Abstract
Background:
Association between C677T polymorphism of the methylenetetrahydrofolate reductase (MTHFR), a key enzyme involved in folate metabolism and DNA methylation, and breast cancer risk are inconsistent. We investigated in a case-control study, possible effect of the common MTHFR C677T polymorphism on breast cancer risk in a sample of Iranian patients.
Materials and Methods:
The study subjects comprised of 123 breast cancer cases and 110 cancer-free control, who were matched for age and body mass index (BMI). C677T genotypes were determined by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay. Lipid profile was measured in all subjects by standard method.
Results:
The genotypes distributions (CC, CT, and TT) were 55.3, 39, and 5.7% in breast cancer cases and 51.8, 44.5, and 3.6% in controls. Chi square analysis revealed that there was no significant association between breast cancer risk and MTHFR genotypes and alleles. Additionally, no significant association was observed between C677T genotypes and biochemistry parameters. A multinomial logistic regression model with MTHFR genotypes, lipid profiles, BMI and age as covariates revealed that there is no significant association between MTHFR genotypes and risk of breast cancer, but higher values of LDL and HDL significantly increase risk of breast cancer.
Conclusions:
Our findings do not support the hypothesis that genetic variation in the MTHFR C677T polymorphism is implicated in the breast cancer risk in a sample of Iranian patients.
Key Words: Brest Cancer, MTHFR C677T polymorphism, PCR-RFLP
INTRODUCTION
Folate, a group of water-soluble B-vitamins, has an important role in DNA methylation, synthesis and repair of DNA, and might protect against cancer. Epidemiological evidence indicating that low intake of folate may increase the risk for neoplasia, including breast cancer.[1,2] The molecular mechanisms linking between folate insufficiency and cancer development could include purine and thymidine depletion and misincorporation of uracil into DNA synthesis, increased DNA strand breaks, aberrations in DNA methylation and disruption of DNA repair.[3,4]
The N5, N10_methylenetetrahydrofolate reductase (MTHFR) is a critical enzyme in folate metabolism, which catalyzes irreversible reaction of N5, N10_methylenetetrahydrofolate (N5, N10_methylene-THF) to N5_methyltetrahydrofolate (N5_methyl_THF), the predominant circulatory form of folate and a one-carbon donor for re-methylation of homocysteine to methionine. Folate that is not converted through this pathway enters another pathway that leads to purine and thymidylate synthesis.[5]
Two common single nucleotide polymorphisms (SNPs) in the MTHFR gene that affect the efficiency of folate metabolism have been described as MTHFR C677T (NCBI SNP ID: rs1801133) transition substitution in exon 4 and MTHFR 1298 A > C transversion substitution in exon 7).[6] The C677T SNP of MTHFR is common at the folate binding site of the MTHFR gene which results in alanine to valine substitution at codon 222.[7,8] In vitro analysis of the MTHFR activity demonstrated that heterozygous and homozygous bearing of the 677T allele variant have a 30–40% and 60–70% reduced enzyme activity, respectively.[7,9,10] Many studies have been found that these low-activity genotypes of MTHFR associated with the risk of a variety of cancers, such as colorectal[11,12], gastric[13,14], endometrial[15], lung cancer[16] and acute leukemia.[17] In addition, numerous case-control studies assessed the association between MTHFR C677T SNP and breast cancer risk, but the findings have been controversial.[18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] Some of them reported a positive association between the 677TT genotype of MTHFR and breast cancer risk[19,22,29,32], whereas no association was noted in other studies.[18,20,21,23,24,25,26,27,28,30,31,32,33,34] Moreover, in another study, an increased risk of breast cancer was found in a selected population of BRCA1 mutation carriers with MTHFR 677TT genotype.[35] We conducted a case-control study in a sample of Iranian women in order to investigate the association between MTHFR C677T genotypes and breast cancer risk.
MATERIALS AND METHODS
Study population
The study population consisted of patients (n = 123) with histologically confirmed breast cancer, admitted to the Ahvaz Medical Faculty and the department of radiation and oncology of Golestan University Hospital, Ahvaz, Iran. The control subjects (n = 110) were recruited from the same geographic area during the same period and were matched to the cases by age and BMI. The control subjects were randomly selected among the people admitted to the same hospital. Anthropometric indices and clinical parameters were measurement by standard methods, as previously described.[36]
MTHFR genotyping
In order to DNA extraction, blood samples were collected into K3-EDTA-treated tube from both patients and controls, and were stored at -20°C. Total genomic DNA was extracted from peripheral blood leukocytes and was dissolved in sterile TBE buffer. The variant MTHFR C677T was genotyped by using PCR-RFLP analysis. The PCR primers were synthesized by primer 3 software and their sequences were as follows: Forward, 5’-CCTGACTGTCATCCCTATTGGC–3′ and reverse 5’- GGAGCTTATGGGCTCTCCTG–3′. Conditions for PCR amplification were 12.5 μl commercially available PCR premix (AccuPower PCR PremiX; BIONEER, Daejeon, Korea) containing (dNTP, TaqDNA polymerase, MgCl2, buffer), 2.0 μl (20 pmol/μl) forward and reverse primers, 2.0 μl (50 ng/μl) template DNA, and 6.5 μl sterile nuclease free water. The thermal cycling conditions were as follows: Initial denaturation at 94°C for 5 min, 35 cycles of denaturation at 94°C for 60 s, annealing at 53°C for 45 s, and extension at 72°C for 60 seconds, with a final extension of 5 min at 72°C. The PCR amplified products were scored in 248-bp in a mixture reaction consisting of: PCR products (10 μl), 10 × buffer (2 μl), 10 units HinfI (New England Bio labs, USA) restriction enzyme, and sterile nuclease free water (18 μl). The reaction mixture was kept overnight at 37°C for 1-16 h. The fragments were separated by electrophoresis on 3% agarose gel, stained with ethidium bromide and results were recorded with photographs of gels in UV light. The C677T substation introduces a new HinfI restriction site which results in the digestion of the 248-bp PCR product into 100 and 148-bp fragments. After electrophoresis of digested DNA fragments, homozygous C allele was represented by a DNA band sized at 248, whereas homozygous T allele was represented by a DNA band sized at 100 and 148-bp and heterozygotes sized at 248, 100 and 148-bp [Figure 1].
Figure 1.
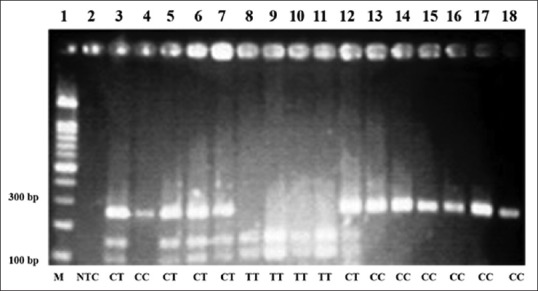
Representative example of MTHFR C677T polymorphism products by PCR-RFLP on agarose gel electrophoresis. Lane 1 shows 100-bp DNA Ladder; Lane 2 shows Non-template control; lanes 3, 5, 6, 7 and 12, show heterozygote CT genotype (248,148 and 100-bp); lanes 4, 13,14,15,16, 17, and 18, show wild type CC genotype (248-bp); lanes 8,9,10 and 11 show mutant TT genotype (148 and 100-bp)
Statistical analyses
Data are expressed as mean ± standard deviation, and all statistical analyses were performed using SPSS software for Windows version 20.0 (IBM Corporation New York, USA). Anthropometric indices and biochemical parameters were compared between breast cancer cases and controls using independent sample t- test, and one way analysis of variance (ANOVA) were used to compare those variables between MTHFR genotypes. All frequencies were estimated by gene counting. The observed genotype frequencies in the breast cancer cases and controls were tested for the Hardy-Weinberg equilibrium (HWE). The statistical significance of the C677T genotype distributions between cases and controls was determined by Chi square analysis. In order to estimate odds ratios (ORs) for breast cancer risk and the corresponding 95% confidence intervals (CI) logistic regression model was used. Multinomial logistic regression analysis was also determined, and results were expressed as P- value, odds ratio (OR) and 95% confidence intervals (95% CI). A P < 0.05 was considered as the criterion for statistical significance.
RESULTS
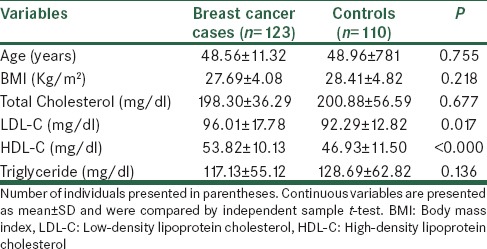
Comparisons of anthropometric indices and biochemical characteristics between breast cancer cases and controls.
Anthropometric indices and biochemical characteristics of breast cancer cases and controls are summarized in Table 1. There were no statistically significant differences between the breast cancer cases and controls for age and BMI (P = 0.755; P = 0.218, respectively). In addition, there were no statistically significant differences between two groups for the means of biochemical characteristics including total cholesterol, triglyceride. However, there was a statistically significant difference between two groups for the means of HDL (P < 0.001) and LDL (P = 0.017).
Table 1.
Comparison the means of age, BMI and lipid profile between breast cancer cases and controls
MTHFR C677T genotype analysis
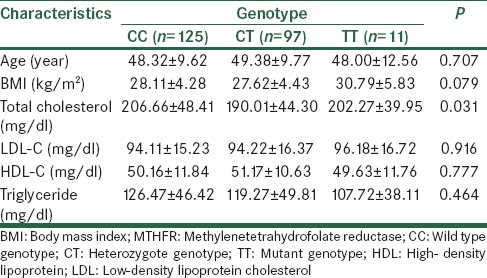
Genotype and allele frequencies of MTHFR C677T polymorphism were compared between breast cancer cases and controls [Table 2]. The observed allele and genotype frequencies in the both breast cancer cases and controls for MTHFR C677T were in accordance with the Hardy-Weinberg lows of equilibrium. The frequencies of the CC, CT, and TT genotypes were 55.3%, 39%, and 5.7% in breast cancer cases and 51.8%, 44.5%, and 3.6% in controls, respectively. Between two study groups, the frequency of genotypes were not different [Table 2, χ2 = 1.075, P = 0.584]. Likewise, the allele frequency, which for C and T alleles were 77.6% 22.4% in cases, and 75.9 and 24.1 in controls, respectively, no significant difference between two groups was observed [Table 2, χ2 = 0.196, P = 0.658]. Overall, there was no association between breast cancer risk and MTHFR C677T genotype and alleles. Conversely, there was an association between CC genotype of this polymorphism and higher mean total cholesterol level [Table 3].
Table 2.
Genotype distribution of MTHFR C677T polymorphism in breast cancer cases and controls
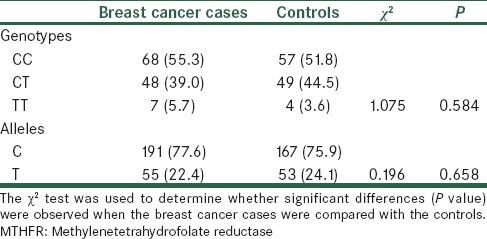
Table 3.
Comparison the means of age, BMI and lipid profile according to the MTHFR C677T genotypes
Risk factors for breast cancer
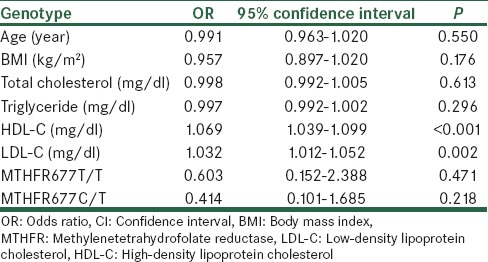
In order to determine predictors of breast cancer we used multinomial logistic regression model, with the dependent variable being breast cancer, and the independent potentially confounding variables being age, BMI, LDL-C, HDL-C, total cholesterol and triglyceride levels and MTHFR C677T genotypes [Table 4]. Among the inherited risk factors, neither homozygosity for MTHFR C677T (OR = 0.603; 95% CI = 0.152-2.388, P = 0.471) nor heterozygosity for MTHFR C677T (OR = 0.414; 95% CI = 0.101-1.680, P = 0.218) were not associated with having breast cancer [Table 4]. Among the non-inherited risk factors, HDL-C (OR = 1.069; 95% CI = 1.039-1.099, P < 0.001), and LDL-C (OR = 1.032; 95% CI = 1.012-1.052, P = 0.002) were associated with having breast cancer [Table 4].
Table 4.
Results of multinomial logistic regression analysis
DISCUSSION
In this case-control study we found no association between a commonly occurring polymorphism of MTHFR C677T and breast cancer risk in a sample of Iranian women. The frequency of the T allele in the cancer-free control of Iranian women (22.4%) seemed to be slightly lower than the reports on other populations, including 35% in Greece[37], 39% in Korea[25], 30% Sothern England[38] or 27-29% in the USA.[38,39,40,41] However, the frequency of the T allele in the whole population (unselected for sex) shows considerable differences in its distribution model in the worldwide, ranging from 10% in African American[42] to 63% in northern China.[43] This variation may account for the basis of the differences observed regarding the association of the C677T polymorphism with cancer risk in studies from different geographical regions.
Previous efforts to investigate the relationship between the common polymorphism of MTHFR C677T and breast cancer have yielded conflicting results.[18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] Although, the results obtained from two meta-analyses, with large sample size of breast cancer cases and controls, showed that the MTHFR C677T polymorphism had low effect on the development of breast cancer.[44,45] However, a strong inverse association has been constantly observed between the MTHFR 677TT genotype and colorectal cancer, particularly in subjects with high levels of folate intake and low levels of alcohol conception.[12]
A study conducted by Marchand et al. reported a significant inverse association between MTHFR 677TT genotype and breast cancer risk among postmenopausal women who were on hormone replacement therapy (HRT) at baseline.[28] They suggested that the MTHFR 677TT genotype may confer a 40% decreased breast cancer risk in postmenopausal women using HRT. And this result is consistent with the role of MTHFR in facilitating the flow of folate for thymidylate and purine synthesis and with the increased nucleic acid need resulting from the hyper proliferative effect of HRT on mammary epithelial cells.
Another study reported opposite effects of these two SNPs on the risk of breast cancer. Results from Long Island Breast Cancer Study demonstrated that the 677T mutant allele was associated with an increased risk of breast cancer and the 1298C mutant allele was associated with a decreased risk of breast cancer.[29] The authors hypothesized that the inverse correlations were caused by the linkage disequilibrium between C677T and A1298C that links a low activity genotype at one locus to a high activity genotype at the other locus.[12,45]
There are many factors that could explain the conflicting results from different studies, including different population characteristics (sample size and ethnic differences), different familial genetic background that may modify breast cancer risk such as BRCA1/2 mutations,[35] steroid hormone administrations, reproductive history, and more critically, menopausal status and folate intake.
Some studies[20,25,28,29] which stratified the population by menopausal status found with various results. A study conducted by Semenza et al.[18] reported a significantly increased risk for breast cancer in premenopausal women with the MTHFR 677 TT genotype, also Chen et al.,[29] observed a higher MTHFR 677 TT frequency in breast cancer cases than in controls, though this difference was borderline significant. Moreover, Ergul et al.[20] investigated a population of premenopausal women and reported a significant positive association (P = 0.016) between MTHFR 677TT genotype and breast cancer risk, although Forsti et al.[24] evaluated a postmenopausal women population with no confirmation of a significant difference between two groups. In addition, Lee et al.[25] Marchand et al.,[28] and chen et al.[29] showed no statistical significant association based on menopausal status, even though the two latter studies were weak powered to detect a difference, due to the small sample of premenopausal compared with postmenopausal women.
The critical function of folate metabolism in breast cancer risk is also supported by several studies which pointed the influence of folate intake in the assessment of MTHFR C677T SNP and breast cancer risk.[18,23,29,30] Although in the first published paper assessment the interactive effect between MTHFR C677T genotypes and folate intake, Sharp et al. did not detected any statistically significant association, probably due to the small sample size[18], but, Shrubsole et al. reported that low intake of folate was significantly associated with an increased risk of breast cancer among all MTHFR C677T genotypes and particularly in subjects with the TT genotype.[23] Chen et al. similarly found an increased but not significant risk of breast cancer in 677TT subjects with the lowest levels of folate intake in comparison to 677 wild type subjects with high levels of folate intake. They also found a significantly increased risk for breast cancer among non-supplement users with MTHFR 677TT genotype.[29] Finally, Chou et al.[34] evaluated the interaction between plasma folate levels and combined genotypes in MTHFR gene. In particular, they analyzed A1298C SNP other than C677T and reported a more pronounced reduction in breast cancer risk among women with lower plasma folate levels and the compound heterozygote and homozygote variants (677CT/TT and 1298AC/CC).
In conclusion our findings showed that higher serum levels of HDL and LDL are significantly associated with breast cancer risk but MTHFR C677T genotypes and alleles did not associate with breast cancer.
ACKNOWLEDGEMENT
This Research Project has been financially supported by Hyperlipidemia Research Center, Ahvaz Jundishapur University of Medical Sciences (grant no. HLRC-9201). We would like to appreciate the staffs of Radiation and Clinical Oncology Department of Ahvaz Golestan Hospital for their friendly cooperation.
Footnotes
Source of Support: This research project has been financially supported by Ahvaz Jundishapur University of Medical sciences (grant no. HLRC-9201), Ahvaz, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Kim YI. Folate and carcinogenesis: Evidence, mechanisms, and implications. J Nutr Biochem. 1999;10:66–88. doi: 10.1016/s0955-2863(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 2.Mason JB, Choi SW. Folate and carcinogenesis: Developing a unifying hypothesis. Enzyme Regul. 2000;40:127–41. doi: 10.1016/s0065-2571(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 3.Bailey LB, Gregory JF., 3rd Polymorphisms of methylenetetrahydrofolate reductase and other enzymes: Metabolic significance, risks and impact on folate requirement. J Nutr. 1999;129:919–22. doi: 10.1093/jn/129.5.919. [DOI] [PubMed] [Google Scholar]
- 4.Choi SW, Mason JB. Folate and carcinogenesis: An integrated scheme. J Nutr. 2000;130:129–32. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 5.Robien K, Ulrich CM, Bigler J, Yasui Y, Gooley T, Bruemmer B, et al. Methylenetetrahydrofolate reductase genotype affects risk of relapse after hematopoietic cell transplantation for chronic myelogenous leukemia. Clin Cancer Res. 2004;10:7592–8. doi: 10.1158/1078-0432.CCR-04-1057. [DOI] [PubMed] [Google Scholar]
- 6.Fodinger M, Horl WH, Sunder-Plassmann G. Molecular biology of 5, 10-methylenetetrahydrofolate reductase. J Nephrol. 2000;13:20–33. [PubMed] [Google Scholar]
- 7.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 8.Goyette P, Sumner JS, Milos R, Duncan AM, Rosenblatt DS, Matthews RG, et al. Human methylenetetrahydrofolate reductase: Isolation of cDNA, mapping and mutation identification. Nat Genet. 1994;7:195–200. doi: 10.1038/ng0694-195. [DOI] [PubMed] [Google Scholar]
- 9.Weisberg IS, Jacques PF, Selhub J, Bostom AG, Chen Z, Curtis ER, et al. The 1298A->C polymorphism in methylenetetrahydrofolate reductase (MTHFR): In vitro expression and association with homocysteine. Atherosclerosis. 2001;156:409–15. doi: 10.1016/s0021-9150(00)00671-7. [DOI] [PubMed] [Google Scholar]
- 10.Sohn KJ, Croxford R, Yates Z, Lucock M, Kim YI. Effect of the methylenetetrahydrofolate reductase C677T polymorphism on chemo sensitivity of colon and breast cancer cells to 5- fluorouracil and methotrexate. J Natl Cancer Inst. 2004;96:134–44. doi: 10.1093/jnci/djh015. [DOI] [PubMed] [Google Scholar]
- 11.Thomas P, Fenech M. Methylenetetrahydrofolate reductase, common polymorphisms, and relation to disease. Vitam Horm. 2008;79:375–392. doi: 10.1016/S0083-6729(08)00413-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Ma J, Stampfer MJ, Palomeque C, Selhub J, Hunter DJ. Linkage disequilibrium between the 677C>T and 1298A>C polymorphisms in human methylenetetrahydrofolate reductase gene and their contributions to risk of colorectal cancer. Pharmacogen. 2002;12:339–342. doi: 10.1097/00008571-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Boccia S, Hung R, Ricciardi G, Gianfagna F, Ebert MP, Fang JY, et al. Metaand pooled analyses of the methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and gastric cancer risk: A huge-GSEC review. Am J Epidemiol. 2008;167:505–16. doi: 10.1093/aje/kwm344. [DOI] [PubMed] [Google Scholar]
- 14.Graziano F, Kawakami K, Ruzzo A, Watanabe G, Santini D, Pizzagalli F, et al. Methylenetetrahydrofolate reductase 677C/T gene polymorphism, gastric cancer susceptibility and genomic DNA hypomethylation in an at-risk Italian population. Int J Cancer. 2006;118:628–32. doi: 10.1002/ijc.21397. [DOI] [PubMed] [Google Scholar]
- 15.Esteller M, Garcia A, Martinez-Palones JM, Xercavins J, Reventos J. Germ line polymorphisms in cytochrome- P450 1A1 (C4887 CYP1A1) and methylenetetrahydrofolate reductase (MTHFR) genes and endometrial cancer susceptibility. Carcinogenesis. 1997;18:2307–11. doi: 10.1093/carcin/18.12.2307. [DOI] [PubMed] [Google Scholar]
- 16.Siemianowicz K, Gminski J, Garczorz W, Slabiak N, Goss M, Machalski M, et al. Methylenetetrahydrofolate reductase gene C677T and A1298C polymorphisms in patients with small cell and non-small cell lung cancer. Oncol Rep. 2003;10:1341–4. [PubMed] [Google Scholar]
- 17.Skibola CF, Smith MT, Kane E, Roman E, Rollinson S, Cartwright RA, et al. Polymorphisms in the methylenetetrahydrofolate reductase gene are associated with susceptibility to acute leukemia in adults. Proc Natl Acad Sci USA. 1999;96:12810–5. doi: 10.1073/pnas.96.22.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharp L, Little J, Schofield AC, Pavlidou E, Cotton SC, Miedzybrodzka Z, et al. Folate and breast cancer: The role of polymorphisms in methylenetetrahydrofolate reductase (MTHFR) Cancer Lett. 2002;181:65–71. doi: 10.1016/s0304-3835(02)00030-7. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T, Matsuo K, Hirose K, Hiraki A, Kawase T, Watanabe M, et al. One-carbon metabolism-related gene polymorphisms and risk of breast cancer. Carcinogenesis. 2008;29:356–362. doi: 10.1093/carcin/bgm295. [DOI] [PubMed] [Google Scholar]
- 20.Semenza JC, Delfino RJ, Ziogas A, Anton-Culver H. Breast cancer risk and methylenetetrahydrofolate reductase polymorphism. Breast Cancer Res Treat. 2003;77:217–23. doi: 10.1023/a:1021843019755. [DOI] [PubMed] [Google Scholar]
- 21.Langsenlehner U, Krippl P, Renner W, Yazdani-Biuki B, Wolf G, Wascher TC, et al. The common 677C>T gene polymorphism of methylenetetrahydrofolatereductase gene is not associated with breast cancer risk. Breast Cancer Res Treat. 2003;81:169–72. doi: 10.1023/A:1025752420309. [DOI] [PubMed] [Google Scholar]
- 22.Maruti SS, Ulrich CM, Jupe ER, White E. MTHFRC677T and postmenopausal breast cancer risk by intakes of one-carbon metabolism nutrients: A nested case-control study. Breast Cancer Res. 2009;11:R91. doi: 10.1186/bcr2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrubsole MJ, Gao YT, Cai Q, Shu XO, Dai Q, Hebert JR, et al. MTHFR polymorphisms, dietary folate intake, and breast cancer risk: Results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2004;13:190–6. doi: 10.1158/1055-9965.epi-03-0273. [DOI] [PubMed] [Google Scholar]
- 24.Forsti A, Angelini S, Festa F, Sanyal S, Zhang Z, Grzybowska E, et al. Single nucleotide polymorphisms in breast cancer. Oncol Rep. 2004;11:917–22. [PubMed] [Google Scholar]
- 25.Lee SA, Kang D, Nishio H, Lee MJ, Kim DH, Han W, et al. Methylenetetrahydrofolate reductase polymorphism, diet, and breast cancer in Korean women. Exp Mol Med. 2004;36:116–21. doi: 10.1038/emm.2004.17. [DOI] [PubMed] [Google Scholar]
- 26.Grieu F, Powell B, Beilby J, Iacopetta B. Methylenetetrahydrofolate reductase and thymidylate synthasepolymorphisms are not associated with breast cancer risk or phenotype. Anticancer Res. 2004;24:3215–9. [PubMed] [Google Scholar]
- 27.Lin WY, Chou YC, Wu MH, Huang HB, Jeng YL, Wu CC, et al. The MTHFR C677T polymorphism, estrogen exposure and breast cancer risk: A nested case-control study in Taiwan. Anticancer Res. 2004;24:3863–8. [PubMed] [Google Scholar]
- 28.Le Marchand L, Haiman CA, Wilkens LR, Kolonel LN, Henderson BE. MTHFR polymorphisms, diet, HRT, and breast cancer risk: The multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2004;13:2071–7. [PubMed] [Google Scholar]
- 29.Chen J, Gammon MD, Chan W, Palomeque C, Wetmur JG, Kabat GC, et al. One-carbon metabolism, MTHFR polymorphisms, and risk of breast cancer. Cancer Res. 2005;65:1606–14. doi: 10.1158/0008-5472.CAN-04-2630. [DOI] [PubMed] [Google Scholar]
- 30.Kalemi TG, Lambropoulos AF, Gueorguiev M, Chrisafi S, Papazisis KT, Kotsis A. The association of p53 mutations and p53 codon 72, Her 2 codon 655 and MTHFR C677T polymorphisms with breast cancer in Northern Greece. Cancer Lett. 2005;222:57–65. doi: 10.1016/j.canlet.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Hekim N, Ergen A, Yaylim I, Yilmaz H, Zeybek U, Ozturk O, et al. No association between methylenetetrahydrofolate reductase C677T polymorphism and breast cancer. Cell Biochem Funct. 2007;25:115–7. doi: 10.1002/cbf.1274. [DOI] [PubMed] [Google Scholar]
- 32.Ericson U, Sonestedt E, Ivarsson MI, Gullberg B, Carlson J, Olsson H, Wirfält E. Folate intake, methylenetetrahydrofolate reductase polymorphisms, and breast cancer risk in women from the Malmö Diet and Cancer cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:1101–10. doi: 10.1158/1055-9965.EPI-08-0401. [DOI] [PubMed] [Google Scholar]
- 33.Justenhoven C, Hamann U, Pierl CB, Rabstein S, Pesch B, Harth V, et al. One-carbon metabolism and breast cancer risk: No association of MTHFR, MTR, and TYMS polymorphisms in the GENICA study from Germany. Cancer Epidemiol Biomarkers Prev. 2005;14:3015–18. doi: 10.1158/1055-9965.EPI-05-0592. [DOI] [PubMed] [Google Scholar]
- 34.Chou YC, Wu MH, Yu JC, Lee MS, Yang T, Shih HL, et al. Genetic polymorphisms of methylenetetrahydrofolate reductase gene, plasma folate levels, and breast cancer susceptibility: A case-control study in Taiwan. Carcinogenesis. 2006;27:2295–300. doi: 10.1093/carcin/bgl108. [DOI] [PubMed] [Google Scholar]
- 35.Jakubowska A, Gronwald J, Menkiszak J, Gorski B, HuzarskiT, Byrski T, et al. Methylenetetrahydrofolate reductase polymorphisms modify BRCA1-associated breast and ovarian cancer risks. Breast Cancer Res Treat. 2007;104:299–308. doi: 10.1007/s10549-006-9417-3. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadzadeh G, Ghaffari MA, Bafandeh A, Hosseini SA. Association of serum soluble leptin receptor and leptin levels with breast cancer. J Res Med Sci. 2014;19:433–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Antoniadi T, Hatzis T, Kroupis C, Economou-Petersen E and Petersen MB. Prevalence of factor V leiden, prothrombin G20210A, and MTHFR C677T mutations in a Grece population of blood donors. Am J Hematol. 1999;61:265–7. doi: 10.1002/(sici)1096-8652(199908)61:4<265::aid-ajh8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Campbell IG, Baxter SW, Eccles DM, Choong DYH. Methylenetetrahydrofolate reductase polymorphism and susceptibility to breast cancer. Breast Cancer Res. 2002;4:R14. doi: 10.1186/bcr457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piyathilake CJ, Macaluso M, Johanning GL, Whiteside M, Heimburger DC, Giuliano A. Methylenetetrahydrofolate reductase (MTHFR) polymorphism increases the risk of cervical intraepithelial neoplasia. Anticancer Res. 2000;20:1751–7. [PubMed] [Google Scholar]
- 40.Goodman MT, Mc Duffie K, Hernandez B, Wilkens LR, Bertram CC, Killeen J, et al. Association of methylenetetrahydrofolate reductase polymorphism C677T and dietary folate with the risk of cervical dysplasia. Cancer Epidemiol Biomarkers Prev. 2001;10:1275–80. [PubMed] [Google Scholar]
- 41.Gerhard DS, Nguyen LT, Zhang ZY, Borecki IB, Coleman BI, Rader JS. A relationship between methylenetetrahydrofolate reductase variants and the development of invasive cervical cancer. Gynecol Oncol. 2003;90:560–65. doi: 10.1016/s0090-8258(03)00368-8. [DOI] [PubMed] [Google Scholar]
- 42.Keku T, Millikan R, Worley K, Winkel S, Eaton A, Biscocho L, et al. 5,10-Methylenetetrahydrofolate reductase codon 677 and 1298 polymorphisms and colon cancer in African Americans and whites. Cancer Epidemiol Biomarkers Prev. 2002;11:1611–21. [PubMed] [Google Scholar]
- 43.Zhang J, Zotz RB, Li Y, Wang R, Kiel S, Schulz WA, et al. Methylenetetrahydrofolate reductase C677T polymorphism and predisposition towards esophageal squamous cell carcinoma in a German Caucasian and a northern Chinese population. J Cancer Res Clin Oncol. 2004;130:574–80. doi: 10.1007/s00432-004-0585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Qiu LX, Wang ZH, Wu XH, Liu XJ, Wang BY, et al. MTHFRC677T polymorphism associated with breast cancer susceptibility: A meta-analysis involving 15,260 cases and 20,411 controls. Breast Cancer Res Treat. 2010;123:549–55. doi: 10.1007/s10549-010-0783-5. [DOI] [PubMed] [Google Scholar]
- 45.Qi X, Ma X, Yang X, Fan L, Zhang Y, Zhang F, et al. Methylenetetrahydrofolate reductase polymorphism and breast cancer risk: A meta-analysis from 41 studies with 16,480 cases and 22,388 controls. Breast Cancer Res Treat. 2010;123:499–506. doi: 10.1007/s10549-010-0773-7. [DOI] [PubMed] [Google Scholar]


