Abstract
Importance
Additional information is needed on the role of dietary sodium on health outcomes in older adults.
Objective
To examine the association between dietary sodium intake and mortality, incident cardiovascular disease (CVD), and incident heart failure (HF) in older adults.
Design, Setting, and Participants
We analyzed 10-year follow-up data from 2,642 older adults (age 71-80) participating in a community-based, prospective cohort study (inception 1997-98).
Exposure
Dietary sodium intake at baseline was assessed by a food frequency questionnaire (FFQ). We examined sodium intake both as a continuous and as a categorical variable (<1500mg/d [N=291; 11.0%]; 1500–2300mg/d [N=779; 29.5%]; and >2300mg/d [N=1572; 59.5%].
Main Outcomes
Adjudicated death, incident CVD, and incident HF over 10-years of follow-up. Analysis of incident CVD was restricted to those without prevalent CVD (N=1981) at baseline.
Results
Average age of participants was 73.6±2.9 years; 51.2% were women; 61.7% white; and 38.3% black. After 10 years, 881 participants had died, 572 developed CVD and 398 developed HF. In adjusted Cox proportional hazards models, sodium intake was not associated with mortality (HR per 1g, 1.03; 95%CI 0.98–1.09; P=0.27). Ten-year mortality was nonsignificantly lower in the 1500–2300-mg group (30.7%) compared to the <1500-mg (33.8%) and >2300-mg (35.2%) groups; P=0.074. Sodium intake >2300mg/d was associated with nonsignificantly higher mortality in adjusted models (HR vs. 1500–2300 mg/d, 1.15; 95%CI 0.99–1.35; P=0.072). Indexing sodium intake for caloric intake and body mass index did not materially affect the results. Adjusted HR for mortality was 1.20 (95%CI 0.93–1.54; P=0.16) per mg/kcal sodium and 1.11 (95%CI 0.96–1.28; P=0.17) per 100mg/kg/m2 sodium. In adjusted models accounting for the competing risk of death, sodium intake was not associated with risk for CVD (HR per 1g, 1.03; 95%CI 0.95–1.11; P=0.47) or HF (HR per 1g, 1.00; 95%CI 0.92–1.08; P=0.92). There were no consistent interactions with gender, race, or hypertensive status for any outcome.
Conclusions
In older adults, FFQ-assessed sodium intake was not associated with 10-year mortality, incident CVD, or incident HF, albeit there was a trend towards higher mortality among those consuming >2300 mg/d sodium.
Excess dietary sodium intake is associated with risk factors for cardiovascular disease (CVD) and heart failure (HF); most prominently with elevated blood pressure,1-3 but also with worse renal function,4-6 left ventricular hypertrophy,7-9 and increased arterial stiffness.10-12 Therefore, limiting sodium intake might be an important intervention to reduce risk for CVD and HF.
Based on the effects of sodium reduction on blood pressure and the current levels of sodium intake in the population, simulation studies have projected substantial benefits on outcomes with stricter dietary sodium control (1500 mg).13, 14 However, these projections (1) are based on extrapolation from small studies with higher baseline sodium intake (>3000 mg/day) in pre-hypertensive and hypertensive but not normotensive populations and (2) assume no or beneficial effects on other risk factors. Moreover, sodium restriction may exert unfavorable effects on insulin resistance,15, 16 serum lipids,17 and neurohormonal activation,17, 18 factors that predispose to CVD and HF. The uncertain net effect of these opposing forces is highlighted by recent observational studies. In a large European cohort study investigating the genetic background of hypertension,19 middle-aged persons in the lower sodium stratum had higher cardiovascular mortality despite lower blood pressure. In a post-hoc analysis from two large randomized trials with an angiotensin II receptor antagonist,20 the association between urine sodium excretion and cardiovascular events was J-shaped. More recently, a U-shaped association between urine sodium excretion and HF risk was demonstrated in a longitudinal study of healthy middle-aged men and women,21 with the lowest incidence observed in the 3000-3400 mg/day group. A similar association between sodium intake and outcomes (mortality and cardiovascular disease) was demonstrated in a recent meta-analysis of 23 cohort studies and 2 clinical trial follow-ups.22
In addition to the concerns raised by these recent reports, inadequate caloric intake and interaction with medications are additional concerns with very low sodium intake in older adults.23-25 Data on the effects of sodium restriction is scarce in this population, especially for those with blood pressure at target. Also, achieving 1500 mg/day sodium intake is difficult, particularly in older adults with long-held dietary habits.26 Thus, the incremental benefit of restricting sodium intake to lower targets (1500 mg daily) instead of the general 2300 mg/day recommendation would need to be prospectively evaluated, as suggested by the recent Institute of Medicine report on the evidence for sodium intake recommendations at the population level.27 In this direction, data from cohort studies can provide useful insights and facilitate the design of future prospective studies.
In this study, we investigated the association between sodium intake, as assessed in year 2 with a food frequency questionnaire (FFQ), and risk for (1) all-cause mortality; (2) incident CVD; and (3) incident HF in the Health ABC Study using subsequent 10-year follow-up data. In secondary analyses, we (1) evaluated for interactions with gender, race, and hypertensive status at baseline and (2) repeated analyses with sodium intake indexed for body mass index and total caloric intake.
METHODS
Study Population
The Health ABC Study enrolled 3075 well-functioning, community dwelling individuals aged 70 to 79 years between April 1997 and June 1998. Participants were identified from a random sample of white Medicare beneficiaries and all age-eligible black community residents in designated zip code areas surrounding Pittsburgh and Memphis. Exclusion criteria included difficulties with walking, stair climbing, or activities of daily living, obvious cognitive impairment, inability to communicate, anticipated move within 3 years, or participation in a trial involving lifestyle intervention. At the year 2 visit, which is herein referred to as our study baseline, participants were asked to complete an FFQ. Data on dietary sodium intake were available for 2713 of 2732 participants (99.3%) who were alive and attended the year 2 visit. This analysis includes data on 2642 participants; we excluded 63 participants with known HF at year 2 (because we assumed that these participants received education to follow low-sodium diet) and 8 participants because of implausibly low sodium intake values (<300 mg/d). We used adjudicated 10-year follow up data for outcomes. Complete 10-year follow up data were available in 2628 of 2642 participants (99.5%). For incident CVD analyses, we considered only the subset of participants without prevalent CVD at baseline (N=1981).
Food Frequency Questionnaire
Food intake was recorded at year 2, during the first annual follow-up visit, with a 108-item FFQ designed specifically for the Health ABC Study by Block Dietary Data Systems (Berkeley, CA). The questionnaire was based on reported intakes of non-Hispanic white and black residents of the Northeast and South older than 65 years in the 3rd National Health and Nutrition Examination Survey. The reference period was the preceding year. A trained dietary interviewer administered the FFQ, and interviews were periodically monitored to assure quality and consistency. Wood blocks, real food models, and flash cards were used to help participants estimate portion sizes. Nutrient and food group intakes were determined by Block Dietary Data Systems. The Block family of FFQs has undergone extensive validation, including early studies in middle-aged and older adults28 and more recently, validation of caloric and sodium components in later versions for special populations.29 In the latter study, median energy intake by FFQ was 115 kcal (5%) higher than that of the 24-hour recalls and sodium intake was 220 mg (8%) higher.
Study Definitions
Race was self-defined. Hypertensive status was defined as self-reported history of hypertension accompanied by use of antihypertensive medications. Diabetes mellitus was based on self-report of positive history or use of anti-hyperglycemic medication. Smoking was classified as current, past (≥100 lifetime cigarettes), or never. Physical activity was ascertained using a standardized questionnaire designed by the Health ABC study. Kilocalories per week expended in common exercise activities (e.g., walking for exercise, exercise classes, weightlifting) and lifestyle activities (e.g., gardening, housework, non-exercise walking) were collected and a summary variable of kcal/week was derived. Major ECG abnormalities included: (1) atrioventricular or ventricular conduction defects; (2) rhythm irregularity; (3) left ventricular hypertrophy; (4) Q-wave and major T-wave and ST-segment abnormalities. Minor abnormalities were defined as any minor ST-segment or T-wave abnormalities.
Prevalent CVD was defined as prevalent: (1) coronary heart disease (history of myocardial infarction, angina treated with medications, or coronary revascularization); (2) cerebrovascular disease (history of stroke, transient ischemic attack, or carotid endarterectomy); or (3) peripheral vascular disease (history of intermittent claudication or vascular bypass or angioplasty) at year 2. These definitions follow the definitions used in previous Health ABC Study publications.30, 31
Study Outcomes
Surveillance was conducted by in-person examination alternating with a telephone interview every 6 months. Participants were asked to report any hospitalizations and were directly asked about new CVD and HF events during planned telephone interviews and in-person examinations. The Health ABC Diagnosis & Disease Ascertainment Committee reviewed hospital records, death certificates, informant interviews, and autopsy data to adjudicate immediate and underlying causes of death. A panel of clinicians verified diagnoses based on hospital records, interviews, and death certificates. Medical records for overnight hospitalizations were reviewed at each site by local adjudicators. CVD events were identified and adjudicated using the surveillance and adjudication process described above. Incident CVD was defined as new (1) coronary heart disease (myocardial infarction, angina, or coronary revascularization); (2) cerebrovascular disease (stroke, transient ischemic attack, or symptomatic carotid artery disease); (3) peripheral arterial disease; or (4) death due to cardiovascular causes. Incident HF was defined as a first admission with overnight stay confirmed to be related to HF, based on symptoms, signs, chest radiograph, and echocardiographic findings, using criteria similar to those used in the Cardiovascular Health Study.32, 33
Statistical Analysis
We examined sodium intake as (1) a continuous variable and (2) a categorical variable using the recommendation level cut-off points, i.e. <1500, 1500-2300, and >2300 mg/day. In continuous-variable analysis, we examined for nonlinear associations using restricted cubic splines.34 For baseline characteristics, we used values from the year 2 visit whenever available; smoking status, physical activity, creatinine, albumin, and ECG abnormalities were carried over from year 1. We used the non-parametric test for trend to examine for differences in characteristics across sodium intake categories. To examine the association between baseline sodium and 10-year mortality, we used Cox regression models. In multivariable analyses, we adjusted for demographics and factors previously associated with mortality in the Health ABC Study,31 including age, gender, race, baseline hypertensive status, body mass index, smoking, physical activity, prevalent CVD, lung disease, diabetes, depression, blood pressure, heart rate, ECG abnormalities, and serum glucose, albumin, creatinine, and cholesterol levels. Covariate values were complete in 98.3% of subjects. Confidence intervals were calculated with bootstrapping (normal-based, 1000 replications). The proportional hazards assumption was evaluated with the Schoenfeld residuals. The power to detect a 20% increase in mortality risk per 1g of sodium intake (assuming a linear association) was 80.5% at the two sided α=0.05 level. For CVD, we used the Fine and Grey competing-risks extension of the Cox model in the subset of participants without prevalent CVD at baseline. The competing-risks model accounts for competing non-cardiovascular death (because cardiovascular death was included in the CVD endpoint), which is considerable in older adults. In multivariable analyses, we adjusted for the risk factors described above; these include risk factors for CVD previously identified in Health ABC.31, 35 We followed the same approach for sodium intake and HF risk (with death as a competing risk) and adjusted for the same risk factors, which include previously identified HF risk predictors in the Health ABC Study.36 Proportional hazards in competing-risks models were evaluated using interaction terms with time. We repeated the analyses after entering sodium intake as a continuous variable indexed for (1) body mass index (“indexed sodium intake”) and (2) total caloric intake (“sodium density”). In secondary analyses, we evaluated (1) the association of sodium intake with self-reported appetite grade (5-point Likert scale ranging from very good to very poor) and (2) self-reported adoption of low-salt diet at year 2 visit; and for potential confounding effects from these characteristics. In exploratory analyses, we examined the association of sodium intake with outcomes using alternative, binary definitions for high intake (3000 mg and 4000 mg dally). Analyses were performed with STATA 12.1 (StataCorp LP, College Station, TX).
RESULTS
Baseline characteristics
The average age in the cohort (N=2,642) was 73.6±2.9 years; 48.8% were men; 61.7% were white; and 38.3% were black. The baseline characteristics according to sodium intake at baseline are presented in Table 1. Men (median, 2850 mg; 25th–75th percentile, 2140–3640) consumed considerably more sodium than women (2320 mg; 1760–2950), P<0.001 for the difference. Whites, and participants with diabetes were also more likely to consume more sodium, whereas participants with hypertension had lower intake. Higher sodium intake was associated with higher albumin and creatinine levels but lower cholesterol levels.
Table 1.
Baseline participant characteristics according to sodium intake at baseline
| <1500 mg/day | 1500-2300 mg/d | >2300 mg/d | P value a | |
|---|---|---|---|---|
| N | 291 | 779 | 1572 | |
| Age, years | 74.4 (2.9) | 74.6 (2.9) | 74.6 (2.9) | 0.17 |
| Male sex, N (%) | 88 (30.2) | 315 (40.4) | 887 (56.4) | <0.001 |
| Race | 0.028 | |||
| Blacks, N (%) | 137 (47.1) | 285 (36.6) | 590 (37.5) | |
| Whites, N (%) | 154 (52.9) | 494 (63.4) | 982 (62.5) | |
| Body mass index, kg/m2 | 27.7 (5.0) | 27.2 (4.8) | 27.1 (4.8) | 0.14 |
| Indexed daily sodium, mg/kg/m2 b | 45 (36, 52) | 71 (62, 83) | 120 (99, 148) | <0.001 |
| Smoking | 0.91 | |||
| Current smokers, N (%) | 32 (11.0) | 65 (8.3) | 149 (9.5) | |
| Past smokers, N (%) | 131 (45.0) | 359 (46.1) | 728 (46.3) | |
| Physical activity, kcal/kg/week | 65 (40, 102) | 68 (40, 108) | 66 (39, 110) | 0.42 |
| Total caloric intake, kcal/day | 940 (790, 1090) | 1400 (1230, 1600) | 2130 (1800, 2590) | <0.001 |
| Dietary sodium density, mg/kcal c | 1.24 (1.12, 1.45) | 1.37 (1.22, 1.52) | 1.50 (1.36, 1.66) | <0.001 |
| Coronary heart disease, N (%) | 55 (18.9) | 140 (18.0) | 284 (18.1) | 0.81 |
| Cerebrovascular disease, N (%) | 27 (9.3) | 61 (7.8) | 103 (6.6) | 0.069 |
| Peripheral vascular disease, N (%) | 17 (5.8) | 35 (4.5) | 78 (5.0) | 0.79 |
| Any cardiovascular disease, N (%) | 74 (25.4) | 203 (26.1) | 384 (24.4) | 0.49 |
| Pulmonary disease, N (%) | 39 (13.4) | 81 (10.4) | 180 (11.5) | 0.69 |
| Diabetes mellitus, N (%) | 38 (13.1) | 138 (17.7) | 297 (18.9) | 0.028 |
| Hypertension, N (%) | 167 (57.4) | 427 (54.8) | 803 (51.1) | 0.019 |
| Uncontrolled hypertension, N (%) d | 94 (32.2) | 214 (27.5) | 419 (26.6) | 0.081 |
| Depression, N (%) | 31 (10.7) | 75 (9.6) | 163 (10.4) | 0.88 |
| Systolic blood pressure, mmHg | 136 (22) | 133 (20) | 134 (21) | 0.36 |
| Diastolic blood pressure, mmHg | 71 (12) | 70 (11) | 70 (12) | 0.43 |
| Heart rate, beats/min | 64.7 (9.5) | 64.7 (10.7) | 64.9 (11.1) | 0.98 |
| ECG – major abnormalities, N (%) e | 56 (19.2) | 172 (22.1) | 326 (20.7) | 0.96 |
| ECG – minor abnormalities, N (%) f | 57 (19.7) | 129 (16.6) | 260 (16.6) | 0.30 |
| Glucose, mg/dl | 97.8 (23.8) | 102.3 (31.3) | 103.0 (29.6) | 0.019 |
| Albumin, g/dl | 3.96 (0.30) | 3.98 (0.32) | 4.00 (0.31) | 0.042 |
| Creatinine, mg/dl | 1.04 (0.37) | 1.04 (0.40) | 1.05 (0.37) | 0.007 |
| Total cholesterol, mg/dl | 212 (38) | 208 (39) | 204 (38) | <0.001 |
| Antihypertensive medications | ||||
| Beta-blocker, N (%) | 45 (15.5) | 107 (13.7) | 230 (14.6) | 0.99 |
| ACE inhibitor or ARB, N (%) | 57 (19.6) | 135 (17.3) | 319 (20.3) | 0.31 |
| Ca++ channel inhibitor, N (%) | 70 (24.0) | 209 (26.8) | 333 (21.2) | 0.024 |
| Thiazide diuretic, N (%) | 64 (22.0) | 161 (20.7) | 285 (18.1) | 0.061 |
| K+ sparing diuretic, N (%) | 38 (13.1) | 80 (10.3) | 117 (7.4) | 0.001 |
| Loop diuretic, N (%) | 19 (6.5) | 36 (4.6) | 91 (5.8) | 0.88 |
Continuous variables are presented as mean (standard deviation) or median (25th percentile, 75th percentile); categorical variables are presented as number (percentage). ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker.
Nonparametric test for trend
Daily sodium intake divided by body mass index
Daily sodium intake divided by daily caloric intake
Systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg
Major Q or QS abnormality, major ST or T wave abnormality, left ventricular hypertrophy, or ventricular conduction defects.
Minor Q or QS abnormality or ST or T wave abnormalities.
Mortality
After 10 years, 881 participants had died (Kaplan-Meier mortality, 33.7%). The association of baseline sodium intake with 10-year mortality was approximately linear; although the spline model suggested a U-shaped association (Supplemental Figure 1), the gain in fit did not justify the added model complexity. Table 2 presents the association of baseline sodium intake with mortality as a continuous variable in the entire cohort and in the subgroups of interest. The hazard ratio (HR) for mortality per 1g of sodium intake was 1.09 (95%CI 1.04–1.16; P=0.001) in the crude and 1.03 (95%CI 0.98–1.09; P=0.27) in the adjusted model. The main confounder was gender, attenuating the unadjusted estimate by 48%. The crude association of sodium intake with mortality was stronger in women and those without baseline hypertension (P=0.069 and P=0.10 for the corresponding interaction terms), but these trends were attenuated in adjusted models (P=0.48 and P=0.11, respectively, for interaction). There was no evidence of differential association across race. No association in subgroups retained significance in adjusted models.
Table 2.
Association of baseline sodium intake with 10-year mortality
| N events / N participants | Unadjusted | Adjusted a | |||
|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | ||
| All | |||||
| Sodium, per 1g | 881 / 2642 | 1.09 (1.04-1.16) | 0.001 | 1.03 (0.98-1.09) | 0.27 |
| Sodium <1500 mg/d | 97 / 291 | 1.11 (0.88-1.40) | 0.37 | 1.12 (0.89-1.42) | 0.33 |
| Sodium 1500-2300 mg/d | 236 / 779 | 1.00 | Reference | 1.00 | Reference |
| Sodium >2300 mg/d | 548 / 1572 | 1.19 (1.02-1.39) | 0.025 | 1.15 (0.99-1.35) | 0.072 |
| Men | |||||
| Sodium, per 1g | 510 / 1290 | 1.01 (0.94-1.09) | 0.74 | 1.01 (0.94-1.09) | 0.76 |
| Sodium <1500 mg/d | 39 / 88 | 1.11 (0.78-1.59) | 0.56 | 1.12 (0.78-1.60) | 0.54 |
| Sodium 1500-2300 mg/d | 127 / 315 | 1.00 | Reference | 1.00 | Reference |
| Sodium >2300 mg/d | 344 / 887 | 0.95 (0.78-1.17) | 0.66 | 1.04 (0.84-1.29) | 0.72 |
| Women | |||||
| Sodium, per 1g | 371 / 1352 | 1.13 (1.03-1.25) | 0.011 | 1.06 (0.96-1.17) | 0.27 |
| Sodium <1500 mg/d | 58 / 203 | 1.25 (0.91-1.72) | 0.17 | 1.12 (0.80-1.55) | 0.51 |
| Sodium 1500-2300 mg/d | 109 / 464 | 1.00 | Reference | 1.00 | Reference |
| Sodium >2300 mg/d | 204 / 685 | 1.33 (1.06-1.66) | 0.014 | 1.26 (0.99-1.59) | 0.055 |
| Whites | |||||
| Sodium, per 1g | 498 / 1630 | 1.08 (1.01-1.17) | 0.032 | 1.03 (0.94-1.12) | 0.50 |
| Sodium <1500 mg/d | 39 / 154 | 0.85 (0.60-1.20) | 0.36 | 0.84 (0.58-1.22) | 0.36 |
| Sodium 1500-2300 mg/d | 145 / 494 | 1.00 | Reference | 1.00 | Reference |
| Sodium >2300 mg/d | 314 / 982 | 1.12 (0.93-1.35) | 0.24 | 1.06 (0.86-1.31) | 0.58 |
| Blacks | |||||
| Sodium, per 1g | 383 / 1012 | 1.09 (1.01-1.17) | 0.020 | 1.03 (0.95-1.12) | 0.42 |
| Sodium <1500 mg/d | 58 / 137 | 1.36 (0.98-1.89) | 0.065 | 1.41 (0.97-2.05) | 0.071 |
| Sodium 1500-2300 mg/d | 91 / 285 | 1.00 | Reference | 1.00 | Reference |
| Sodium >2300 mg/d | 234 / 590 | 1.31 (1.03-1.67) | 0.029 | 1.29 (0.98-1.69) | 0.066 |
| Hypertension | |||||
| Sodium, per 1g | 515 / 1397 | 1.05 (0.98-1.13) | 0.14 | 1.01 (0.93-1.09) | 0.84 |
| Sodium <1500 mg/d | 69 / 167 | 1.29 (0.97-1.70) | 0.078 | 1.21 (0.89-1.66) | 0.22 |
| Sodium 1500-2300 mg/d | 144 / 427 | 1.00 | Reference | 1.00 | Reference |
| Sodium >2300 mg/d | 302 / 803 | 1.14 (0.93-1.39) | 0.21 | 1.10 (0.90-1.34) | 0.36 |
| No hypertension | |||||
| Sodium, per 1g | 366 / 1245 | 1.15 (1.07-1.25) | <0.001 | 1.07 (0.98-1.17) | 0.13 |
| Sodium <1500 mg/d | 28 / 124 | 0.83 (0.55-1.26) | 0.38 | 0.86 (0.56-1.31) | 0.48 |
| Sodium 1500-2300 mg/d | 92 / 352 | 1.00 | Reference | 1.00 | Reference |
| Sodium >2300 mg/d | 246 / 769 | 1.30 (1.02-1.65) | 0.035 | 1.19 (0.91-1.57) | 0.21 |
CI: confidence interval; HR: hazard ratio
* Adjusted for age, gender, race, baseline hypertensive status, body mass index, smoking, physical activity, prevalent cardiovascular disease, lung disease, diabetes, depression, blood pressure, heart rate, ECG abnormalities, and serum glucose, albumin, creatinine, and cholesterol levels.
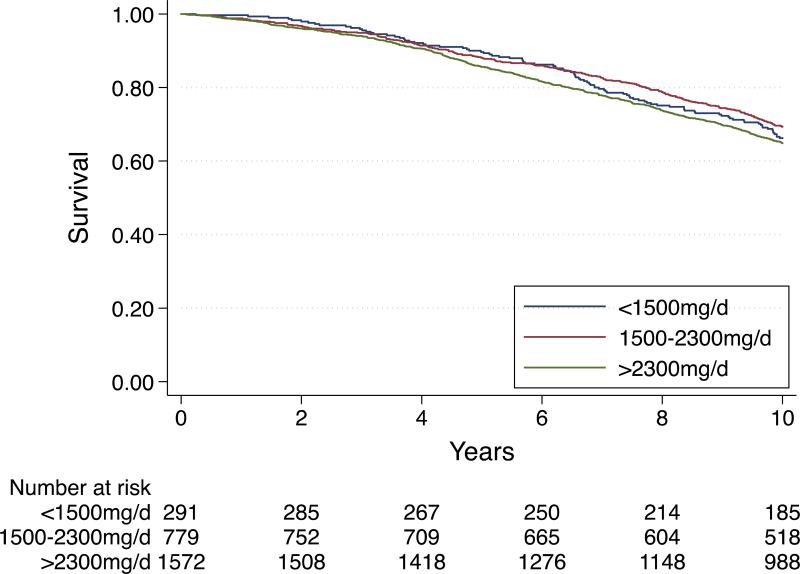
Mortality was 33.8%, 30.7%, and 35.2% among participants consuming <1500, 1500-2300, and >2300mg/d sodium, respectively (log-rank P=0.074), Figure 1. In crude models, sodium intake >2300 mg/d was associated with higher mortality compared to 1500-2300mg/d, driven by the higher risk in women, blacks, and those without hypertension (Table 2). However, none of these findings retained significance in adjusted models.
Figure 1.
Ten-year all-cause mortality in the Health ABC Study according to baseline sodium intake. The log-rank chi-square test was 5.22 with d.f. =2; P=0.074.
When sodium density was examined (sodium intake indexed for caloric intake), the results were not materially different; HR per mg/kcal was 1.34 (95%CI 1.06–1.70; P=0.014) in crude and 1.20 (95%CI 0.93–1.54; P=0.16) in adjusted models. There was no significant interaction with the major subgroups of interest (data not shown). Indexing sodium intake for body mass index yielded similar results; HR per 100mg/kg/m2 sodium was 1.36 (95%CI 1.19–1.55; P<0.001) in crude and 1.11 (95%CI 0.96–1.28; P=0.17) in adjusted models without significant interactions with the major subgroups of interest (data not shown).
Incident Cardiovascular Disease
Among the 1981 participants without CVD at baseline, 572 (28.9%) developed CVD after 10 years of follow up. The linear form best represented the association between baseline sodium intake and CVD. In models taking the competing risk of death into account, the crude HR for CVD per 1g of sodium intake was 1.09 (95%CI 1.01–1.16; P=0.023) and the adjusted HR was 1.03 (95%CI 0.95–1.11; P=0.47). The main confounder was gender, attenuating the unadjusted estimate by 61%. There was no differential association in the subgroups examined (Table 3).
Table 3.
Association of baseline sodium intake with 10-year incident cardiovascular disease and heart failure
| Incident Cardiovascular Disease |
Incident Heart Failure |
|||||
|---|---|---|---|---|---|---|
| Adjusted* | Adjusted* | |||||
| N events / N participants | sHR (95%CI) | P | N events / N participants | sHR (95%CI) | P | |
| All | ||||||
| Sodium, per 1g | 572 / 1981 | 1.03 (0.95-1.11) | 0.47 | 398 / 2642 | 1.00 (0.92-1.08) | 0.92 |
| Sodium <1500 mg/d | 63 / 217 | 1.05 (0.79-1.41) | 0.73 | 45 / 291 | 1.11 (0.77-1.61) | 0.57 |
| Sodium 1500-2300 mg/d | 161 / 576 | 1.00 | Reference | 111 / 779 | 1.00 | Reference |
| Sodium >2300 mg/d | 348 / 1188 | 1.02 (0.84-1.24) | 0.82 | 242 / 1572 | 1.08 (0.86-1.36) | 0.52 |
| Men | ||||||
| Sodium, per 1g | 312 / 900 | 0.98 (0.89-1.08) | 0.73 | 213 / 1290 | 1.00 (0.91-1.11) | 0.94 |
| Sodium <1500 mg/d | 23 / 59 | 1.13 (0.72-1.77) | 0.59 | 18 / 88 | 1.50 (0.84-2.68) | 0.17 |
| Sodium 1500-2300 mg/d | 77 / 208 | 1.00 | Reference | 51 / 315 | 1.00 | Reference |
| Sodium >2300 mg/d | 212 / 633 | 0.91 (0.69-1.18) | 0.47 | 144 / 887 | 1.10 (0.78-1.54) | 0.58 |
| Women | ||||||
| Sodium, per 1g | 260 / 1081 | 1.12 (0.98-1.28) | 0.086 | 185 / 1352 | 0.99 (0.87-1.13) | 0.92 |
| Sodium <1500 mg/d | 40 / 158 | 1.04 (0.71-1.54) | 0.83 | 27 / 203 | 0.91 (0.56-1.48) | 0.71 |
| Sodium 1500-2300 mg/d | 84 / 368 | 1.00 | Reference | 60 / 464 | 1.00 | Reference |
| Sodium >2300 mg/d | 136 / 555 | 1.12 (0.85-1.49) | 0.42 | 98 / 685 | 1.11 (0.80-1.54) | 0.55 |
| Whites | ||||||
| Sodium, per 1g | 353 / 1217 | 0.98 (0.88-1.09) | 0.72 | 229 / 1630 | 1.00 (0.89-1.13) | 0.99 |
| Sodium <1500 mg/d | 37 / 116 | 1.15 (0.80-1.67) | 0.45 | 19 / 154 | 0.80 (0.47-1.37) | 0.42 |
| Sodium 1500-2300 mg/d | 98 / 375 | 1.00 | Reference | 69 / 494 | 1.00 | Reference |
| Sodium >2300 mg/d | 218 / 726 | 1.03 (0.80-1.33) | 0.81 | 141 / 982 | 0.95 (0.71-1.28) | 0.74 |
| Blacks | ||||||
| Sodium, per 1g | 219 / 764 | 1.08 (0.97-1.20) | 0.15 | 169 / 1012 | 1.01 (0.89-1.13) | 0.92 |
| Sodium <1500 mg/d | 26 / 101 | 0.82 (0.52-1.32) | 0.42 | 26 / 137 | 1.65 (0.96-2.85) | 0.072 |
| Sodium 1500-2300 mg/d | 63 / 201 | 1.00 | Reference | 42 / 285 | 1.00 | Reference |
| Sodium >2300 mg/d | 130 / 462 | 0.92 (0.67-1.27) | 0.63 | 101 / 590 | 1.33 (0.90-1.97) | 0.16 |
| Hypertension | ||||||
| Sodium, per 1g | 302 / 951 | 1.02 (0.93-1.12) | 0.68 | 255 / 1397 | 0.98 (0.89-1.09) | 0.74 |
| Sodium <1500 mg/d | 33 / 113 | 0.87 (0.57-1.31) | 0.50 | 32 / 167 | 1.06 (0.68-1.66) | 0.80 |
| Sodium 1500-2300 mg/d | 90 / 286 | 1.00 | Reference | 75 / 427 | 1.00 | Reference |
| Sodium >2300 mg/d | 179 / 552 | 1.02 (0.78-1.32) | 0.91 | 148 / 803 | 1.01 (0.76-1.35) | 0.94 |
| No hypertension | ||||||
| Sodium, per 1g | 270 / 1030 | 1.06 (0.93-1.20) | 0.38 | 143 / 1245 | 1.04 (0.90-1.20) | 0.63 |
| Sodium <1500 mg/d | 30 / 104 | 1.23 (0.82-1.84) | 0.32 | 13 / 124 | 1.10 (0.57-2.12) | 0.78 |
| Sodium 1500-2300 mg/d | 71 / 290 | 1.00 | Reference | 36 / 352 | 1.00 | Reference |
| Sodium >2300 mg/d | 169 / 636 | 1.02 (0.77-1.37) | 0.87 | 94 / 769 | 1.15 (0.77-1.72) | 0.49 |
CI: confidence interval; sHR: subhazard ratio (hazard ratio conditional on the competing risk of death)
a Adjusted for age, gender, race, baseline hypertensive status, body mass index, smoking, physical activity, prevalent cardiovascular disease (for heart failure events), lung disease, diabetes, depression, blood pressure, heart rate, ECG abnormalities, and serum glucose, albumin, creatinine, and cholesterol levels.
The cumulative incidence of CVD over 10 years, accounting for the competing risk of death, was 28.5%, 28.2%, and 29.7% for <1500, 1500-2300, and >2300mg/d sodium intake, respectively. Using the 1500–2300-mg category as reference (lowest incidence), the adjusted subhazard ratio (sHR) was 1.05 (95%CI 0.79–1.41; P=0.73) for <1500-mg and 1.02 (95%CI 0.84–1.24; P=0.82) for >2300-mg intake.
In sodium density models, sHR per mg/kcal sodium for CVD was 1.15 (95%CI 0.85–1.56; P=0.36) in crude and 1.00 (95%CI 0.72–1.38; P=0.98) in adjusted models. In body mass-indexed sodium models, sHR per 100mg/kg/m2 sodium was 1.13 (95%CI 0.94–1.35; P=0.20) in crude and 1.10 (95%CI 0.90–1.34; P=0.36) in adjusted models.
Incident Heart Failure
Among the 2642 participants (no participant had HF at baseline by design), 398 (28.9%) developed HF after 10 years of follow up. The association between baseline sodium intake and HF was linear. In competing-risks models, the crude sHR for HF per 1g of sodium intake was 1.03 (95%CI 0.95–1.12; P=0.50) and the adjusted sHR was 1.00 (95%CI 0.92–1.08; P=0.92). There was no differential association in the subgroups examined (Table 3).
The cumulative incidence of HF over 10 years, accounting for the competing risk of death, was 15.7%, 14.3%, and 15.5% for <1500, 1500-2300, and >2300mg/d sodium, respectively. Using the 1500–2300-mg category as reference (lowest incidence), the adjusted sHR was 1.11 (95%CI 0.77–1.61; P=0.57) for <1500-mg and 1.08 (95%CI 0.86–1.36; P=0.52) for >2300-mg intake.
In sodium density models, sHR per mg/kcal sodium for HF was 1.28 (95%CI 0.87–1.89; P=0.21) in crude and 1.03 (95%CI 0.70–1.51; P=0.89) in adjusted models. In body mass-indexed sodium models, sHR per 100mg/kg/m2 sodium was 0.94 (95%CI 0.75–1.18; P=0.60) in crude and 1.00 (95%CI 0.80–1.25; P=0.99) in adjusted models.
Self-Reported Appetite and Self-Reported Low-Salt Diet Adoption
At year 2 visit, participants were asked to rate their appetite on a 5-point Likert scale (with higher grade representing worse appetite). Among 2637 of 2642 responders (99.8%), only 3.5% rated their appetite as poor (3.0%) or very poor (0.5%). The correlation between sodium intake and appetite grade was significant but weak (Spearman ρ=−0.05; P=0.009). Worse appetite was independently associated with higher mortality and heart failure (HF) but not cardiovascular disease (CVD) risk. However, appetite grade did not confound or modify the association of sodium intake with outcomes of interest.
Based on year 2 questionnaire data, 538 of 2642 participants (20.4%) reported adoption of a low-salt diet. However, FFQ-estimated sodium intake among low-salt diet adopters (median, 2520 mg; 25th–75th percentile, 1900–3370) was similar to non-adopters (2540 mg; 1920–3330), P=0.69 for the difference. Self-reported low-salt diet was not associated with mortality (adjusted HR 0.94; 95%CI 0.79–1.12; P=0.50), CVD (adjusted sHR 1.11; 95%CI 0.90–1.38; P=0.34), or HF (adjusted sHR 1.16; 95%CI 0.91–1.48; P=0.22) risk and did not confound or modify the association of sodium intake with outcomes of interest (data not shown).
Alternative Definitions of High Sodium Intake
In exploratory analyses, we have examined two alternative binary definitions for high sodium intake (3000 mg and 4000 mg), Supplemental Table 1. Sodium intake >4000 mg was associated with more consistent estimates for mortality and incident CVD and HF risk in unadjusted analyses. However, the strength of the association is considerably dampened in adjusted analyses.
DISCUSSION
In our study, we did not observe an association between FFQ-determined dietary sodium intake and 10-year mortality or incident CVD and HF among the older adults participating in the Health ABC Study. Compared with baseline sodium intake of 1500-2300-mg daily, there was no signal of benefit with <1500 mg daily sodium. There was however a signal for potential harm with >2300-mg daily sodium intake, driven mainly by women and black participants, but this finding needs further confirmation because of borderline significance and multiple subgroup testing. Also, there was no signal for association of sodium intake with incident CVD and HF in this older adult population.
Adults over age 65 currently comprise 13% of the Unites States population37 but account for the majority of incident and prevalent CVD and HF cases.38 This population segment is projected to double by year 2050,37 leading to almost doubling of new CVD cases39 and 50% more HF cases40 in this age group. Currently, sodium intake <1500 mg is recommended for adults over age 50 as a means to prevent CVD,41 though this recommendation has been debated.27 In a recent analysis from the National Health and Nutrition Examination Survey (NHANES), only 1.3% of adults over age 50 were consuming <1500 mg sodium daily, whereas the average consumption was 3100 mg.42 In a recent report from a Canadian cohort of older adults,43 sodium intake by FFQ was similar to our study. These findings highlight the difficulties in the implementation of strict sodium intake, especially in older adults,26 and the tremendous efforts that would be required at the industry, community, interpersonal, and individual level to achieve this level of sodium intake. Considering our and other data, there is a pressing need to evaluate the dose-response association of dietary sodium with cardiovascular outcomes in older adults using data from well-designed cohort studies in order to inform the design of outcome trials and make recommendations specific to older adults.
A recent meta-analysis of 13 prospective studies reported that a 2000-mg higher sodium intake was associated with a 14% higher risk for CVD.44 The estimates in our study were lower (approximately 6% higher risk per 2000-mg higher sodium intake) and did not reach significance. However, the Health ABC Study included only older adults age 70-79 years at the time of inception and the average sodium intake was much lower compared to that meta-analysis, emphasizing the importance of the population under investigation and the absolute levels of sodium intake where the potential treatment effect is estimated. In the post hoc analysis from the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) and Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) trials (N=28,880), a significant association between sodium urinary excretion and CVD was not observed until excretion exceeded 6500 mg per day, a much higher threshold than that recommended by national guidelines (2300 mg for the general population or 1500 mg for high-risk individuals). In our exploratory analysis with alternative thresholds, the signal towards risk appeared more consistent with sodium intake >4000 mg, although not significant in adjusted analysis.
Previous individual prospective cohort studies have either reported a positive association, no association, or an inverse relationship between sodium intake and mortality.19, 45, 46 Moreover, it is vexing that in some observational studies the reductions in blood pressure achieved by dietary means did not translate into lower CVD rates.47 Discrepant findings of previous studies are likely due to differences in ranges of sodium intake, study populations, methods of sodium assessment, and failure to explore nonlinear associations.20, 44, 48 The non-linearity issue was highlighted in a recent meta-analysis including 23 cohort studies and 2 clinical trial follow-ups.22 In that analysis, increased risk for adverse outcomes was observed at intakes <2600 mg and >4900 mg. Recently, long-term outcomes from the control arms of the Trials of Hypertension Prevention (TOHP), during which 24-h urinary sodium excretion was repeatedly assessed, were reported.49 In the follow up, which started 10 and 5 years after the end of TOHP I and II respectively, sodium excretion <2300 mg was associated with lower CVD risk in middle-aged adults. Of note, there was no clear risk gradient between the <2300-mg and 2300–3600-mg groups and linear trends were not significant.49 However, in contrast with previous studies, our study population included older adults who are inherently at higher risk for CVD and HF and therefore the effect of sodium intake may be more difficult to ascertain.
A number of mostly short-term trials have shown that reduction of sodium excretion to levels consistent with current guidelines lowers blood pressure in participants with either prehypertension or hypertension and also in normotensive participants, although the effect is attenuated in the latter group.50 However, trials in younger participants with high-normal blood pressure did not report a difference in CVD risk events on initial follow-up, and only during an extended observational follow-up of 10 to 15 years a non-significant benefit was reported, which became statistically significant only after multivariable adjustment.51 In line with these findings, a recent Cochrane review of randomized controlled trials evaluating reduced sodium intake did not detect a significant reduction in CVD risk or mortality.46 In another meta-analysis of 4 primary prevention trials (2 in hypertension and 2 in hypertension), a marginally significant reduction in CVD events in those randomized to reduced sodium intake was reported.52 Therefore, these studies are suggestive but not conclusive of a benefit from sodium reduction to very low intake targets in a primary prevention population. This benefit might even more difficult to demonstrate in older adults because of higher comorbidity burden and concomitant medications that interact with sodium metabolism. It becomes evident from these discrepancies that randomized trials evaluating reduced sodium intake for primary and secondary prevention of cardiovascular outcomes are needed. In addition, considering the special case of older adults, in whom inadequate caloric intake and interaction with medications are additional concerns with very low sodium intake,23, 24 the effect of sodium restriction should probably be tested explicitly in this population before implementing a generalized recommendation for very low (<1500 mg daily) sodium intake target. In the interim, a more conservative approach to sodium restriction, e.g. targeting <2300 mg daily, might be appropriate for older adults.
Our study has a number of limitations. First, the accuracy of FFQ for estimation of sodium intake, albeit adequate from a broad epidemiological perspective, it is less accurate at the individual participant level compared to other methods such as 24h urinary sodium excretion. Sodium intake estimates by FFQ are reasonably reproducible but poorly correlated with 24h excretion estimates, with ρ values of 0.2 or less, and underestimate sodium intake.53 As a consequence, the association between sodium intake and outcomes is probably attenuated in our study. Also, participants at greatest risk may be more susceptible to underestimation of sodium intake. However, self-reported adoption of low-salt diet was not associated with significantly higher risk of events. Second, Health ABC was not designed to specifically answer this question. Secondary data analyses associating sodium intake with outcomes have several methodological drawbacks.54, 55 Third, study participants were selected on the basis of voluntary participation and good functional capacity. This sample may therefore not fully reflect the general older adult population. However, individuals without mobility disability represent approximately 60% of the population aged 70–79 in the United States.56 In addition, our results may not apply to younger, lower-risk populations. Fourth, confounders not included in our study may have affected estimates. Although we have comprehensively adjusted for risk factors previously identified in this cohort, we cannot exclude unobserved confounding. In addition, the lack of association between sodium intake and systolic blood pressure or hypertensive status, one of the main mechanisms by which sodium intake leads to increased CVD and HF risk, might be an indication of reverse causality or, alternatively, of higher sodium intake thresholds for blood pressure effects in this population. Fifth, considering the 10-year horizon between sodium intake assessment and outcomes, we cannot exclude the possibility of regression dilution. Finally, our approach has probably reduced power in the individual sodium intake groups (compared to a quantile-based approach) and may have led to unstable relative risk estimates. However, we have opted for recommendation-based cut-offs of sodium intake (<1500, 1500-2300, >2300 mg) for categorical analysis instead of quantiles (e.g. tertiles), in an attempt to facilitate clinical interpretation.
In conclusion, we observed that sodium intake estimated by FFQ was not associated with mortality or risk for CVD and HF in a cohort of adults 70 years or older. These findings extended to gender- and race-based subgroups and in participants with and without hypertension at baseline. Our data emphasizes the need for stronger evidence, preferably from rigorous controlled trials testing additional thresholds for sodium intake, before applying a policy of further sodium restriction beyond the current recommendation for the general adult population (2300 mg) to older adults.
Supplementary Material
Acknowledgments
Funding Sources: This study was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging (NIA), and contracts, N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant: R01-AG028050, and NINR grant R01-NR012459; and by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript that has been accepted for publication in Journal of the American Medical Association Internal Medicine. – Accepted 7/7/2014. Copyright Journal of the American Medical Association Internal Medicine. This manuscript may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, US Code) without permission of the copyright owner, ISPAH. The final copyedited article, which is the version of record, can be found at: http://journals.humankinetics.com/JPAH. The ISPAH disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. Citation: Kalogeropoulos AP, Georgiopoulou VV, Murphy RA, Newman AB, Bauer DC, Harris TB, Yang Z, Applegate WB, Kritchevsky SB. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: the Health, Aging, and Body Composition (Health ABC) Study. JAMA Intern Med. 2015 Mar;175(3):410-9. PMID:25599120
Authors’ contributions:
- Conception and design of analysis, data analysis, interpretation, and drafting: APK
- Drafting, revisions, and critical intellectual content: VVG, RAM, DCB, ZY, WBA
- Design of main study, acquisition of funding and data, interpretation, and drafting: ABN, TBH, SBK
Conflict of interest disclosures: None
REFERENCES
- 1.Intersalt: an international study of electrolyte excretion and blood pressure Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297(6644):319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Midgley JP, Matthew AG, Greenwood CM, Logan AG. Effect of reduced dietary sodium on blood pressure: a meta-analysis of randomized controlled trials. JAMA. 1996;275(20):1590–1597. doi: 10.1001/jama.1996.03530440070039. [DOI] [PubMed] [Google Scholar]
- 3.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 4.Cianciaruso B, Bellizzi V, Minutolo R, et al. Salt intake and renal outcome in patients with progressive renal disease. Miner Electrolyte Metab. 1998;24(4):296–301. doi: 10.1159/000057385. [DOI] [PubMed] [Google Scholar]
- 5.Verhave JC, Hillege HL, Burgerhof JG, et al. Sodium intake affects urinary albumin excretion especially in overweight subjects. J Intern Med. 2004;256(4):324–330. doi: 10.1111/j.1365-2796.2004.01390.x. [DOI] [PubMed] [Google Scholar]
- 6.du Cailar G, Ribstein J, Mimran A. Dietary sodium and target organ damage in essential hypertension. Am J Hypertens. 2002;15(3):222–229. doi: 10.1016/s0895-7061(01)02287-7. [DOI] [PubMed] [Google Scholar]
- 7.Schmieder RE, Messerli FH, Garavaglia GE, Nunez BD. Dietary salt intake. A determinant of cardiac involvement in essential hypertension. Circulation. 1988;78(4):951–956. doi: 10.1161/01.cir.78.4.951. [DOI] [PubMed] [Google Scholar]
- 8.Liebson PR, Grandits G, Prineas R, et al. Echocardiographic correlates of left ventricular structure among 844 mildly hypertensive men and women in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1993;87(2):476–486. doi: 10.1161/01.cir.87.2.476. [DOI] [PubMed] [Google Scholar]
- 9.Schmieder RE, Langenfeld MR, Friedrich A, Schobel HP, Gatzka CD, Weihprecht H. Angiotensin II related to sodium excretion modulates left ventricular structure in human essential hypertension. Circulation. 1996;94(6):1304–1309. doi: 10.1161/01.cir.94.6.1304. [DOI] [PubMed] [Google Scholar]
- 10.Simon G, Illyes G, Csiky B. Structural vascular changes in hypertension: role of angiotensin II, dietary sodium supplementation, blood pressure, and time. Hypertension. 1998;32(4):654–660. doi: 10.1161/01.hyp.32.4.654. [DOI] [PubMed] [Google Scholar]
- 11.Avolio A, Deng F, Li W, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71(2):202–210. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- 12.Avolio A, Clyde K, Beard T, Cooke H, Ho K, O'Rourke M. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis. 1986;6(2):166–169. doi: 10.1161/01.atv.6.2.166. [DOI] [PubMed] [Google Scholar]
- 13.Smith-Spangler CM, Juusola JL, Enns EA, Owens DK, Garber AM. Population strategies to decrease sodium intake and the burden of cardiovascular disease: a cost-effectiveness analysis. Ann Intern Med. 2010;152(8):481–487, W170-483. doi: 10.7326/0003-4819-152-8-201004200-00212. [DOI] [PubMed] [Google Scholar]
- 14.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362(7):590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman RD, Schmidt ND. Moderate dietary salt restriction increases vascular and systemic insulin resistance. Am J Hypertens. 1999;12(6):643–647. doi: 10.1016/s0895-7061(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 16.Petrie JR, Morris AD, Minamisawa K, et al. Dietary sodium restriction impairs insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83(5):1552–1557. doi: 10.1210/jcem.83.5.4835. [DOI] [PubMed] [Google Scholar]
- 17.Graudal NA, Galloe AM, Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. JAMA. 1998;279(17):1383–1391. doi: 10.1001/jama.279.17.1383. [DOI] [PubMed] [Google Scholar]
- 18.Grassi G, Dell'Oro R, Seravalle G, Foglia G, Trevano FQ, Mancia G. Short- and long-term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation. 2002;106(15):1957–1961. doi: 10.1161/01.cir.0000033519.45615.c7. [DOI] [PubMed] [Google Scholar]
- 19.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305(17):1777–1785. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306(20):2229–2238. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 21.Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Estimated urinary sodium excretion and risk of heart failure in men and women in the EPIC-Norfolk study. Eur J Heart Fail. 2014 doi: 10.1002/ejhf.56. [DOI] [PubMed] [Google Scholar]
- 22.Graudal N, Jurgens G, Baslund B, Alderman MH. Compared With Usual Sodium Intake, Low- and Excessive-Sodium Diets Are Associated With Increased Mortality: A Meta-Analysis. Am J Hypertens. 2014 doi: 10.1093/ajh/hpu028. [DOI] [PubMed] [Google Scholar]
- 23.Nakasato M, Strunk CM, Guimaraes G, Rezende MV, Bocchi EA. [Is the low-sodium diet actually indicated for all patients with stable heart failure?]. Arq Bras Cardiol. 2010;94(1):92–101. doi: 10.1590/s0066-782x2010000100015. [DOI] [PubMed] [Google Scholar]
- 24.Fray JC, Johnson MD, Barger AC. Renin release and pressor response to renal arterial hypotension: effect of dietary sodium. Am J Physiol. 1977;233(2):H191–195. doi: 10.1152/ajpheart.1977.233.2.H191. [DOI] [PubMed] [Google Scholar]
- 25.Laederach-Hofmann K, Weidmann P, Ferrari P. Hypovolemia contributes to the pathogenesis of orthostatic hypotension in patients with diabetes mellitus. Am J Med. 1999;106(1):50–58. doi: 10.1016/s0002-9343(98)00367-2. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein AM, Willett WC. Trends in 24-h urinary sodium excretion in the United States, 1957-2003: a systematic review. Am J Clin Nutr. 2010;92(5):1172–1180. doi: 10.3945/ajcn.2010.29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sodium Intake in Populations: Assessment of Evidence. The National Academies Press; 2013. [PubMed] [Google Scholar]
- 28.Mares-Perlman JA, Klein BE, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr. 1993;123(3):489–501. doi: 10.1093/jn/123.3.489. [DOI] [PubMed] [Google Scholar]
- 29.Block G, Wakimoto P, Jensen C, Mandel S, Green RR. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis. 2006;3(3):A77. [PMC free article] [PubMed] [Google Scholar]
- 30.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study). Am J Cardiol. 2003;92(5):522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 31.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 32.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 33.Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165(21):2460–2466. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 34.Binder H, Sauerbrei W, Royston P. Comparison between splines and fractional polynomials for multivariable model building with continuous covariates: a simulation study with continuous response. Stat Med. 2013;32(13):2262–2277. doi: 10.1002/sim.5639. [DOI] [PubMed] [Google Scholar]
- 35.Butler J, Rodondi N, Zhu Y, et al. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47(8):1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 36.Butler B, Kalogeropoulos A, Georgiopoulou V, et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1(2):125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincent Grayson K., Velkoff Victoria A. Current Population Reports. U.S. Census Bureau; Washington, DC.: 2010. THE NEXT FOUR DECADES, The Older Population in the United States: 2010 to 2050; pp. P25–1138. [Google Scholar]
- 38.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the United States. Am J Med. 2011;124(9):827–833. e825. doi: 10.1016/j.amjmed.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vigen R, Maddox TM, Allen LA. Aging of the United States population: impact on heart failure. Curr Heart Fail Rep. 2012;9(4):369–374. doi: 10.1007/s11897-012-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.United States Department of Agriculture [10/26/2011];Dietary Guidelines for Americans. 2010 2010; http://www.cnpp.usda.gov/DGAs2010-PolicyDocument.htm.
- 42.Usual sodium intakes compared with current dietary guidelines --- United States, 2005--2008. MMWR Morb Mortal Wkly Rep. 2011:601413–1417. [PubMed] [Google Scholar]
- 43.Fiocco AJ, Shatenstein B, Ferland G, et al. Sodium intake and physical activity impact cognitive maintenance in older adults: the NuAge Study. Neurobiol Aging. 2012;33(4):829, e821–828. doi: 10.1016/j.neurobiolaging.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009:339b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuomilehto J, Jousilahti P, Rastenyte D, et al. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet. 2001;357(9259):848–851. doi: 10.1016/S0140-6736(00)04199-4. [DOI] [PubMed] [Google Scholar]
- 46.Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;(7):CD009217. doi: 10.1002/14651858.CD009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimazu T, Kuriyama S, Hozawa A, et al. Dietary patterns and cardiovascular disease mortality in Japan: a prospective cohort study. Int J Epidemiol. 2007;36(3):600–609. doi: 10.1093/ije/dym005. [DOI] [PubMed] [Google Scholar]
- 48.O'Donnell MJ, Mente A, Smyth A, Yusuf S. Salt intake and cardiovascular disease: why are the data inconsistent? Eur Heart J. 2013;34(14):1034–1040. doi: 10.1093/eurheartj/ehs409. [DOI] [PubMed] [Google Scholar]
- 49.Cook NR, Appel LJ, Whelton PK. Lower Levels of Sodium Intake and Reduced Cardiovascular Risk. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.113.006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013:4CD004937. doi: 10.1002/14651858.CD004937.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007;334(7599):885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet. 2011;378(9789):380–382. doi: 10.1016/S0140-6736(11)61174-4. [DOI] [PubMed] [Google Scholar]
- 53.McKeown NM, Day NE, Welch AA, et al. Use of biological markers to validate self-reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort. Am J Clin Nutr. 2001;74(2):188–196. doi: 10.1093/ajcn/74.2.188. [DOI] [PubMed] [Google Scholar]
- 54.Cobb LK, Anderson CA, Elliott P, et al. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the american heart association. Circulation. 2014;129(10):1173–1186. doi: 10.1161/CIR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 55.Whelton PK, Appel LJ, Sacco RL, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126(24):2880–2889. doi: 10.1161/CIR.0b013e318279acbf. [DOI] [PubMed] [Google Scholar]
- 56.Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older Americans: National Health And Nutrition Examination Surveys, 1988-1994 and 1999-2004. Am J Public Health. 2010;100(1):100–107. doi: 10.2105/AJPH.2008.157388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.