Abstract
The NF-E2-related factor 2 (Nrf2)-mediated signaling pathway provides living organisms an efficient and pivotal line of defensive to counteract environmental insults and endogenous stressors. Nrf2 coordinates the basal and inducible expression of antioxidant and phase II detoxification enzymes to adapt to different stress conditions. The stability and cellular distribution of Nrf2 is tightly controlled by its inhibitory binding protein Kelch-like ECH-associated protein 1 (Keap1). Nrf2 signaling is also regulated by post-translational, transcriptional, translational and epigenetic mechanisms, as well as by other protein partners, including p62, p21 and IQ motif-containing GTPase activating protein 1 (IQGAP1). Many studies have demonstrated that Nrf2 is a promising target for preventing carcinogenesis and other chronic diseases, including cardiovascular diseases, neurodegenerative diseases, and pulmonary injury. However, constitutive activation of Nrf2 in advanced cancer cells may confer drug resistance. Here, we review the molecular mechanisms of Nrf2 signaling, the diverse classes of Nrf2 activators, including bioactive nutrients and other chemicals and the cellular functions and disease relevance of Nrf2 and discuss the dual role of Nrf2 in different contexts.
Keywords: NF-E2-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1 (Keap1), nutrients, activator, antioxidant, ROS
1. Introduction
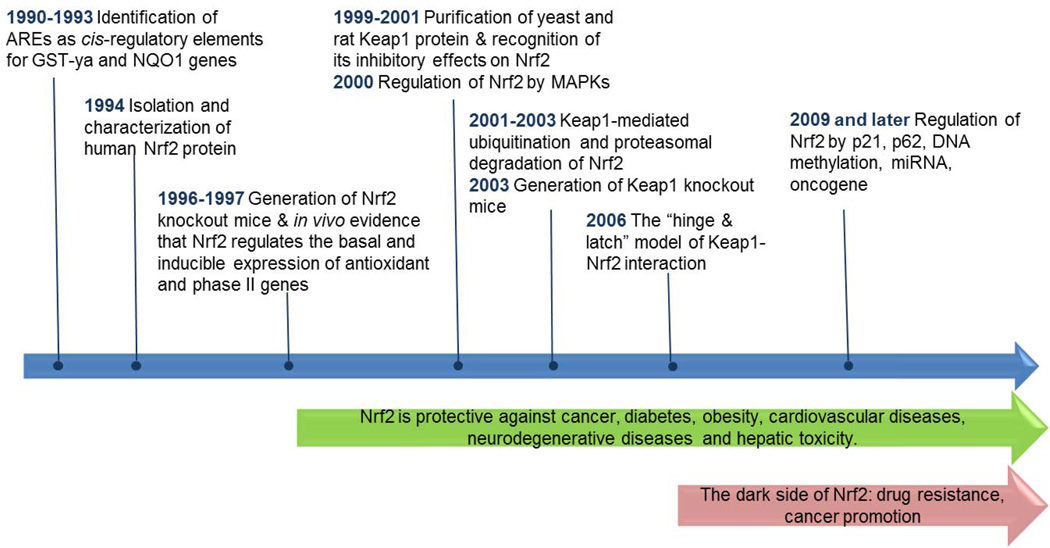
Understanding of the NF-E2-related factor 2 (Nrf2) pathway has been greatly advanced in the last two decades (Figure 1). Nrf2 is a master regulator of the antioxidant response and xenobiotic metabolism through the regulation of a wide range of antioxidant and phase II detoxification genes [1, 2]. Nrf2 protects cells from stressors, including endogenous substances, reactive oxygen species, radiation, environmental toxins, and xenobiotics from food. Therefore, activation of the Nrf2 pathway might be a promising strategy for chemoprevention. This view is supported by a number of studies demonstrating that Nrf2 is essential for chemopreventive agents, such as sulforaphane and oltipraz, to block carcinogenesis and that Nrf2-deficient mice are more prone to chemical-induced cancer development [3–5]. Recent studies have demonstrated that Nrf2 also protects against chronic diseases such as cardiovascular disease, neurodegenerative diseases, and pulmonary injury [6–8]. However, the role of Nrf2 in human diseases is more complicated than originally thought. For example, Nrf2 promotes cancer cell survival and confers resistance to chemotherapeutics and radiotherapy, which has been coined as the “dark side of Nrf2” [9]. The paradox is not yet resolved and stimulates more research to rationalize the dual role of Nrf2 at different contexts.
Figure 1.
Timeline of research advances in the Nrf2 pathway
Many structurally distinct chemicals are inducers of antioxidant and phase II detoxification enzymes (Table 1). Their common features include their electrophilicity and reactivity to sulfhydryl groups [10]. With the discovery of the antioxidant response element (ARE), Nrf2 and Kelch-like ECH-associated protein 1 (Keap1), the molecular mechanisms of Nrf2 activation have been unraveled [1, 11–14]. Some Nrf2 activators exhibit promising efficacy in preventing or treating chronic diseases, including cancer. However, the “dark side” of Nrf2 and off-target side effects of Nrf2 inducers must be taken into consideration when applying them in clinical trials.
Table 1.
Selected Nrf2 activators
| Compound | Structure | Class | Source | Mechanisms of Nrf2 induction | Reference |
|---|---|---|---|---|---|
| Sulforaphane | Isothiocyanates | Cruciferous vegetables |
|
[15–20] | |
| Curcumin | 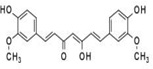 |
phenols | ginger |
|
[21–23] |
| DATS | organosulfur | garlic |
|
[24, 25] | |
| EGCG | 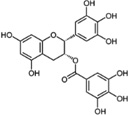 |
polyphenol | tea |
|
[26–30] |
| geneistein | 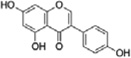 |
isoflavone | lupin, fava beans, soybeans, kudzu, coffee and psoralea |
|
[31, 32] |
| CDDO-Im | 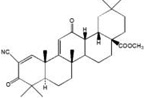 |
Michael reaction acceptors |
Synthetic modification of natural product |
|
[33–35] |
| Oltipraz | 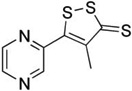 |
Dithiolethiones | Synthetic |
|
[36] |
| tBHQ | 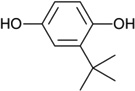 |
Oxidizable diphenols | Synthetic |
|
[17, 34, 37–40] |
| Arsenic trioxide | As3+ | Trivalent arsenicals |
Environment al toxicant |
|
[41–44] |
Specific position of cysteine is not clear.
Both dependence and independent on Keap1-cys151 were reported, probably due to different experimental conditions such as cell cultures, treatment time and choice of amino acid mutation.
2. Regulation of the Nrf2 pathway
2.1 The classical Nrf2-Keap1 signaling pathway
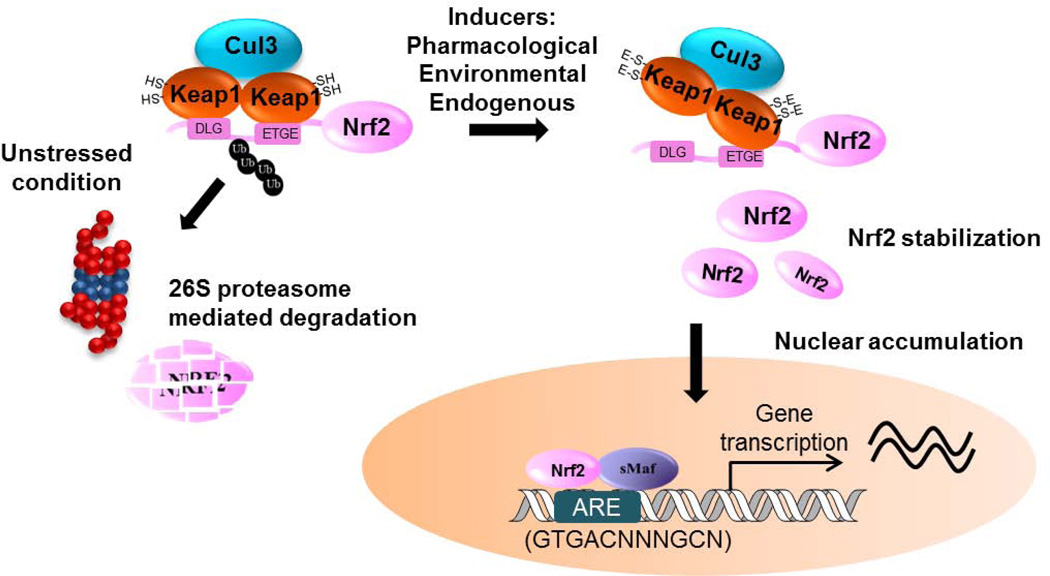
As depicted in Figure 2, Keap1 is a key Nrf2 repressor and plays a pivotal role in regulating the Nrf2 signaling pathway [12, 13, 45, 46]. Nrf2 has two binding motifs in the Neh2 domain, the ETGE and DLG motifs, and recruits two molecules of Keap1 in the absence of stimuli [47, 48]. Keap1 serves as a bridge between Nrf2 and ubiquitination ligase Cullin-3 (Cul-3), which is required for the ubiquitination of lysines in the Neh2 domain and subsequent proteasomal degradation [45, 46, 49, 50]. The negative regulation of Nrf2 by Keap1 has been confirmed in mouse models. Keap1-knockout mice express constitutively high levels of Nrf2, and select heterozygous Keap1 mutations abrogate the repressive effects of wild-type Keap1 on Nrf2 [51, 52].
Figure 2.
Nrf2 signaling pathway schema diagram
Oxidative stressors or electrophiles inhibit the ubiquitination-dependent degradation and increase nuclear accumulation of Nrf2. As a cysteine-rich protein, Keap1 is an excellent sensor for chemical inducers [53]. Accumulating evidence lead to the “cysteine code” hypothesis, which proposes that different classes of Nrf2 activators have unique preferences in modifying specific cysteines (reviewed in [54]). Sulforaphane and tBHQ activate Nrf2 in a Cys151-dependent manner, whereas endogenous alkenal metabolites prefer Cys273/288 [17, 34, 55]. Cysteine modifications alter the proper conformation of the Keap1-Nrf2-Cul3 complex but do not dissociate Keap1 from the Neh2 domain of Nrf2 [56]. In the “hinge & latch” model, Nrf2 activators disrupt the relatively weak interaction between Keap1 and the DLG motif, but not the one between Keap1 and the ETGE motif [47, 48]. The switch from two-site to one-site binding inhibits Nrf2 ubiquitination, thereby rescuing Nrf2 from degradation. Nrf2 accumulates in the nucleus, where it dimerizes with small Maf proteins and binds to the ARE cis-regulatory sequences to trigger transcriptional expression [1, 57]. A large number of genes have been identified as downstream targets of Nrf2, including NAD(P)H:Quinone Oxidoreductase 1 (NQO1) and certain glutathione S-transferases (GSTs) (reviewed in [58]).
2.2 Posttranslational regulation of Nrf2
Phosphorylation of Nrf2 can be detected by radioactive 32P labeling, phosphorylation-specific antibodies and mass spectrometry [59, 60]. A number of studies have examined the upstream regulators of Nrf2 phosphorylation, including protein kinase C (PKC), mitogen-activated protein kinases (MAPKs), PKR-like endoplasmic reticulum kinase (PERK), phosphatidylinositol 3-kinase (PI3K), and glycogen synthase kinase-3 (GSK-3). PKC phosphorylates Nrf2 at Ser40, a residue located in the Neh2 domain that binds Keap1. PKC activates the Nrf2 pathway, likely through disturbing the Nrf2-Keap1 interaction [60–62]. The effects of MAPKs on Nrf2 signaling appear to depend on the specific MAPK. In general, activation of JNK1 and ERK2 promotes the Nrf2 pathway, whereas activation of p38 is inhibitory [63]. Multiple sites in Nrf2 are phosphorylated by various forms of MAPKs (JNK1/2, ERK2, p38, and MEKK3/4) in HEK293T cells, but the Nrf2-mediated ARE response is unaffected by mutation of these sites [60]. This finding raises the question whether MAPK-mediated activation of Nrf2 occurs through direct phosphorylation. PERK enhances the nuclear accumulation of Nrf2 under endoplasmic reticulum stress [64]. PI3K also promotes the nuclear translocation of Nrf2 and induces the expression of ARE-containing genes [65]. By contrast, GSK-3, negatively regulated by PI3K, inhibits Nrf2 by promoting its degradation [66–69]. GSK-3 catalyzes the phosphorylation of Nrf2 at the Neh6 domain and decreases its stability independent of Keap1-mediated degradation [68]. Taken together, protein kinases play a crucial role in Nrf2 signaling. However, it remains unclear whether the phosphorylation of critical Nrf2 residues directly affects Nrf2 signaling. Protein kinases might regulate the Nrf2 pathway through direct phosphorylation or indirect signaling cascades, which need to be dissected in the future.
2.3 Regulation of Nrf2 by p62, p21 and IQGAP1
Several studies have revealed the important role of p62 in the Nrf2-Keap1 pathway. The p62 protein, also named as sequestosome 1 (SQSTM1), is involved in autophagy by regulating the formation of protein aggregates [70]. p62 competes with Nrf2 for binding with Keap1 through its STGE motif [71–74]. p62 also sequesters Keap1 in the autophagosome, leading to the autophagy-dependent degradation of Keap1 [71–75]. Consequently, Keap1-mediated Nrf2 ubiquitination is attenuated, and Nrf2 stability is increased. Overexpression of p62 in HEK293 cells increases both the protein level of Nrf2 and the mRNA expression of its target genes HO-1 and NQO1 [73]. In the livers of autophagy-deficient mice where p62 levels are high, the expression of Nrf2 and its downstream genes are markedly elevated [71, 76]. Persistent activation of p62 and Nrf2 contributes to diseases such as liver dysfunction and hepatocellular carcinoma, possibly through abnormal autophagy [71, 75–77]. Interestingly, arsenic induces Nrf2 in a p62-dependent manner, whereas sulforaphane and tBHQ are independent of p62 [44, 72]. The crosstalk between p62 and the Nrf2-Keap1 pathway provides an emerging pathway to examine the role of Nrf2 in carcinogenesis.
Direct interaction between Nrf2 and p21Cip1/AF1 contributes to the basal and inducible antioxidant response [78]. Chen et al. revealed the binding of p21 to Nrf2 at the DLG and ETGE domains, both of which are required for Keap1-mediated ubiquitination and degradation [78]. In addition, the authors reported that ubiquitination of Nrf2 is enhanced in p21-deficient HCT116 cells under both basal and tBHQ treated conditions, leading to decreased stability of Nrf2. It is likely that p21 alters the conformational structure of the Keap1-Nrf2 complex. Loss of p21 substantially reduces the basal and induced levels of Nrf2 protein and its target genes in HCT116 cells and mouse livers.
We recently identified the IQ motif-containing GTPase-activating protein 1 (IQGAP1) as a binding partner of Nrf2 through the IQGAP1 IQ domain (amino acids 699–905) [79, 80]. IQGAP1 is a scaffold protein that interacts with proteins such as actin, E-cadherin, β-cadherin, and calmodulin [81]. IQGAP1 is an important component in maintaining cytoskeletal architecture, cell-cell adhesion, and Ca2+ signaling [81]. We demonstrated that ectopically expressed IQGAP1 enhances the stability of GFP-Nrf2, and conversely, IQGAP-1 deficiency decreases Nrf2 stability [79]. Treatment with Ca2+, an agonist for IQGAP1 signaling, promotes the nuclear accumulation of the IQGAP1-Nrf2 complex and induces the expression Nrf2 target genes, such as GSTpi, GCLC and NQO1. We also demonstrated that MEK-ERK-mediated Nrf2 phosphorylation is attenuated in IQGAP1 knockdown cells [80]. Given that a direct interaction between IQGAP1 and both MEK and ERK has been reported [82], we speculate that IQGAP1 might provide a platform for the crosstalk between MAPK and Nrf2 signaling.
2.4 Transcriptional regulation
Regulation of Nrf2 at the transcriptional level is far less studied compared to its regulation at the protein level. DeNicola et al. demonstrated that oncogenic alleles of Kras, Braf and Myc induce mRNA expression of Nrf2 and its target genes [83]. The transcriptional upregulation may involve the binding of Myc and Jun to the Nrf2 promoter, although detailed molecular mechanisms are not clear. Enhanced Nrf2 expression leads to the reduction of intracellular ROS and may provide a more favorable environment for cancer cell proliferation.
2.5 Translational regulation
We have reported the translational machinery that controls protein synthesis of Nrf2 [84]. Human Nrf2 mRNA contains an internal ribosomal entry site (IRES) at the 5’ untranslated region that is required to initiate the internalization of Nrf2 mRNA into ribosomes for protein synthesis. In addition, an inhibitory element (IE) exists upstream of the IRES, blocking ribosomal internalization of mRNA. Hydrogen peroxide and sulforaphane treatment induces the entry of Nrf2 mRNA into polysomal fractions and augments its translation in an IRES-dependent manner. These findings suggest that the Nrf2 translation efficiency is low in normal conditions and markedly increases under stress such that cells can consume energy efficiently for protein synthesis and degradation.
2.6 Epigenetic regulation
Accumulating research demonstrates that the transcriptional expression of some specific genes is controlled by epigenetic modification involving chromatin structural alterations. Epigenetic modulations can be caused by DNA methylation, histone modifications, and microRNAs [85]. DNA methylation is catalyzed by DNA methyltransferases (DNMTs) that transfer a methyl group to the 5′ position of the cytosine residue within CpG dinucleotides [86, 87]. Inappropriate DNA methylation of CpG islands in tumor suppressor genes and oncogenes has been observed in many cancer cells and is one of the potential carcinogenic mechanisms during the development of human cancers [85, 88–91].
Our group has reported that the transcription of Nrf2 is suppressed in prostate tumors of TRAMP mice and tumorigenic TRAMP-C1 cells due to the hypermethylation of select CpGs in the promoter of Nrf2 [92]. Repressive proteins, such as methyl-CpG-binding protein 2 (MBD2) and trimethyl-histone H3 (Lys9), are enriched in this region. Because DNA methylation is reversible, combined treatment with 5-aza-29-deoxycytidine (a DNMT inhibitor) and trichostatin A (a histone deacetylase inhibitor) restores Nrf2 expression in TRAMP-C1 cells [92]. Recently, a variety of bioactive nutrients, e.g. curcumin [21], tocopherols [93], sulforaphane [19, 20], 3,3′-diindolylmethane (DIM) [94] were found to modulate DNA methylation and/or histone modification, effectively restoring Nrf2 expression.
Recently, several microRNAs have been found to regulate the Nrf2-Keap1 pathway. MicroRNAs, transcribed from genetic loci, are small (20–22 nucleotides) non-protein-coding RNAs [95]. MicroRNAs regulate gene expression by inhibiting translation or inducing degradation of their target mRNAs [95]. Overexpression of four microRNAs, including miR-144, miR-153, miR-27a, and miR-142-5, either individually or as a group, can reduce Nrf2 mRNA and protein levels, leading to reduced glutathione production in neuronal SH-SY5Y cells [96]. MiR-144 and miR-28 mediate the degradation of Nrf2 mRNA by targeting the 3’-untranslated region (3’-UTR) [97, 98]. Moreover, miR-34a is elevated in the livers of aged rats, which is associated with suppressed expression of Nrf2 [99]. The negative regulation of miR-34a on Nrf2 was further confirmed by the transfection of miR-34a in HEK293 cells. In addition, miR-200a mediates the degradation of Keap1 mRNA in breast cancer cells, and the reduction of Keap1 activates the Nrf2 pathway [100].
3. Nrf2 activators: diverse structures and distinct mechanisms
Nrf2 activators comprise a range of structurally diverse chemicals, classified as isothiocyanates, Michael reaction acceptors, dithiolethiones, oxidizable diphenols/phenylenediamines/quinones, thiocarbamates, polyenes, hydroperoxides, trivalent arsenicals, heavy metals and dimercaptans (Table 1) [10, 101]. Keap1 is the primary target of Nrf2 activators, most of which are electrophilic or reactive to thiol groups. Select Keap1 cysteine residues undergo covalent modifications depending on the nature of the Nrf2 inducers [15, 36, 37, 53, 56]. It is intriguing whether the position of the modified cysteine affects the subsequent biological effects. In addition to Keap1, some Nrf2 activators also modulate other machinery in Nrf2 signaling, as discussed in the preceding section.
3.1 Nrf2 activation and bioactive nutrients
The human diet provide a wide variety of bioactive nutrients that posses health beneficial effects and able to activate the Nrf2 signaling pathway. Isothiocyanates (cruciferous vegetables) [102], organosulfur compounds (garlic and onions) [103], polyphenols (green tea and spice turmeric) [28], and isoflavones (soy beans) [104] have been characterized as potent Nrf2 activators. In general, these naturally occurring compounds can stimulate various upstream kinases, interfere the Keap1-Nrf2 interaction, and/or disturb cellular redox balance, resulting in the activation of the Nrf2 pathway. Administration of these compounds, i.e., sulforaphane [105], curcumin [106, 107], daillyl trisulfide (DATS) [24], epigallocatechin-3-gallate(EGCG) [108, 109] and genistein [31], have been reported to be protective against carcinogenesis, neurodegeneration, cardiovascular disease and diabetic neuropathy in rodent models in part through activation of the Nrf2 pathway. Consuming sufficient amount of fruits and vegetables is not only to meet the nutritional needs but also to boost defense capacity against many oxidative stress and inflammation-associated disease.
Sulforaphane, an isothiocyanate, can be digested from cooked cruciferous vegetables and a variety of oral supplements containing purified sulforaphane or broccoli sprout extract [82–86]. Sulforaphane activates the Nrf2-Keap1 pathway through direct modifications of critical Keap1 cysteines, especially Cys151, as evidenced in mass spectrometry analysis, site-directed mutagenesis and in vivo experiments [15–17, 34, 110]. Sulforaphane treatment also promotes the ribosomal internalization of Nrf2 mRNA for protein synthesis [84]. Recently, we demonstrated that sulforaphane restores Nrf2 mRNA expression epigenetically through the demethylation of promoter CpGs in TRAMP-C1 and JB6 cells [19, 20]. Following acute or long-term administration of sulforaphane, antioxidant and phase II drug metabolizing enzymes are induced in the liver, intestines, skin, prostate, and blood lymphocytes [111–117]. Sulforaphane has been shown to be protective against carcinogenesis in various types of carcinogen-induced and transgenic cancer models (reviewed [58, 118]). Clinical studies have demonstrated that the consumption of broccoli sprout extract changes the disposition of aflatoxin-DNA adducts and is well-tolerated in humans [119–121].
Curcumin, a polyphenolic phytochemical, is one of the most active components of Curcuma longa (turmeric or curcuma), which is a rhizomatus monocotyledonous perennial herbaceous plant member of the ginger family (Zingiberaceae) [122]. Evidence has demonstrated that curcumin could strongly induce HO-1 and other protein members of Phase II detoxification through the activation of Nrf2/ARE pathway in different tissues [123]. In a long term in vivo study, curcumin showed cancer prevention effect by inducing phase-II antioxidant enzymes via activation of Nrf2 signalling, restoration of tumour suppressor p53 and modulation of inflammatory mediators[106]. After intraperitoneal injection of curcumin to mice, it highly induced Nrf2/ARE activity in intestine, liver, kidney and spleen [107]. Curcumin was found to induce HO-1 expression via activation of Nrf2 by binding to cysteine residue of Keap1 [22]. Curcumin were also found to increase NQO1 expression and the binding activity of Nrf2 to antioxidant response element (ARE) [23]. Our recent epigenetics study found that curcumin restores the expression of Nrf2 via demethylation of its CpGs promoter region [21].
DATS, an organosulfur, is one of the major constituents in garlic oil. In in vitro and in vivo experiments, DATS activates Nrf2 and induces HO-1 and NQO1 expression which appears to be mediated through modification of the Keap1 Cys288 residue [24]. DATS-induced ROS production and the subsequent activation of upstream kinases may also be related to the activation of Nrf2 [24]. When cardiomyocytes exposed to high glucose were treated with DATS, it showed protection against hyperglycemia-induced ROS-mediated apoptosis by increase of HO-1 and Nrf2 expression via upregulating the PI3K/Akt/Nrf2 pathway [25].
EGCG, a polyphenol found abundantly in green tea possesses anti-oxidative stress and chemopreventive activities [124]. Using in vitro and in vivo studies, EGCG has demonstrated its effect in increasing NRF2 expression as a possible chemopreventive agent for cancer [109] or lupus nephritis [108]. Many publications have revealed the mechanism of EGCG on Nrf2 pathway. EGCG has demonstrated its protective effect on human umbilical vein endothelial cells from PM2.5-induced oxidative stress injury by upregulating Nrf2/HO-1 via activation of the p38 MAPK and the ERK1/2 signaling pathways [28]. Na et.al. found that EGCG induces Nrf2-mediated expression of MnSOD and HO-1 and activation of ERK1/2 and PI3K/Akt in MCF10A cells [30]. EGCG could promote the nuclear translocation of Nrf2 and EGCG were found to promote the dissociation of Nrf2 from Keap1 [29]. Kanzaki et.al. reported that SFN and EGCG augmented the nuclear translocation of Nrf2 and the expression of HO-1 in mouse monocytic cell line [26]. Wang et.al. also found EGCG promotes Nrf2 nuclear translocation in normal rat kidney proximal tubular epithelial cell line NRK-52E [27].
Genistein is a major soy isoflavone in soy products. Low dose genistein was demonstrated to exert profound neuroprotection, antioxidant and cognitive function preservation effects in rats via enhanced eNOS activation and upregulation of the Nrf2/HO-1 via increaseing keap1 S-nitrosylation, nuclear accumulation of Nrf2 and enhanced DNA binding activity of Nrf2 [31]. Genistein was found to protect cerebrovascular endothelial cells from oxidative damage by activation of Nrf2 signaling pathway via modulating PI3K activity [32].
3.2 Nrf2 activation and other compounds
Synthetic oleanane triterpenoids such as 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) and its derivatives are the most potent Nrf2 inducers ever discovered, activating Nrf2 signaling at nanomolar concentrations [33, 125]. These compounds belong to the Michael reaction acceptors and are reactive to nucleophiles containing –SH groups when incubated with dithiothreitol (DTT), cysteine, or reduced glutathione [126]. Given the chemical properties of these compounds, they very likely modify one or more Keap1 cysteine residues, although the exact ‘cysteine code’ remains to be elucidated [34, 35]. Oral administration of CDDO-Im, an imidazole derivative of CDDO, elevates the expression of HO-1 and NQO1 in the liver and other organs in mice in an Nrf2-dependent manner [33, 127]. A variety of CDDO derivatives are effective for preventing or treating cancer in preclinical models (reviewed in [128]). In addition, CDDO-Im also provides protection against kidney injury, emphysema and cardiac dysfunctions through the Nrf2 pathway [129, 130].
Oltipraz is a synthetic dithiolethione, which is a class of organosulfur compounds. Dithiolethiones are reactive to thiols and potentially modify Keap1 cysteine residues [131, 132]. 3H-1,2-dithiole-3-thione, a dithiolethione structurally similar to oltipraz, induces intermolecular disulfide bonds between two Keap1 molecules at Cys273 and Cys288 [36]. Other studies suggest that dithiolethiones activate the Nrf2 pathway through the generation of H2O2 or other superoxides, which are another class of Nrf2 inducers [132–134]. Oltipraz stimulates a battery of antioxidant and phase II detoxification enzymes, such as NQO1, GSTs and UGTs, and elevates GSH in vitro and in vivo [3, 4, 135, 136]. Oltipraz, an ancient compound used in early chemoprevention studies in the 1980s [137–140], suppresses tumorigenesis induced by a broad range of carcinogens (reviewed in [131, 141]). Its chemopreventive efficacy in carcinogen-induced models appears to depend on Nrf2, as its efficacy is lost in Nrf2 knockout (KO) mice [3, 4, 135]. A clinical study reported that oltipraz increased aflatoxin-mercapturic acid conjugates in urine in subjects with high exposure to aflatoxin B1, indicating faster excretion of aflatoxin B1 [142].
tBHQ and tBQ are oxidized products from butylated hydroxyanisole (BHA), which is an oxidizable diphenol and commonly used as an antioxidant food preservative. Cys151 of Keap1 is a specific sensor for tBHQ. Nrf2 induction by tBHQ is diminished in cell culture and zebrafish by the ectopic expression of Keap1-C151S mutants, as well as in mouse embryonic fibroblasts (MEFs) and primary peritoneal macrophages derived from transgenic mice expressing the Keap1-C151S mutant [17, 34, 38, 110]. Although tBHQ-Cys151 adduct has not been detected by mass spectrometry, the oxidized quinone metabolite of tBQ forms a covalent adduct with Keap1 at Cys151 in a cell-free system [37]. Furthermore, tBHQ enhances the ubiquitination of Keap1, possibly from the switch of Cul3-mediated ubiquitination from Nrf2 to Keap1, leading to the stabilization of Nrf2 protein [39]. Nrf2 induction by BHA and tBHQ is associated with the activation of JNK1 and ERK2, respectively [40]. BHA regulates a wide array of genes involved in phase II detoxification, ubiquitination, transporters and protein kinases in the small intestine and liver in mice through an Nrf2-dependent pathway [143]. Notably, the in vivo effects of BHA might be due to the combined action of BHA itself and its oxidized metabolites such as tBHQ and tBQ, which are more potent Nrf2 activators [144, 145]. The health concerns of the use of BHA as a food additive have been debated since the 1960s. One early study reported that BHA feeding (0.5% w/w) for 60 weeks inhibited ciprofibrate-induced hepatic tumorigenesis in rats [146]. By contrast, other studies found that BHA increased the toxicity of other chemicals or radiation, but BHA feeding (0.4%) alone for 104 weeks did not lead to carcinogenesis in rats [147, 148].
Arsenic is a ubiquitous environmental toxin, found in ground water, soil, and air. Arsenic exposure leads to prolonged Nrf2 activation through unique mechanisms that are not observed in sulforaphane or tBHQ treatment [41, 44]. Nrf2 induction by arsenic is associated with elevated p62 and the accumulation of autophagosomes, and knockdown of p62 diminishes the induction [44]. The proper interaction of Nrf2 and Keap1 is likely to be disordered due to the Keap1-p62 interaction and the sequestration of Keap1 in autophagosomes during arsenic-induced autophagy deficiency [44, 73]. The half-life of Nrf2 is markedly extended by approximately 10-fold when Nrf2 ubiquitination is inhibited by arsenic [41]. Although Nrf2 deletion rescues liver dysfunction in autophagy-deficient mice, it is not clear whether prolonged Nrf2 activation contributes to arsenic toxicity. By contrast, Nrf2 deficiency exacerbates arsenic toxicity in liver and bladder [149].
4. Nrf2 and cellular functions
4.1 Redox balance and xenobiotic metabolism
Reactive oxygen species (ROS), such as superoxides, hydrogen peroxide, and hydroxyl free radicals, are constantly produced in aerobic organisms. Aerobic respiration, during which some electrons leak from the electron transport chain and activate oxygen molecules, is the major source of ROS. Exposure to radiation, ultraviolet light, tobacco smoke, or metal can also trigger ROS production. Nitric oxide (NO), a reactive nitrogen species (RNS) generated by a group of NO synthases, has important physiological functions in regulating muscle contraction, inflammatory response, vasodilation, and platelet aggregation. Balanced cellular ROS/RNS is essential to maintain normal physiological processes, whereas excess amounts of ROS/RNS are harmful to intracellular macromolecules and are associated with chronic diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), diabetes, cardiovascular disease, inflammatory diseases, and cancer [150]. Mammalian cells have evolved with complicated antioxidant systems for survival, consisting of non-enzymatic antioxidants such as vitamin C and E and inducible antioxidant enzymes. Nrf2 coordinates the expression of ARE-containing genes, including superoxide dismutases (SODs), glutathione peroxidase (GPx), NQO1, heme oxygenase-1 (HO-1), and many enzymes involved in glutathione production, which can respond quickly to oxidative stress and maintain a balanced redox state in cells [151].
Humans also face carcinogenic and mutagenic stresses caused by xenobiotics from environmental pollutants. In general, xenobiotics undergo metabolism through two distinct phases: phase I metabolism that includes oxidation, reduction and hydrolyses, and phase II metabolism that comprises conjugation reactions such as glucuronidation, glutathione conjugation and sulfation [152]. Phase I metabolism of xenobiotics often produces carcinogenic intermediates, as exemplified by benzo[a]pyrene and aflatoxin [153, 154]. Phase II metabolism often yields more water-soluble and fewer toxic metabolites. Nrf2 controls many phase II metabolizing enzymes, such as the GST family, the sulfotransferase 3A family, and the UDP-glucuronosyl transferase (UGT) family [58, 151]. Indeed, Nrf2 deficiency predisposes the toxicity of various carcinogens such as benzo[a]pyrene and aflatoxin [5, 135].
4.2 Inflammation
Excessive and chronic inflammation contributes to many acute and chronic diseases, including autoimmune, neurological and cardiovascular diseases and cancer [155, 156]. Increasing evidence suggests that Nrf2 may also protect against inflammation as well as oxidative stress [157–163]. Nrf2 mitigates chemical-induced pulmonary injury and inflammation [164, 165]. Severe tobacco smoke-induced emphysema, airway inflammation and asthma in mice with genetic ablation of Nrf2 may be caused by the reduction of antioxidant gene expression and the induction of interleukin (IL)-4 and IL-13 in bronchoalveolar lavage fluid and in splenocytes [166, 167]. Nrf2 is also involved in the modulation of the innate immune response, as demonstrated in Nrf2-deficient MEFs [168]. Nrf2 may block lipopolysaccharide (LPS)-induced ROS generation of tumor necrosis factor alpha (TNF-α), IL-6 and chemokines (Mip2 and Mcp-1) in mice peritoneal neutrophils [169]. Compared with wild-type mice, LPS stimulates a high level of inflammatory-related signals, such as TNF-α, IL-1, cyclooxygenase 2 (COX-2), and iNOS, in primary peritoneal macrophages in Nrf2-KO mice [170, 171]. More severe DSS-induced colitis was observed in the colon tissues of Nrf2-KO mice than in wild-type mice, and a lower induction of phase II antioxidant and detoxification enzymes and a higher induction of pro-inflammatory biomarkers were observed in Nrf2-KO mice [160]. This finding suggests that Nrf2 may indirectly protect cells from inflammatory damage through antioxidant activation [169, 172]. NF-κB is a key regulator in the innate immune/inflammatory pathway, and the activation of NF-κB has been demonstrated in many cancer types [173, 174]. When cells are exposed to various stimuli, such as TNF-α, IL-1, H2O2, LPS, or microbial infection, the induced proteasome-mediated degradation of IκB proteins leads to the translocation of NF-κB from the cytoplasm to the nucleus [175, 176]. Activated NF-κB may further trigger the expression of downstream target genes, including various inflammatory cytokines and chemokines, adhesion molecules, COX-2, and NO synthase as well as other stress response genes [176–179]. This suggests that there is cross-talk among Nrf2, NF-κB and inflammation [180]. The Nrf2-ARE signaling pathway may be downregulated by pro-inflammatory signaling, as NF-κB could block the binding between CREB-binding protein (CBP) and Nrf2 or promote the interaction of HDAC3 with either CBP or MafK [181]. Additionally, higher activation of NF-κB in response to LPS and TNF-α has been observed in Nrf2-deficient MEFs compared with wild type MEFs [168]. NF-κB activation can also be affected by Nrf2 target genes such as HO-1, NQO1 and thioredoxin (TRX) [182–185].
4.3 Transporters and drug resistance
Transporters mediate the deposition of endogenous substances and xenobiotics, including drugs. For example, efflux transporters in the intestine and brain limit the permeability across the gastrointestinal tract and blood-brain barrier [186]. Overexpression of efflux transporters is a common mechanism of drug resistance, causing failure of cancer chemotherapy (reviewed in [187–189]). One superfamily of efflux transporters is the ATP-binding cassette (ABC) family, which uses the energy of ATP hydrolysis to pump xenobiotics out of cells against a concentration gradient.
A growing body of evidence suggests that Nrf2 is involved in regulating the expression of efflux transporters, especially those belonging to the ABC family. ARE-like sequences are identified in the promoters of genes encoding multidrug resistance-associated proteins (MRP), including Mrp1, Mrp2, Mrp3, Mrp4, and Abcg2 [190–193]. The binding of Nrf2 to AREs has been confirmed in chromatin immunoprecipitation (ChIP) or ChIP-seq experiments [190–194]. In addition, the Nrf2 activators tBHQ, butylated hydroxyanisole (BHA), oltipraz, and ethoxyquin induce the expression of MRPS in cultured cells as well as in mouse and rat livers [190, 193, 195–197]. Nrf2-knockout mice exhibit lower basal expression of Mrp3 and Mrp4 and are less sensitive to BHA and oltipraz-induced expression of Mrp2, Mrp3 and Mrp4 compared with Nrf2 wild-type mice [193, 198]. Constitutive overexpression of Nrf2 confers chemoresistance in some advanced cancer cells, including human lung adenocarcinoma A549 cells and pancreatic carcinoma Panc1 and Colo357 cells. The knockdown of Nrf2 using siRNA or Nrf2 inhibitors sensitizes cellular responses to common chemotherapeutic drugs or natural compounds [199–202]. Collectively, Nrf2 is a potential target to circumvent chemoresistance.
4.4 Metabolic reprogramming
The distinct patterns of energy metabolism in normal and cancer cells have long been observed, but until recently, very little was known regarding the role of Nrf2 in this metabolic switch. Cancer cells tend to generate ATP through anabolic glycolysis of glucose rather than mitochondrial oxidative phosphorylation, a phenomena known as the Warburg effect [203]. Despite the reduced efficiency of energy production through glycolysis, the Warburg effect provides a variety of glycolytic intermediates that are needed to synthesize nucleosides and amino acids for cell proliferation and division [204, 205]. Recently, Mitsuishi et al. found that Nrf2 redirects glucose and glutamine metabolism in favor of the production of ribose-5-phosphate and NADPH through anabolic pathways [206]. In their study, several metabolic genes were identified as Nrf2 targets. Nrf2 activates glucose-6-phosphate dehydrogenase (G6PD), phosphogluconate dehydrogenase (PGD), transketolase (TKT), transaldolase 1 (TALDO1), and malic enzyme 1 (ME-1) by binding their AREs. These findings provide mechanistic understanding of how Nrf2 supports cancer proliferation.
Substantial evidence has linked the impaired Krebs cycle (tricarboxylic acid cycle) to Nrf2 activation via the metabolite fumarate [207–209]. In the normal Krebs cycle, fumarate is converted to malate by fumarate hydratase (FH) and remains at a low cellular concentration. Accumulation of fumarate can form an adduct with Keap1 on its cysteine residues and provoke Nrf2 activation [207, 208]. In hereditary type 2 papillary renal cell carcinoma, the high level of fumarate caused by a loss-of-function mutation in FH is associated with sustained activation of Nrf2 [207, 208]. The disrupted Krebs cycle triggers metabolic reprogramming toward glycolysis [210]. It would be interesting to elucidate the detailed molecular mechanisms of Nrf2 in the metabolic switch.
5. Nrf2 and diseases
5.1 The protective role of Nrf2 in chronic diseases
The protective role of Nrf2 in carcinogenesis has been demonstrated in animal models of different types of cancer. In early chemoprevention studies, it was observed that chemopreventive agents modulate the disposition of carcinogens and inhibit carcinogenesis through induction of phase II metabolizing enzymes [138, 211, 212]. Nrf2 is required for sulforaphane and oltipraz to exert chemopreventive effects, as shown by comparing their efficacy in Nrf2 WT and KO mice [3, 4, 213]. Furthermore, Nrf2 deletion markedly increases susceptibility to carcinogen-induced tumor development in various organs, including colon, skin, breast, bladder and liver [5]. Nrf2-KO mice exhibit more pronounced inflammation in a dextran sulfate sodium-induced colitis model [160, 214, 215]. These results strongly suggest that Nrf2 protects against tumorigenesis by modulating the disposition of carcinogens and inflammatory response.
The impact of Nrf2 in neurodegenerative diseases has been evaluated using mouse models of PD and AD. Nrf2-KO mice were more vulnerable to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced loss of dopaminergic neurons, which is a key characteristic of PD [6, 216, 217]. Similar results were observed in the 6-hydroxydopamine-induced PD model [218]. Conversely, astrocyte-specific overexpression of Nrf2 abolished MPTP-induced neurotoxicity [6]. In a chronic neuroinflammation mouse model induced by long-term administration of MPTP, the levels inflammatory makers such as COX-2, iNOS, IL-6, and TNF-α were higher in the brains of Nrf2-KO mice than WT mice [219]. In transgenic APP/PS1 AD mice, the overexpression of Nrf2 in the hippocampus significantly improved learning and memory ability [220].
A number of studies have demonstrated that Nrf2 protects against cardiovascular diseases. Compared with WT mice, Nrf2-KO mice exhibit exaggerated cardiac hypertrophy, overt heart failure, and increased mortality caused by transverse aortic constriction [221]. Nrf2-KO mice exhibit more severe cardiac oxidative stress and cardiac hypertrophy after sustained administration of angiotensin II [7]. CDDO-Im treatment relieved smoke-induced cardiac dysfunction in Nrf2-WT mice, but the beneficial effects were not observed in Nrf2-KO mice [129]. Enhancing Nrf2 is a promising strategy to prevent the exacerbation of chronic obstructive pulmonary disease (COPD). Features of COPD include persistent inflammation and oxidative damage [8]. Nrf2-KO mice are more susceptible to cigarette smoke-induced emphysema [222]. Genetic ablation of Nrf2 enhances bronchoalveolar and airway inflammation in cigarette smoke-and ovalbumin-induced mouse models [215, 222]. Nrf2-KO mice also exhibit more severe pulmonary inflammation and lung tissue injury in mouse models of COPD exacerbation triggered by bacterial or viral infection [223–225]. Furthermore, sulforaphane enhances bacteria clearance and reduces pulmonary inflammation, which requires the involvement of Nrf2 [223].
5.2 The dark side of Nrf2 in carcinogenesis
Given the protective role of Nrf2 in counteracting different stressors and toxins, it is logical to speculate that it also provides protection for cancer cells. Indeed, an increasing body of evidence demonstrates that Nrf2 is constitutively elevated in many types of cancer cells or tumor samples from cancer patients [161, 226–232]. Overexpression of Nrf2 is associated with poor prognosis in cancer patients [233–235]. A recent study reported that urethane-induced lung tumors from Nrf2-KO mice failed to engraft in nude mice, whereas those from Nrf2 WT mice grew progressively [236]. Knockdown of Nrf2 led to cell cycle arrest at the G1 phase in A549 and NCI-H292 lung cancer cells [201]. Nrf2 might be required to sustain proliferative signaling and reprogram energy metabolism, which are hallmarks of cancer [201, 206, 237].
Several molecular mechanisms have been elucidated for the constitutive expression of Nrf2 in cancer. Somatic mutations in Keap1 or Nrf2 occur frequently in lung, ovarian, gallbladder, liver and gastric cancer (reviewed in [151, 238, 239]). Loss-of-function mutations in Keap1 weaken its binding affinity to Nrf2 and attenuate its repressive effect on Nrf2 [226, 227]. It is noteworthy that mutations in Nrf2 occur predominantly in the DLG or ETGE motif in the Neh2 binding domain, thereby enabling Nrf2 to escape from Keap1-Cul3-mediated ubiquitination and degradation [202]. In addition, epigenetic silencing of Keap1 due to CpG hypermethylation in its promoter has been observed in some types of cancer [229, 240, 241]. Furthermore, defective autophagy in cancer causes abnormal autophagic degradation of Keap1 and impaired Keap1-Nrf2 interaction with the involvement of p62 [71–75]. Additionally, endogenous expression of the oncogenes Kras, Braf and Myc stimulates the transcriptional expression of Nrf2 in MEFs, which may also occur during tumorigenesis [83]. Collectively, the normal Keap1-Nrf2 axis is severely disturbed by multiple factors in cancer.
6. The complexity of the Nrf2 pathway: Strength and duration of activation, disease stages, and multiple targets of Nrf2 activators
Transcription factor activity is commonly a double-edged sword. The strength of its action is a crucial factor in determining which side is predominant. Kensler et al. proposed a U-shaped relationship of Nrf2 expression and cancer risk [242]. According this paradigm, extreme low or high Nrf2 levels increase disease risk. The U-shaped relationship effectively describes the observations that Nrf2 deletion increases susceptibility to carcinogens, neuronal toxins and bacterial and viral infection, whereas Nrf2 overexpression promotes drug resistance and cancer cell proliferation. Therefore, effective prevention occurs within a range between the biologically effective dose (BED) and the maximal-tolerated dose (MTD).
The duration of Nrf2 activation, which is determined by the underlying mechanism for activation, has profound biological consequences. For instance, chemical inducers and genetic modifications exhibit different response-time profiles for Nrf2 induction. Chemical inducers often exhibit a transient effect on Nrf2 pathway activation. We performed a pharmacokinetic-pharmacodynamic study of sulforaphane in rats and found that the expression of Nrf2-target genes peaks at 1–2 hours in blood lymphocytes after intravenous injection and returns to basal level after 24 hours [117]. By contrast, some chemical inducers have a prolonged effect on Nrf2 induction, likely through mechanisms other than the classical Nrf2-Keap1 interaction. One such example is arsenic, which recruits p62 and traps Keap1 in autophagosomes [44]. Furthermore, genetic factors such as Keap1 deletion, Keap1 mutation, Nrf2 mutation, and oncogenes generally lead to persistent activation of Nrf2, which is linked to the “dark side” of Nrf2 [239]. Transient Nrf2 activation through an intact Nrf2-Keap1 axis appears beneficial, whereas persistent Nrf2 activation might be harmful.
The dual role of Nrf2 is exemplified in different stages of tumorigenesis. Nrf2 blocks or delays tumorigenesis in normal and premalignant cells by relieving oxidative, mutagenic and inflammatory damage and by modulating carcinogen disposition. However, Nrf2 becomes undesirable when the defensive effects are hijacked by the malignant cells. The dual role is demonstrated in a recent study comparing WT and Nrf2-KO mice with urethane exposure [236]. Nrf2 prevents lung cancer initiation but enhances its progression in the late stage.
Many Nrf2 activators concomitantly exert multiple effects, such as inhibition of the NF-κB pathway and modulation of epigenetics, which appear to contribute to protective effects in addition to Nrf2 activation. For example, sulforaphane, 3H-1,2-dithiole-3-thione, and synthetic triterpenoid CDDO-Me repress the NF-κB mediated pro-inflammatory response by inhibiting the binding of NF-κB to DNA and the degradation of the NF-κB inhibitory protein IKK [171, 243–245]. In addition, several Nrf2 activators regulate epigenetic changes by affecting the enzymatic activity of HDACs and DNMTs. Class I, II and IV HDACs are zinc metalloproteins whose activity is dependent on Zn2+ [246]. Some Nrf2 activators interfere with HDAC activity, possibly through chemical characteristics (i.e., isothiocyanates, organosulfides and phenols) that may chelate Zn2+ [247]. Various Nrf2 activators inhibit the expression or the activity of DNMTs (i.e., sulforaphane and curcumin) or deplete the cellular pool of methyl donors (i.e., catechol polyphenols), resulting in altered DNA methylation [20, 21, 248]. The beneficial effects of Nrf2 activators may be due to a combination of Nrf2 activation, NF-κB inhibition and epigenetic regulation.
7. Conclusions and future prospects
Nrf2 plays a profound role in physiological and pathological processes. Nrf2 not only acts as a master regulator in the antioxidant response and xenobiotic disposition but also modulates the inflammatory response, metabolic programming, cell proliferation and survival. Nrf2 protects against many chronic diseases other than cancer, although the mechanism is not yet completely understood. Nutritional dietary phytochemicals such as sulforaphane, curcumin, DATS, EGCG and genistein have been reported to possess health beneficial effects and protective against carcinogenesis, neurodegeneration, cardiovascular disease and diabetic neuropathy through activation of the Nrf2 pathway. Future studies on the cross-talk between Nrf2 and other signaling pathways are needed. In addition, the dual role of Nrf2 in carcinogenesis raises concerns for the use of Nrf2 inducers in chemoprevention, although there is no evidence demonstrating that Nrf2 activation through an intact Nrf2-Keap1 axis initiates tumorigenesis. The difference between transient and constitutive activation of Nrf2 needs to be distinguished. Substantial evidence indicates that transient Nrf2 activation by pharmacological agents is beneficial. However, inhibition of the constitutive expression of Nrf2 may be a potential target to overcome drug resistance in cancer therapeutics. Furthermore, the structural information of Nrf2 remains to be unraveled, which will be helpful in designing better Nrf2 activators with high potency and reduced off-targets effects.
Acknowledgments
The authors express sincere gratitude to all of the members of Dr. Tony Kong’s laboratory for their helpful discussions. This work was supported in part by institutional funds and by R01-CA118947 and R01-CA152826 from the National Cancer Institute (NCI), R01AT007065 from the National Center for Complementary and Alternative Medicines (NCCAM) and the Office of Dietary Supplements (ODS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical and biophysical research communications. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 2.McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, et al. The Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer research. 2001;61:3299–3307. [PubMed] [Google Scholar]
- 3.Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, Kawai K, et al. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer research. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 4.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwak MK, Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicology and applied pharmacology. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, et al. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Zhang C, Xing Y, Janicki JS, Yamamoto M, Wang XL, et al. Up-regulation of p27(kip1) contributes to Nrf2-mediated protection against angiotensin II-induced cardiac hypertrophy. Cardiovascular research. 2011;90:315–324. doi: 10.1093/cvr/cvr010. [DOI] [PubMed] [Google Scholar]
- 8.Biswal S, Thimmulappa RK, Harvey CJ. Experimental therapeutics of Nrf2 as a target for prevention of bacterial exacerbations in COPD. Proceedings of the American Thoracic Society. 2012;9:47–51. doi: 10.1513/pats.201201-009MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacological research : the official journal of the Italian Pharmacological Society. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prestera T, Holtzclaw WD, Zhang Y, Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes & development. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhakshinamoorthy S, Jaiswal AK. Functional characterization and role of INrf2 in antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene. 2001;20:3906–3917. doi: 10.1038/sj.onc.1204506. [DOI] [PubMed] [Google Scholar]
- 14.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. The Journal of biological chemistry. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 15.Eggler AL, Luo Y, van Breemen RB, Mesecar AD. Identification of the highly reactive cysteine 151 in the chemopreventive agent-sensor Keap1 protein is method-dependent. Chemical research in toxicology. 2007;20:1878–1884. doi: 10.1021/tx700217c. [DOI] [PubMed] [Google Scholar]
- 16.Hu C, Eggler AL, Mesecar AD, van Breemen RB. Modification of keap1 cysteine residues by sulforaphane. Chemical research in toxicology. 2011;24:515–521. doi: 10.1021/tx100389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Molecular and cellular biology. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakubikova J, Sedlak J, Mithen R, Bao Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochemical pharmacology. 2005;69:1543–1552. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Su ZY, Khor TO, Shu L, Kong AN. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochemical pharmacology. 2013;85:1398–1404. doi: 10.1016/j.bcp.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su ZY, Zhang C, Lee JH, Shu L, Wu TY, Khor TO, et al. Requirement and epigenetics reprogramming of Nrf2 in suppression of tumor promoter TPA-induced mouse skin cell transformation by sulforaphane. Cancer prevention research. 2014;7:319–329. doi: 10.1158/1940-6207.CAPR-13-0313-T. [DOI] [PubMed] [Google Scholar]
- 21.Khor TO, Huang Y, Wu TY, Shu L, Lee J, Kong AN. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochemical pharmacology. 2011;82:1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 22.Farombi EO, Shrotriya S, Na H-K, Kim S-H, Surh Y-J. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food and Chemical Toxicology. 2008;46:1279–1287. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Li Q, Wang X, Yu S, Li L, Wu X, et al. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PloS one. 2013;8:e59843. doi: 10.1371/journal.pone.0059843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S, Lee HG, Park SA, Kundu JK, Keum YS, Cha YN, et al. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PloS one. 2014;9:e85984. doi: 10.1371/journal.pone.0085984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai CY, Wang CC, Lai TY, Tsu HN, Wang CH, Liang HY, et al. Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. International journal of cardiology. 2013;168:1286–1297. doi: 10.1016/j.ijcard.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Kanzaki H, Shinohara F, Itohiya-Kasuya K, Ishikawa M, Nakamura Y. Nrf2 activation attenuates both orthodontic tooth movement and relapse. Journal of dental research. 2015;94:787–794. doi: 10.1177/0022034515577814. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Liu N, Su X, Zhou G, Sun G, Du F, et al. Epigallocatechin-3-gallate attenuates transforming growth factor-beta1 induced epithelial-mesenchymal transition via Nrf2 regulation in renal tubular epithelial cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2015;70:260–267. doi: 10.1016/j.biopha.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Yang GZ, Wang ZJ, Bai F, Qin XJ, Cao J, Lv JY, et al. Epigallocatechin-3-Gallate Protects HUVECs from PM2.5-Induced Oxidative Stress Injury by Activating Critical Antioxidant Pathways. Molecules. 2015;20:6626–6639. doi: 10.3390/molecules20046626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang J, Mo ZC, Yin K, Zhao GJ, Lv YC, Ouyang XP, et al. Epigallocatechin-3-gallate prevents TNF-alpha-induced NF-kappaB activation thereby upregulating ABCA1 via the Nrf2/Keap1 pathway in macrophage foam cells. International journal of molecular medicine. 2012;29:946–956. doi: 10.3892/ijmm.2012.924. [DOI] [PubMed] [Google Scholar]
- 30.Na HK, Kim EH, Jung JH, Lee HH, Hyun JW, Surh YJ. (−)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Archives of biochemistry and biophysics. 2008;476:171–177. doi: 10.1016/j.abb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Wang R, Tu J, Zhang Q, Zhang X, Zhu Y, Ma W, et al. Genistein attenuates ischemic oxidative damage and behavioral deficits via eNOS/Nrf2/HO-1 signaling. Hippocampus. 2013;23:634–647. doi: 10.1002/hipo.22126. [DOI] [PubMed] [Google Scholar]
- 32.Xi YD, Yu HL, Ding J, Ma WW, Yuan LH, Feng JF, et al. Flavonoids protect cerebrovascular endothelial cells through Nrf2 and PI3K from beta-amyloid peptide-induced oxidative damage. Current neurovascular research. 2012;9:32–41. doi: 10.2174/156720212799297092. [DOI] [PubMed] [Google Scholar]
- 33.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer research. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 34.Takaya K, Suzuki T, Motohashi H, Onodera K, Satomi S, Kensler TW, et al. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free radical biology & medicine. 2012;53:817–827. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eggler AL, Small E, Hannink M, Mesecar AD. Cul3-mediated Nrf2 ubiquitination and antioxidant response element (ARE) activation are dependent on the partial molar volume at position 151 of Keap1. The Biochemical journal. 2009;422:171–180. doi: 10.1042/BJ20090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abiko Y, Miura T, Phuc BH, Shinkai Y, Kumagai Y. Participation of covalent modification of Keap1 in the activation of Nrf2 by tert-butylbenzoquinone, an electrophilic metabolite of butylated hydroxyanisole. Toxicology and applied pharmacology. 2011;255:32–39. doi: 10.1016/j.taap.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, et al. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Molecular and cellular biology. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong F, Sekhar KR, Freeman ML, Liebler DC. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. The Journal of biological chemistry. 2005;280:31768–31775. doi: 10.1074/jbc.M503346200. [DOI] [PubMed] [Google Scholar]
- 40.Yu R, Tan TH, Kong AN. Butylated hydroxyanisole and its metabolite tert-butylhydroquinone differentially regulate mitogen-activated protein kinases. The role of oxidative stress in the activation of mitogen-activated protein kinases by phenolic antioxidants. The Journal of biological chemistry. 1997;272:28962–28970. doi: 10.1074/jbc.272.46.28962. [DOI] [PubMed] [Google Scholar]
- 41.He X, Chen MG, Lin GX, Ma Q. Arsenic induces NAD(P)H-quinone oxidoreductase I by disrupting the Nrf2 x Keap1 x Cul3 complex and recruiting Nrf2 x Maf to the antioxidant response element enhancer. The Journal of biological chemistry. 2006;281:23620–23631. doi: 10.1074/jbc.M604120200. [DOI] [PubMed] [Google Scholar]
- 42.Wang XJ, Sun Z, Chen W, Li Y, Villeneuve NF, Zhang DD. Activation of Nrf2 by arsenite and monomethylarsonous acid is independent of Keap1-C151: enhanced Keap1-Cul3 interaction. Toxicology and applied pharmacology. 2008;230:383–389. doi: 10.1016/j.taap.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He X, Ma Q. Critical cysteine residues of Kelch-like ECH-associated protein 1 in arsenic sensing and suppression of nuclear factor erythroid 2-related factor 2. The Journal of pharmacology and experimental therapeutics. 2010;332:66–75. doi: 10.1124/jpet.109.160465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau A, Zheng Y, Tao S, Wang H, Whitman SA, White E, et al. Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner. Molecular and cellular biology. 2013;33:2436–2446. doi: 10.1128/MCB.01748-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. The Journal of biological chemistry. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Molecular and cellular biology. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. The Journal of biological chemistry. 2006;281:24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- 48.Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Molecular and cellular biology. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Molecular and cellular biology. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Molecular and cellular biology. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nature genetics. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T, Maher J, Yamamoto M. Select heterozygous Keap1 mutations have a dominant-negative effect on wild-type Keap1 in vivo. Cancer research. 2011;71:1700–1709. doi: 10.1158/0008-5472.CAN-10-2939. [DOI] [PubMed] [Google Scholar]
- 53.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends in pharmacological sciences. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 55.McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen T, Sherratt PJ, Nioi P, Yang CS, Pickett CB. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. The Journal of biological chemistry. 2005;280:32485–32492. doi: 10.1074/jbc.M503074200. [DOI] [PubMed] [Google Scholar]
- 58.Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxidants & redox signaling. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 59.Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Z, Huang Z, Zhang DD. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PloS one. 2009;4:e6588. doi: 10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Numazawa S, Ishikawa M, Yoshida A, Tanaka S, Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. American journal of physiology Cell physiology. 2003;285:C334–C342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 62.Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. The Journal of biological chemistry. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- 63.Lee JH, Khor TO, Shu L, Su ZY, Fuentes F, Kong AN. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacology & therapeutics. 2013;137:153–171. doi: 10.1016/j.pharmthera.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. The Journal of biological chemistry. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 65.Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Molecular pharmacology. 2002;62:1001–1010. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- 66.Jain AK, Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. The Journal of biological chemistry. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 67.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Molecular and cellular biology. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rojo AI, Sagarra MR, Cuadrado A. GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. Journal of neurochemistry. 2008;105:192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- 70.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. The Journal of cell biology. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nature cell biology. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 72.Copple IM, Lister A, Obeng AD, Kitteringham NR, Jenkins RE, Layfield R, et al. Physical and functional interaction of sequestosome 1 with Keap1 regulates the Keap1-Nrf2 cell defense pathway. The Journal of biological chemistry. 2010;285:16782–16788. doi: 10.1074/jbc.M109.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Molecular and cellular biology. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. The Journal of biological chemistry. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, et al. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13561–13566. doi: 10.1073/pnas.1121572109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. The Journal of cell biology. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 78.Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, et al. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Molecular cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim JH, Xu EY, Sacks DB, Lee J, Shu L, Xia B, et al. Identification and functional studies of a new Nrf2 partner IQGAP1: a critical role in the stability and transactivation of Nrf2. Antioxidants & redox signaling. 2013;19:89–101. doi: 10.1089/ars.2012.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheung KL, Lee JH, Shu L, Kim JH, Sacks DB, Kong AN. The Ras GTPase-activating-like protein IQGAP1 mediates Nrf2 protein activation via the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)-ERK pathway. The Journal of biological chemistry. 2013;288:22378–22386. doi: 10.1074/jbc.M112.444182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Briggs MW, Sacks DB. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS letters. 2003;542:7–11. doi: 10.1016/s0014-5793(03)00333-8. [DOI] [PubMed] [Google Scholar]
- 82.Roy M, Li Z, Sacks DB. IQGAP1 binds ERK2 and modulates its activity. The Journal of biological chemistry. 2004;279:17329–17337. doi: 10.1074/jbc.M308405200. [DOI] [PubMed] [Google Scholar]
- 83.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li W, Thakor N, Xu EY, Huang Y, Chen C, Yu R, et al. An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic acids research. 2010;38:778–788. doi: 10.1093/nar/gkp1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghavifekr Fakhr M, Farshdousti Hagh M, Shanehbandi D, Baradaran B. DNA Methylation Pattern as Important Epigenetic Criterion in Cancer. Genetics research international. 2013;2013:317569. doi: 10.1155/2013/317569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21:5427–5240. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- 87.Issa JP, Kantarjian HM. Targeting DNA methylation. Clin Cancer Res. 2009;15:3938–3946. doi: 10.1158/1078-0432.CCR-08-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 89.Arasaradnam RP, Commane DM, Bradburn D, Mathers JC. A review of dietary factors and its influence on DNA methylation in colorectal carcinogenesis. Epigenetics. 2008;3:193–198. doi: 10.4161/epi.3.4.6508. [DOI] [PubMed] [Google Scholar]
- 90.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 91.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 92.Yu S, Khor TO, Cheung KL, Li W, Wu TY, Huang Y, et al. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PloS one. 2010;5:e8579. doi: 10.1371/journal.pone.0008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang Y, Khor TO, Shu L, Saw CL, Wu TY, Suh N, et al. A gamma-tocopherol-rich mixture of tocopherols maintains Nrf2 expression in prostate tumors of TRAMP mice via epigenetic inhibition of CpG methylation. The Journal of nutrition. 2012;142:818–823. doi: 10.3945/jn.111.153114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu TY, Khor TO, Su ZY, Saw CL, Shu L, Cheung KL, et al. Epigenetic modifications of Nrf2 by 3,3’-diindolylmethane in vitro in TRAMP C1 cell line and in vivo TRAMP prostate tumors. The AAPS journal. 2013;15:864–874. doi: 10.1208/s12248-013-9493-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Narasimhan M, Patel D, Vedpathak D, Rathinam M, Henderson G, Mahimainathan L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PloS one. 2012;7:e51111. doi: 10.1371/journal.pone.0051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sangokoya C, Telen MJ, Chi JT. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2010;116:4338–4348. doi: 10.1182/blood-2009-04-214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang M, Yao Y, Eades G, Zhang Y, Zhou Q. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast cancer research and treatment. 2011;129:983–991. doi: 10.1007/s10549-011-1604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li N, Muthusamy S, Liang R, Sarojini H, Wang E. Increased expression of miR-34a and miR-93 in rat liver during aging, and their impact on the expression of Mgst1 and Sirt1. Mechanisms of ageing and development. 2011;132:75–85. doi: 10.1016/j.mad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 100.Eades G, Yang M, Yao Y, Zhang Y, Zhou Q. miR-200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cells. The Journal of biological chemistry. 2011;286:40725–40733. doi: 10.1074/jbc.M111.275495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochemical pharmacology. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 102.Keum YS, Jeong WS, Kong AN. Chemopreventive functions of isothiocyanates. Drug news & perspectives. 2005;18:445–451. doi: 10.1358/dnp.2005.18.7.939350. [DOI] [PubMed] [Google Scholar]
- 103.Ho CY, Cheng YT, Chau CF, Yen GC. Effect of diallyl sulfide on in vitro and in vivo Nrf2-mediated pulmonic antioxidant enzyme expression via activation ERK/p38 signaling pathway. Journal of agricultural and food chemistry. 2012;60:100–107. doi: 10.1021/jf203800d. [DOI] [PubMed] [Google Scholar]
- 104.Perez Diaz MF, Acosta M, Mohamed FH, Ferramola ML, Oliveros LB, Gimenez MS. Protective effect of soybeans as protein source in the diet against cadmium-aorta redox and morphological alteration. Toxicology and applied pharmacology. 2013;272:806–815. doi: 10.1016/j.taap.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 105.Lin H, Wei B, Li G, Zheng J, Sun J, Chu J, et al. Sulforaphane reverses glucocorticoid-induced apoptosis in osteoblastic cells through regulation of the Nrf2 pathway. Drug design, development and therapy. 2014;8:973–982. doi: 10.2147/DDDT.S65410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Das L, Vinayak M. Long Term Effect of Curcumin in Restoration of Tumour Suppressor p53 and Phase-II Antioxidant Enzymes via Activation of Nrf2 Signalling and Modulation of Inflammation in Prevention of Cancer. PloS one. 2015;10:e0124000. doi: 10.1371/journal.pone.0124000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Balstad TR, Carlsen H, Myhrstad MC, Kolberg M, Reiersen H, Gilen L, et al. Coffee, broccoli and spices are strong inducers of electrophile response element-dependent transcription in vitro and in vivo - studies in electrophile response element transgenic mice. Molecular nutrition & food research. 2011;55:185–197. doi: 10.1002/mnfr.201000204. [DOI] [PubMed] [Google Scholar]
- 108.Tsai PY, Ka SM, Chang JM, Chen HC, Shui HA, Li CY, et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free radical biology & medicine. 2011;51:744–754. doi: 10.1016/j.freeradbiomed.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 109.Zhang ZM, Yang XY, Yuan JH, Sun ZY, Li YQ. Modulation of NRF2 and UGT1A expression by epigallocatechin-3-gallate in colon cancer cells and BALB/c mice. Chinese medical journal. 2009;122:1660–1665. [PubMed] [Google Scholar]
- 110.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Molecular and cellular biology. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, et al. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer letters. 2006;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 112.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer research. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 113.Khor TO, Hu R, Shen G, Jeong WS, Hebbar V, Chen C, et al. Pharmacogenomics of cancer chemopreventive isothiocyanate compound sulforaphane in the intestinal polyps of ApcMin/+ mice. Biopharmaceutics & drug disposition. 2006;27:407–420. doi: 10.1002/bdd.522. [DOI] [PubMed] [Google Scholar]
- 114.Xu C, Huang MT, Shen G, Yuan X, Lin W, Khor TO, et al. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer research. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 115.Nair S, Barve A, Khor TO, Shen GX, Lin W, Chan JY, et al. Regulation of Nrf2- and AP-1-mediated gene expression by epigallocatechin-3-gallate and sulforaphane in prostate of Nrf2-knockout or C57BL/6J mice and PC-3 AP-1 human prostate cancer cells. Acta pharmacologica Sinica. 2010;31:1223–1240. doi: 10.1038/aps.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Keum YS, Khor TO, Lin W, Shen G, Kwon KH, Barve A, et al. Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharmaceutical research. 2009;26:2324–2331. doi: 10.1007/s11095-009-9948-5. [DOI] [PubMed] [Google Scholar]
- 117.Wang H, Khor TO, Yang Q, Huang Y, Wu TY, Saw CL, et al. Pharmacokinetics and pharmacodynamics of phase II drug metabolizing/antioxidant enzymes gene response by anticancer agent sulforaphane in rat lymphocytes. Molecular pharmaceutics. 2012;9:2819–2827. doi: 10.1021/mp300130k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, Chen JG, et al. Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Topics in current chemistry. 2013;329:163–177. doi: 10.1007/128_2012_339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutrition and cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 120.Egner PA, Chen JG, Wang JB, Wu Y, Sun Y, Lu JH, et al. Bioavailability of Sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res (Phila) 2011;4:384–395. doi: 10.1158/1940-6207.CAPR-10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Munoz A, et al. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis. 2012;33:101–107. doi: 10.1093/carcin/bgr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Trujillo J, Chirino YI, Molina-Jijon E, Anderica-Romero AC, Tapia E, Pedraza-Chaverri J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox biology. 2013;1:448–456. doi: 10.1016/j.redox.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Molecular neurobiology. 2011;44:192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, et al. Green tea and cancer chemoprevention. Mutation research. 1999;428:339–344. doi: 10.1016/s1383-5742(99)00059-9. [DOI] [PubMed] [Google Scholar]
- 125.Thimmulappa RK, Fuchs RJ, Malhotra D, Scollick C, Traore K, Bream JH, et al. Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils. Antioxidants & redox signaling. 2007;9:1963–1970. doi: 10.1089/ars.2007.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 127.Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, McCulloch CC, et al. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 2009;30:1024–1031. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liby KT, Sporn MB. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacological reviews. 2012;64:972–1003. doi: 10.1124/pr.111.004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, et al. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu M, Reddy NM, Higbee EM, Potteti HR, Noel S, Racusen L, et al. The Nrf2 triterpenoid activator, CDDO-imidazolide, protects kidneys from ischemia-reperfusion injury in mice. Kidney international. 2014;85:134–141. doi: 10.1038/ki.2013.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kensler TW, Groopman JD, Sutter TR, Curphey TJ, Roebuck BD. Development of cancer chemopreventive agents: oltipraz as a paradigm. Chemical research in toxicology. 1999;12:113–126. doi: 10.1021/tx980185b. [DOI] [PubMed] [Google Scholar]
- 132.Jia Z, Zhu H, Trush MA, Misra HP, Li Y. Generation of superoxide from reaction of 3H-1,2-dithiole-3-thione with thiols: implications for dithiolethione chemoprotection. Molecular and cellular biochemistry. 2008;307:185–191. doi: 10.1007/s11010-007-9598-z. [DOI] [PubMed] [Google Scholar]
- 133.Velayutham M, Villamena FA, Navamal M, Fishbein JC, Zweier JL. Glutathione-mediated formation of oxygen free radicals by the major metabolite of oltipraz. Chemical research in toxicology. 2005;18:970–975. doi: 10.1021/tx049687h. [DOI] [PubMed] [Google Scholar]
- 134.Holland R, Navamal M, Velayutham M, Zweier JL, Kensler TW, Fishbein JC. Hydrogen peroxide is a second messenger in phase 2 enzyme induction by cancer chemopreventive dithiolethiones. Chemical research in toxicology. 2009;22:1427–1434. doi: 10.1021/tx900110n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, Kensler TW. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis. 2003;24:461–467. doi: 10.1093/carcin/24.3.461. [DOI] [PubMed] [Google Scholar]
- 136.Buetler TM, Gallagher EP, Wang C, Stahl DL, Hayes JD, Eaton DL. Induction of phase I and phase II drug-metabolizing enzyme mRNA, protein, and activity by BHA, ethoxyquin, and oltipraz. Toxicology and applied pharmacology. 1995;135:45–57. doi: 10.1006/taap.1995.1207. [DOI] [PubMed] [Google Scholar]
- 137.Wattenberg LW, Bueding E. Inhibitory effects of 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (Oltipraz) on carcinogenesis induced by benzo[a]pyrene, diethylnitrosamine and uracil mustard. Carcinogenesis. 1986;7:1379–1381. doi: 10.1093/carcin/7.8.1379. [DOI] [PubMed] [Google Scholar]
- 138.Kensler TW, Egner PA, Trush MA, Bueding E, Groopman JD. Modification of aflatoxin B1 binding to DNA in vivo in rats fed phenolic antioxidants, ethoxyquin and a dithiothione. Carcinogenesis. 1985;6:759–763. doi: 10.1093/carcin/6.5.759. [DOI] [PubMed] [Google Scholar]
- 139.Helzlsouer KJ, Kensler TW. Cancer chemoprotection by oltipraz: experimental and clinical considerations. Preventive medicine. 1993;22:783–795. doi: 10.1006/pmed.1993.1072. [DOI] [PubMed] [Google Scholar]
- 140.Kensler TW, Egner PA, Dolan PM, Groopman JD, Roebuck BD. Mechanism of protection against aflatoxin tumorigenicity in rats fed 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (oltipraz) and related 1,2-dithiol-3-thiones and 1,2-dithiol-3-ones. Cancer research. 1987;47:4271–4277. [PubMed] [Google Scholar]
- 141.Zhang Y, Munday R. Dithiolethiones for cancer chemoprevention: where do we stand? Molecular cancer therapeutics. 2008;7:3470–3479. doi: 10.1158/1535-7163.MCT-08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang JS, Shen X, He X, Zhu YR, Zhang BC, Wang JB, et al. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People’s Republic of China. Journal of the National Cancer Institute. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- 143.Nair S, Xu C, Shen G, Hebbar V, Gopalakrishnan A, Hu R, et al. Pharmacogenomics of phenolic antioxidant butylated hydroxyanisole (BHA) in the small intestine and liver of Nrf2 knockout and C57BL/6J mice. Pharmaceutical research. 2006;23:2621–2637. doi: 10.1007/s11095-006-9099-x. [DOI] [PubMed] [Google Scholar]
- 144.Wang XJ, Hayes JD, Higgins LG, Wolf CR, Dinkova-Kostova AT. Activation of the NRF2 signaling pathway by copper-mediated redox cycling of para- and ortho-hydroquinones. Chemistry & biology. 2010;17:75–85. doi: 10.1016/j.chembiol.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 145.Dinkova-Kostova AT, Wang XJ. Induction of the Keap1/Nrf2/ARE pathway by oxidizable diphenols. Chemico-biological interactions. 2011;192:101–106. doi: 10.1016/j.cbi.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 146.Rao MS, Lalwani ND, Watanabe TK, Reddy JK. Inhibitory effect of antioxidants ethoxyquin and 2(3)-tert-butyl-4-hydroxyanisole on hepatic tumorigenesis in rats fed ciprofibrate, a peroxisome proliferator. Cancer research. 1984;44:1072–1076. [PubMed] [Google Scholar]
- 147.Kahl R. Synthetic antioxidants: biochemical actions and interference with radiation, toxic compounds, chemical mutagens and chemical carcinogens. Toxicology. 1984;33:185–228. doi: 10.1016/0300-483x(84)90038-6. [DOI] [PubMed] [Google Scholar]
- 148.Hirose M, Takesada Y, Tanaka H, Tamano S, Kato T, Shirai T. Carcinogenicity of antioxidants BHA, caffeic acid, sesamol, 4-methoxyphenol and catechol at low doses, either alone or in combination, and modulation of their effects in a rat medium-term multi-organ carcinogenesis model. Carcinogenesis. 1998;19:207–212. doi: 10.1093/carcin/19.1.207. [DOI] [PubMed] [Google Scholar]
- 149.Jiang T, Huang Z, Chan JY, Zhang DD. Nrf2 protects against As(III)-induced damage in mouse liver and bladder. Toxicology and applied pharmacology. 2009;240:8–14. doi: 10.1016/j.taap.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 151.Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends in biochemical sciences. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 152.Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Archives of pharmacal research. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 153.Nebert DW, Shi Z, Galvez-Peralta M, Uno S, Dragin N. Oral benzo[a]pyrene: understanding pharmacokinetics, detoxication, and consequences--Cyp1 knockout mouse lines as a paradigm. Molecular pharmacology. 2013;84:304–313. doi: 10.1124/mol.113.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Eaton DL, Gallagher EP. Mechanisms of aflatoxin carcinogenesis. Annual review of pharmacology and toxicology. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- 155.Bosma-den Boer MM, van Wetten ML, Pruimboom L. Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels and medication prevent our body from recovering. Nutrition & metabolism. 2012;9:32. doi: 10.1186/1743-7075-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC medicine. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004;10:879–891. doi: 10.2174/1381612043452901. [DOI] [PubMed] [Google Scholar]
- 158.Yang H, Magilnick N, Ou X, Lu SC. Tumour necrosis factor alpha induces co-ordinated activation of rat GSH synthetic enzymes via nuclear factor kappaB and activator protein-1. The Biochemical journal. 2005;391:399–408. doi: 10.1042/BJ20050795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, et al. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290:H1862–H1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 160.Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer research. 2006;66:11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 161.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS medicine. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochemical pharmacology. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 163.Yates MS, Kensler TW. Chemopreventive promise of targeting the Nrf2 pathway. Drug News Perspect. 2007;20:109–117. doi: 10.1358/dnp.2007.20.2.108343. [DOI] [PubMed] [Google Scholar]
- 164.Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. Faseb J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 165.Ishii Y, Itoh K, Morishima Y, Kimura T, Kiwamoto T, Iizuka T, et al. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J Immunol. 2005;175:6968–6975. doi: 10.4049/jimmunol.175.10.6968. [DOI] [PubMed] [Google Scholar]
- 166.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, et al. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochemical and biophysical research communications. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lin W, Wu RT, Wu T, Khor TO, Wang H, Kong AN. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochemical pharmacology. 2008;76:967–973. doi: 10.1016/j.bcp.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. The Journal of biological chemistry. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 172.Khor TO, Yu S, Kong AN. Dietary cancer chemopreventive agents - targeting inflammation and Nrf2 signaling pathway. Planta Med. 2008;74:1540–1547. doi: 10.1055/s-0028-1088303. [DOI] [PubMed] [Google Scholar]
- 173.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 174.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor perspectives in biology. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Inoue J, Kerr LD, Kakizuka A, Verma IM. I kappa B gamma, a 70 kd protein identical to the C-terminal half of p110 NF-kappa B: a new member of the I kappa B family. Cell. 1992;68:1109–1120. doi: 10.1016/0092-8674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- 176.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 177.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 178.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 179.Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free radical biology & medicine. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu GH, Qu J, Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 182.Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, et al. Distinct roles of thioredoxin in the cytoplasm and in the nucleus A two-step mechanism of redox regulation of transcription factor NF-kappaB. The Journal of biological chemistry. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 183.Jun CD, Kim Y, Choi EY, Kim M, Park B, Youn B, et al. Gliotoxin reduces the severity of trinitrobenzene sulfonic acid-induced colitis in mice: evidence of the connection between heme oxygenase-1 and the nuclear factor-kappaB pathway in vitro and in vivo. Inflammatory bowel diseases. 2006;12:619–629. doi: 10.1097/01.ibd.0000225340.99108.8a. [DOI] [PubMed] [Google Scholar]
- 184.Iskander K, Li J, Han S, Zheng B, Jaiswal AK. NQO1 and NQO2 regulation of humoral immunity and autoimmunity. The Journal of biological chemistry. 2006;281:30917–30924. doi: 10.1074/jbc.M605809200. [DOI] [PubMed] [Google Scholar]
- 185.Seldon MP, Silva G, Pejanovic N, Larsen R, Gregoire IP, Filipe J, et al. Heme oxygenase-1 inhibits the expression of adhesion molecules associated with endothelial cell activation via inhibition of NF-kappaB RelA phosphorylation at serine 276. J Immunol. 2007;179:7840–7851. doi: 10.4049/jimmunol.179.11.7840. [DOI] [PubMed] [Google Scholar]
- 186.Ayrton A, Morgan P. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica; the fate of foreign compounds in biological systems. 2001;31:469–497. doi: 10.1080/00498250110060969. [DOI] [PubMed] [Google Scholar]
- 187.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 188.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–757. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 189.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. Journal of the National Cancer Institute. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 190.Vollrath V, Wielandt AM, Iruretagoyena M, Chianale J. Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. The Biochemical journal. 2006;395:599–609. doi: 10.1042/BJ20051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ji L, Li H, Gao P, Shang G, Zhang DD, Zhang N, et al. Nrf2 pathway regulates multidrug-resistance-associated protein 1 in small cell lung cancer. PloS one. 2013;8:e63404. doi: 10.1371/journal.pone.0063404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Singh A, Wu H, Zhang P, Happel C, Ma J, Biswal S. Expression of ABCG2 (BCRP) is regulated by Nrf2 in cancer cells that confers side population and chemoresistance phenotype. Molecular cancer therapeutics. 2010;9:2365–2376. doi: 10.1158/1535-7163.MCT-10-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- 194.Hirotsu Y, Katsuoka F, Funayama R, Nagashima T, Nishida Y, Nakayama K, et al. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic acids research. 2012;40:10228–10239. doi: 10.1093/nar/gks827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug metabolism and disposition: the biological fate of chemicals. 2005;33:956–962. doi: 10.1124/dmd.105.003798. [DOI] [PubMed] [Google Scholar]
- 196.Adachi T, Nakagawa H, Chung I, Hagiya Y, Hoshijima K, Noguchi N, et al. Nrf2-dependent and -independent induction of ABC transporters ABCC1, ABCC2, and ABCG2 in HepG2 cells under oxidative stress. Journal of experimental therapeutics & oncology. 2007;6:335–348. [PubMed] [Google Scholar]
- 197.Yeh CT, Yen GC. Induction of hepatic antioxidant enzymes by phenolic acids in rats is accompanied by increased levels of multidrug resistance-associated protein 3 mRNA expression. The Journal of nutrition. 2006;136:11–15. doi: 10.1093/jn/136.1.11. [DOI] [PubMed] [Google Scholar]
- 198.Anwar-Mohamed A, Degenhardt OS, El Gendy MA, Seubert JM, Kleeberger SR, El-Kadi AO. The effect of Nrf2 knockout on the constitutive expression of drug metabolizing enzymes and transporters in C57Bl/6 mice livers. Toxicology in vitro : an international journal published in association with BIBRA. 2011;25:785–795. doi: 10.1016/j.tiv.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 199.Kweon MH, Adhami VM, Lee JS, Mukhtar H. Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. The Journal of biological chemistry. 2006;281:33761–33772. doi: 10.1074/jbc.M604748200. [DOI] [PubMed] [Google Scholar]
- 200.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res. 2009;15:3423–3432. doi: 10.1158/1078-0432.CCR-08-2822. [DOI] [PubMed] [Google Scholar]
- 202.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, et al. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 204.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 206.Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 207.Kinch L, Grishin NV, Brugarolas J. Succination of Keap1 and activation of Nrf2-dependent antioxidant pathways in FH-deficient papillary renal cell carcinoma type 2. Cancer cell. 2011;20:418–420. doi: 10.1016/j.ccr.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ashrafian H, Czibik G, Bellahcene M, Aksentijevic D, Smith AC, Mitchell SJ, et al. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell metabolism. 2012;15:361–371. doi: 10.1016/j.cmet.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sudarshan S, Sourbier C, Kong HS, Block K, Valera Romero VA, Yang Y, et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species. Molecular and cellular biology. 2009;29:4080–4090. doi: 10.1128/MCB.00483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Primiano T, Egner PA, Sutter TR, Kelloff GJ, Roebuck BD, Kensler TW. Intermittent dosing with oltipraz: relationship between chemoprevention of aflatoxin-induced tumorigenesis and induction of glutathione S-transferases. Cancer research. 1995;55:4319–4324. [PubMed] [Google Scholar]
- 212.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Osburn WO, Karim B, Dolan PM, Liu G, Yamamoto M, Huso DL, et al. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. International journal of cancer Journal international du cancer. 2007;121:1883–1891. doi: 10.1002/ijc.22943. [DOI] [PubMed] [Google Scholar]
- 215.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. The Journal of experimental medicine. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Clark J, Simon DK. Transcribe to survive: transcriptional control of antioxidant defense programs for neuroprotection in Parkinson’s disease. Antioxidants & redox signaling. 2009;11:509–528. doi: 10.1089/ars.2008.2241. [DOI] [PubMed] [Google Scholar]
- 217.Innamorato NG, Jazwa A, Rojo AI, Garcia C, Fernandez-Ruiz J, Grochot-Przeczek A, et al. Different susceptibility to the Parkinson’s toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PloS one. 2010;5:e11838. doi: 10.1371/journal.pone.0011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jakel RJ, Townsend JA, Kraft AD, Johnson JA. Nrf2-mediated protection against 6-hydroxydopamine. Brain research. 2007;1144:192–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rojo AI, Innamorato NG, Martin-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia. 2010;58:588–598. doi: 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- 220.Kanninen K, Heikkinen R, Malm T, Rolova T, Kuhmonen S, Leinonen H, et al. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 222.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. The Journal of clinical investigation. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Science translational medicine. 2011;3:78ra32. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yageta Y, Ishii Y, Morishima Y, Masuko H, Ano S, Yamadori T, et al. Role of Nrf2 in host defense against influenza virus in cigarette smoke-exposed mice. Journal of virology. 2011;85:4679–4690. doi: 10.1128/JVI.02456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, et al. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. American journal of respiratory and critical care medicine. 2009;179:138–150. doi: 10.1164/rccm.200804-535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, et al. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Molecular cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 227.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer research. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 228.Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, et al. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. The Journal of pathology. 2010;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 229.Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, et al. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Molecular cancer therapeutics. 2010;9:336–346. doi: 10.1158/1535-7163.MCT-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jiang T, Chen N, Zhao F, Wang XJ, Kong B, Zheng W, et al. High levels of Nrf2 determine chemoresistance in type II endometrial cancer. Cancer research. 2010;70:5486–5496. doi: 10.1158/0008-5472.CAN-10-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rushworth SA, Zaitseva L, Murray MY, Shah NM, Bowles KM, MacEwan DJ. The high Nrf2 expression in human acute myeloid leukemia is driven by NF-kappaB and underlies its chemo-resistance. Blood. 2012;120:5188–5198. doi: 10.1182/blood-2012-04-422121. [DOI] [PubMed] [Google Scholar]
- 232.Lister A, Nedjadi T, Kitteringham NR, Campbell F, Costello E, Lloyd B, et al. Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy. Molecular cancer. 2011;10:37. doi: 10.1186/1476-4598-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sasaki H, Suzuki A, Shitara M, Hikosaka Y, Okuda K, Moriyama S, et al. Genotype analysis of the NRF2 gene mutation in lung cancer. International journal of molecular medicine. 2013;31:1135–1138. doi: 10.3892/ijmm.2013.1324. [DOI] [PubMed] [Google Scholar]
- 234.Hu XF, Yao J, Gao SG, Wang XS, Peng XQ, Yang YT, et al. Nrf2 overexpression predicts prognosis and 5-fu resistance in gastric cancer. Asian Pacific journal of cancer prevention : APJCP. 2013;14:5231–5235. doi: 10.7314/apjcp.2013.14.9.5231. [DOI] [PubMed] [Google Scholar]
- 235.Solis LM, Behrens C, Dong W, Suraokar M, Ozburn NC, Moran CA, et al. Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res. 2010;16:3743–3753. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer research. 2013;73:4158–4168. doi: 10.1158/0008-5472.CAN-12-4499. [DOI] [PubMed] [Google Scholar]
- 237.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 238.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes & development. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang R, An J, Ji F, Jiao H, Sun H, Zhou D. Hypermethylation of the Keap1 gene in human lung cancer cell lines and lung cancer tissues. Biochemical and biophysical research communications. 2008;373:151–154. doi: 10.1016/j.bbrc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 241.Hanada N, Takahata T, Zhou Q, Ye X, Sun R, Itoh J, et al. Methylation of the KEAP1 gene promoter region in human colorectal cancer. BMC cancer. 2012;12:66. doi: 10.1186/1471-2407-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 244.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. The Journal of biological chemistry. 2006;281:35764–35769. doi: 10.1074/jbc.M607160200. [DOI] [PubMed] [Google Scholar]
- 245.Karuri AR, Huang Y, Bodreddigari S, Sutter CH, Roebuck BD, Kensler TW, et al. 3H-1,2-dithiole-3-thione targets nuclear factor kappaB to block expression of inducible nitric-oxide synthase, prevents hypotension, and improves survival in endotoxemic rats. The Journal of pharmacology and experimental therapeutics. 2006;317:61–67. doi: 10.1124/jpet.105.096396. [DOI] [PubMed] [Google Scholar]
- 246.Marks PA, Xu WS. Histone deacetylase inhibitors: Potential in cancer therapy. Journal of cellular biochemistry. 2009;107:600–608. doi: 10.1002/jcb.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Seidel C, Schnekenburger M, Dicato M, Diederich M. Histone deacetylase modulators provided by Mother Nature. Genes & nutrition. 2012;7:357–367. doi: 10.1007/s12263-012-0283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. The Journal of nutrition. 2007;137:223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]