Abstract
Background:
This study was undertaken to evaluate and establish the role of total sialic acid (TSA) and highly sensitive C-reactive protein (hs-CRP) in type 2 diabetes mellitus (T2DM) and its correlation with complications such as diabetic nephropathy.
Materials and Methods:
One hundred fifty-seven patients with T2DM with nephropathy (DN) and 162 patients of T2DM without nephropathy (DM) along with 165 unrelated age and sex-matched healthy controls were included in the study. Serum glucose (fasting and postprandial) levels, renal profile, and lipid profile were done as per standard protocol. Serum TSA test levels and hs-CRP level were evaluated using thiobarbituric acid assay and immunoturbidimetric method respectively.
Results:
We observed a higher concentration of serum TSA (82.67 ± 6.63 mg/dl) and hs-CRP (3.2 ± 1.44 mg/L) in diabetic nephropathy than the diabetes mellitus group (73.83 ± 6.90 mg/dl and 2.07 ± 1.32 mg/L, respectively). Both TSA and hs-CRP levels were found significantly correlated with fasting and postprandial blood sugar, hemoglobin A1c, and urine microalbumin levels in both DM and DN groups. Multinomial logistic regression analysis showed that both TSA and hs-CRP was independently associated with diabetic nephropathy.
Conclusion:
High serum TSA and hs-CRP levels may increase the microangiopathic (diabetic nephropathy) complications of T2DM.
Keywords: Diabetic nephropathy, highly sensitive C-reactive protein, total sialic acid, type 2 diabetes mellitus
INTRODUCTION
Diabetes mellitus (DM) has become a pandemic, most common noncommunicable disease, and around 347 million people worldwide have diabetes.[1] WHO projects that diabetes will be the 7th leading cause of death in 2030.[2]
Type 2 diabetes comprises 90% of people with diabetes around the world,[3] and is largely the result of excess body weight and physical inactivity. The long-term damage is caused by chronic hyperglycemia and results in dysfunction and failure of various organs especially the eyes, kidneys, nerves, heart, and blood vessels.[4] Diabetic nephropathy is one of the three major microangiopathies of diabetes mellitus and occurs in approximately 30% of type 2 DM (T2DM) patients.[5] Diabetic nephropathy is usually first recognized as proteinuria. Urinary albumin excretion may be then, an indicator of renal disease in T2DM patients and, in fact, may reflect a state of generalized vascular damage occurring throughout the body.
Acute inflammation is the immediate and early response to an injurious agent. T2DM is frequently associated with an inflammatory status; there is a cytokine associated acute phase reaction, part of the innate immune response.[6] Among several markers of inflammation, highly sensitive C-reactive protein (hs-CRP) is found to be significant in people with diabetes.[7] Several studies demonstrate that hs-CRP remained a significant predictor of diabetes risk even after adjusting with body mass index, family history of diabetes mellitus, smoking, and other factors.[8,9,10]
Sialic acid is a generic term for a family of acetylated derivatives of neuraminic acid. It is an essential component of glycoproteins and glycolipids. It is located in the terminal nonreducing ends of carbohydrate chains being linked to other sugars most commonly galactose and N-acetyl galactosamine. It acts as a cofactor of many cell surface receptors, e.g., insulin receptor and is positively associated with most of the serum acute phase reactants. In human plasma, large quantity of sialic acid is found as a component of orosomucoid, alpha-1-antitrypsin, haptoglobin, ceruloplasmin, fibrinogen, complement proteins, and transferrin.[11,12] Some of these sialylated glycoproteins are called acute phase reactants, and such substances rapidly increase in concentration after the onset of an inflammatory reaction or injury. Hypothesis has also been made that a cytokine-induced acute phase response is an integral part of the pathophysiology of T2DM, which leads to elevated serum sialic acid level.[12,13]
In diabetic nephropathy, there is a greater increase in sialic acid due to the damage of the vascular endothelial cells of the kidney and it is considered as a newly established potential risk factor for the development of diabetic nephropathy.[14]
Therefore, the aim of our study was to see the association of markers of acute phase response to serum total sialic acid (TSA) and hs-CRP levels in T2DM patients with and without nephropathy and compared them with controls.
MATERIALS AND METHODS
Subjects
Between July 2011 and Jun 2013, we recruited 157 patients with T2DM with nephropathy (DN) and 162 patients of T2DM without nephropathy (DM) from Outpatient Department of Medicine, M. Y. Hospital, Indore, Madhya Pradesh, India. This study was conducted with the approval of the Institutional Ethical Committee. Written informed consent was taken from the subjects prior to the study.
The inclusion criteria for patients were the onset of diabetes after the age of 35 years and no episodes of ketoacidosis. Patients on any kind of multivitamin, lipid lowering agents, anti-inflammatory drugs, analgesics, anticoagulants like aspirin, pregnant or lactating women, alcoholics, smokers and individuals with tobacco or drug addiction, past or present history of chronic illness like tuberculosis, rheumatoid arthritis other autoimmune disorders and patients of juvenile and type 1 DM were excluded from the study group.
Diabetic nephropathy is clinically defined by the presence of persistent microalbuminuria (>30 mg/day) in a diabetic patient in the absence of clinical or laboratory evidence of other kidney or urinary tract disease. For comparison, we recruited 165 unrelated age and sex matched healthy controls.
Fasting venous blood sample was drawn from all the subjects in EDTA tube (2 ml) and plain tube (5 ml), the serum was carefully separated and transferred to microtubes and stored at −20°C until analysis. Postprandial venous blood sample was collected 2 h after the meal. Total serum cholesterol, triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), fasting, and postprandial glucose were analyzed on the fully automated analyzer (Biosystems A25, Barcelona, Spain). Serum hs-CRP levels were analyzed by Turbidimetry Analysers (Quantimate, Tulip, India). Serum TSA levels were estimated using thiobarbituric acid assay method as described by Warren.[15]
Statistical analysis
All the data were entered in SPSS 20.0 (IBM Statistics, SPSS Inc., Chicago, IL, USA). One-way ANOVA followed by Bonferroni test was applied to see the difference in means of various biochemical parameters in DM, DN, and control groups. Karl Pearson correlation test was applied to find out the linear correlation of different parameters. The multinomial logistic analysis was performed with bootstrapping of 1000 samples to see the factors independently associated with diabetic nephropathy.
RESULTS
The mean age of DM and DN patients was 53.18 ± 10.4 and 54.59 ± 7.85 years, respectively. There was no significant difference in age and sex between all groups [Table 1].
Table 1.
Demographic and biochemical profile in three groups
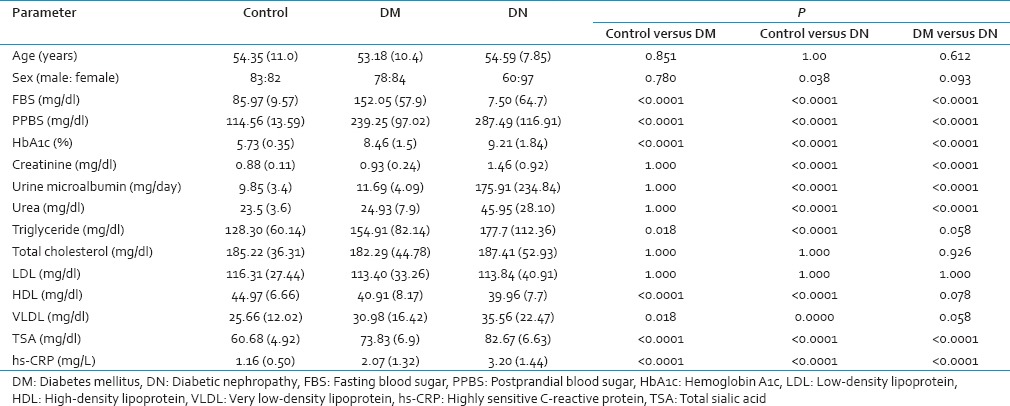
We observed a significant higher fasting and postprandial blood glucose, as well as hemoglobin A1c (HbA1c) in DN group as compared to DM and control groups. The mean duration of diabetes was 5.00 ± 2.2 and 6.55 ± 2.7 years in DM and DN groups, respectively.
TG levels were found significantly different in all three groups [Table 1], and it was highest in diabetic nephropathy group (177.7 ± 112.36 mg/dl). Total cholesterol and LDL levels were similar in all three groups. However, HDL cholesterol levels were found significantly low in DM (P < 0.0001) and DN (P < 0.0001) group when compared from controls.
TSA was found significantly raised in DN group (82.67 ± 6.63 mg/dl) when compared from controls (60.68 ± 4.92 mg/dl). TSA was also found significantly higher in DM group as compared to controls (P < 0.0001) but it was within the normal limits.
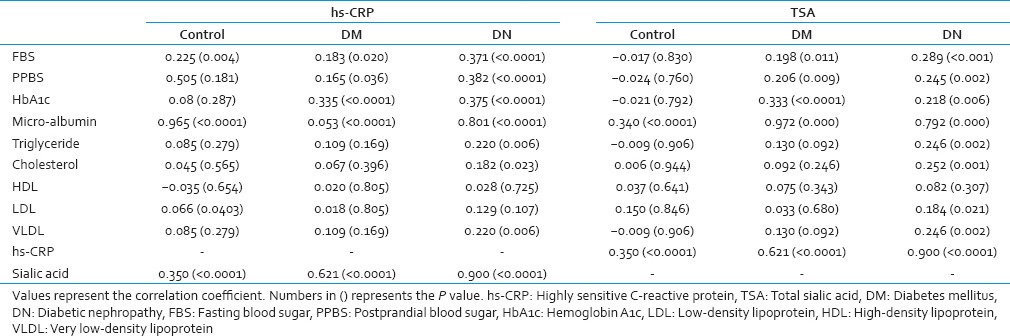
TSA was found significantly correlated with fasting blood sugar (FBS), postprandial blood sugar (PPBS), HbA1c, urine microalbumin, and hs-CRP in both DM and DN groups [Table 2]. However, no correlation of serum TSA was observed with FBS, PPBS, and HbA1c in the control groups. Similarly, hs-CRP levels were also found significantly correlated with FBS, PPBS, HbA1c, urine microalbumin and total sialic acid in both DM and DN groups [Table 2].
Table 2.
Correlation of hs-CRP and TSA with other parameters
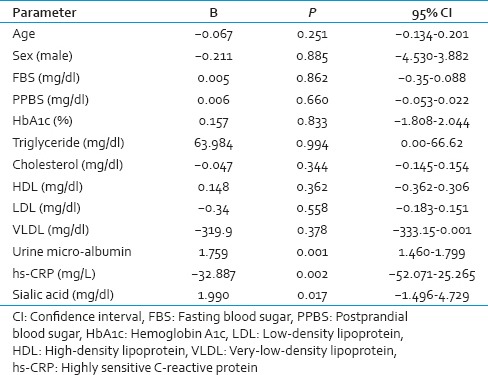
Multinomial logistic regression analysis showed that both TSA and hs-CRP was independently associated with diabetic nephropathy [Table 3].
Table 3.
Multinomial logistic regression analysis
DISCUSSION
Inflammation plays a major role in the pathogenesis of T2DM and its complications. Hence inflammatory markers or acute phase markers have gained the importance as indicators and predictors of the diabetic process.
It is perceived that chronic low-grade inflammation as evidenced by elevated hs-CRP might potentially be a cause underlying the etiology and manifestation of T2DM, although the exact mechanisms are still not well understood. Martha and Fernando[7] in their study found that hyperglycemia is an associated factor to the increase of serum CRP levels; in uncontrolled type 2 diabetes subjects. Lima et al.[16] and Amanullah et al.[17] in their study found that hypertensive patients with T2DM had higher levels of hs-CRP than normal subjects. This finding suggests that patients with two associated diseases have a more active inflammatory state. Several studies demonstrate that hs-CRP remained a significant predictor of diabetes risk even after adjusting with BMI, family history of DM, smoking, and other factors.[8] Chiriboga et al.,[18] Jager et al.[19] and Pfützner and Forst[20] in their studies showed that in people with diabetes, CRP levels in highest tertile (>0.28 mg/dl) were associated with a two-fold increase in cardiovascular mortality after adjusting for age, sex, and glucose tolerance tests. Similar to these previous studies hs-CRP levels were found raised in diabetic mellitus with or without nephropathy. Patients with diabetic nephropathy have significant higher hs-CRP than diabetic mellitus.
TSA was also found raised in both diabetes mellitus with or without nephropathy. Similar to our study K Prajna et al. observed significantly increased TSA levels in diabetes mellitus without any complication and in diabetes mellitus with nephropathy as compared to control.[21] It was also fond positively correlated with blood glucose levels in both the groups. Similar to our study Masuda et al.[22] have shown that serum TSA reflects the status of blood glucose control and the progression of the ischemic disease of the lower extremities in T2DM. Crook et al. shows that serum TSA is a newly established potential risk factor for the development of macrovascular and microvascular complications of diabetes.[23]
Similar findings have been stated by Chen et al.,[13] Nayak and Bhaktha[14] and Krishnamurthy et al.[24] Shahid and Mahboob in their study found increased serum TSA as a potential risk factor for the development of macro and microvascular complications of diabetes.[25] In conclusion serum hs-CRP and TSA may be used as a predictive biomarkers of diabetic nephropathy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Status Report on Noncommunicable Diseases 2010. Geneva: World Health Organization; 2011. [Google Scholar]
- 3.World Health Organization. Part 1: Diagnosis and Classification of Diabetes Mellitus. (WHO/NCD/NCS/99.2) Geneva: World Health Organization; 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. [Google Scholar]
- 4.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35:S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar R, Sharma RK, Agarwal S. Genetic predisposition for development of nephropathy in type 2 diabetes mellitus. Biochem Genet. 2013;51:865–75. doi: 10.1007/s10528-013-9613-x. [DOI] [PubMed] [Google Scholar]
- 6.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, et al. Low-grade systemic inflammation and the development of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes. 2003;52:1799–805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 7.Martha RM, Fernando GR. Increased levels of CRP in non-controlled type II diabetic subjects. J Diabetes Complications. 1999;13:211–5. doi: 10.1016/s1056-8727(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 9.Mahajan A, Tabassum R, Chavali S, Dwivedi OP, Bharadwaj M, Tandon N, et al. High-sensitivity C-reactive protein levels and type 2 diabetes in urban North Indians. J Clin Endocrinol Metab. 2009;94:2123–7. doi: 10.1210/jc.2008-2754. [DOI] [PubMed] [Google Scholar]
- 10.Shahid HS, Kurdi MI, Zohair AA. Serum high-sensitivity C-reactive protein and lipoprotein (a) levels: A comparison between diabetic and non-diabetic patients with coronary artery disease. Med J Malaysia. 2011;66:113–6. [PubMed] [Google Scholar]
- 11.Kishore BK, Gejyo F, Arakawa M. Altered glycosylation and sialylation of serum proteins and lipid bound sialic acids in chronic renal failure. Postgrad Med J. 1983;59:551–5. doi: 10.1136/pgmj.59.695.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–23. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Gall MA, Yokoyama H, Jensen JS, Deckert M, Parving HH. Raised serum sialic acid concentration in NIDDM patients with and without diabetic nephropathy. Diabetes Care. 1996;19:130–4. doi: 10.2337/diacare.19.2.130. [DOI] [PubMed] [Google Scholar]
- 14.Nayak SB, Bhaktha G. Relationship between sialic acid and metabolic variables in Indian type 2 diabetic patients. Lipids Health Dis. 2005;4:15. doi: 10.1186/1476-511X-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234:1971–5. [PubMed] [Google Scholar]
- 16.Lima LM, Carvalho MD, Soares AL, Sabino Ade P, Fernandes AP, Novelli BA, et al. High-sensitivity C-reactive protein in subjects with type 2 diabetes mellitus and/or high blood pressure. Arq Bras Endocrinol Metabol. 2007;51:956–60. doi: 10.1590/s0004-27302007000600010. [DOI] [PubMed] [Google Scholar]
- 17.Amanullah S, Jarari A, Govindan M, Basha MI, Khatheeja S. Association of hs-CRP with diabetic and non-diabetic individuals. Jordan J Biol Sci. 2010;3:7–12. [Google Scholar]
- 18.Chiriboga DE, Ma Y, Li W, Stanek EJ, 3rd, Hébert JR, Merriam PA, et al. Seasonal and sex variation of high-sensitivity C-reactive protein in healthy adults: A longitudinal study. Clin Chem. 2009;55:313–21. doi: 10.1373/clinchem.2008.111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Yudkin JS, Nijpels G, et al. von Willebrand factor, C-reactive protein, and 5-year mortality in diabetic and nondiabetic subjects: The Hoorn Study. Arterioscler Thromb Vasc Biol. 1999;19:3071–8. doi: 10.1161/01.atv.19.12.3071. [DOI] [PubMed] [Google Scholar]
- 20.Pfützner A, Forst T. High-sensitivity C-reactive protein as cardiovascular risk marker in patients with diabetes mellitus. Diabetes Technol Ther. 2006;8:28–36. doi: 10.1089/dia.2006.8.28. [DOI] [PubMed] [Google Scholar]
- 21.KP, Kumar JA, Rai S, Shetty SK, Rai T, Shrinidhi, et al. Predictive value of serum sialic Acid in type-2 diabetes mellitus and its complication (nephropathy) J Clin Diagn Res. 2013;7:2435–7. doi: 10.7860/JCDR/2013/6210.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masuda H, Wakabayashi R, Wakabayashi I. Serum sialic acid and ankle versus brachial arterial-pressure ratio in NIDDM. Scand J Clin Lab Invest. 1998;58:433–39. doi: 10.1080/00365519850186427. [DOI] [PubMed] [Google Scholar]
- 23.Crook M, Lumb P, Andrews V, Swaminathan R. Serum total sialic acid, a reputed cardiovascular risk factor, and its relationship to lipids, plasma fasting insulin, blood pressure and body mass index in normal individuals. Clin Sci (Lond) 1998;95:53–7. doi: 10.1042/cs0950053. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamurthy U, Halyal SS, Murthy DS. Serum sialic acid and microalbuminuria in non-insulin dependent diabetes mellitus. Biomed Res. 2011;22:31–4. [Google Scholar]
- 25.Shahid SM, Mahboob T. Clinical correlation between frequent risk factors of diabetic nephropathy and serum sialic acid. Int J Diabetes Metab. 2006;14:138–42. [Google Scholar]


