Abstract
Objective
To longitudinally characterize non-invasive markers of liver disease in HIV-infected youth.
Design
HIV infection, without viral hepatitis co-infection, may contribute to liver disease. Non-invasive markers of liver disease [FIB-4 (Fibrosis-4) and APRI (aspartate aminotransferase-to-platelet ratio index)] have been evaluated in adults with concomitant HIV and hepatitis C, but are less studied in children.
Methods
In prospective cohorts of HIV-infected and HIV-uninfected youth, we used linear regression models to compare log-transformed FIB-4 and APRI measures by HIV status based on a single visit at ages 15–20 years. We also longitudinally modeled trends in these measures in HIV-infected youth with ≥2 visits to compare those with behavioral vs perinatal HIV infection (PHIV) using mixed effect linear regression, adjusting for age, gender, body mass index, and race/ethnicity.
Results
Of 1785 participants, 41% were male, 57% black non-Hispanic and 27% Hispanic. More HIV-infected than uninfected youth had an APRI score >0.5 (13% vs 3%, p<0.001). Among 1307 HIV-infected participants with longitudinal measures, FIB-4 scores increased 6% per year (p<0.001) among all HIV-infected youth, whereas APRI scores increased 2% per year (p=0.007) only among PHIV youth. The incidence rates (95% CI) of progression of APRI to >0.5 and >1.5 were 7.5 (6.5–8.7) and 1.4 (1.0–1.9) cases per 100 person-years of follow up, respectively. The incidence of progression of FIB-4 to >1.5 and >3.25 were 1.6 (1.2–2.2) and 0.3 (0.2–0.6) cases per 100 person-years, respectively.
Conclusions
APRI and FIB-4 scores were higher among HIV-infected youth. Progression to scores suggesting subclinical fibrosis or worse was common.
Keywords: Adolescents, Non-Invasive Liver Disease Markers, HIV infection
INTRODUCTION
Combination antiretroviral therapy (cART) has led to a reduction in AIDS-associated morbidities and mortality, but a parallel rise in illnesses and deaths from non-AIDS causes including cardiovascular, hepatic and renal disease has emerged in adults[1] and children[2, 3]. Preliminary data suggest that HIV infection and ensuing inflammation may play a significant role in this process. HIV infection is associated with many hepatobiliary disorders, including hepatomegaly, steatosis and elevated serum liver enzymes[4–7]. There is evidence to suggest that HIV interacts directly with multiple liver cell types[8–17]. Furthermore, some studies in adults have shown an association between control of HIV replication and favorable effect on liver fibrosis either by histopathology[18], or by novel approaches such as transient elastography[19], leading to speculation that these findings may be partly explained by interactions between hepatic stellate cells and HIV glycoproteins resulting in stimulation of collagen production[20].
Concomitant hepatitis B and C virus infections significantly increase the risk of progressive liver dysfunction and end-stage liver outcomes like cirrhosis, hepatocellular carcinoma (HCC) and death in HIV-infected patients[21, 22]. The risks and low acceptability of liver biopsy for histopathologic diagnosis and monitoring of liver disease and fibrosis have prompted exploration of alternative non-invasive approaches. Non-invasive markers of liver disease such as the Fibrosis-4 (FIB-4) score [based on the serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), the platelet count and patient age] and the AST-to-platelet ratio index (APRI), have both been validated for identifying liver fibrosis and cirrhosis in adults with viral hepatitis[23–31]. While experience with these makers is extensive in adults, only a single study has compared APRI and liver biopsy in children[28] and there is little experience with these markers in children with HIV infection[32, 33].
We undertook this study to evaluate and characterize non-invasive biomarkers of liver fibrosis among HIV-uninfected and HIV-infected youth. We hypothesized that there would be differences in these markers by HIV status and by route of infection among youth with HIV.
METHODS
Study Population
Reaching for Excellence in Adolescent Care and Health (REACH) was a prospective observational cohort study aimed at improving the understanding and management of HIV disease progression and co-morbidities in HIV-infected and uninfected at-risk youth adolescents 12–18 years old in which sequential behavioral and biomedical assessments, including biological specimens, were obtained every 6 months from March 1996 through November 1999. Pediatric AIDS Clinical Trials Group (PACTG) 219/219C was a prospective, multicenter cohort study designed to assess the long-term consequences of HIV-1 infection and its treatment in infants, children, and adolescents, and of in utero and neonatal exposure to antiretroviral therapy (ART) drugs in HIV-1–exposed but uninfected infants born to women enrolled in PACTG clinical trials to prevent mother-to-child HIV-1 transmission. PACTG 219/219C also performed serial biomedical assessments and collected biological specimens from April 1993 through May 2007. This analysis included four separate cohorts for comparison: (A) uninfected youth from REACH; (B) behaviorally HIV-infected youth from REACH; (C) behaviorally HIV-infected youth from P219/219C; and (D) perinatally HIV-infected youth from P219/219C, with all four cohorts including only those with liver biomarker measurements available between the ages of 15 and 20 years. Subjects with known hepatitis B or C infection were excluded from this analysis, though routine testing was not required by the study protocols.
Determination of FIB-4 and APRI scores
The Fibrosis-4 (FIB-4) index is calculated as follows:
The AST-to-platelet ratio index (APRI) is calculated as follows
^ULN = upper limit of normal
The relevant clinical thresholds suggestive of fibrosis have been previously validated in adults: FIB-4 scores >1.45 and >3.25 and APRI scores of >0.5 and >1.5 are suggestive of mild-to-moderate and advanced fibrosis, respectively[26–28, 30, 31]. Because both FIB-4 and APRI are functions of AST/platelets, a direct numerical relationship between the two measures can be expressed as FIB-4=K * APRI, where K=(Age*AST ULN)/(100*√ALT).
Statistical Analysis
The majority of HIV-uninfected youth in the REACH cohort had only a single measurement of liver biomarkers and the uninfected youth from P219/219C were too young to meet eligibility criteria. Thus, we first conducted a cross-sectional comparison of FIB-4 and APRI measures across all four cohorts defined by study, HIV infection status, and route of infection. Because the 219C perinatally HIV-infected (PHIV) youth (cohort D) tended to be younger than other groups, we based this cross-sectional comparison on the latest available measurement before or at age 20 years in this cohort, and the earliest measurement at age 15 years or older in the other three cohorts. FIB-4 and APRI measures were log-transformed for all analyses to more closely approximate a normal distribution.
Secondly, among HIV-infected youth, an analysis of the longitudinal measures between ages 15 and 20 years was conducted using repeated measures mixed effect linear regression models to estimate trends in log-transformed scores, adjusting for age, gender, exposure (behavioral or perinatal route of infection) category, and body mass index z-score (BMIZ). Specifically, this objective was addressed by fitting a model for each liver biomarker (FIB-4 and APRI) as a function of age at visit, with a random effect for participant to account for within-subject correlations. The slope of the age coefficient was evaluated via a Wald test to determine whether there was a significant increase (or decrease) in each liver biomarker over time. Interaction terms between age and route of exposure were added to the model to evaluate whether the trends over age differed between perinatally vs behaviorally infected youth.
Since FIB-4 and APRI have not traditionally been used in children under 18 years of age, two analyses were conducted to evaluate the internal consistency and agreement between these measures in a younger population. Concordance between the log-transformed FIB-4 and APRI scores was assessed by calculating Pearson correlation coefficients, overall and for each year of age between 15 and 20 years, and additional evaluation of these two biomarkers for this age range was conducted by evaluating within-person correlations for each separate liver biomarker to assess reproducibility. For the purposes of this longitudinal analysis, baseline is defined the earliest visit available for participants between ages 15 to 20 years.
Among HIV-infected youth with ≥2 visits, in those with low baseline scores (APRI ≤0.5 or FIB ≤ 1.5), we estimated and compared incidence rates of progression to higher scores during follow-up using Poisson regression models. In addition, the effect of cART on liver score progression was evaluated by fitting a mixed effect linear regression model similar to that described above. This model included an effect for cART initiation as a time-varying covariate, reflecting current use of cART at the time of liver biomarker measurement. These models were also employed to evaluate the association of CD4 count and viral load with longitudinally measured FIB-4 and APRI scores, again including a random effect of participant and a fixed effect of age to reflect trends over time. To evaluate the clinical utility of FIB-4 and APRI scores, Cox proportional hazards models were fit with the liver biomarkers as predictors (based on the earliest measure) of time to death or clinical progression, where clinical progression was based on transitioning to a CDC Class C[34] among those without such prior classifications. All models were fit using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Population Characteristics and Correlation Study
Of 4088 potential participants, 1785 met criteria for inclusion (Fig. 1). Characteristics of the sub-groups at the time the liver function tests (LFTs) were obtained are shown in Table 1. The REACH cohorts had a higher percentage of females than did the 219/219C cohorts, with higher mean BMIZ. PHIV youth in PACTG 219/219C were more often on PI-containing cART than the other groups, and had the best virologic and immunologic parameters.
Fig. 1.
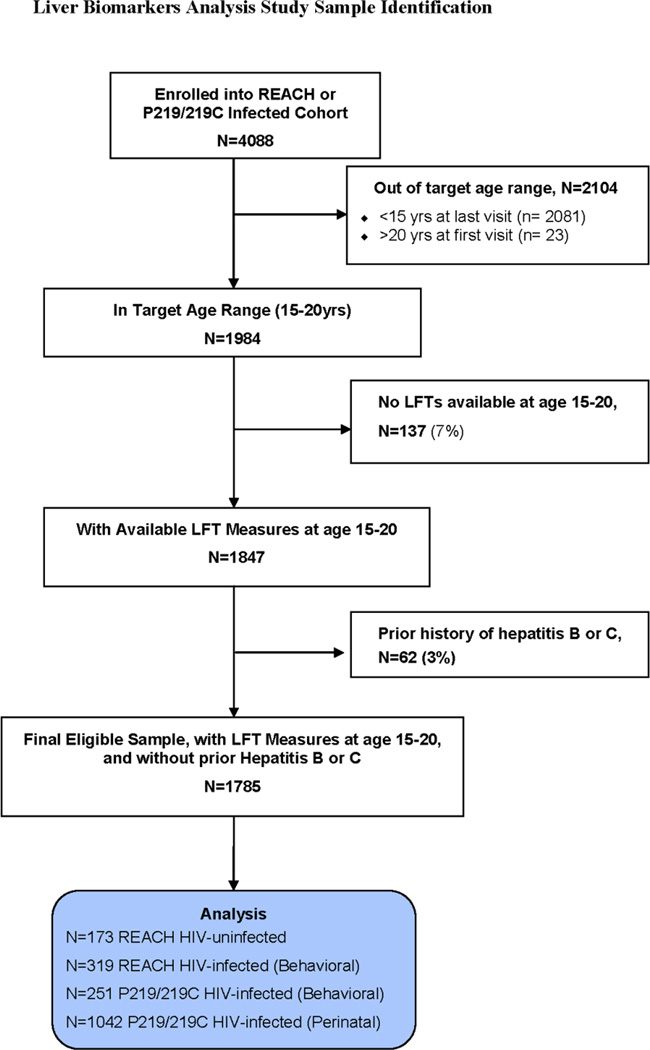
Study population determination for liver biomarker analysis
Table 1.
Demographic and HIV-related Characteristics among REACH and PACTG 219/219C Participants with Liver Function Tests (LFTs) between age 15 and 20 years
| Cohort |
|||||
|---|---|---|---|---|---|
| Characteristic | Total (N=1785) |
(A) REACH: HIV uninfected (N=173) |
(B) REACH: Behaviorally HIV-infected (N=319) |
(C) 219/219C: Behaviorally HIV-infected (N=251) |
(D) 219/219C: Perinatally HIV-infected (N=1042) |
| Age at LFT (median, IQR) | 17.3 (16.1, 18.8) | 17.6 (16.5, 18.2) | 17.9 (17.0, 18.5) | 17.9 (15.8, 19.5) | 17.1 (16.0, 19.0) |
| Number with at least 2 LFTs | 1307 | 38 | 282 | 192 | 795 |
| Follow-up time (median, IQR) | 2.01 (1.08, 3.04) | 1.38 (1.00, 2.03) | 1.99 (1.09, 2.84) | 1.93 (1.00, 3.00) | 2.09 (1.12, 3.55) |
| Female sex | 1,055 (59%) | 135 (78%) | 242 (76%) | 146 (58%) | 532 (51%) |
| Race/Ethnicity | |||||
| White Non-Hispanic | 227 (13%) | 12 (7%) | 10 (3%) | 57 (23%) | 148 (14%) |
| Black Non-Hispanic | 1,019 (57%) | 106 (61%) | 231 (72%) | 130 (52%) | 552 (53%) |
| Other Non-Hispanic | 50 (3%) | 12 (7%) | 19 (6%) | 6 (2%) | 13 (1%) |
| Hispanic | 488 (27%) | 43 (25%) | 58 (18%) | 58 (23%) | 329 (32%) |
| BMI Z-score, mean (SD) | 0.41 (1.17) | 0.66 (1.19) | 0.74 (1.08) | 0.39 (1.23) | 0.27 (1.16) |
| Liver Biomarker Measures | |||||
| FIB-4 Score > 1.45 | 40 (2%) | 2 (1%) | 2 (1%) | 7 (3%) | 29 (3%) |
| FIB-4 Score > 3.25 | 9 (1%) | 0 (0%) | 1 (<1%) | 1 (<1%) | 7 (1%) |
| APRI score > 0.5 | 209 (12%) | 6 (3%) | 21 (7%) | 39 (16%) | 143 (14%) |
| APRI score > 1.5 | 37 (2%) | 2 (1%) | 2 (1%) | 7 (3%) | 26 (2%) |
| HIV Disease Severity measures and Characteristics among HIV-infected participants | |||||
| ARV regimen type | |||||
| HAART w/PI | 744 (46%) | --- | 60 (19%) | 66 (26%) | 618 (59%) |
| HAART w/out PI | 170 (11%) | --- | 16 (5%) | 60 (24%) | 94 (9%) |
| Non-HAART ARV | 258 (16%) | --- | 82 (26%) | 28 (11%) | 148 (14%) |
| Not on ARVs | 440 (27%) | --- | 161 (50%) | 97 (39%) | 182 (17%) |
| Current didanosine use | 225 (14%) | --- | 12 (4%) | 25 (10%) | 188 (18%) |
| Current stavudine use | 342 (21%) | --- | 16 (5%) | 43 (17%) | 283 (27%) |
| Viral load (copies/mL) | |||||
| ≤ 400 | 545 (34%) | --- | 75 (24%) | 80 (32%) | 390 (37%) |
| 401 - <1000 | 94 (6%) | --- | 22 (7%) | 8 (3%) | 64 (6%) |
| 1,000 - <10,000 | 348 (22%) | --- | 115 (36%) | 42 (17%) | 191 (18%) |
| 10,000 or greater | 442 (27%) | --- | 106 (33%) | 57 (23%) | 279 (27%) |
| CD4 count (cells/mm3) | |||||
| Median (IQR) | 483 (292, 702) | --- | 487 (365, 667) | 474 (283, 652) | 483 (266, 726) |
| <200 | 251 (16%) | --- | 28 (9%) | 37 (15%) | 186 (18%) |
| 200 - <350 | 253 (16%) | --- | 49 (15%) | 46 (18%) | 158 (15%) |
| 350 - <500 | 310 (19%) | --- | 90 (28%) | 52 (21%) | 168 (16%) |
| 500 or greater | 747 (47%) | --- | 152 (48%) | 112 (44%) | 483 (46%) |
IQR=interquartile range (expressed as 25th percentile, 75th percentile); SD=standard deviation, BMI=body mass index; LFT=liver function test; ARV=antiretroviral; HAART=highly active antiretroviral therapy (at least 3 drugs from at least 2 ARV drug classes); PI=protease inhibitor.
Characteristics reflected status at earliest measurement within age 15–20 years for REACH and 219C behaviorally HIV-infected cohorts and latest measures within age 15–20 years for 219C perinatally HIV-infected participants. Measurements or characteristics were unavailable for some participants, including race/ethnicity (n=1), BMI z-score (n=102), viral load (n=183), and CD4 count (n=51).
The HIV-infected cohorts were followed for a median of 2 years. Based on evaluation of the repeated measurements from ages 15 to 20 years, the within-subject correlations for FIB-4 and APRI were 0.60 and 0.56, respectively, indicating similar repeatability of each liver biomarker over time. In addition, and as expected given their proportional relationship, there was a high correlation between the log-transformed APRI and FIB-4 measures (r=0.85), which did not vary by age (data not shown).
Association of HIV Infection with FIB-4 and APRI Scores
The cross-sectional analysis characterizing the distribution of FIB-4 and APRI scores by relevant clinical thresholds indicated that a higher percentage of HIV-infected than uninfected youth had APRI scores > 0.5, suggesting at least mild to moderate fibrosis (13% vs 3%, p<0.001) (Table 1). Elevated FIB-4 was less common in both infected and uninfected youth (2% vs 1%, p=0.42). In adjusted models, among the entire sample, being HIV-infected, male, and having a low BMIZ were each associated with an APRI > 0.5 (each p<0.02); among only HIV-infected participants, male gender, low CD4 count (<350 cells/µl) and unsuppressed VL (> 400 copies/mL) were each associated with APRI > 0.5 (each p<0.02).
In the cross-sectional analysis exploring the associations of HIV infection and other covariates with continuous (log-transformed) FIB-4 and APRI scores (Table 2), being HIV-infected and PHIV were each associated with significantly higher scores for both log APRI and log FIB-4. These differences also persisted after adjustment for potential confounders, including calendar year. Youth of older age, male gender, non-white race, low BMIZ, low CD4 count, unsuppressed viral load, and prior or current didanosine use had significantly higher log FIB-4 scores; these findings also held for log APRI scores except that age, race or BMIZ were not significantly associated with APRI scores. Finally, prior or current stavudine use was not associated with either log FIB-4 or APRI scores.
Table 2.
Adjusted Geometric Means for Non-Invasive Serum Biomarkers within Specific Subgroups, Based on Cross-Sectional Analysis*
| APRI | FIB-4 | |||||
|---|---|---|---|---|---|---|
| Characteristic/Level | Adjusted Geometric Mean (SE) |
Relative Increase (vs reference category) |
P-value ╪ | Adjusted Geometric Mean (SE) |
Relative Increase (vs reference category) |
P-value ╪ |
|
Including all participants from all cohorts | ||||||
| Cohort | <0.001 | 0.011 | ||||
| REACH HIV-uninfected | 0.20 (0.07) | REF | 0.36 (0.04) | REF | ||
| REACH Behaviorally HIV+ | 0.26 (0.04) | 31.3% | 0.42 (0.02) | 15.1% | ||
| P219/C Behaviorally HIV+ | 0.25 (0.05) | 27.5% | 0.39 (0.03) | 7.9% | ||
| P219/C Perinatally HIV+ | 0.27 (0.02) | 38.5% | 0.41 (0.01) | 11.8% | ||
| HIV Infection Status | <0.001 | 0.003 | ||||
| HIV-uninfected | 0.20 (0.07) | REF | 0.36 (0.04) | REF | ||
| HIV-infected | 0.27 (0.02) | 35.1% | 0.41 (0.01) | 12.1% | ||
| Age group | 0.19 | <0.001 | ||||
| 15–16 | 0.26 (0.03) | REF | 0.36 (0.02) | REF | ||
| 17–18 | 0.25 (0.03) | −1.1% | 0.41 (0.02) | 14.5% | ||
| 19–20 | 0.27 (0.04) | 5.7% | 0.47 (0.02) | 30.9% | ||
| Gender | <0.001 | <0.001 | ||||
| Female | 0.24 (0.03) | REF | 0.38 (0.01) | REF | ||
| Male | 0.30 (0.03) | 26.3% | 0.44 (0.02) | 13.6% | ||
| Race/Ethnicity | 0.71 | 0.048 | ||||
| White NH | 0.26 (0.05) | REF | 0.37 (0.03) | REF | ||
| Black NH | 0.26 (0.02) | 0.3% | 0.41 (0.01) | 10.6% | ||
| Other NH | 0.27 (0.11) | 5.3% | 0.41 (0.06) | 12.0% | ||
| Hispanic | 0.27 (0.04) | 3.6% | 0.41 (0.02) | 10.6% | ||
| BMI Z-score | 0.09 | <0.001 | ||||
| >2 | 0.25 (0.07) | REF | 0.35 (0.04) | REF | ||
| +1 to +2 | 0.25 (0.04) | 0.8% | 0.38 (0.02) | 7.5% | ||
| −1 to 1 | 0.26 (0.02) | 3.1% | 0.41 (0.01) | 17.2% | ||
| < −1 | 0.29 (0.05) | 14.0% | 0.45 (0.03) | 28.9% | ||
| Restricted to HIV-Infected Participants | ||||||
| Route of HIV Infection | 0.006 | 0.64 | ||||
| Behavioral | 0.25 (0.04) | REF | 0.40 (0.02) | REF | ||
| Perinatal | 0.27 (0.02) | 9.2% | 0.41 (0.01) | 1.3% | ||
| CD4 Count | <0.001 | <0.001 | ||||
| 350 or more | 0.25 (0.02) | REF | 0.39 (0.01) | REF | ||
| <350 | 0.30 (0.04) | 16.1% | 0.45 (0.02) | 15.1% | ||
| HIV RNA viral load | <0.001 | <0.001 | ||||
| <400 | 0.24 (0.04) | REF | 0.37 (0.02) | REF | ||
| 400 – 10,000 | 0.27 (0.03) | 14.5% | 0.42 (0.02) | 12.8% | ||
| 10,000 or more | 0.30 (0.03) | 27.6% | 0.44 (0.02 | 18.5% | ||
| ARV Regimen | 0.96 | 0.75 | ||||
| HAART w/PI | 0.26 (0.03) | REF | 0.41 (0.02) | REF | ||
| HAART w/out PI | 0.27 (0.06) | 1.4% | 0.39 (0.03) | −3.0% | ||
| Non-HAART ARV | 0.27 (0.05) | 2.3% | 0.41 (0.03) | 1.5% | ||
| Not on ARVs | 0.27 (0.04) | 1.2% | 0.41 (0.02) | 1.8% | ||
| Didanosine use | 0.011 | 0.003 | ||||
| Did not use ddI | 0.26 (0.02) | REF | 0.40 (0.01) | REF | ||
| Used ddI | 0.29 (0.05) | 11.3% | 0.45 (0.03) | 11.2% | ||
Cross-sectional analysis based on first LFT within age 15–20 years for REACH and 219C behaviorally HIV-infected, and most recent within age 15–20 years for perinatally HIV-infected.
P-value is from linear regression model for log-transformed biomarker, comparing means across covariate categories. Means within overall population are adjusted for age, sex, race/ethnicity, BMI z-score, and HIV status, and among analyses restricted to HIV-infected participant are adjusted for age, sex, race/ethnicity, BMI z-score, route of exposure, CD4 count, and viral load.
Progression to FIB-4 and APRI Scores Suggestive of Fibrosis More Common than Expected among this HIV-infected Youth Cohort
During the study, the incidence of progression to FIB-4 scores suggesting mild to moderate fibrosis was 1.6 cases (95% CI: 1.2, 2.2) per 100 person-years, whereas the incidence for progression to APRI scores suggesting mild to moderate fibrosis was 7.5 cases (95% CI: 6.5, 8.7) (Supplemental Table 1). The incidence of progression to more advanced levels of fibrosis was 0.3 (95% CI: 0.2, 0.6) for FIB-4 (defined as FIB-4>3.25) and 1.4 cases per 100 person-years (95% CI: 1.0, 1.9) for APRI (defined as APRI >1.5). Having a CD4 count of less than 350 cells/µl at the first visit between ages 15 and 20 years was associated with higher incidence rates of progression for each threshold evaluated for FIB-4 and APRI, with incidence rate ratios ranging from 2 to over 7 (Supplemental Table 1). Incidence rates did not vary by any of the four sub-cohorts or baseline viral load (data not shown).
Predicted Mean Log FIB-4 and APRI Increased Over Time but Were Attenuated by Improvements in Measures of HIV Disease Activity
Longitudinal trends in mean log transformed FIB-4 (Fig. 2) and APRI scores (Fig. 3) were estimated, adjusting for potential confounders. The mean log FIB-4 scores, which are a function of age, increased by 6% per year of age, regardless of exposure category (p<0.001)(Fig. 2a). In contrast, the mean log APRI scores only increased significantly among those with perinatal HIV infection, by 2% per year of age (p=0.007) (Fig. 3a). For both biomarkers, there was no association with calendar year and increases with age persisted after adjustment for calendar year.
Fig. 2.
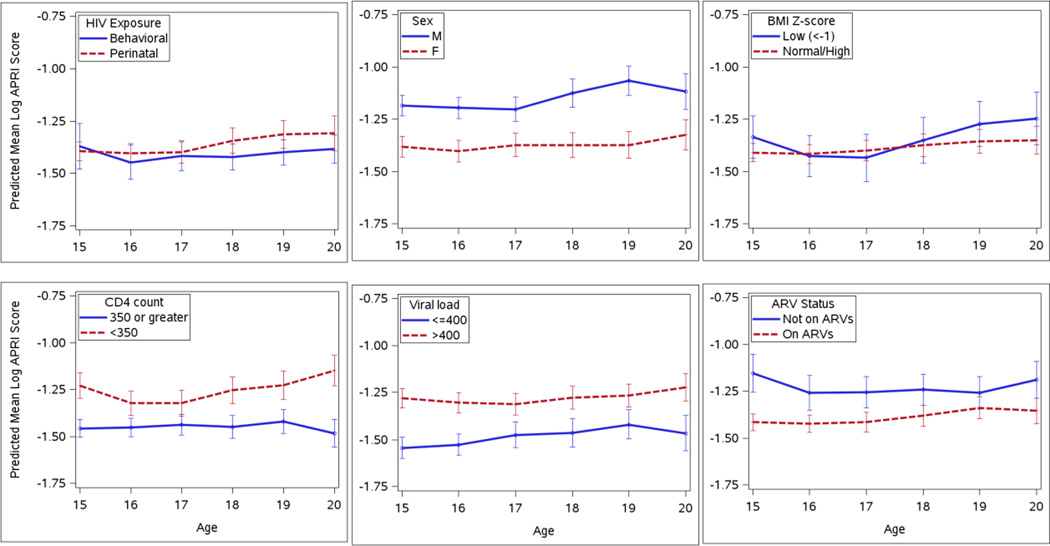
Predicted log APRI scores over time among HIV-infected youth aged 15 to 20 years old, by various risk factors including route of HIV infection, sex, body mass index (BMI) z-score, CD4 cell count, HIV RNA viral load level, and antiretroviral treatment (ARV) status. Predicted scores were based on mixed effect models with a random effect for each participant to account for within-subject correlation over time. APRI scores were estimated to increase by 2% per year for perinatally infected youth only, were 24% higher for males than females, 21% higher for those with CD4<350 vs >350 cells/ul, 23% higher for those with VL>400 copies/mL vs <400 copies, and 17% higher for those on no ARVs vs on ARVs.
Fig. 3.
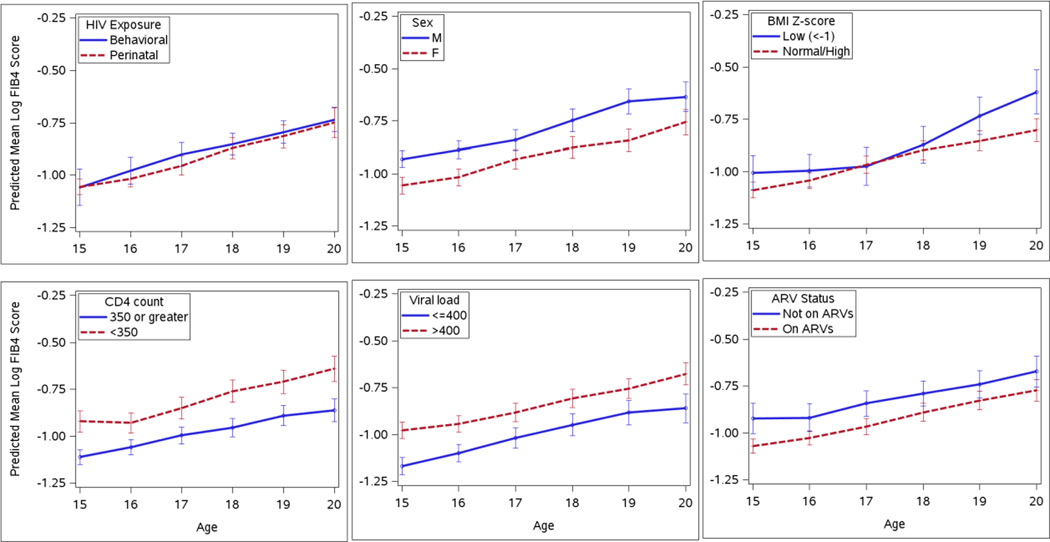
Predicted log FIB-4 scores over time among HIV-infected youth aged 15 to 20 years old, by various risk factors including route of HIV infection, sex, body mass index (BMI) z-score, CD4 cell count, HIV RNA viral load level, and antiretroviral treatment (ARV) status. Predicted scores were based on mixed effect models with a random effect for each participant to account for within-subject correlation over time. APRI scores were estimated to increase by 6% per year, were 13% higher for males than females, 19% higher for those with CD4<350 vs >350 cells/ul, 17% higher for those with VL>400 copies/mL vs <400 copies, and 12% higher for those on no ARVs vs on ARVs.
When FIB-4 and APRI score trajectories were further evaluated longitudinally by the demographic and clinical characteristics described, there were clear and statistically significant differences among all parameters but BMIZ (Figs. 2c and 3c). FIB-4 score trajectories were, on average, higher by about 13% in males (p<0.001) (Fig. 2b), 19% in those with CD4 counts < 350 cells/µl (p<0.001) (Fig. 2d), 17% in those with unsuppressed VL (p<0.001) (Fig. 2e) and 12% in those not on any antiretrovirals (ARVs) (p<0.001) (Fig. 2f). APRI score trajectories were, on average, higher by about 24% in males (p<0.001) (Fig. 3b), 21% in those with CD4 counts < 350 cells/µl (p<0.001) (Fig. 3d), 23% in those with unsuppressed VL (p<0.001) (Fig. 3e) and 17% in those not on any ARVs (p<0.001) (Fig. 3f). No interactions were observed between any of these characteristics and increasing subject age.
Progression to CDC class C disease and/or death was associated with lower BMIZ, higher HIV viral load and lower CD4 counts in unadjusted models, with an approximate 2-fold higher risk of progression for each log increase in either baseline FIB-4 or APRI score (Supplemental Table 2). However, once adjusted for BMIZ, CD4 count, viral load and receipt of ART (variables that are more tightly associated with disease activity) these associations were attenuated and no longer significant.
DISCUSSION
We demonstrate, in the largest cohort of its kind and in a young population likely free of liver comorbidities like viral hepatitis, diabetes and substance use, that HIV infection is an important and independent contributor to liver fibrosis score elevation and progression, and that factors associated with uncontrolled HIV replication were predictive of higher APRI and FIB-4 scores over time.
Our findings were generally consistent with those from other studies[23, 25, 29]. In their evaluation of FIB-4 markers in HIV mono-infected women, the associations of low CD4 count, detectable viral load and ART use in the study of Blackard, et al were consistent with our findings[23]. Among those who evaluated specific ART backbones, DallaPiazza, et al did not find an association between either stavudine or didanosine use and an elevated APRI among HIV-mono-infected adults, in contrast to our finding of an association with didanosine but not stavudine[25]; however, small numbers may contribute to these findings. The association of higher APRI scores in males has also been previously reported[29, 35, 36] and includes attribution to possible greater alcohol use among men than women, as well as other biological, environmental and psychosocial influences. Our study is limited in exploring this as our larger sub-cohort (PACTG 219/C) did not systematically collect data on substance use. Finally, the association of higher FIB-4 scores with low BMIZ probably reflects underlying poor control of HIV disease activity, though this should be interpreted cautiously since it was not consistently found with APRI scores.
To understand the clinical relevance of our findings, we evaluated the prevalence of liver marker score elevation among our cohort using thresholds previously established by others to be suggestive of varying degrees of fibrosis. Congruent with our above findings, the prevalence of evidence of at least mild to moderate fibrosis or worse (APRI > 0.5 or FIB-4 >1.45) was also consistently higher among HIV infected participants compared to their uninfected counterparts, though only statistically significant for APRI. Our prevalence rate of APRI >0.5 and >1.5 was 10% and 2%, respectively which was comparable to another domestic study of HIV-infected children whose rates were 6.5% and 0.8%[33], respectively and slightly lower compared to a Latin American study of HIV-infected children whose rate of APRI > 1.5 was 3.2%[32]. The smaller evaluable sample for FIB-4 showed a similar trend that did not achieve statistical significance. Only male gender, low CD4 count and detectable HIV viral load were found to be independently associated with achievement of an APRI score suggestive of mild to moderate fibrosis or worse, which is consistent with findings from the Latin American pediatric cohort with perinatally transmitted HIV-infection[32].
Few studies have evaluated the incidence of progression of non-invasive markers of liver disease over time[29, 33] and only one was in HIV-infected children. This pediatric study only evaluated APRI and, while their rate of progression to an APRI > 0.5 was comparable to ours, that of APRI >1.5, which is suggestive of significant and advanced fibrosis, was almost 3-fold higher among our cohort. FIB-4 has not been previously evaluated in children. Among the Italian adult cohort, the rates of progression to APRI > 0.5 and > 1.5, and to FIB-4 > 1.45 and >3.25 were very comparable to our findings for APRI but were several fold higher for FIB-4 among the adult group[29]. This latter difference may be in part due to the age factor in the FIB-4 formula, something that is currently being evaluated as part of a separate analysis. The finding that a low CD4 T cell count, but not a detectable HIV viral load, at the first visit between age 15 to 20 years, was predictive of progression to higher APRI and FIB-4 scores probably reflects the greater contribution of a more stable measure of HIV disease activity like CD4 count than would be of a more dynamic indicator like viral load.
Finally, no studies have examined the longitudinal trajectories of APRI or FIB-4. After adjustments, the predicted mean log transformed scores significantly increased over time for APRI and FIB-4; however, the magnitude of the increase was lower for APRI than FIB-4 and only significant among the perinatal cohort for APRI, which again suggests that the age in the FIB-4 formula may be playing a role in these differences. When looking at these scores by demographic and clinical characteristics, the predicted trajectories for the mean log APRI and FIB-4 scores were, on average, 13–28% higher for parameters mainly associated with poor control of HIV disease activity. Taken together all of these data suggest that active, uncontrolled HIV replication for prolonged periods which leads to immunodepletion and worsening of disease activity, results in increased surrogate markers of liver disease and that ART receipt mitigates these outcomes.
Our study does have some notable limitations. Liver histopathology was unavailable to validate our results. We could not ascertain any potential contribution of nonalcoholic fatty liver disease (NAFLD)[37], though it is reassuring that the only association we found was among those with the lowest BMIZ and higher FIB-4. Also, the experience with FIB-4 markers has been limited to adult patients with liver disease and/or HIV infection and has not been evaluated or validated in children. Additionally, FIB-4 and APRI depend on platelet counts which, in general, are not as affected in our younger population (e.g. in contrast to long-standing advanced liver disease and cirrhosis seen in adults) and thus, the scores in our study may potentially underestimate the histopathologic severity of fibrosis. Data on the presence of diabetes and substance use were also not available. Finally, the absence of routine hepatitis virus surveillance could also be a potential limitation; However, given that routine liver function testing has been a standard of care for HIV infected children (e.g. every 3 months), it would be unusual that a diagnosis of subclinical hepatitis infection would have been missed among an otherwise highly scrutinized research cohort.
In conclusion, the PACTG 219/219c and REACH studies have provided a unique opportunity to conduct an extensive, longitudinal evaluation of APRI and FIB-4 in a large cohort of children. Future studies might include liver stiffness assessments, novel biochemical markers and validation with biopsy, to determine cause/effect relationships associated with therapeutic interventions or confounding variables and their impact on clinical outcomes. The validation of these non-invasive markers for identifying and monitoring liver disease would be particularly important for youth with perinatally acquired HIV infection who face a lifetime of HIV, ART and other potential risk factors for liver disease.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to the Pediatric HIV/AIDS Cohort Study (PHACS) which provided support for the conduct of this analysis (Dr. George Seage, PI of Data and Operations Center, U01HD052102, and Dr. Russell Van Dyke, PI of Clinical Coordinating Center, U01HD052104). REACH was supported by U01HD32842 through the Adolescent Medicine HIV/AIDS Research Network (AMHARN). The authors thank investigators and staff [listed in J Adolesc Health Sept 2001, Vol 29, Issue 3 (supplement 1): 5–6] of AMHARN (1994–2001) and the youth who participated in the REACH project for their valuable contributions. PACTG 219/219C was supported through the following cooperative agreements: Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by U01AI068632. The Statistical and Data Analysis Center at Harvard School of Public Health was supported under cooperative agreement U01AI41110 with the PACTG and under U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the Eunice Kennedy Shriver NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C). The authors thank investigators and staff [listed in AIDS July 2013, Vol 27, Issue 12: 1959–70] of the Pediatric AIDS Clinical Trials Group (PACTG) 219/219C protocols (1993–2007) and the youth who participated in these protocols for their valuable contributions. We also would like to thank all investigators from the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN), PHACS and IMPAACT who were involved in the review of this study for their invaluable expertise and input.
Funding:
This work was supported by the following main entities
PACTG 219/219C
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases [U01 AI068632] and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) International and Domestic Pediatric and Maternal HIV Clinical Trials Network supported by the NICHD [contract N01-3-3345 and HHSN267200800001C]. Additional support was provided by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group.
REACH
The Reaching for Excellence in Adolescent Care and Heath cohort was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [U01HD32842] through the Adolescent Medicine HIV/AIDS Research Network (AMHARN).
PHACS
The analyses for this work were funded by the Pediatric HIV/AIDS Cohort Study (PHACS) which was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health [HD052102] and the Tulane University School of Medicine [HD052104].
Source of Funding:
RBVD: NIH-funded investigator in PHACS and IMPAACT networks, including support to travel to network meetings; PF: NIH-funded investigator in PHACS and IMPAACT networks; also receives support for consultancy to Merck’s Safety Monitoring Committee and royalties for the publication of UpToDate; EL, PLW: NIH-funded Data and Operations center for the Pediatric HIV/AIDS Cohort Study and IMPAACT, under cooperative agreements U01 HD052102 and #1 U01 AI068616, respectively.
The REACH and PACTG 219/219C studies received support from the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institutes of Health.
Footnotes
Conflicts of Interest
The remaining authors had nothing to declare.
References
- 1.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 2.Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner WM, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapogiannis BG, Soe MM, Nesheim SR, Abrams EJ, Carter RJ, Farley J, et al. Mortality Trends in the U.S Perinatal AIDS Collaborative Transmission Study (1986–2004) Clin Infect Dis. 2011;53:1024–1034. doi: 10.1093/cid/cir641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonacini M. Hepatobiliary complications in patients with human immunodeficiency virus infection. Am J Med. 1992;92:404–411. doi: 10.1016/0002-9343(92)90271-c. [DOI] [PubMed] [Google Scholar]
- 5.Ingiliz P, Valantin MA, Duvivier C, Medja F, Dominguez S, Charlotte F, et al. Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology. 2009;49:436–442. doi: 10.1002/hep.22665. [DOI] [PubMed] [Google Scholar]
- 6.Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339:33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 7.Keaveny AP, Karasik MS. Hepatobiliary and pancreatic infections in AIDS: Part one. AIDS Patient Care STDS. 1998;12:347–357. doi: 10.1089/apc.1998.12.347. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian A, Ganju RK, Groopman JE. Hepatitis C virus and HIV envelope proteins collaboratively mediate interleukin-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem. 2003;278:35755–35766. doi: 10.1074/jbc.M302889200. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee R, Sperber K, Pizzella T, Mayer L. Inhibition of HIV-1 productive infection in hepatoblastoma HepG2 cells by recombinant tumor necrosis factor-alpha. Aids. 1992;6:1127–1131. doi: 10.1097/00002030-199210000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Blackard JT, Sherman KE. HCV/ HIV co-infection: time to re-evaluate the role of HIV in the liver? J Viral Hepat. 2008;15:323–330. doi: 10.1111/j.1365-2893.2008.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, Ho DD. Identification and quantitation of HIV-1 in the liver of patients with AIDS. Aids. 1992;6:65–70. doi: 10.1097/00002030-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Cao YZ, Friedman-Kien AE, Huang YX, Li XL, Mirabile M, Moudgil T, et al. CD4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. J Virol. 1990;64:2553–2559. doi: 10.1128/jvi.64.6.2553-2559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaldson YK, Bell JE, Ironside JW, Brettle RP, Robertson JR, Busuttil A, et al. Redistribution of HIV outside the lymphoid system with onset of AIDS. Lancet. 1994;343:383–385. doi: 10.1016/s0140-6736(94)91222-x. [DOI] [PubMed] [Google Scholar]
- 14.Housset C, Lamas E, Brechot C. Detection of HIV1 RNA and p24 antigen in HIV1-infected human liver. Res Virol. 1990;141:153–159. doi: 10.1016/0923-2516(90)90017-d. [DOI] [PubMed] [Google Scholar]
- 15.Housset C, Lamas E, Courgnaud V, Boucher O, Girard PM, Marche C, et al. Presence of HIV-1 in human parenchymal and non-parenchymal liver cells in vivo. J Hepatol. 1993;19:252–258. doi: 10.1016/s0168-8278(05)80579-3. [DOI] [PubMed] [Google Scholar]
- 16.Munshi N, Balasubramanian A, Koziel M, Ganju RK, Groopman JE. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis. 2003;188:1192–1204. doi: 10.1086/378643. [DOI] [PubMed] [Google Scholar]
- 17.Vlahakis SR, Villasis-Keever A, Gomez TS, Bren GD, Paya CV. Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4. J Infect Dis. 2003;188:1455–1460. doi: 10.1086/379738. [DOI] [PubMed] [Google Scholar]
- 18.Brau N, Salvatore M, Rios-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriguez-Orengo JF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Grunhage F, Wasmuth JC, Herkenrath S, Vidovic N, Goldmann G, Rockstroh J, et al. Transient elastography discloses identical distribution of liver fibrosis in chronic hepatitis C between HIV-negative and HIV-positive patients on HAART. Eur J Med Res. 2010;15:139–144. doi: 10.1186/2047-783X-15-4-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno R, Galastri S, Sacchi P, Cima S, Caligiuri A, DeFranco R, et al. gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut. 2010;59:513–520. doi: 10.1136/gut.2008.163287. [DOI] [PubMed] [Google Scholar]
- 21.Merwat SN, Vierling JM. HIV infection and the liver: the importance of HCV-HIV coinfection and drug-induced liver injury. Clin Liver Dis. 2011;15:131–152. doi: 10.1016/j.cld.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49:S138–S145. doi: 10.1002/hep.22883. [DOI] [PubMed] [Google Scholar]
- 23.Blackard JT, Welge JA, Taylor LE, Mayer KH, Klein RS, Celentano DD, et al. HIV mono-infection is associated with FIB-4 - A noninvasive index of liver fibrosis - in women. Clin Infect Dis. 2011;52:674–680. doi: 10.1093/cid/ciq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cales P, Halfon P, Batisse D, Carrat F, Perre P, Penaranda G, et al. Comparison of liver fibrosis blood tests developed for HCV with new specific tests in HIV/HCV co-infection. J Hepatol. 2010;53:238–244. doi: 10.1016/j.jhep.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 25.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V., 3rd Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis. 2010;10:116. doi: 10.1186/1471-2334-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loko MA, Castera L, Dabis F, Le Bail B, Winnock M, Coureau G, et al. Validation and comparison of simple noninvasive indexes for predicting liver fibrosis in HIV-HCV-coinfected patients: ANRS CO3 Aquitaine cohort. Am J Gastroenterol. 2008;103:1973–1980. doi: 10.1111/j.1572-0241.2008.01954.x. [DOI] [PubMed] [Google Scholar]
- 27.Macias J, Gonzalez J, Ortega E, Tural C, Cabrero E, Burgos A, et al. Use of simple noninvasive biomarkers to predict liver fibrosis in HIV/HCV coinfection in routine clinical practice. HIV Med. 2010;11:439–447. doi: 10.1111/j.1468-1293.2009.00812.x. [DOI] [PubMed] [Google Scholar]
- 28.McGoogan KE, Smith PB, Choi SS, Berman W, Jhaveri R. Performance of the AST-to-platelet ratio index as a noninvasive marker of fibrosis in pediatric patients with chronic viral hepatitis. J Pediatr Gastroenterol Nutr. 2010;50:344–346. doi: 10.1097/MPG.0b013e3181aed725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendeni M, Foca E, Gotti D, Ladisa N, Angarano G, Albini L, et al. Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without hepatitis C and B infection. Clin Infect Dis. 2011;52:1164–1173. doi: 10.1093/cid/cir071. [DOI] [PubMed] [Google Scholar]
- 30.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 31.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 32.Siberry GK, Cohen RA, Harris DR, Cruz ML, Oliveira R, Peixoto MF, et al. Prevalence and predictors of elevated aspartate aminotransferase-to-platelet ratio index in Latin American perinatally HIV-infected children. Pediatr Infect Dis J. 2014;33:177–182. doi: 10.1097/INF.0b013e3182a01dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siberry GK, Patel K, Pinto JA, Puga A, Mirza A, Miller TL, et al. Elevated aspartate aminotransferase-to-platelet ratio index in perinatally HIV-infected children in the United States. Pediatr Infect Dis J. 2014;33:855–857. doi: 10.1097/INF.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MMWR. 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. 1992 [PubMed] [Google Scholar]
- 35.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 36.Ioannou GN, Weiss NS, Boyko EJ, Kahn SE, Lee SP. Contribution of metabolic factors to alanine aminotransferase activity in persons with other causes of liver disease. Gastroenterology. 2005;128:627–635. doi: 10.1053/j.gastro.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Morse CG, McLaughlin M, Matthews L, Proschan M, Thomas F, Gharib AM, et al. Nonalcoholic Steatohepatitis and Hepatic Fibrosis in HIV-1-Monoinfected Adults With Elevated Aminotransferase Levels on Antiretroviral Therapy. Clin Infect Dis. 2015 doi: 10.1093/cid/civ101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


