SUMMARY
piRNAs engage Piwi proteins to suppress transposons and are essential for fertility in diverse organisms. An interesting feature of piRNAs is that, while piRNA lengths are stereotypical within a species, they can differ widely between species. For example piRNAs are mainly 29 and 30 nucleotides in humans, 24 to 30 nucleotides in D. melanogaster, and uniformly 21 nucleotides in C. elegans. However, how piRNA length is determined and whether length impacts function remains unknown. Here we show that C. elegans deficient for PARN-1, a conserved ribonuclease, accumulate untrimmed piRNAs with 3′ extensions. Surprisingly, these longer piRNAs are stable and associate with the Piwi protein, PRG-1, but fail to robustly recruit downstream silencing factors. Our findings identify PARN-1 as a key regulator of piRNA length in C. elegans and suggest that length is regulated to promote efficient transcriptome surveillance.
INTRODUCTION
Piwi proteins and Piwi-interacting RNAs (piRNAs) are essential for germline development and fertility in animals (Carmell et al., 2007; Cox et al., 1998; Houwing et al., 2007; Lin and Spradling, 1997). Although the genomic origins, lengths, and sequences of piRNAs are diverse between organisms, biogenesis mechanisms are remarkably conserved (Luteijn and Ketting, 2013; Weick and Miska, 2014). In worms, flies, and mice, for example, piRNA production involves primary piRNA pathways and amplification pathways that ensure robust silencing of their targets (Ishizu et al., 2012; Malone and Hannon, 2009; Senti and Brennecke, 2010; Siomi et al., 2011). Primary piRNA production requires 5′ and 3′ processing of piRNA precursors in each organism.
In flies and mice, primary piRNAs derive from piRNA clusters transcribed as single-stranded transcripts (Brennecke et al., 2007; Li et al., 2013; Malone et al., 2009). The endonucleases Zucchini (flies) and MitoPLD (mice) are thought to generate the 5′ ends of piRNAs (Ipsaro et al., 2012; Nishimasu et al., 2012; Olivieri et al., 2010; Pane et al., 2007). Precursor piRNA transcripts are bound by Piwi, which is thought to direct Zucchini/MitoPLD to cleave the primary transcript at a position 3′ downstream of the PIWI-bound region. In flies, Zucchini often generates ~26 nucleotide (nt) intermediates that are compatible with Piwi binding (Han et al., 2015; Mohn et al., 2015). By contrast, piRNA intermediates generated by MitoPLD cleavage are 3 to 10 nt longer than mature piRNAs (Han et al., 2015; Mohn et al., 2015). The 3′ ends of intermediates thus are trimmed to the optimal length accommodated by Piwi (Kawaoka et al., 2011; Saxe et al., 2013; Vourekas et al., 2012). This trimming process requires an unknown exonuclease and the conserved Tudor-domain protein Papi/Tdrkh (Honda et al., 2013; Liu et al., 2011a; Saxe et al., 2013). The final step of piRNAs maturation is the 2′–O–methylation at their 3′ termini that is catalyzed by the conserved methyltransferase Hen1 (Horwich et al., 2007; Kirino and Mourelatos, 2007; Saito et al., 2007).
The C. elegans Piwi protein PRG-1 binds piRNAs (termed 21U-RNAs) that derive from thousands of genomic locations, including two large clusters of piRNA genes on chromosome IV (Batista et al., 2008; Das et al., 2008; Gu et al., 2012; Ruby et al., 2006). 21U-RNAs have a uniform length of 21 nt, a 5′ monophosphorylated (5′-monoP) uridine and a 2′–O–methylated 3′ residue (Batista et al., 2008; Billi et al., 2012; Das et al., 2008; Kamminga et al., 2012; Montgomery et al., 2012; Ruby et al., 2006). 21U-RNAs are processed from 25 to 29 nt capped small (cs) RNA precursors, which are transcribed by RNA polymerase II and initiate precisely two nucleotides upstream of the 5′ end of mature 21U-RNAs (Cecere et al., 2012; Gu et al., 2012; Weick et al., 2014). Thus, to generate mature 21 nt piRNAs, the 5′ cap and first two nucleotides of a csRNA must be removed, and extra nucleotides must be trimmed from the 3′ end. The enzymes that mediate the 5′ and 3′ processing of C. elegans piRNAs are unknown.
PRG-1 and its genomically-encoded piRNAs scan for foreign sequences while allowing mismatched pairing with the targets (Ashe et al., 2012; Bagijn et al., 2012; Lee et al., 2012a; Shirayama et al., 2012). For example, PRG-1 initiates epigenetic silencing on transgenes expressing the jellyfish green fluorescent protein (gfp), despite the fact that there are no perfectly matched piRNA target sites within the gfp sequence (Shirayama et al., 2012). “Self” mRNAs are protected from PRG-1 by the CSR-1 Argonaute pathway (Conine et al., 2013; Seth et al., 2013; Wedeles et al., 2013). Upon targeting, PRG-1 recruits RNA-dependent RNA polymerase (RdRP) to initiate the biogenesis of secondary siRNAs (22G-RNAs). 22G-RNAs are then loaded onto worm-specific Argonautes (WAGOs) to maintain and propagate epigenetic silencing (Ashe et al., 2012; Bagijn et al., 2012; Lee et al., 2012a; Shirayama et al., 2012).
Here we show that PARN-1, a conserved exonuclease, is required for 3′-end processing of piRNA in C. elegans. Recombinant PARN-1 protein possesses 3′-to-5′ exonuclease activity in vitro. Depletion of PARN-1 specifically compromises 3′ trimming of piRNAs while 5′ end processing and methylation of 3′ ends are unaffected. PARN-1 is restricted to the adult germline and co-localizes with PRG-1 in germline nuage structures called P granules. Intriguingly, in parn-1 mutants, untrimmed piRNAs associate with PRG-1 and accumulate to levels similar to those observed for wild-type (WT) piRNAs. Although the expression levels of piRNAs remain unchanged in parn-1 animals, Piwi-dependent mRNA silencing is reduced, as is the production of Piwi-dependent 22G-RNAs on mRNA targets. Together, our findings demonstrate that the highly conserved PARN-1 protein is required for piRNA trimming, and suggest that piRNA length is precisely regulated to promote Piwi/piRNA surveillance functions in the C. elegans germline.
RESULTS
PARN-1 is required for piRNA trimming
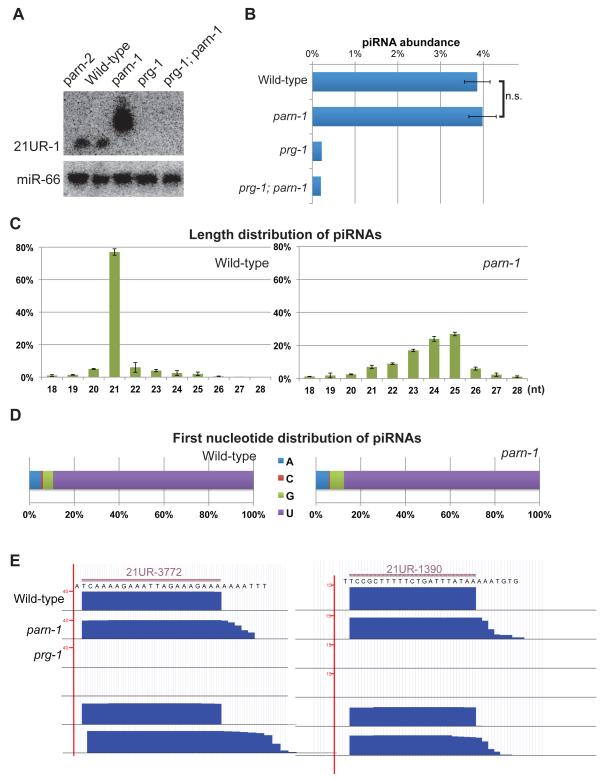
To identify ribonucleases responsible for 3′ end trimming, we took a candidate approach. We used Northern blotting to search for changes in piRNA length in animals depleted for expression of candidate nucleases. This search identified PARN-1 as specifically required for piRNA expression in C. elegans (Figures 1A and S1A). Animals bearing a presumptive null allele of parn-1(tm869) that deletes most of the catalytic domain and shifts the open reading frame (Nousch et al., 2013), failed to express mature 21U-RNAs, and instead produced piRNA species several nucleotides longer (Figures 1A and S1B). Mutations in another parn gene, parn-2, had no effect on piRNAs and failed to enhance the piRNA defect of parn-1 mutants (Figures 1A and S1B). Expression of a parn-1::gfp transgene restored expression of piRNAs of appropriate length (Figure S1B).
Figure 1. PARN-1 is required for 3′ trimming of piRNAs.
(A) Northern blot analysis of 21U-RNA-1 and miR-66 from total RNA prepared from wild type, parn-1, parn-2, prg-1, and parn-1; prg-1 double mutant strains.
(B) The expression profile for the bulk population of piRNAs as determined by small RNA sequencing. Plotted for each library is the percent of reads that represented piRNAs after normalized to non-structural RNAs. For wild-type and parn-1 samples, the average of three replicates is shown, error bars represent standard error of mean (SEM). One set of small RNA libraries from prg-1 and prg-1; parn-1 mutants is shown. n.s.=not significant (Student's t-test).
(C, D) Length distribution (C) and first nucleotide distribution (D) of piRNA reads from wild type and parn-1 mutant small RNA libraries. The average of three experiments is shown and error bars represent SEM.
(E) A browser view of representative piRNA loci. Small RNA reads mapped the 21U-RNA-3772 and 21U-RNA-1390 genomic loci.
See also Figure S1.
Previous studies showed that piRNA accumulation depends on PRG-1, suggesting that PRG-1 protects and stabilizes piRNAs in wild-type animals (Batista et al., 2008; Das et al., 2008; Wang and Reinke, 2008). Consistent with the idea that the longer parn-1 mutant piRNAs are also stabilized by PRG-1, our Northern blots revealed that prg-1; parn-1 double mutants lack piRNAs (Figure 1A). To look more broadly at small RNA biogenesis in parn-1 mutants, we cloned and deep sequenced small RNAs. We employed three different small RNA cloning methods. To enrich for 5′-monoP species such as miRNAs and piRNAs, we employed a direct ligation method to add a 5′ adaptor. To recover small RNAs with capped or 5′ triphosphorylated (5′ triP) ends, we applied two additional protocols, one involving pre-treatment with 5′ Polyphosphatase which removes the γ and β phosphates from 5′-triP RNA, and a second involving pre-treatment with Tobacco Acid Pyrophosphatase (TAP) to convert 5′ capped or polyphosphate RNA into 5′-monoP RNA. Using all of these methods we found that overall piRNA abundance was comparable between WT and parn-1 strains (Figures 1B and S1C). Interestingly, the piRNAs in parn-1 mutants were extended by 2 to 4 nucleotides at their 3′ ends but exhibited 5′ ends that map precisely to the 5′ uridine of wild-type mature piRNAs (Figures 1C and 1D). This finding was further confirmed by inspecting specific piRNA loci (Figure 1E). No changes in the length distribution of other small RNA species, such as microRNAs, were observed (Figures 1A and S1D). As expected, piRNA levels were dramatically reduced in prg-1 single mutant and prg-1; parn-1 double mutant animals (Figures 1A, 1B and S1B). Although our Northern blots suggest that untrimmed piRNAs are more abundant than wild-type piRNAs (Figures 1A and 2C), our deep-sequencing data do not support this conclusion. It is possible that the stronger signals on Northern blots do not reflect increased levels but rather stronger hybridization, perhaps due to pairing between the additional 3′ nucleotides in the longer piRNAs and the flanking sequences present in the Starfire probes (IDT). Altogether, our findings suggest that PARN-1 is required for 3′ trimming, but not for 5′ processing.
Figure 2. Untrimmed piRNAs are loaded onto PRG-1 and possess 2′–O–methylation.
(A) Bar plots showing the change in small RNA reads matching indicated genome annotations between input and PRG-1 IP samples prepared from wild type and parn-1 strains.
(B) Correlation analysis of the piRNA level. Libraries were prepared from PRG-1 IP samples from WT and parn-1 strains. Data from reads for each piRNA were normalized to total reads in the same sample. For a perfect correlation, the Spearman rank correlation coefficient (r) = 1 or −1, and for no correlation, r = 0.
(C) Oxidation and β–elimination followed by Northern blot analysis of RNA prepared from WT, parn-1 and henn-1 strains. RNA samples were not treated (−) or β–elimination treated (+), and probed for 21UR-1949. Probing for miRNA-66 served as controls for loading and β–elimination reactions.
(D) Fold enrichment of piRNA reads was calculated by comparing β–eliminated and non-treated samples. Small RNA libraries were generated from β–eliminated and untreated RNAs prepared from WT, parn-1, henn-1 strains.
See also Figure S2.
To ask if the untrimmed piRNAs associate with PRG-1, we recovered PRG-1 immunoprecipitation (IP) complexes from parn-1 extracts and analyzed PRG-1-bound small RNAs by deep sequencing. We found that the longer piRNAs co-precipitated with PRG-1 and confirmed their extended 3′ ends and their 5′ uridine (Figures 2A, S2A and S2B). The majority of piRNA species exhibited significant enrichment in the PRG-1 IP complex and the levels of the untrimmed piRNA species correlated well with that of their corresponding wild-type counterparts (Figures 2B, S2C and S2D). Similarly, we found that the expression levels of PRG-1 protein appeared similar in WT and parn-1 mutant animals as assayed by Western blotting (Figure S2E).
To examine the methylation status of piRNA 3′ ends, we performed β–elimination experiments. While RNAs containing 2′ and 3′ hydroxyl groups react with sodium periodate, RNA species with terminal 2′–O–methylation are resistant to this treatment. The β–elimination reaction removes the last nucleotide leaving a phosphate group at the 3′ terminus. The resulting size change can be monitored by polyacrylamide gel electrophoresis. Interestingly, we found that piRNAs were methylated in WT and parn-1 animals, but not in the henn-1 methyltransferase mutant (Figure 2C) (Billi et al., 2012; Kamminga et al., 2012; Montgomery et al., 2012). A control microRNA known to have terminal 2′ hydroxyl showed the expected size shift in both wild type and in parn-1 mutants (Figure 2C). To determine if resistance to sodium periodate treatment is a general feature of these longer piRNAs, we deep sequenced both β–eliminated and untreated small RNAs harvested from WT, parn-1, and henn-1 strains. This treatment leaves a 2′–phosphate at the 3′ end of RNAs containing 2′ and 3′ hydroxyl groups rendering them poor substrates for 3′ adaptor ligation. Thus non-modified RNA species are eliminated while RNAs with terminal 2′–O–methylation are expected to be enriched in deep-sequencing libraries generated from periodate treated samples. We found that periodate treatment enriched the abundance of piRNAs by 5 to 6 fold in both wild type and parn-1 animals when compared to untreated samples (Figure 2D). These enrichments were not observed in henn-1 mutants (Figure 2D). Thus, although untrimmed at their 3′ ends, the piRNAs in parn-1 animals are properly loaded onto PRG-1 and exhibit 5′- and 3′- end structures typical of wild-type piRNAs.
PARN-1 localizes in germline nuage
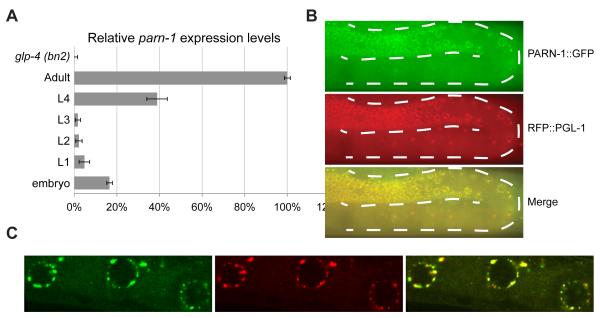
We next examined parn-1 mRNA expression and PARN-1 protein localization. Using quantitative PCR, we found that parn-1 mRNA was expressed at low levels at the L1 to L3 larval stages, but began to accumulate at the L4 stage, and reached its highest expression level at the gravid adult stage (Figure 3A). parn-1 mRNA was not detected in RNA samples isolated from germline-deficient glp-4 (bn2) animals (Beanan and Strome, 1992), suggesting that expression of parn-1 is restricted to the germline (Figure 3A). PRG-1 protein was previously shown to localize in P-granules (Batista et al., 2008; Wang and Reinke, 2008), where surveillance of nascent transcripts as well as piRNA maturation may take place (Klattenhoff and Theurkauf, 2008; van der Heijden et al., 2010). We found that a rescuing PARN-1::GFP substantially co-localized with PRG-1 and with the P granule component, PGL-1 (Figures 3B and 3C) (Kawasaki et al., 1998). In some instances, we observed PARN-1 localization adjacent to PRG-1 rather than fully overlapping with PRG-1 foci (Figure 3C). PRG-1 localization appeared to be wild-type in parn-1 mutants (Figure S3A), and similarly PARN-1::GFP localization was not altered in PRG-1 mutants (Figure S3B). Thus PRG-1 and PARN-1 can both independently localize to P-granules.
Figure 3. PARN-1 is expressed in the germline and localizes in P granules.
(A) Quantitative RT-PCR of parn-1 mRNA from total RNA isolated from synchronized wild-type populations at the indicated developmental stages and germline-deficient glp-4 (bn2) mutants at the adult stage. Expression of act-3 served as the internal control. Data were collected from three independent biological replicates. Error bars represent standard deviation.
(B) Fluorescence micrographs showing PARN-1::GFP expression (green) from an adult hermaphrodite. Expression RFP::PGL-1(red) served the P granule marker. The dashed lines outline the position of germline,
(C) Immunostaining of PRG-1 (red) and PARN-1::GFP (green) in dissected gonad arms from the parn-1 ::gfp rescue line.
See also Figure S3.
Although the proteins co-localize in germline nuage, we failed to detect physical interactions between PRG-1 and PARN-1::GFP in co-immunoprecipitation (co-IP) assays (Figure S3C). These findings suggest that the PARN-1-mediated piRNA trimming either occurs prior to PRG-1 loading or is very transient.
PARN-1 is a 3′ to 5′ exoribonuclease
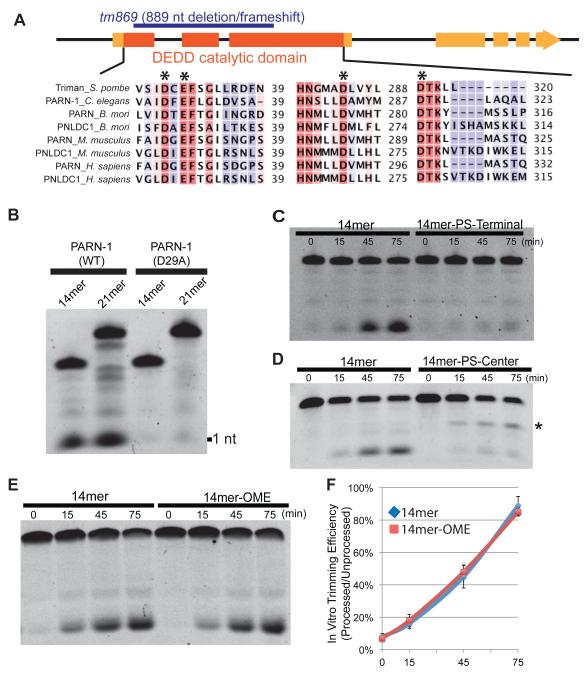
PARN-1 belongs to a highly conserved ribonuclease family that contains the DEDD catalytic domain (Figure 4A) (Goldstrohm and Wickens, 2008; Korner et al., 1998; Zuo and Deutscher, 2001). To directly test if PARN-1 functions as a ribonuclease, we purified the PARN-1 protein in Escherichia coli (Figure S4A). We found that recombinant wild-type PARN-1 processed 5′-end-labeled synthetic RNA substrates of 14 and 21 nts into variable sized fragments with a prominent product of one nucleotide (Figure 4B). To rule out the possibility that the observed activity was catalyzed by a contaminating bacterial nuclease, we also expressed a parn-1 mutant in which the conserved aspartic acid residue (D29) was mutated to alanine. As expected, this D29A catalytic mutant showed greatly reduced nuclease activity in our in vitro assay (Figure 4B). To test whether PARN-1 is an exoribonuclease, we used 14-mer oligonucleotides with phosphorothioate (PS) linkages. PS bonds are known to stabilize oligonucleotides against nuclease degradation (Kawaoka et al., 2011; Ren et al., 2004). Incorporation of PS linkages at the 3′ end should therefore render the substrate resistant to 3′ exoribonuclease trimming, but should leave it sensitive to endoribonucleases. We found that the 14mer-PS-Terminal substrate exhibited a significant reduction in processing compared to an unmodified control (Figures 4C). Consistent with the idea that PARN-1 acts as a 3′-5′ exoribonuclease, when the PS bond was instead placed between positions 7 and 8 (14mer-PS-Center), trimming of the substrate was blocked specifically at the phosphorothioate site (Figure 4D).
Figure 4. Recombinant PARN-1 possesses 3′-to-5′ exonuclease activity in vitro.
(A) Schematic of the parn-1 genes showing exons (boxes) and intron (lines) with the catalytic ribonuclease domain shaded in dark orange. The deletion allele (tm869) is highlighted in blue. The alignment shows human, mouse, C. elegans, B. mori, and S. pombe PARN and poly(A)-specific ribonuclease-like domain containing (PNLDC) protein with conserved catalytic residues (asterisk).
(B) Electrophoresis analysis on 21nt and 14nt 5′ Fluorescein labeled RNA substrates incubated with purified PARN-1 (WT and D29A) proteins.
(C-E) Electrophoresis analysis of 14-mer RNAs containing 3′ hydroxyl (14mer) and phosphorothioate (PS) bonds from positions 10 to 14 (14mer-PS-Terminal) (C), 14-mer RNAs with PS bond between positions 7 to 8 (14mer-PS-Center). Asterisk represents the degradation intermediate (D), and 14-mers containing 3′ hydroxyl and 2′–O–methylated 3′ termini (14mer-OME) (E).
(F) Quantification of the ratio between processed and unprocessed products among biological replicates. The average of six replicates for 14mer and the average of three replicates for 14mer-OME are shown. Error bars represent standard deviation.
See also Figure S4.
By incubating PARN-1 with varying concentrations of substrate, we determined its Michaelis-Menten parameter Km value to be 18 nM. To test if the enzyme is a processive enzyme, different amounts of the substrate (18 and 108 nM, corresponding to concentrations at or in 6-fold excess of the Km value) were subjected to the trimming assay. Two findings indicate PARN-1 trims RNA in a processive mode: (i), in the time course experiment, both RNA substrate and fully processed product are present at some time points; (ii), more importantly, the time point for the appearance of the first fully processed product is independent of the amount of RNA substrate in the reaction (Figure S4B).
We next wished to ask whether recombinant PARN-1 could process untrimmed piRNAs associated with PRG-1. We purified PRG-1 complexes by PRG-1 IP and by FLAG IP from parn-1 mutant extracts and incubated these purified complexes with recombinant PARN-1. To monitor for potential inhibitory factors present in the reaction that might inactivate the recombinant ribonuclease, we included a labeled ‘naked’ RNA substrate as a positive control. While the ‘naked’ substrate was efficiently processed, we failed to detect any significant trimming of the piRNAs associated with PRG-1 immune complexes (Figure S4C). To ask if the methylated 3′ termini of these PRG-1 bound piRNAs block 3′ trimming, we conducted trimming experiments using PRG-1 complexes purified from henn-1; parn-1 double mutants. We found that unmethylated piRNAs in these PRG-1 complexes were also resistant to trimming by recombinant PARN-1 (Figure S4C). Moreover, we found that when incubated with a ‘naked’ RNA containing a 3′ terminal methylation, purified PARN-1 digested the substrate at a rate similar to that observed for an unmodified substrate (Figures 4E and 4F). These findings suggest that 2′–O–methylation does not protect piRNAs from PARN-1 processing. Instead, the 3′ ends of the untrimmed piRNAs appear to be protected by PRG-1 binding, perhaps by association with the PAZ domain of PRG-1 (Simon et al., 2011; Tian et al., 2011).
PARN-1 is required for fertility and acts in the PRG-1 pathway
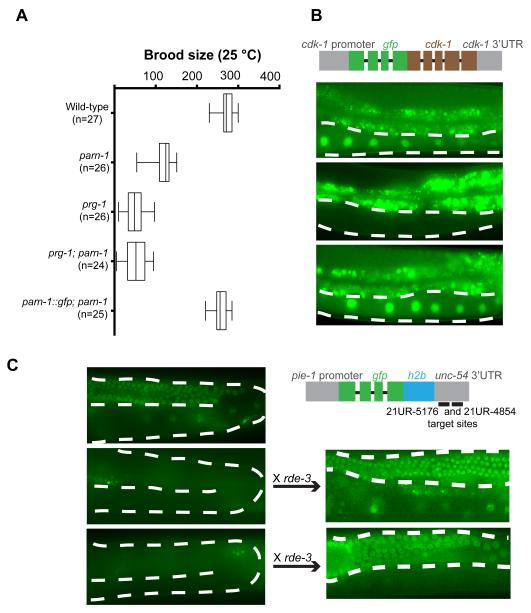
Mutations in Piwi pathway components cause reduced fertility in C. elegans (Batista et al., 2008; Das et al., 2008; Wang and Reinke, 2008). In prg-1 animals this sterility phenotype is progressive and fertility declines over generations (Barberan-Soler et al., 2014; Simon et al., 2014). To accurately measure fertility in different mutant backgrounds, we outcrossed the prg-1 and parn-1 strains with wild type four times, and freshly generated the prg-1; parn-1 double mutants prior to scoring brood sizes. Consistent with findings from a previous study (Nousch et al., 2013), we found that parn-1 animals display a reduced brood size at elevated temperature (25°C) as compared to WT (Figure 5A). Fertility was rescued to wild-type levels by expression of a parn-1::gfp transgene (Figure 5A). The reduced fertility observed in parn-1 mutants was less than that observed in prg-1 mutants (Figure 5A).
Figure 5. PARN-1 is required for fertility and epigenetic silencing of single-copy transgenes.
(A) Brood size counts in WT, prg-1, parn-1, prg-1; parn-1 double, and parn-1 rescue animals at 25 °C. n=Number of parental adults scored.
(B, C) Schematics of the gfp::cdk-1 reporter (B), and of the h2b::gfp::21U-RNA-target-site reporter (C), that were injected into wild-type, parn-1 and prg-1 worms to establish single copy transgenic lines. Images of GFP fluorescence signals in the resulting strains are shown. Oocyte nuclei that either express, or fail to express the construct are indicated by arrowheads. The dashed lines outline the position of germline. Bright signals outside of the germline are from gut granule autofluorescence. The gfp::h2b::21U-RNA-target-site reporter established in wild-type and parn-1 animals was crossed to the rde-3 mutant strain. n=number of independent transgenic lines exhibiting the pattern of expression shown.
PARN-1 homologs regulate gene expression independently of piRNAs in other species (Berndt et al., 2012; Copeland and Wormington, 2001; Lee et al., 2012b), thus it was possible that the fertility defect of parn-1 is not caused by the observed defect in trimming piRNAs. We therefore used a genetic epistasis test to ask if PARN-1 acts in the prg-1 pathway. If the fertility defects observed in parn-1 animals result from defects in functions unrelated to piRNA processing, the phenotype of the double mutant would be expected to exhibit an additive fertility deficit. However, in contrast to this possibility and consistent with their function within the same pathway, we found that parn-1; prg-1 double mutants exhibited fertility levels similar to those observed in prg-1 single mutants (Figure 5A). These findings suggest that PARN-1 acts in the Piwi pathway and is required for fertility, and furthermore that the PRG-1-bound untrimmed piRNAs must retain at least partial function.
parn-1 mutants are partially defective in transgene silencing and 22G-RNA production
To ask if parn-1 mutants are defective in transgene silencing, we first tested a gfp::cdk-1 transgene that is exceptionally prone to silencing in wild-type animals (Shirayama et al., 2012). As expected, we found that introduction of this transgene into wild-type animals invariably resulted in silencing (n=5), while introduction into prg-1 mutant animals resulted in uniform and robust expression of the nuclear GFP::CDK-1 protein (n=4, Figure 5B) (Shirayama et al., 2012). Strikingly, and consistent with a role for parn-1 in the PRG-1 pathway, we found that newly introduced copies of the gfp::cdk-1 transgene were stably expressed in all of the independently generated parn-1 mutant transgenic strains analyzed (n=6, Figure 5B).
We next asked if parn-1 mutants were defective in piRNA guided silencing of a perfectly matched target. For this assay, we used a gfp::h2b reporter engineered to contain sites perfectly complementary to two abundant piRNAs (Figure 5C). As previously reported (Lee et al., 2012a), this transgene was silenced when introduced into wild-type animals, but was active upon introduction into prg-1 mutants (Figure 5C). Interestingly, however, parn-1 mutants were able to silence this gfp::h2b transgene (Figure 5C). The polynucleotide polymerase RDE-3 is required for the accumulation of WAGO 22G-RNAs (Chen et al., 2005; Gu et al., 2009), and as expected, we found that crossing the silent reporter from wild type or parn-1 mutants into the rde-3 animals resulted in fully restored transgene expression (Figure 5C). Taken together these data suggest that untrimmed piRNAs have reduced activity, but can, nevertheless, recognize perfectly complementary sequences to initiate 22G-RNA biogenesis and target silencing.
In addition to stimulating 22G-RNA production on transgenes with foreign sequences, PRG-1 also triggers 22G-RNA production on endogenous RNA targets (Bagijn et al., 2012; Lee et al., 2012a). We therefore examined whether depletion of PARN-1 affects synthesis of these PRG-1-dependent 22G-RNAs on endogenous genes. There are 1,478 genes that exhibit a depletion of 22G-RNAs in prg-1 mutants when compared to wild type (Figure 6A and Table S2). Strikingly, a significant overlap was found between genes with depleted 22G-RNAs in parn-1 animals and genes previously identified as prg-1 targets (Figure 6A and Table S2). Consistent with parn-1 and prg-1 functioning together on these targets, parn-1; prg-1 double mutants also exhibited a very significant overlap with prg-1 single mutants (Figure 6A and Table S2). Finally we found that 22G-RNAs at predicted target sites were significantly reduced in parn-1 mutant animals (Figure 6B and Table S3). Depletion of 22G-RNAs was not observed at randomly selected sites (Figures S5A and S5B). Consistent with the idea that the longer piRNAs produced in parn-1 mutants are partially functional, we found that, although reduced relative to wild type, 22G-RNAs were nevertheless enriched relative to prg-1 mutants near predicted piRNA target sites (Figure 6C). Taken together these findings suggest that untrimmed piRNAs, despite their robust loading onto PRG-1, exhibit reduced activity in both 22G-RNA induction and target silencing.
Figure 6. Trimming of piRNAs is important for 22G-RNAs production.
(A) Venn diagram summarizing the overlap in the number of genes whose 22G-RNAs levels decrease by two fold when compared to wild type. Gene sets from prg-1, parn-1, and prg-1; parn-1 double mutants are plotted (p-values < 0.0001; Hypergenomic Test).
(B) Box and whisker plot showing fold change of 22G-RNAs at predicted target sites in wild type and parn-1 mutants as compared to prg-1 mutants. Asterisks indicate statistical significance (p-value = 6.26 × e−227, Wilcoxon Rank-Sum Test).
(C) Density of 22G-RNAs in a 100 nt interval around predicted piRNA target sites in the wild type, parn-1, and prg-1 mutants. The plots are centered on piRNAs.
(D) Model illustrating the role of PARN-1 in piRNA biogenesis and functions.
DISCUSSION
A conserved role for PARN family members in piRNA processing
PARN was initially identified as a poly(A)–specific 3′ exoribonuclease (Astrom et al., 1992; Korner and Wahle, 1997; Korner et al., 1998). In C. elegans the Ccr4-Not deadenylase complex, rather than PARN-1 appears to be the key regulator of poly (A) tail length (Nousch et al., 2013). Here we have shown that the C. elegans PARN ortholog, PARN-1 is the bona fide 3′ trimmer for piRNAs in C. elegans. Both the Human and mouse genomes contain PARN and a second homolog named Poly(A)-specific Ribonuclease-Like Domain Containing 1 (PNLDC1) (Figure 4A). While mouse PARN appears to be ubiquitously expressed in all tissues, PNLDC1 is enriched in mouse testes (Brawand et al., 2011; Petryszak et al., 2014), making it a good candidate for a role in piRNA processing. Finally, an independent study has identified the PARN family member PNLDC1 from silkworm Bombyx mori as a ribonuclease required for piRNA trimming (Izumi et al., co-submitted). Together, these findings suggest that despite the many differences in piRNA gene organization, and the different lengths of mature piRNAs in mammals, insects and nematodes, the machinery for defining piRNA length may nevertheless be conserved.
The PARN family of ribonucleases belongs to the DEDD exonuclease superfamily whose members have diverse functions in processing both RNA and DNA (Goldstrohm and Wickens, 2008; Zuo and Deutscher, 2001). Several members of the DEDD family play important roles in small RNA silencing. For example, in worms MUT-7 is required for RNA interference, although its biochemical function is unknown (Gu et al., 2009; Ketting et al., 1999; Tabara et al., 1999). In flies, Nibbler modulates the lengths of some microRNAs and piRNAs (Feltzin et al., 2015; Han et al., 2011; Liu et al., 2011b). In fission yeast, Triman, a PARN family member, processes Dicer-independent small RNAs that are involved in heterochromatin establishment (Marasovic et al., 2013). In vertebrates, PARN mediates 3′-end formation of an Ago2-cleaved miRNA (Yoda et al., 2013). Given the diversity of both proteins themselves and distinct functions of their small RNA substrates, these examples could represent convergent evolution rather than conservation of a role in small RNA processing.
What determines piRNA size?
Our findings support a model for piRNA biogenesis in C. elegans in which the exonuclease PARN-1 mediates 3′ trimming of piRNA precursors prior to, or independently, from PRG-1 loading (Figure 6D). In vitro reconstitution in silkworm cell extracts suggested that 3′ trimming occurs on Piwi-bound piRNA precursors (Kawaoka et al., 2011). Consistent with this notion, depletion of Tdrkh/Papi, a Tudor domain protein that presumably recruits the trimmer, leads to longer piRNAs that are associated with Piwi proteins (Han et al., 2015; Mohn et al., 2015; Saxe et al., 2013). Our findings demonstrate that once loaded on PRG-1, untrimmed piRNAs are as stable as wild-type piRNAs, are at least partially functional, and have the terminal 2′–O–methyl modification normally found on mature piRNAs. Thus in wild-type animals PARN-1 mediated trimming prevents longer piRNAs from being loaded and stabilized on PRG-1. The finding that recombinant PARN-1 processes ‘naked’ RNA down to single nucleotides suggests that PRG-1 or other factors may be required to limit PARN-1 processing in vivo.
Properties of untrimmed piRNAs
Compared to their wild-type counterparts, piRNAs in parn-1 mutants are only slightly (2 to 4 nts) longer. Yet, these longer piRNAs are defective in several Piwi pathway activities. Firstly, parn-1 animals have reduced fertility at elevated temperature. Secondly, the silencing of foreign sequences, such as the gfp::cdk-1 transgene, is compromised. Lastly, the accumulation of PRG-1–dependent 22G-RNAs is significantly reduced. For miRNAs, base-pairing within the 3′ region can supplement pairing within the seed region (nucleotides 2-8) (Bartel, 2009; Brennecke et al., 2005; Friedman et al., 2009). In vitro studies suggest that release of the target RNA is a slow step in Argonaute-guided catalysis (Deerberg et al., 2013; Haley and Zamore, 2004; Salomon et al., 2015). Extended base-pairing between targets and the longer piRNAs in parn-1 may over-stabilize target interactions, reducing the ability of PRG-1 to efficiently scan germline transcripts. Considering the uniformity of piRNA length in wild-type animals, our findings reveal a surprising versatility in the ability of PRG-1 to accommodate and protect piRNAs of different length. Thus the stereotypical size of piRNAs in C. elegans, and perhaps in other animals, may reflect precise regulation of the trimming machinery rather than a limitation imposed by physical attributes of the Piwi proteins themselves. Our findings suggest that the optimal sizing of piRNAs is important for both fertility and gene silencing.
EXPERIMENTAL PROCEDURES
Strains and genetics
C. elegans culture and genetics were performed as described (Brenner, 1974). The Bristol strain N2 was used as the standard wild-type strain. Alleles used in this study listed by chromosome: LGI: prg-1(tm872), glp-4(bn2); LGII: parn-2(tm1339), neSi[cb-unc-119(+); wago1p::parn-1::gfp], neSi[cb-unc-119(+); flag::prg-1]; LGIII: henn-1(tm4477); LGV: parn-1(tm869); unmapped: rfp::pgl-1. Depletion of candidate nucleases was conducted by feeding worms bacteria expressing dsRNA as described (Conte et al., 2015).
Generation of transgenic lines by MosSCI
The gfp::cdk-1 (Shirayama et al., 2012) and gfp::h2b (Lee et al., 2012a) reporters were injected into recipient strains EG4322 Mos1(ttTi5605) II; unc-119(ed3) III, TW35 Mos1(ttTi5605) II; unc-119(ed3) III; parn-1(tm869) V and TW60 Mos1(ttTi5605) II; unc-119(ed3) III; prg-1 (tm872) I using a direct insertion method (Frokjaer-Jensen et al., 2008).
To generate the MosSCI donor vector for parn-1::gfp integration, wago-1 promoter sequences (1159 bp), sequences of wago-1 3′ untranslated region (360 bp) parn-1 open reading frame, and gfp coding sequences were sequentially cloned to pBluescript SK (+). PARN-1 mutants (D29A and D29A; E31A) were generated by Q5 site-directed mutagenesis kit (NEB). The fragment was subcloned into the MosSCI vector pCFJ151. The transgenic lines were generated as described (Frokjaer-Jensen et al., 2008).
Small RNA cloning
Total RNA was extracted from adult N2 and outcrossed mutants at generation twelve using TRI Reagent (MRC). Small RNA was enriched using MirVana Kit (Life Technologies). RNAs ranging from 18 to 40 nt were further purified from the 15% polyacrylamide/7M urea gel. RNAs from WT, parn-1, prg-1, parn-1; prg-1 double mutants, input and IP samples were subjected to TAP (Epicentre) treatment, RNA 5′ polyphosphatase (Epicentre) treatment, or mock treatment. All small RNAs were ligated to the miRNA cloning linker 1 (5′ rAppCTGTAGGCACCATCAAT/3ddC/ 3′, IDT) and a 5′ linker containing a 4 nt barcode using T4 RNA ligase (Takara). Ligated RNA products were converted to cDNA using Superscript III Reverse Transcriptase (Life Technologies). Libraries were amplified and sequenced using HiSeq sequencing system (Illumina) at the UMass Medical School Deep Sequencing Core.
β–elimination reaction
50 μg small RNA enriched by the MirVana Kit (Life Technologies) was incubated with borax/boric acid buffer (0.06 M borax, 0.06 M boric acid, pH 8.6) containing 25 mM NaIO4 at room temperature for 1 h. 20 μl of 50% glycerol was added to quench extra NaIO4. RNA was collected by ethanol precipitation. The pellet was dissolved in 100 μl borax/boric/NaOH buffer (0.055 M borax, 0.055 M boric acid, 0.055 M NaOH, pH 9.5) and incubated at 45 °C for 90 min. RNA was recovered by ethanol precipitation and subjected to small RNA cloning and Northern analysis.
Protein purification
The cDNA encoding truncated PARN-1 containing the catalytic domain (residue 1-403) were cloned into the pGEX-4T-3 vector (GE Healthcare) and the mutated PARN-1 (D29A) was generated by Q5 site-directed mutagenesis kit (NEB). Proteins were expressed as the glutathione-S transferase fusion protein in ArcticExpress cells (Agilent Technologies) at 12°C overnight and purified using Glutathione Sepharose 4B (GE Healthcare). Recombinant proteins were dialyzed against RNA trimming buffer (20 mM HEPES-KOH pH 7.5, 50 mM KCl, 0.25 mM MgCl2, 1 mM DTT), supplemented with 20% glycerol, and stored at −80°C. Protein purity was examined by SDS-PAGE followed by Coomassie blue staining.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Y. Tomari for sharing unpublished data; S. Mitani for parn-1 (tm869) and parn-2 (tm1339) strains; J. Kim for the henn-1 (tm4477) mutant; E. Kittler and the UMass Deep Sequencing Core for Illumina sequencing; M. Shirayama for his assistance with molecular cloning and generating parn-1; parn-2 double mutants; W. Gu for bioinformatic analysis; D. Conte for comments on the text, members of the Mello and Victor labs, and Y. Zhang for discussion; Some of the strains were provided by the Caenorhabditis Genetics Center. This work was supported in part by a Hope Funds for Cancer Research Postdoctoral fellowship to W. T.; NIH Pathway to Independence Award (GM108866) to H.-C. L.; NIH grant (HD078253) to Z.W.; NIH grants (GM058800 and HD078253) to C.C.M.; C.C.M. is a Howard Hughes Medical Institute Investigator.
Footnotes
Accession Numbers
All sequencing data have been deposited in the Sequence Read Archive (SRA). The accession number is SRS1021265.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashe A, Sapetschnig A, Weick EM, Mitchell J, Bagijn MP, Cording AC, Doebley AL, Goldstein LD, Lehrbach NJ, Le Pen J, et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150:88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrom J, Astrom A, Virtanen A. Properties of a HeLa cell 3′ exonuclease specific for degrading poly(A) tails of mammalian mRNA. The Journal of biological chemistry. 1992;267:18154–18159. [PubMed] [Google Scholar]
- Bagijn MP, Goldstein LD, Sapetschnig A, Weick EM, Bouasker S, Lehrbach NJ, Simard MJ, Miska EA. Function, targets, and evolution of Caenorhabditis elegans piRNAs. Science. 2012;337:574–578. doi: 10.1126/science.1220952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberan-Soler S, Fontrodona L, Ribo A, Lamm AT, Iannone C, Ceron J, Lehner B, Valcarcel J. Co-option of the piRNA pathway for germline-specific alternative splicing of C. elegans TOR. Cell reports. 2014;8:1609–1616. doi: 10.1016/j.celrep.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Molecular cell. 2008;31:67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beanan MJ, Strome S. Characterization of a germ-line proliferation mutation in C. elegans. Development. 1992;116:755–766. doi: 10.1242/dev.116.3.755. [DOI] [PubMed] [Google Scholar]
- Berndt H, Harnisch C, Rammelt C, Stohr N, Zirkel A, Dohm JC, Himmelbauer H, Tavanez JP, Huttelmaier S, Wahle E. Maturation of mammalian H/ACA box snoRNAs: PAPD5-dependent adenylation and PARN-dependent trimming. Rna. 2012;18:958–972. doi: 10.1261/rna.032292.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billi AC, Alessi AF, Khivansara V, Han T, Freeberg M, Mitani S, Kim JK. The Caenorhabditis elegans HEN1 ortholog, HENN-1, methylates and stabilizes select subclasses of germline small RNAs. PLoS genetics. 2012;8:e1002617. doi: 10.1371/journal.pgen.1002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawand D, Soumillon M, Necsulea A, Julien P, Csardi G, Harrigan P, Weier M, Liechti A, Aximu-Petri A, Kircher M, et al. The evolution of gene expression levels in mammalian organs. Nature. 2011;478:343–348. doi: 10.1038/nature10532. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS biology. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmell MA, Girard A, van de Kant HJ, Bourc'his D, Bestor TH, de Rooij DG, Hannon GJ. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Developmental cell. 2007;12:503–514. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cecere G, Zheng GX, Mansisidor AR, Klymko KE, Grishok A. Promoters recognized by forkhead proteins exist for individual 21U-RNAs. Molecular cell. 2012;47:734–745. doi: 10.1016/j.molcel.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Simard MJ, Tabara H, Brownell DR, McCollough JA, Mello CC. A member of the polymerase beta nucleotidyltransferase superfamily is required for RNA interference in C. elegans. Current biology : CB. 2005;15:378–383. doi: 10.1016/j.cub.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Conine CC, Moresco JJ, Gu W, Shirayama M, Conte D, Jr., Yates JR, 3rd, Mello CC. Argonautes Promote Male Fertility and Provide a Paternal Memory of Germline Gene Expression in C. elegans. Cell. 2013;155:1532–1544. doi: 10.1016/j.cell.2013.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte D, Jr., MacNeil LT, Walhout AJ, Mello CC. RNA Interference in Caenorhabditis elegans. Current protocols in molecular biology. 2015;109:26 23 21–26 23 30. doi: 10.1002/0471142727.mb2603s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland PR, Wormington M. The mechanism and regulation of deadenylation: identification and characterization of Xenopus PARN. Rna. 2001;7:875–886. doi: 10.1017/s1355838201010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes & development. 1998;12:3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PP, Bagijn MP, Goldstein LD, Woolford JR, Lehrbach NJ, Sapetschnig A, Buhecha HR, Gilchrist MJ, Howe KL, Stark R, et al. Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Molecular cell. 2008;31:79–90. doi: 10.1016/j.molcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deerberg A, Willkomm S, Restle T. Minimal mechanistic model of siRNA-dependent target RNA slicing by recombinant human Argonaute 2 protein. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17850–17855. doi: 10.1073/pnas.1217838110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltzin VL, Khaladkar M, Abe M, Parisi M, Hendriks GJ, Kim J, Bonini NM. The exonuclease Nibbler regulates age-associated traits and modulates piRNA length in Drosophila. Aging cell. 2015;14:443–452. doi: 10.1111/acel.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome research. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM. Single-copy insertion of transgenes in Caenorhabditis elegans. Nature genetics. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstrohm AC, Wickens M. Multifunctional deadenylase complexes diversify mRNA control. Nature reviews Molecular cell biology. 2008;9:337–344. doi: 10.1038/nrm2370. [DOI] [PubMed] [Google Scholar]
- Gu W, Lee HC, Chaves D, Youngman EM, Pazour GJ, Conte D, Jr., Mello CC. CapSeq and CIP-TAP identify Pol II start sites and reveal capped small RNAs as C. elegans piRNA precursors. Cell. 2012;151:1488–1500. doi: 10.1016/j.cell.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Shirayama M, Conte D, Jr., Vasale J, Batista PJ, Claycomb JM, Moresco JJ, Youngman EM, Keys J, Stoltz MJ, et al. Distinct argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline. Molecular cell. 2009;36:231–244. doi: 10.1016/j.molcel.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley B, Zamore PD. Kinetic analysis of the RNAi enzyme complex. Nature structural & molecular biology. 2004;11:599–606. doi: 10.1038/nsmb780. [DOI] [PubMed] [Google Scholar]
- Han BW, Hung JH, Weng Z, Zamore PD, Ameres SL. The 3′-to-5′ exoribonuclease Nibbler shapes the 3′ ends of microRNAs bound to Drosophila Argonaute1. Current biology : CB. 2011;21:1878–1887. doi: 10.1016/j.cub.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BW, Wang W, Li C, Weng Z, Zamore PD. Noncoding RNA. piRNA-guided transposon cleavage initiates Zucchini-dependent, phased piRNA production. Science. 2015;348:817–821. doi: 10.1126/science.aaa1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Kirino Y, Maragkakis M, Alexiou P, Ohtaki A, Murali R, Mourelatos Z, Kirino Y. Mitochondrial protein BmPAPI modulates the length of mature piRNAs. Rna. 2013;19:1405–1418. doi: 10.1261/rna.040428.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich MD, Li C, Matranga C, Vagin V, Farley G, Wang P, Zamore PD. The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Current biology : CB. 2007;17:1265–1272. doi: 10.1016/j.cub.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, van den Elst H, Filippov DV, Blaser H, Raz E, Moens CB, et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell. 2007;129:69–82. doi: 10.1016/j.cell.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Ipsaro JJ, Haase AD, Knott SR, Joshua-Tor L, Hannon GJ. The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature. 2012;491:279–283. doi: 10.1038/nature11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizu H, Siomi H, Siomi MC. Biology of PIWI-interacting RNAs: new insights into biogenesis and function inside and outside of germlines. Genes & development. 2012;26:2361–2373. doi: 10.1101/gad.203786.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamminga LM, van Wolfswinkel JC, Luteijn MJ, Kaaij LJ, Bagijn MP, Sapetschnig A, Miska EA, Berezikov E, Ketting RF. Differential impact of the HEN1 homolog HENN-1 on 21U and 26G RNAs in the germline of Caenorhabditis elegans. PLoS genetics. 2012;8:e1002702. doi: 10.1371/journal.pgen.1002702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka S, Izumi N, Katsuma S, Tomari Y. 3′ end formation of PIWI-interacting RNAs in vitro. Molecular cell. 2011;43:1015–1022. doi: 10.1016/j.molcel.2011.07.029. [DOI] [PubMed] [Google Scholar]
- Kawasaki I, Shim YH, Kirchner J, Kaminker J, Wood WB, Strome S. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell. 1998;94:635–645. doi: 10.1016/s0092-8674(00)81605-0. [DOI] [PubMed] [Google Scholar]
- Ketting RF, Haverkamp TH, van Luenen HG, Plasterk RH. Mut-7 of C. elegans, required for transposon silencing and RNA interference, is a homolog of Werner syndrome helicase and RNaseD. Cell. 1999;99:133–141. doi: 10.1016/s0092-8674(00)81645-1. [DOI] [PubMed] [Google Scholar]
- Kirino Y, Mourelatos Z. Mouse Piwi-interacting RNAs are 2′-O-methylated at their 3′ termini. Nature structural & molecular biology. 2007;14:347–348. doi: 10.1038/nsmb1218. [DOI] [PubMed] [Google Scholar]
- Klattenhoff C, Theurkauf W. Biogenesis and germline functions of piRNAs. Development. 2008;135:3–9. doi: 10.1242/dev.006486. [DOI] [PubMed] [Google Scholar]
- Korner CG, Wahle E. Poly(A) tail shortening by a mammalian poly(A)-specific 3′-exoribonuclease. The Journal of biological chemistry. 1997;272:10448–10456. doi: 10.1074/jbc.272.16.10448. [DOI] [PubMed] [Google Scholar]
- Korner CG, Wormington M, Muckenthaler M, Schneider S, Dehlin E, Wahle E. The deadenylating nuclease (DAN) is involved in poly(A) tail removal during the meiotic maturation of Xenopus oocytes. The EMBO journal. 1998;17:5427–5437. doi: 10.1093/emboj/17.18.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Gu W, Shirayama M, Youngman E, Conte D, Jr., Mello CC. C. elegans piRNAs mediate the genome-wide surveillance of germline transcripts. Cell. 2012a;150:78–87. doi: 10.1016/j.cell.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Lee JY, Trembly J, Wilusz J, Tian B, Wilusz CJ. The PARN deadenylase targets a discrete set of mRNAs for decay and regulates cell motility in mouse myoblasts. PLoS genetics. 2012b;8:e1002901. doi: 10.1371/journal.pgen.1002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XZ, Roy CK, Dong X, Bolcun-Filas E, Wang J, Han BW, Xu J, Moore MJ, Schimenti JC, Weng Z, et al. An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Molecular cell. 2013;50:67–81. doi: 10.1016/j.molcel.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Spradling AC. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development. 1997;124:2463–2476. doi: 10.1242/dev.124.12.2463. [DOI] [PubMed] [Google Scholar]
- Liu L, Qi H, Wang J, Lin H. PAPI, a novel TUDOR-domain protein, complexes with AGO3, ME31B and TRAL in the nuage to silence transposition. Development. 2011a;138:1863–1873. doi: 10.1242/dev.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Abe M, Sabin LR, Hendriks GJ, Naqvi AS, Yu Z, Cherry S, Bonini NM. The exoribonuclease Nibbler controls 3′ end processing of microRNAs in Drosophila. Current biology : CB. 2011b;21:1888–1893. doi: 10.1016/j.cub.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luteijn MJ, Ketting RF. PIWI-interacting RNAs: from generation to transgenerational epigenetics. Nature reviews Genetics. 2013;14:523–534. doi: 10.1038/nrg3495. [DOI] [PubMed] [Google Scholar]
- Malone CD, Brennecke J, Dus M, Stark A, McCombie WR, Sachidanandam R, Hannon GJ. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell. 2009;137:522–535. doi: 10.1016/j.cell.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone CD, Hannon GJ. Small RNAs as guardians of the genome. Cell. 2009;136:656–668. doi: 10.1016/j.cell.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasovic M, Zocco M, Halic M. Argonaute and Triman generate dicer-independent priRNAs and mature siRNAs to initiate heterochromatin formation. Molecular cell. 2013;52:173–183. doi: 10.1016/j.molcel.2013.08.046. [DOI] [PubMed] [Google Scholar]
- Mohn F, Handler D, Brennecke J. Noncoding RNA. piRNA-guided slicing specifies transcripts for Zucchini-dependent, phased piRNA biogenesis. Science. 2015;348:812–817. doi: 10.1126/science.aaa1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery TA, Rim YS, Zhang C, Dowen RH, Phillips CM, Fischer SE, Ruvkun G. PIWI associated siRNAs and piRNAs specifically require the Caenorhabditis elegans HEN1 ortholog henn-1. PLoS genetics. 2012;8:e1002616. doi: 10.1371/journal.pgen.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimasu H, Ishizu H, Saito K, Fukuhara S, Kamatani MK, Bonnefond L, Matsumoto N, Nishizawa T, Nakanaga K, Aoki J, et al. Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature. 2012;491:284–287. doi: 10.1038/nature11509. [DOI] [PubMed] [Google Scholar]
- Nousch M, Techritz N, Hampel D, Millonigg S, Eckmann CR. The Ccr4-Not deadenylase complex constitutes the main poly(A) removal activity in C. elegans. Journal of cell science. 2013;126:4274–4285. doi: 10.1242/jcs.132936. [DOI] [PubMed] [Google Scholar]
- Olivieri D, Sykora MM, Sachidanandam R, Mechtler K, Brennecke J. An in vivo RNAi assay identifies major genetic and cellular requirements for primary piRNA biogenesis in Drosophila. The EMBO journal. 2010;29:3301–3317. doi: 10.1038/emboj.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pane A, Wehr K, Schupbach T. zucchini and squash encode two putative nucleases required for rasiRNA production in the Drosophila germline. Developmental cell. 2007;12:851–862. doi: 10.1016/j.devcel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryszak R, Burdett T, Fiorelli B, Fonseca NA, Gonzalez-Porta M, Hastings E, Huber W, Jupp S, Keays M, Kryvych N, et al. Expression Atlas update--a database of gene and transcript expression from microarray- and sequencing-based functional genomics experiments. Nucleic acids research. 2014;42:D926–932. doi: 10.1093/nar/gkt1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren YG, Kirsebom LA, Virtanen A. Coordination of divalent metal ions in the active site of poly(A)-specific ribonuclease. The Journal of biological chemistry. 2004;279:48702–48706. doi: 10.1074/jbc.M403858200. [DOI] [PubMed] [Google Scholar]
- Ruby JG, Jan C, Player C, Axtell MJ, Lee W, Nusbaum C, Ge H, Bartel DP. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell. 2006;127:1193–1207. doi: 10.1016/j.cell.2006.10.040. [DOI] [PubMed] [Google Scholar]
- Saito K, Sakaguchi Y, Suzuki T, Suzuki T, Siomi H, Siomi MC. Pimet, the Drosophila homolog of HEN1, mediates 2′-O-methylation of Piwi- interacting RNAs at their 3′ ends. Genes & development. 2007;21:1603–1608. doi: 10.1101/gad.1563607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon WE, Jolly SM, Moore MJ, Zamore PD, Serebrov V. Single-Molecule Imaging Reveals that Argonaute Reshapes the Binding Properties of Its Nucleic Acid Guides. Cell. 2015;162:84–95. doi: 10.1016/j.cell.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe JP, Chen M, Zhao H, Lin H. Tdrkh is essential for spermatogenesis and participates in primary piRNA biogenesis in the germline. The EMBO journal. 2013;32:1869–1885. doi: 10.1038/emboj.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senti KA, Brennecke J. The piRNA pathway: a fly's perspective on the guardian of the genome. Trends in genetics : TIG. 2010;26:499–509. doi: 10.1016/j.tig.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth M, Shirayama M, Gu W, Ishidate T, Conte D, Jr., Mello CC. The C. elegans CSR-1 Argonaute Pathway Counteracts Epigenetic Silencing to Promote Germline Gene Expression. Developmental cell. 2013 doi: 10.1016/j.devcel.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Seth M, Lee HC, Gu W, Ishidate T, Conte D, Jr., Mello CC. piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell. 2012;150:65–77. doi: 10.1016/j.cell.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon B, Kirkpatrick JP, Eckhardt S, Reuter M, Rocha EA, Andrade-Navarro MA, Sehr P, Pillai RS, Carlomagno T. Recognition of 2′-O-methylated 3′-end of piRNA by the PAZ domain of a Piwi protein. Structure. 2011;19:172–180. doi: 10.1016/j.str.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Simon M, Sarkies P, Ikegami K, Doebley AL, Goldstein LD, Mitchell J, Sakaguchi A, Miska EA, Ahmed S. Reduced insulin/IGF-1 signaling restores germ cell immortality to caenorhabditis elegans Piwi mutants. Cell reports. 2014;7:762–773. doi: 10.1016/j.celrep.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi MC, Sato K, Pezic D, Aravin AA. PIWI-interacting small RNAs: the vanguard of genome defence. Nature reviews Molecular cell biology. 2011;12:246–258. doi: 10.1038/nrm3089. [DOI] [PubMed] [Google Scholar]
- Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- Tian Y, Simanshu DK, Ma JB, Patel DJ. Structural basis for piRNA 2′-O-methylated 3′-end recognition by Piwi PAZ (Piwi/Argonaute/Zwille) domains. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:903–910. doi: 10.1073/pnas.1017762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden GW, Castaneda J, Bortvin A. Bodies of evidence - compartmentalization of the piRNA pathway in mouse fetal prospermatogonia. Current opinion in cell biology. 2010;22:752–757. doi: 10.1016/j.ceb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Vourekas A, Zheng Q, Alexiou P, Maragkakis M, Kirino Y, Gregory BD, Mourelatos Z. Mili and Miwi target RNA repertoire reveals piRNA biogenesis and function of Miwi in spermiogenesis. Nature structural & molecular biology. 2012;19:773–781. doi: 10.1038/nsmb.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Reinke V. A C. elegans Piwi, PRG-1, regulates 21U-RNAs during spermatogenesis. Current biology : CB. 2008;18:861–867. doi: 10.1016/j.cub.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeles CJ, Wu MZ, Claycomb JM. Protection of Germline Gene Expression by the C. elegans Argonaute CSR-1. Developmental cell. 2013 doi: 10.1016/j.devcel.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Weick EM, Miska EA. piRNAs: from biogenesis to function. Development. 2014;141:3458–3471. doi: 10.1242/dev.094037. [DOI] [PubMed] [Google Scholar]
- Weick EM, Sarkies P, Silva N, Chen RA, Moss SM, Cording AC, Ahringer J, Martinez-Perez E, Miska EA. PRDE-1 is a nuclear factor essential for the biogenesis of Ruby motif-dependent piRNAs in C. elegans. Genes & development. 2014;28:783–796. doi: 10.1101/gad.238105.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda M, Cifuentes D, Izumi N, Sakaguchi Y, Suzuki T, Giraldez AJ, Tomari Y. Poly(A)-Specific Ribonuclease Mediates 3′-End Trimming of Argonaute2-Cleaved Precursor MicroRNAs. Cell reports. 2013;5:715–726. doi: 10.1016/j.celrep.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Deutscher MP. Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucleic acids research. 2001;29:1017–1026. doi: 10.1093/nar/29.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.