Abstract
Objective
In virologically suppressed HIV-infected adults, non-communicable diseases (NCDs) have been associated with immune senescence and low CD4/CD8 lymphocyte ratio. Age differences in the relationship between CD4/CD8 ratio and NCDs have not been described.
Design
Observational cohort study.
Methods
We assessed CD4/CD8 ratio and incident NCDs (cardiovascular, cancer, liver, and renal diseases) in HIV-infected adults started on antiretroviral therapy between 1998–2012. Study inclusion began once patients maintained virologic suppression for 12 months (defined as baseline). We examined age and baseline CD4/CD8 ratio and used Cox proportional hazard models to assess baseline CD4/CD8 ratio and NCDs.
Results
This study included 2,006 patients. Low baseline CD4/CD8 ratio was associated with older age, male sex, and low CD4 lymphocyte counts. In models adjusting for CD4 lymphocyte count, CD4/CD8 ratio was inversely associated with age (p <0.01). Among all patients, 182 had incident NCDs, including 46 with coronary artery disease (CAD) events. CD4/CD8 ratio was inversely associated with risk of CAD events (adjusted HR per 0.1 increase in CD4/CD8 ratio = 0.87, 95% CI: 0.76–0.99, p=0.03). This association was driven by those under age 50 years (adjusted HR 0.83 [0.70–0.97], p = 0.02) versus those over age 50 years (adjusted HR = 0.96 [0.79–1.18], p = 0.71). CD4/CD8 ratio was not significantly associated with incident non-cardiac NCDs.
Conclusions
Higher CD4/CD8 ratio after one year of HIV virologic suppression was independently predictive of decreased CAD risk, particularly among younger adults. Advanced immune senescence may contribute to CAD events in younger HIV patients on antiretroviral therapy.
Keywords: HIV, age, CD4, CD8, cardiovascular disease, non-communicable diseases, immune senescence, depression
Introduction
With the success of antiretroviral therapy (ART), the burden of morbidity and mortality among HIV-infected adults is shifting from AIDS-defining illnesses to non-communicable diseases (NCDs) [1–4]. In particular, cardiovascular disease, non-AIDS defining cancers, liver disease, metabolic diseases, and renal diseases occur at high rates and account for a high proportion of deaths in HIV-infected adults [5–7]. Compared to their uninfected peers, HIV-infected adults experience higher rates of these important NCDs and, in some cases, at younger ages [5, 8, 9]. Understanding the pathophysiology, prevention, and treatment of NCDs have become clinical and research priorities to reduce the excess morbidity that persists among HIV-infected adults on ART.
The underlying immunologic effects of chronic HIV infection have been proposed as one mechanism driving the pathogenesis of NCDs. Research has shown that the features of chronic, treated HIV infection that mirror the immunologic changes observed with aging are also associated with NCD outcomes, including T lymphocyte activation and replicative senescence, alteration in CD8 lymphocyte populations, and elevated systemic measures of innate immune activation [3, 4, 10]. However, studies that have examined the association between CD4 lymphocyte counts and NCDs have demonstrated inconsistent results, and a clinically applicable measure of immune senescence as a predictor of NCDs has not yet been identified [11–14].
In HIV-uninfected adults CD4/CD8 ratio increases over the lifespan, and inversion of the CD4/CD8 ratio in the elderly has been associated with risk of frailty and chronic viral infections, such as CMV [15–19]. In HIV-infected adults with virologic suppression, CD4/CD8 ratio inversely correlates with measures of innate and adaptive immune senescence [20–22]. HIV-infected adults with NCDs were more likely to have lower CD4/CD8 ratios than matched controls in recent studies [20, 23]. However, the effect of age on the value of CD4/CD8 ratios for identifying patients at risk for incident NCDs has not been evaluated.
This large observational cohort study among HIV-infected adults on ART aimed to examine the relationship between age, CD4/CD8 ratio, and NCDs. We hypothesized that CD4/CD8 ratio would be correlated with age but remain a predictor of NCD outcomes independent of age.
Methods
The study included adult patients (age ≥ 18 years at cohort entry) who received care at the Vanderbilt Comprehensive Care Clinic (VCCC) in Nashville, Tennessee between 1998 and 2012. The Vanderbilt Comprehensive Care Clinic provides specialty HIV care and primary care to HIV-infected adults. Patients are routinely seen in clinic every three to six months. Research staff systematically abstracts demographic, clinical, and laboratory data from the electronic medical record, including NCD diagnoses and dates of onset. All cardiovascular, hepatic, renal, and oncologic NCD diagnoses were individually validated by research and medical staff.
We included patients who enrolled in clinic and started or were maintained on ART between January 1, 1998, and December 31, 2012. Patient follow-up time for the study began at the time of the earliest date of persistent measurement of virologic suppression (HIV-1 RNA <400 copies/mL) after a 12-month period based on at least two laboratory values no more than 12 months apart obtained at the VCCC. This time point was determined to be the baseline for the start of study follow-up time. Additionally, patients were required to have CD4 and CD8 lymphocyte counts (cells/mm3) obtained within six months before or after the baseline date. CD4 and CD8 lymphocyte values closest to the start of study follow-up time were used as baseline values. Other demographic and clinical variables obtained included sex, race, HIV transmission risk factor, age at baseline, year of follow-up start, duration of ART prior to baseline, history of serious NCDs (defined below) or depression (including bipolar disorder), and hepatitis C and hepatitis B virus infection status at baseline. Laboratory values collected included CD4 lymphocyte nadir at baseline, all CD4 lymphocyte counts, CD8 lymphocyte counts, and all HIV-1 RNA results. Study follow-up time was censored at death, December 31, 2012, or at the last clinic visit if patients had no clinic visits for >12 months or if the last clinic visit was before December 31, 2011.
The outcomes of interest for this study were serious NCDs. These were defined as NCDs with end-organ effects or associated significant morbidity or risk of mortality, including cardiovascular disease events, non-AIDS-defining cancers, cirrhosis, advanced chronic kidney disease, and diabetes. Cardiovascular disease (CVD) included coronary artery disease (CAD) events (myocardial infarctions, coronary artery atherosclerosis, or ischemic cardiomyopathy), cerebrovascular events, and peripheral vascular disease as recorded by health care providers in the medical record. Oncologic NCDs included invasive malignancies, excluding squamous and basal cell skin cancers and AIDS-defining cancers (Kaposi sarcoma, non-Hodgkin lymphoma, and cervical cancer). Hepatic NCDs included cirrhosis due to any cause, as documented by health care providers in the medical record. Renal NCDs included incident stage IV (estimated glomerular filtration rate 15–30 ml/min) or stage V (estimated glomerular filtration rate <15 ml/min) chronic kidney disease as documented in the medical record by health care providers. Incident diabetes was defined as new diagnoses documented in the medical record by health care providers.
The primary predictor of interest was CD4/CD8 ratio after the first 12 months of virologic suppression (baseline), defined as CD4 lymphocyte count (cells/mm3) divided by CD8 lymphocyte count (cells/mm3). As CD4 lymphocyte counts are highly dependent upon HIV-1 RNA, we selected the value after the first year of virologic suppression as a consistent measure of adaptive immune status independent of HIV-1 replication [24]. Baseline CD4/CD8 ratio was categorized by approximate tertiles into low (<0.40), middle (0.40–0.69), and high (≥0.70). Previous studies have observed CD4/CD8 ratio <0.4 as predictive of NCD outcomes in HIV-infected adults on ART [20, 23]. Patient demographic and clinical characteristics were compared between those with low and high baseline CD4/CD8 ratio using Wilcoxon rank sum and Chi squared test for continuous and categorical variables, respectively.
We explored the association between age and CD4/CD8 ratio using linear regression models. We examined the relationship of baseline CD4/CD8 ratio and age using data from all subjects. Age was fit using restricted cubic splines with the number of knots determined by the Akaike information criterion. An interaction term for CD4 lymphocyte counts and age was included. The results of the linear regression models for CD4/CD8 ratio outcomes by age were graphed and stratified by CD4 lymphocyte count categories.
We next examined change in CD4/CD8 ratio over time among patients with persistent measurements of virologic suppression; follow-up was censored at the first occurrence of an HIV-1 RNA level ≥ 400 copies/mL or gap in HIV-1 RNA measurements of more than 6 months. The association between age and change in CD4/CD8 ratio after three years of virologic suppression from baseline was examined using linear regression with restricted cubic splines, including adjustment for and examination of effect modification by baseline CD4 lymphocyte count.
Lastly, we examined the risk of NCDs predicted by baseline CD4/CD8 ratio. We compared the baseline CD4/CD8 ratio between patients with no NCD events during study follow-up to those patients with cardiovascular, oncologic, hepatic or renal NCDs, or incident diabetes diagnosis using the Wilcoxon rank sum test. We calculated the cumulative incidence of each NCD category and a composite NCD outcome by baseline CD4/CD8 ratio tertiles.
To examine the relationship of CD4/CD8 ratio and NCDs over time, we performed Cox proportional hazard models for each NCD category and a composite outcome for any NCD event. For the composite NCD model, patients were censored after their first NCD event. For the models for each NCD category, patients were censored after their first NCD event of that category. Variables for inclusion in adjusted models were selected a priori based upon pathophysiology (including age, sex, and CD4 lymphocyte count as priority covariates) and number of events (degrees of freedom). Age was included using restricted cubic splines (three knots). Adjusted models were repeated with inclusion of an interaction term for age and CD4/CD8 ratio and, when feasible, stratifying by age to assess for effect modification.
As a sensitivity analysis, all statistical studies were repeated excluding those patients with HIV-1 RNA <400 copies/mL at clinic entry as their duration of virologic suppression was unknown and may affect their CD4/CD8 ratio.
The study was approved by the Vanderbilt University Institutional Review Board. Statistical analyses and figures were performed using Stata 12.1 (Stata Corporation, College Station, Texas, USA). All p values are two-sided.
Results
From 1998 to 2012, 2,245 ART-naïve patients were enrolled into care at the clinic, started or maintained on antiretroviral therapy, and had consistent measurements of suppressed HIV-1 RNA (<400 copies/ml) for at least 12 months. Study follow-up began after the first 12 months of sustained virologic suppression (i.e., baseline). Two-hundred and twenty-one patients (10%) had missing CD4 or CD8 lymphocyte count values within 6 months of the baseline date and were excluded, leaving 2,006 patients included in this study. These patients contributed a total of 8,762 person-years of follow-up.
At baseline, the median CD4/CD8 ratio for all subjects was 0.57 (interquartile range: 0.36–0.85). Patient demographic and clinical characteristics by baseline CD4/CD8 ratio are shown in Table 1. Compared to patients with higher CD4/CD8 ratio (≥ 0.7), those in the lowest tertile (ratio <0.4) were older, more likely to be male, had lower CD4 lymphocyte nadir and CD4 lymphocyte count values at baseline, and were more likely to have HIV-1 RNA <400 copies/mL but detectable at baseline but. They were less likely to have virologic suppression at clinic entry. There were slightly longer duration of ART at baseline and longer follow-up time after baseline among patients with the lowest versus highest CD4/CD8 ratios but these differences did not reach statistical significance.
Table 1.
Patient characteristics at baseline by CD4/CD8 ratio category
| Low CD4/CD8 Ratio (< 0.4) | Middle CD4/CD8 Ratio (0.4–0.69) | High CD4/CD8 Ratio (≥ 0.7) | P value comparison of low and high ratio groups | |
|---|---|---|---|---|
| N (%) | 610 (30) | 650 (32) | 746 (37) | |
|
| ||||
| Age, median (IQR) | 44 (38–50) | 41 (34–48) | 40 (32–46) | <0.001a |
|
| ||||
| Sex (%) | ||||
| Male | 520 (85) | 500 (77) | 534 (72) | <0.001b |
| Female | 90 (15) | 150 (23) | 212 (28) | |
|
| ||||
| Non-white race (%) | 257 (42) | 292 (45) | 318 (43) | 0.85b |
|
| ||||
| HIV Risk Factor (%): | ||||
| MSM | 360 (59) | 361 (56) | 395 (53) | 0.028b |
| Injection drug use | 48 (8) | 40 (6) | 46 (6) | |
| Heterosexual | 180 (30) | 228 (35) | 273 (37) | |
| Other/unknown | 22 (4) | 21 (3) | 32 (4) | |
|
| ||||
| Chronic hepatitis C virus infection (%) | 102 (17) | 85 (13) | 101 (14) | 0.10b |
|
| ||||
| Chronic hepatitis B virus infection (%) | 54 (9) | 52 (8) | 44 (6) | 0.037b |
|
| ||||
| History of NCD (%) | 16 (3) | 12 (2) | 9 (1) | 0.054b |
|
| ||||
| History of depression (%) | 183 (30) | 183 (28) | 206 (28) | 0.33b |
|
| ||||
| CD4 lymphocyte nadir (cells/mm3), median (IQR) | 88 (24–175) | 216 (120–323) | 329 (224–503) | <0.001a |
|
| ||||
| CD4 lymphocyte count (cells/mm3), median (IQR) | 274 (189–390) | 462 (360–597) | 651 (504–850) | <0.001a |
|
| ||||
| HIV-1 RNA <400 copies/mL at clinic entry (%) | 89 (15) | 100 (15) | 176 (24) | <0.001b |
|
| ||||
| HIV-1 RNA <400 copies/mL but detectable (%) | 344 (56) | 361 (56) | 332 (45) | <0.001b |
|
| ||||
| Years of ART duration, median (IQR) | 1.4 (1.1–3.1) | 1.4 (1.1–2.8) | 1.3 (1.1–3.0) | 0.064a |
|
| ||||
| Calendar year, median (IQR) | 2007 (2004–2009) | 2007 (2005–2010) | 2007 (2004–2010) | 0.018a |
|
| ||||
| Years of follow-up after baseline, median (IQR) | 3.8 (1.9–6.5) | 3.7 (1.7–6.3) | 3.1 (1.4–6.5) | 0.096a |
Wilcoxon rank sum test
χ2 test
Abbreviations used:
IQR: interquartile range
MSM: men who have sex with men
NCD: non-communicable disease
ART: antiretroviral therapy
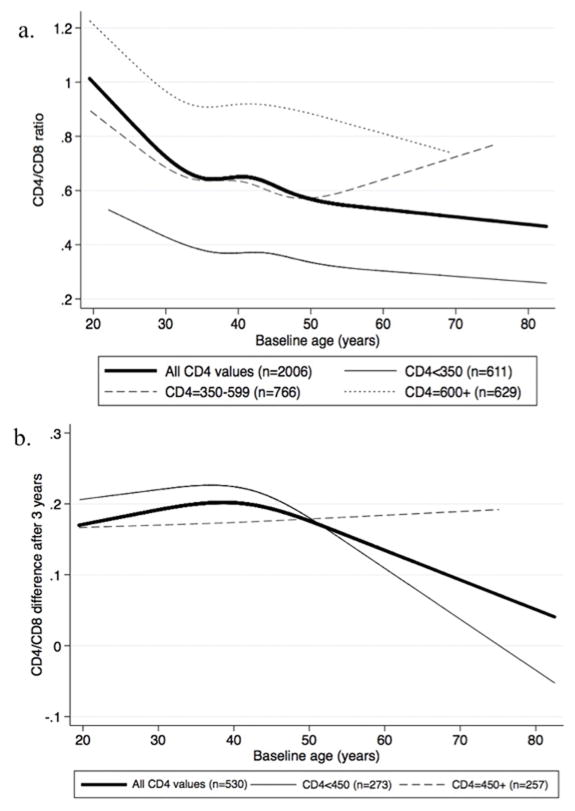
Figure 1a shows the baseline CD4/CD8 ratio by age, including results stratified by CD4 lymphocyte count. Baseline CD4/CD8 ratio was inversely associated with age (likelihood ratio test, p <0.001). The association between CD4/CD8 ratio and age did not statistically differ by CD4 lymphocyte count (test of interaction p=0.80).
Figure 1. Trends in CD4/CD8 ratio (A) and change in CD4/CD8 ratio by age (B), stratified by baseline CD4 lymphocyte count.
We next examined patients with consistent measurements of virologic suppression (HIV-1 RNA <400 copies/mL) at three years after baseline (n=530). Among all patients with sustained virologic suppression, CD4/CD8 ratio values tended to increase over time (overall median CD4/CD8 difference from baseline at three years = 0.17 [interquartile range: 0.06–0.28]). Figure 1b shows the difference in CD4/CD8 ratio over time by age and stratified by CD4 lymphocyte count. Older patients tended to have less CD4/CD8 ratio improvement after three years (likelihood ratio test, p=0.056). The relationship between change in CD4/CD8 ratio and age did not statistically differ by CD4 lymphocyte count (test of interaction, p=0.50).
Of the 2,006 patients included, 50 patients had incident non-AIDS-defining cancers, 49 were diagnosed with type 2 diabetes, 46 had incident CAD events, 22 developed cirrhosis, 18 had incident cerebrovascular disease, nine were diagnosed with peripheral vascular disease, and eight developed stage IV or V chronic kidney disease. Combining coronary artery disease, cerebrovascular disease, and peripheral vascular disease, 69 patients (3%) had incident CVD events. Compared to patients without any NCD events, those with any NCD event were older at baseline (median age 46 vs. 41 years), particularly those with CVD (median age 48 years) and cancer (median age 49 years) events during follow-up.
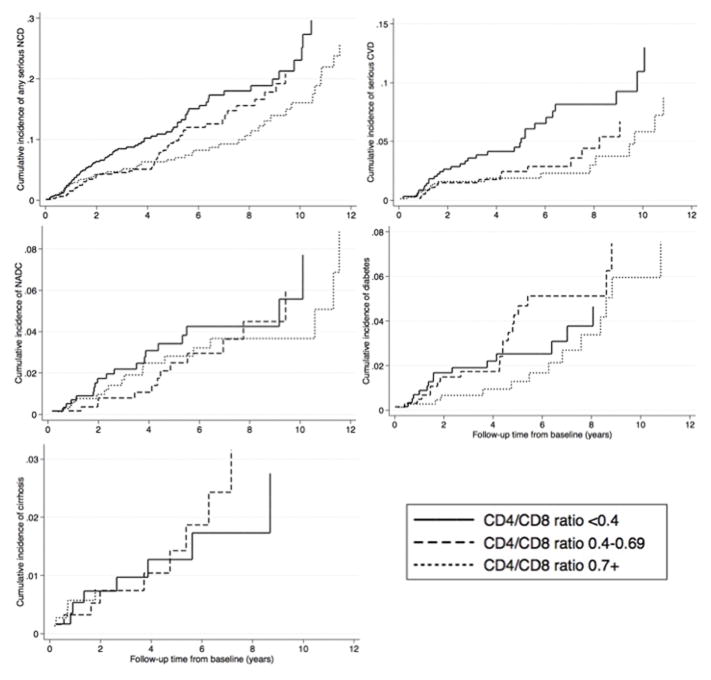
Figure 2 shows the median CD4/CD8 ratio of patients by NCD category. The median baseline CD4/CD8 ratio among patients without any NCD events was 0.58 (interquartile range: 0.37–0.85). In contrast, the CD4/CD8 ratios were significantly lower for patients with CVD events during follow-up (median ratio = 0.42, interquartile range: 0.30–0.71). Of all NCD outcomes, patients with CAD events had the lowest baseline CD4/CD8 ratio (median ratio = 0.37, interquartile range 0.27–0.63) (not shown in Figure 2). There were no statistically significant differences in baseline CD4/CD8 ratio values for those patients with subsequent cancer, diabetes, cirrhosis, or renal outcomes compared to those without any NCD events. Similarly, cumulative incidence of CVD outcomes was highest among patients with the lowest CD4/CD8 ratios (<0.4) compared to those with higher CD4/CD8 ratios at baseline (Figure 3). In contrast, patients with lower CD4/CD8 ratio at baseline did not have a higher incidence of diabetes or cirrhosis compared to patients with higher ratios.
Figure 2. Baseline CD4/CD8 ratio of patients by NCD outcomes.
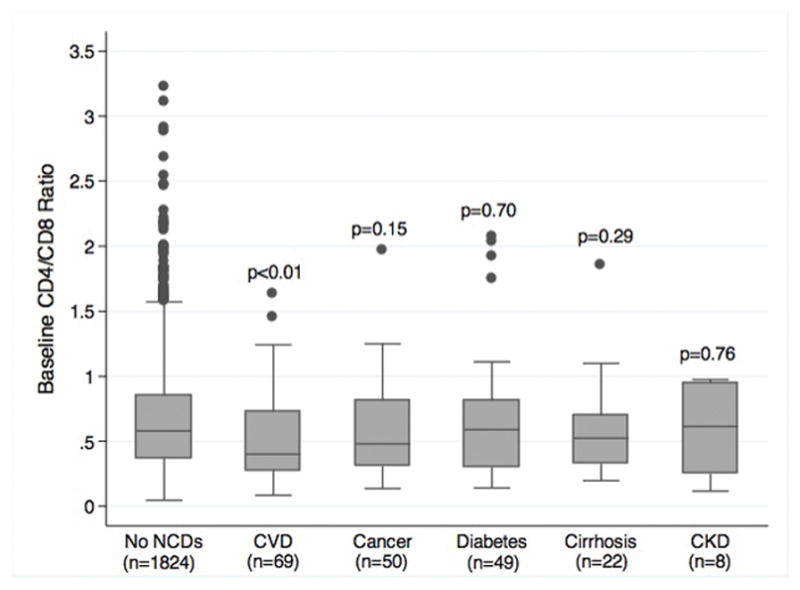
Median values and IQR shown by box plots. P values reflect comparison with CD4/CD8 ratio among patients without any NCD events.
Figure 3. Cumulative incidence of NCD outcomes by baseline CD4/CD8 ratio category.
Death was used as the competing event to calculate cumulative incidence.
We examined CD4/CD8 ratio and age on risk of NCDs in Cox proportional hazard models (Table 2). Using any NCD event as a composite outcome, CD4/CD8 ratio was inversely associated with risk in unadjusted analysis but was no longer statistically significant after adjusting for age, CD4 lymphocyte count, sex, and other covariates. In analyses stratified by NCD category, CD4/CD8 ratio was significantly associated with risk of CVD outcomes in unadjusted analyses and trended toward significance in the adjusted model. However, in an analysis limited to patients with only CAD, increasing CD4/CD8 ratio remained associated with decreased risk of CAD (adjusted hazard ratio [aHR] per 0.1 increase in ratio = 0.87, [95% confidence interval: 0.76–0.99], p = 0.034). The test of interaction between CD4/CD8 ratio and age was not statistically significant (p=0.71). However, the association of CD4/CD8 ratio and CAD events remained significant when analyses were restricted to those patients aged less than 50 years (aHR per 0.1 increase = 0.83 [0.70–0.97], p = 0.019, n=34 events). In contrast, among patients age 50 years or greater CD4/CD8 ratio was not associated with CAD outcomes in unadjusted or adjusted analyses (aHR = 0.96 [0.79–1.18], p = 0.708), though this was limited to few events (n=12). CD4/CD8 ratio was not statistically associated with other NCD outcomes in unadjusted or adjusted models.
Table 2.
Unadjusted and adjusted Cox proportional hazard models for CD4/CD8 ratio and risk of NCDs
| Unadjusted hazard ratio for CD4/CD8 ratio (per 0.1 increase) | Adjusted hazard ratio for CD4/CD8 ratio (per 0.1 increase) | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | aHR | 95% CI | P value | |
| All NCDsa (n=182 events) | 0.95 | 0.91–0.99 | 0.014 | 0.97 | 0.92–1.02 | 0.17 |
| Interaction term for age and CD4/CD8 ratio | 0.35 | |||||
| Cardiovascular diseaseb (n=69 events) | 0.90 | 0.84–0.97 | 0.006 | 0.92 | 0.84–1.01 | 0.096 |
| Interaction term for age and CD4/CD8 ratio | 0.94 | |||||
| Coronary artery disease aloneb (n=46 events) | 0.85 | 0.76–0.94 | 0.002 | 0.87 | 0.76–0.99 | 0.034 |
| Interaction term for age and CD4/CD8 ratio | 0.71 | |||||
| Cancerc (n=50 events) | 0.94 | 0.87–1.02 | 0.16 | 0.98 | 0.89–1.07 | 0.60 |
| Interaction term for age and CD4/CD8 ratio | 1.00 | |||||
| Diabetesb (n=49 events) | 1.00 | 0.94–1.07 | 0.92 | 0.95 | 0.87–1.04 | 0.31 |
| Interaction term for age and CD4/CD8 ratio | 0.56 | |||||
| Cirrhosisd (n=22 events) | 0.95 | 0.84–1.07 | 0.40 | 0.96 | 0.86–1.08 | 0.54 |
| Interaction term for age and CD4/CD8 ratio | 0.87 | |||||
Adjusted model for all NCDs included CD4/CD8 ratio, age (using restricted cubic splines, 3 knots), CD4 lymphocyte count, sex, HCV infection, HBV infection, history of serious NCD at baseline, history of depression at baseline, calendar year at baseline, and total ART duration.
Adjusted model for cardiovascular disease, coronary artery disease, and diabetes events included CD4/CD8 ratio, age (using restricted cubic splines, 3 knots), CD4 lymphocyte count, race, sex, and history of depression.
Adjusted model for cancer included included CD4/CD8 ratio, age (using restricted cubic splines, 3 knots), CD4 lymphocyte count, race, and sex.
Adjusted model for cirrhosis included CD4/CD8 ratio and age (using restricted cubic splines, 3 knots)
Abbreviations used:
NCD: non-communicable disease
HR: hazard ratio
aHR: adjusted hazard ratio
CI: confidence interval
In the Cox proportional hazard models, history of depression was noted to be an independent predictor of certain NCD outcomes. History of depression was associated with an increased risk of any NCD (aHR= 1.66 [1.22–2.27], p=0.001), CVD (aHR = 2.35 [1.44–3.85], p=0.001), CAD (aHR = 1.69 [0.93–3.10], p=0.088), and diabetes outcomes (aHR = 1.98 [1.11–3.54], p=0.021), after adjusting for notable covariates. There was no association of depression and cirrhosis or cancer observed. The inclusion of depression in the models did not affect the observed associations of CD4/CD8 ratio and the NCD outcomes. As a sensitivity analysis, we repeated all statistical studies excluding the 365 patients with HIV-1 RNA <400 copies/mL at the time of clinic entry. The results of the association of age and CD4/CD8 ratio as well as CD4/CD8 ratio and the NCD outcomes were unaffected (data not shown).
Discussion
In this observational cohort study, we found that low CD4/CD8 ratio after the first year of virologic suppression in HIV-infected adults was independently associated with serious CAD outcomes. This association was strongest among patients aged <50 years, though analyses were limited by small numbers of events in older patients. There was no statistical interaction between CD4/CD8 ratio and age on risk of any NCDs. In the setting of successful ART and regardless of CD4 lymphocyte values, CD4/CD8 ratio was highly associated with age, supporting the observation that CD4/CD8 ratio may be a marker of aging-associated immune senescence in HIV-infected adults.
In our study, low CD4/CD8 was strongly associated with older age in HIV-infected adults on stable ART. Our study is novel in its exploration of the relationship between age and CD4/CD8 ratio as prior studies have largely used case-control designs that have matched on age. Chronic HIV infection has been observed to cause immunologic changes similar to those observed in natural aging, including decreased naïve T lymphocyte populations, increased T lymphocyte activation, T lymphocyte replicative senescence, and increased immune activation, among others [25–30]. Previous studies have demonstrated that CD4/CD8 ratio correlates with a number of these adaptive immune senescence changes in HIV-infected adults on ART [20, 21, 31]. In older HIV-infected adults on ART, the immune senescent effects of HIV and aging are often additive [32–34]. Furthermore, CD4/CD8 ratio also inversely correlates with measures of innate immune activation including soluble CD14, CRP, and IL-6 in HIV-infected adults on ART [20]. In aging HIV-uninfected adults, CD4/CD8 ratio increases over the lifespan and inversion of the ratio is associated with immunologic effects of chronic viral infections, particularly CMV [19, 35]. The strong association between age and CD4/CD8 ratio in our study of HIV-infected adults on ART supports the conclusion that CD4/CD8 ratio reflects the cumulative immune senescent effects of chronic viral infections, such as HIV or herpes viruses. Our study found a trend towards reduced CD4/CD8 improvement over time in older adults, after adjustment for CD4 lymphocyte count. Formal testing of interaction terms for age and CD4 lymphocyte count were not statistically significant. Further study of the effects of age and CD4 lymphocyte count on CD4/CD8 ratio over time is needed.
This study demonstrated an association of CD4/CD8 ratio and CAD outcomes. In adjusted models, higher CD4/CD8 ratio was significantly associated with decreased risk incident CAD events. CD4/CD8 ratio has recently been observed in a number of cohorts to not only be associated with immunologic measures of senescence but atherosclerosis in HIV-infected adults [20, 23, 36, 37]. In particular, clinical studies that have used computed tomography of coronary angiography or carotid artery intima-media thickness have found low CD4/CD8 ratio associated with measures of subclinical atherosclerosis [36, 37]. While markers of systemic inflammation and monocyte activation have been strongly associated CVD outcomes in HIV-infected adults, T lymphocyte activation and senescence has also been linked to measures of carotid artery stiffness in HIV-infected adults [4, 10, 38, 39]. The hypothesized immunologic mechanisms of cardiovascular disease in HIV-infected adults are multiple, including activation of endothelial cells, immune activation, and alteration in lipid function [40]. While some studies have shown either CD4 or CD8 lymphocyte measures are independent predictors of cardiovascular disease outcomes, others have found no association [13, 41–45]. Of note, CD4/CD8 ratio has been observed to show less intra-individual variability over time compared to either CD4 or CD8 lymphocyte counts [20]. Given its association with immune senescence and routine measurement in HIV clinical care, CD4/CD8 ratio may serve as important biomarker for CVD in treated HIV-infected adults. Furthermore, in our study, CD4/CD8 ratio was most strongly associated with CAD in patients <50 years of and its association was attenuated when peripheral vascular disease and cerebrovascular disease (diseases more prevalent at older ages) were added in CVD models. This observation suggests that the utility of CD4/CD8 ratio as a potential clinical biomarker for CVD risk and as a measure of adaptive immune senescence may differ across the lifespan of the HIV-infected adult on ART.
Finally, our study unexpectedly found a significant association between depression and risk of CVD and diabetes, independent of age, sex, race, CD4/CD8 ratio, and CD4 cell count. Depression has been observed to increase the risk of CVD in HIV-uninfected populations and is of growing interest in HIV research as a risk factor for CVD outcomes [46–48]. The results of our study add to this growing body of literature and further study of depression as a potentially modifiable risk factor for CVD is needed.
This study has some limitations. We used the CD4/CD8 ratio after one year of virologic suppression as our primary predictor of interest rather than time-updated values in order to limit the effect of episodic HIV viremia [24]. The immune response following the first few years of ART has been shown in other studies to be a reliable reflection of changes over time during ART and has been associated with NCD outcomes [4, 49]. However, we are unable to draw conclusions about the role of time-varying changes in CD4/CD8 ratio and risk of future NCDs based upon results of our study. Our study is also limited by its observational design. While we controlled for the confounders of CD4 lymphocyte count, age, and sex in our multivariate models assessing CD4/CD8 ratio and NCD outcomes, we cannot exclude that unmeasured confounders may have affected our results. For example, adaptive immune senescence due to CMV infection has been associated with CVD outcomes [18, 50]. As CMV antibody titers are not routinely collected in clinical HIV practice, we could not examine if CMV titers or serology differed by CD4/CD8 ratio, age, or clinical outcomes. Similarly, we did not have available tobacco, alcohol, or socioeconomic data on our patients. However, studies to date have not examined if these important social and behavioral factors affect measures of immune senescence, such as CD4/CD8 ratio, in HIV-infected adults.
In this cohort analysis of HIV-infected adults on ART and NCD outcomes, we found CD4/CD8 ratio to be an important measure of aging-associated immune senescence and a significant, independent predictor of CAD events. Further studies to examine its role as clinical biomarker of cardiovascular disease outcomes are needed in prospective studies and in larger cohorts. Additionally, the role of variation in CD4/CD8 ratio over time and the underlying mechanisms associated with premature immune senescence in younger HIV-infected adults warrant further exploration. As the causes of morbidity and mortality in HIV-infected adults increasingly shift to those of NCDs, the need for further understanding and prevention of these outcomes grows more urgent.
Acknowledgments
Funding Sources:
National Institutes of Health: K24AI65298 (JLC, TRS, MT, SB, JL), K12HD043483 (JLC), K23AI00700 (JK)
Tennessee Center for AIDS Research, P30AI110527 (BES, TRS)
We thank Peter Rebeiro, Daniel Rasbach, Paul No, Henry Heaton, and Fernanda Maruri for their contributions toward the collection and validation of NCDs and William Dupont for his biostatistical consultation. This study was supported by the NIH: K24AI65298 (JLC, TRS, MT, SB, JL), K12HD043483 (JLC), K23AI00700 (JK), and P30AI110527 (BES, TRS).
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.McComsey GA, Kitch D, Sax PE, Tierney C, Jahed NC, Melbourne K, et al. Associations of inflammatory markers with AIDS and non-AIDS clinical events after initiation of antiretroviral therapy: AIDS clinical trials group A5224s, a substudy of ACTG A5202. J Acquir Immune Defic Syndr. 2014;65:167–174. doi: 10.1097/01.qai.0000437171.00504.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble Markers of Inflammation and Coagulation but Not T-Cell Activation Predict Non-AIDS-Defining Morbid Events During Suppressive Antiretroviral Treatment. J Infect Dis. 2014 doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 7.Stewart A, Chan Carusone S, To K, Schaefer-McDaniel N, Halman M, Grimes R. Causes of Death in HIV Patients and the Evolution of an AIDS Hospice: 1988–2008. AIDS Res Treat. 2012;2012:390406. doi: 10.1155/2012/390406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–1797. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 9.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010;153:452–460. doi: 10.1059/0003-4819-153-7-201010050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karim R, Mack WJ, Kono N, Tien PC, Anastos K, Lazar J, et al. T-cell activation, both pre- and post-HAART levels, correlates with carotid artery stiffness over 6. 5 years among HIV-infected women in the WIHS. J Acquir Immune Defic Syndr. 2014;67:349–356. doi: 10.1097/QAI.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore RD, Gebo KA, Lucas GM, Keruly JC. Rate of comorbidities not related to HIV infection or AIDS among HIV-infected patients, by CD4 cell count and HAART use status. Clin Infect Dis. 2008;47:1102–1104. doi: 10.1086/592115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker JV, Peng G, Rapkin J, Krason D, Reilly C, Cavert WP, et al. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J Acquir Immune Defic Syndr. 2008;48:541–546. doi: 10.1097/QAI.0b013e31817bebb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achhra AC, Petoumenos K, Law MG. Relationship between CD4 cell count and serious long-term complications among HIV-positive individuals. Curr Opin HIV AIDS. 2014;9:63–71. doi: 10.1097/COH.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 14.Ingle SM, May MT, Gill MJ, Mugavero MJ, Lewden C, Abgrall S, et al. Impact of risk factors for specific causes death in the first and subsequent years of ART among HIV-infected patients. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–1283. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, Kang L, Lu HZ, Pan X, Lin Q, Pan Q, et al. Normal values for CD4 and CD8 lymphocyte subsets in healthy Chinese adults from Shanghai. Clin Diagn Lab Immunol. 2004;11:811–813. doi: 10.1128/CDLI.11.4.811-813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan J, Greer JM, Hull R, O'Sullivan JD, Henderson RD, Read SJ, et al. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7:4. doi: 10.1186/1742-4933-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luz Correa B, Ornaghi AP, Cerutti Muller G, Engroff P, Pestana Lopes R, Gomes da Silva Filho I, et al. The inverted CD4:CD8 ratio is associated with cytomegalovirus, poor cognitive and functional states in older adults. Neuroimmunomodulation. 2014;21:206–212. doi: 10.1159/000356827. [DOI] [PubMed] [Google Scholar]
- 19.Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121:187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 20.Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10:e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sainz T, Serrano-Villar S, Diaz L, Gonzalez Tome MI, Gurbindo MD, de Jose MI, et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS. 2013;27:1513–1516. doi: 10.1097/QAD.0b013e32835faa72. [DOI] [PubMed] [Google Scholar]
- 22.Serrano-Villar S, Gutierrez C, Vallejo A, Hernandez-Novoa B, Diaz L, Abad Fernandez M, et al. The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect. 2013;66:57–66. doi: 10.1016/j.jinf.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Serrano-Villar S, Perez-Elias MJ, Dronda F, Casado JL, Moreno A, Royuela A, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One. 2014;9:e85798. doi: 10.1371/journal.pone.0085798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz L, Mendez-Lagares G, Correa-Rocha R, Pacheco YM, Ferrando-Martinez S, Ruiz-Mateos E, et al. Detectable viral load aggravates immunosenescence features of CD8 T-cell subsets in vertically HIV-infected children. J Acquir Immune Defic Syndr. 2012;60:447–454. doi: 10.1097/QAI.0b013e318259254f. [DOI] [PubMed] [Google Scholar]
- 25.Aberg JA. Aging, inflammation, and HIV infection. Top Antivir Med. 2012;20:101–105. [PMC free article] [PubMed] [Google Scholar]
- 26.Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Exp Gerontol. 2007;42:432–437. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des. 2013;19:1680–1698. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choremi-Papadopoulou H, Viglis V, Gargalianos P, Kordossis T, Iniotaki-Theodoraki A, Kosmidis J. Downregulation of CD28 surface antigen on CD4+ and CD8+ T lymphocytes during HIV-1 infection. J Acquir Immune Defic Syndr. 1994;7:245–253. [PubMed] [Google Scholar]
- 29.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dock JN, Effros RB. Role of CD8 T Cell Replicative Senescence in Human Aging and in HIV-mediated Immunosenescence. Aging Dis. 2011;2:382–397. [PMC free article] [PubMed] [Google Scholar]
- 31.Tinago W, Coghlan E, Macken A, McAndrews J, Doak B, Prior-Fuller C, et al. Clinical, Immunological and Treatment-Related Factors Associated with Normalised CD4+/CD8+ T-Cell Ratio: Effect of Naive and Memory T-Cell Subsets. PLoS One. 2014;9:e97011. doi: 10.1371/journal.pone.0097011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allers K, Bosel D, Epple HJ, Karcher H, Schmidt W, Kunkel D, et al. Effect of age on the CD4(+) T-cell impairment in HIV-infected persons without and with cART. J Acquir Immune Defic Syndr. 2014;66:7–15. doi: 10.1097/QAI.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 33.Kalayjian RC, Landay A, Pollard RB, Taub DD, Gross BH, Francis IR, et al. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J Infect Dis. 2003;187:1924–1933. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 34.Kalayjian RC, Spritzler J, Matining RM, Fiscus SA, Gross BH, Francis IR, et al. Older HIV-infected patients on antiretroviral therapy have B-cell expansion and attenuated CD4 cell increases with immune activation reduction. AIDS. 2013;27:1563–1571. doi: 10.1097/QAD.0b013e32835fabc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37:445–453. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 36.Serrano-Villar S, Moreno S, Fuentes-Ferrer M, Sanchez-Marcos C, Avila M, Sainz T, et al. The CD4:CD8 ratio is associated with markers of age-associated disease in virally suppressed HIV-infected patients with immunological recovery. HIV Med. 2014;15:40–49. doi: 10.1111/hiv.12081. [DOI] [PubMed] [Google Scholar]
- 37.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217:207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merlini E, Luzi K, Suardi E, Barassi A, Cerrone M, Martinez JS, et al. T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One. 2012;7:e46073. doi: 10.1371/journal.pone.0046073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanni MV, Grinspoon SK. HIV-specific immune dysregulation and atherosclerosis. Curr HIV/AIDS Rep. 2012;9:200–205. doi: 10.1007/s11904-012-0123-y. [DOI] [PubMed] [Google Scholar]
- 41.Manner IW, Troseid M, Oektedalen O, Baekken M, Os I. Low nadir CD4 cell count predicts sustained hypertension in HIV-infected individuals. J Clin Hypertens (Greenwich) 2013;15:101–106. doi: 10.1111/jch.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helleberg M, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Obel N, et al. CD4 decline is associated with increased risk of cardiovascular disease, cancer, and death in virally suppressed patients with HIV. Clin Infect Dis. 2013;57:314–321. doi: 10.1093/cid/cit232. [DOI] [PubMed] [Google Scholar]
- 43.Lang S, Mary-Krause M, Simon A, Partisani M, Gilquin J, Cotte L, et al. HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin Infect Dis. 2012;55:600–607. doi: 10.1093/cid/cis489. [DOI] [PubMed] [Google Scholar]
- 44.Sabin CA, Ryom L, De Wit S, Mocroft A, Phillips AN, Worm SW, et al. Associations between immune depression and cardiovascular events in HIV infection. AIDS. 2013;27:2735–2748. doi: 10.1097/01.aids.0000432457.91228.f3. [DOI] [PubMed] [Google Scholar]
- 45.Badejo OA, Chang CC, So-Armah KA, Tracy RP, Baker JV, Rimland D, et al. CD8+ T-cells count in acute myocardial infarction in HIV disease in a predominantly male cohort. Biomed Res Int. 2015;2015:246870. doi: 10.1155/2015/246870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang CJ, Hsieh MH, Hou WH, Liu JC, Jeng C, Tsai PS. Depression, antidepressants, and the risk of coronary heart disease: a population-based cohort study. Int J Cardiol. 2013;168:4711–4716. doi: 10.1016/j.ijcard.2013.07.173. [DOI] [PubMed] [Google Scholar]
- 47.White JR, Chang CC, So-Armah KA, Stewart JC, Gupta SK, Butt AA, et al. Depression and Human Immunodeficiency Virus Infection Are Risk Factors for Incident Heart Failure Among Veterans: Veterans Aging Cohort Study. Circulation. 2015;132:1630–1638. doi: 10.1161/CIRCULATIONAHA.114.014443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parruti G, Vadini F, Sozio F, Mazzott E, Ursini T, Polill E, et al. Psychological factors, including alexithymia, in the prediction of cardiovascular risk in HIV infected patients: results of a cohort study. PLoS One. 2013;8:e54555. doi: 10.1371/journal.pone.0054555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright ST, Petoumenos K, Boyd M, Carr A, Downing S, O'Connor CC, et al. Ageing and long-term CD4 cell count trends in HIV-positive patients with 5 years or more combination antiretroviral therapy experience. HIV Med. 2013;14:208–216. doi: 10.1111/j.1468-1293.2012.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, et al. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014;192:2143–2155. doi: 10.4049/jimmunol.1301721. [DOI] [PMC free article] [PubMed] [Google Scholar]