. PLoS One. 2011;6:e20458. doi: 10.1371/journal.pone.0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannidis P, Papaventsis D, Karabela S, Nikolaou S, Panagi M, Raftopoulou E, et al. Cepheid GeneXpert MTB/RIF assay for Mycobacterium tuberculosis detection and rifampin resistance identification in patients with substantial clinical indications of tuberculosis and smear-negative microscopy results. J Clin Microbiol. 2011;49:3068–70. doi: 10.1128/JCM.00718-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MB, Popowitch EB, Backlund MG, Ager EP. Performance of Xpert MTB/RIF RUO assay and IS6110 real-time PCR for Mycobacterium tuberculosis detection in clinical samples. J Clin Microbiol. 2011;49:3458–62. doi: 10.1128/JCM.05212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teo J, Jureen R, Chiang D, Chan D, Lin R. Comparison of two nucleic acid amplification assays, the Xpert MTB/ RIF and the amplified Mycobacterium tuberculosis direct assay, for the detection of Mycobacterium tuberculosis in respiratory and non-respiratory specimens. J Clin Microbiol. 2011;49:3659–62. doi: 10.1128/JCM.00211-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicol MP, Workman L, Isaacs W, Munro J, Black F, Eley B, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: A descriptive study. Lancet Infect Dis. 2011;11:819–24. doi: 10.1016/S1473-3099(11)70167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson DA, Basu I, Bower J, Freeman JT, Henderson G, Roberts SA. An evaluation of the Xpert MTB/RIF assay and detection of false-positive rifampicin resistance in Mycobacterium tuberculosis. Diagn Microbiol Infect Dis. 2012;74:207–9. doi: 10.1016/j.diagmicrobio.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Carriquiry G, Otero L, González-Lagos E, Zamudio C, Sánchez E, Nabeta P, et al. A diagnostic accuracy study of Xpert® MTB/RIF in HIV-positive patients with high clinical suspicion of pulmonary tuberculosis in Lima, Peru. PLoS One. 2012;7:e44626. doi: 10.1371/journal.pone.0044626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurbatova EV, Kaminski DA, Erokhin VV, Volchenkov GV, Andreevskaya SN, Chernousova LN, et al. Performance of Cepheid® Xpert MTB/RIF® and TB-Biochip® MDR in two regions of Russia with a high prevalence of drug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2013;32:735–43. doi: 10.1007/s10096-012-1798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedrich SO, von Groote-Bidlingmaier F, Diacon AH. Xpert MTB/RIF assay for diagnosis of pleural tuberculosis. J Clin Microbiol. 2011;49:4341–2. doi: 10.1128/JCM.05454-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229–37. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balcells ME, García P, Chanqueo L, Bahamondes L, Lasso M, Gallardo AM, et al. Rapid molecular detection of pulmonary tuberculosis in HIV-infected patients in Santiago, Chile. Int J Tuberc Lung Dis. 2012;16:1349–53. doi: 10.5588/ijtld.12.0156. [DOI] [PubMed] [Google Scholar]
- 39.Hanif SN, Eldeen HS, Ahmad S, Mokaddas E. GeneXpert® MTB/RIF for rapid detection of Mycobacterium tuberculosis in pulmonary and extra-pulmonary samples. Int J Tuberc Lung Dis. 2011;15:1274–5. doi: 10.5588/ijtld.11.0394. [DOI] [PubMed] [Google Scholar]
- 40.Barnard M, Gey van Pittius NC, van Helden PD, Bosman M, Coetzee G, Warren RM. The diagnostic performance of the GenoType MTBDRplus version 2 line probe assay is equivalent to that of the Xpert MTB/RIF assay. J Clin Microbiol. 2012;50:3712–6. doi: 10.1128/JCM.01958-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Rie A, Page-Shipp L, Hanrahan CF, Schnippel K, Dansey H, Bassett J, et al. Point-of-care Xpert® MTB/RIF for smear-negative tuberculosis suspects at a primary care clinic in South Africa. Int J Tuberc Lung Dis. 2013;17:368–72. doi: 10.5588/ijtld.12.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanrahan CF, Selibas K, Deery CB, Dansey H, Clouse K, Bassett J, et al. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PLoS One. 2013;8:e65421. doi: 10.1371/journal.pone.0065421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciftçi IH, Aslan MH, Asik G. Evaluation of Xpert MTB/RIF results for the detection of Mycobacterium tuberculosis in clinical samples. Mikrobiyol Bul. 2011;45:43–7. [PubMed] [Google Scholar]
- 44.Scott LE, McCarthy K, Gous N, Nduna M, Van Rie A, Sanne I, et al. Comparison of Xpert MTB/RIF with other nucleic acid technologies for diagnosing pulmonary tuberculosis in a high HIV prevalence setting: A prospective study. PLoS Med. 2011;8:e1001061. doi: 10.1371/journal.pmed.1001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bunsow E, Ruiz-Serrano MJ, López Roa P, Kestler M, Viedma DG, Bouza E. Evaluation of GeneXpert MTB/RIF for the detection of Mycobacterium tuberculosis and resistance to rifampin in clinical specimens. J Infect. 2014;68:338–43. doi: 10.1016/j.jinf.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Hillemann D, Rüsch-Gerdes S, Boehme C, Richter E. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol. 2011;49:1202–5. doi: 10.1128/JCM.02268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawn SD, Brooks SV, Kranzer K, Nicol MP, Whitelaw A, Vogt M, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: A prospective study. PLoS Med. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Grady J, Bates M, Chilukutu L, Mzyece J, Cheelo B, Chilufya M, et al. Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting where tuberculosis and HIV infection are highly endemic. Clin Infect Dis. 2012;55:1171–8. doi: 10.1093/cid/cis631. [DOI] [PubMed] [Google Scholar]
- 49.Walters E, Gie RP, Hesseling AC, Friedrich SO, Diacon AH, Gie RP. Rapid diagnosis of pediatric intrathoracic tuberculosis from stool samples using the Xpert MTB/RIF Assay: A pilot study. Pediatr Infect Dis J. 2012;31:1316. doi: 10.1097/INF.0b013e318266c21c. [DOI] [PubMed] [Google Scholar]
- 50.Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2011;49:4138–41. doi: 10.1128/JCM.05434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Safianowska A, Walkiewicz R, Nejman-Gryz P, Grubek-Jaworska H. The use of selected commercial molecular assays for the microbiological diagnosis of tuberculosis. Pneumol Alergol Pol. 2012;80:6–12. [PubMed] [Google Scholar]
- 52.World Health Organization. [Last accessed on 2015 Jun 26]. Available from: http://www.who.int/tb/laboratory/mtbrifrollout/en/
- 53.Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, et al. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol. 2010;48:2495–501. doi: 10.1128/JCM.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miotto P, Bigoni S, Migliori GB, Matteelli A, Cirillo DM. Early tuberculosis treatment monitoring by Xpert(R) MTB/RIF. Eur Respir J. 2012;39:1269–71. doi: 10.1183/09031936.00124711. [DOI] [PubMed] [Google Scholar]
- 55.Flores LL, Pai M, Colford JM, Jr, Riley LW. In-house nucleic acid amplification tests for the detection of Mycobacterium tuberculosis in sputum specimens: meta-analysis and meta-regression. BMC Microbiol. 2005;5:55. doi: 10.1186/1471-2180-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7–10. [PubMed] [Google Scholar]
- 57.Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: A meta-analysis. Eur Respir J. 2008;32:1165–74. doi: 10.1183/09031936.00061808. [DOI] [PubMed] [Google Scholar]
- 58.Nahid P, Kim PS, Evans CA, Alland D, Barer M, Diefenbach J, et al. Clinical research and development of tuberculosis diagnostics: Moving from silos to synergy. J Infect Dis. 2012;205(Suppl 2):S159–68. doi: 10.1093/infdis/jis194. [DOI] [PMC free article] [PubMed] [Google Scholar]