Abstract
Background
Controversy exists over whether resection of the primary tumor in stage IV colorectal cancer with inoperable metastases improves patient outcomes.
Objective
To evaluate whether resection of the primary tumor without metastasectomy in patients with stage IV colorectal cancer is associated with improved overall survival compared to patients undergoing chemotherapy and/or radiation therapy alone.
Design
The 2003–2006 National Cancer Data Base was reviewed to identify patients with stage IV adenocarcinoma of the colon or rectum who underwent palliative treatment without curative intent, either in the form of surgical resection of the primary tumor without metastasectomy consisting of a colectomy or rectal resection with or without chemotherapy and/or radiation, or chemotherapy and/or radiation alone. Groups were compared for baseline characteristics. Overall survival was compared using Kaplan-Meier analysis before and after propensity matching with a 1:1 nearest neighbor algorithm.
Results
Of the 1446 patients included in the analysis, 231 (16%) underwent surgical resection of the primary tumor without metastasectomy. Surgical resection was associated with a significant survival benefit upon unadjusted analysis (median survival: 9.2 months vs 7.6 months, p<0.01). After propensity matching to adjust for non-random treatment selection, surgical resection continued to be associated with a significant survival benefit (median survival 9.2 months vs 7.3 months p<0.01).
Limitations
Potential for selection bias regarding which patients received surgical resection. Lack of data regarding indication for operation, specifically whether a patient was symptomatic or asymptomatic prior to resection. Inability to account for tumor size or grade among patients who did not receive surgical resection.
Conclusions
Surgical resection of the primary tumor without metastasectomy in patients with metastatic colorectal cancer is associated with improved survival as compared to chemotherapy/radiation therapy alone. Further research is necessary to determine which patients may benefit from this intervention.
Keywords: Colon Cancer, Rectal Cancer, Palliative Care, Survival
Introduction
Colorectal cancer (CRC) is the third most common cancer among both men and women, and is the third most common cause of cancer-related deaths in the United States.1 Fortunately, both the incidence of CRC as well as the associated mortality have been decreasing steadily, likely secondary to lifestyle modifications and endoscopic surveillance leading to earlier detection of tumors and pre-cancerous lesions.1–3 Nonetheless, 20% of patients still present with stage IV disease; and although survival has improved amongst such patients, median survival remains at approximately 14 months.4,5 Coupled with resection of the primary tumor and adjuvant therapy, resection of metastases limited to the lung or liver has been associated with significant improvements in survival.6,7 However, other sites of metastatic spread of disease or unresectable tumor burden usually precludes the use of curative surgical resection.
Nevertheless, over the past decade, there have been a number of studies demonstrating that resection of a primary colorectal tumor, despite the presence of unresectable metastatic disease, may be associated with improved overall survival.8–10 For example, Ruo and colleagues reviewed 209 patients with stage IV CRC from a single institution including both symptomatic and asymptomatic patients, and found that patients undergoing resection of their primary tumor had a median survival of 16 months as compared to 9 months for unresected patients.9 In a larger population-based analysis, Tarantino and colleagues investigated 37,793 patients with stage IV CRC in the Surveillance, Epidemiology, and End Results database between 1998 and 2009, and again demonstrated that primary cancer resection was associated with significant improvements in overall and cancer-specific survival.8
Unfortunately, population-based analyses have the potential for substantial bias, as it can be difficult to determine that a patient had “incurable” disease (eg that metastatic disease was not limited to the liver or lung). Beginning in 2003, the National Cancer Data Base (NCDB) began including a variable to define care as that provided “in an effort to palliate or alleviate symptoms,” which could potentially be used to better ensure that patients are not receiving surgical resection with an intent to cure.11 Therefore, taking into account this variable, we performed an analysis in the NCDB which aimed to examine the association between resection of the primary tumor in unresectable metastatic CRC and survival.
Materials and Methods
Data Source
The NCDB is a clinical oncology database collecting information from over 1500 committee on cancer (COC) accredited hospitals.12 Current estimates demonstrate that roughly 70% of newly diagnosed cancers within the United States are captured in this database. Collected data include patient demographics and comorbidities, treatment regimens, post-operative length of stay and readmission, and long-term overall survival. Approval from the Duke University Institutional Review Board (IRB) was obtained prior to data analysis.
Patient Population
The NCDB was queried from 2003–2006 for patients 18 years of age or older who presented with Stage IV adenocarcinoma of the colon and rectum, using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) topography and histology codes. This time period was selected as these were the only years during which both the palliative care variable and long-term survival were available at the time of analysis. Patients with known resection of metastases at other sites (such as liver or lung) were excluded (using the “Surgery at Other Site” variable). In order to ensure that included patients were not receiving therapy with the intention to cure, only patients listed as having underwent either surgery, chemotherapy, or radiation as palliative treatment were included in the analysis. In order to ensure that only patients who had a colonic or rectal resection (and not a surgical biopsy or bypass procedure) were included in the surgery group, the surgical group was limited to patients with surgery of primary site codes 30–80 (which included all procedures consistent with or more extensive than at least a partial colectomy or rectal resection). Any patient who underwent a surgical procedure which could not be classified was also excluded.
Statistical Analysis
Patients were grouped based on palliative resection with surgery (with/without chemotherapy and/or radiation) versus palliation with chemotherapy and/or radiation alone. Groups were compared with regards to baseline characteristics, treatment characteristics, and outcomes. Multivariable logistic regression was then performed in order to identify patient factors associated with undergoing surgical resection of the primary tumor. Variables included in this model were chosen a priori and included age, sex, race, private insurance status (as a surrogate for socioeconomic status), Charlson/Deyo comorbidity index (0 vs 1 vs 2+), academic facility status, and tumor location (rectal vs colon). Continuous variables were plotted against the logit of undergoing surgery in order to determine linearity. If non-linear, they were categorized into groups or transformed as appropriate.
In order to determine the association between palliative surgical resection and overall survival, Kaplan-Meier analysis and the log-rank test were performed. Follow-up time was begun at the time of diagnosis (and not surgery) in order to allow comparison between the surgical and non-surgical groups. In order to account for non-random treatment selection, a 1:1 nearest-neighbor propensity matched analysis was performed. Patients were matched based on a priori determined variables including age, sex, race, private insurance status, Charlson/Deyo comorbidity index, academic center status, and tumor location (rectal vs colon). Tumor characteristics such as size and pathologic grade could not be included as these results are based on pathologic specimens in the NCDB, and therefore were missing among the majority of non-surgical patients. The success of the match was evaluated via two methods. The first involved comparing the variation in propensity score between groups both before and after matching. The second involved comparing the standardized difference between baseline characteristics both before and after the match. Following matching, overall survival was again compared using Kaplan-Meier methods and the log-rank test. In order to demonstrate how survival differed by treatment based on tumor location, Kaplan-Meier methods were also used to evaluate the association of palliative surgical resection and overall survival among only patients with colon tumors (excluding rectal tumors).
When unadjusted statistical comparisons were made between groups, continuous variables were evaluated with the Wilcoxon rank sum test while categorical variables were evaluated with the Chi square test or the Fisher exact test as appropriate. Complete case analysis was used for all adjusted models. A p-value of <0.05 was used to define statistical significance. All statistical analyses were performed using R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
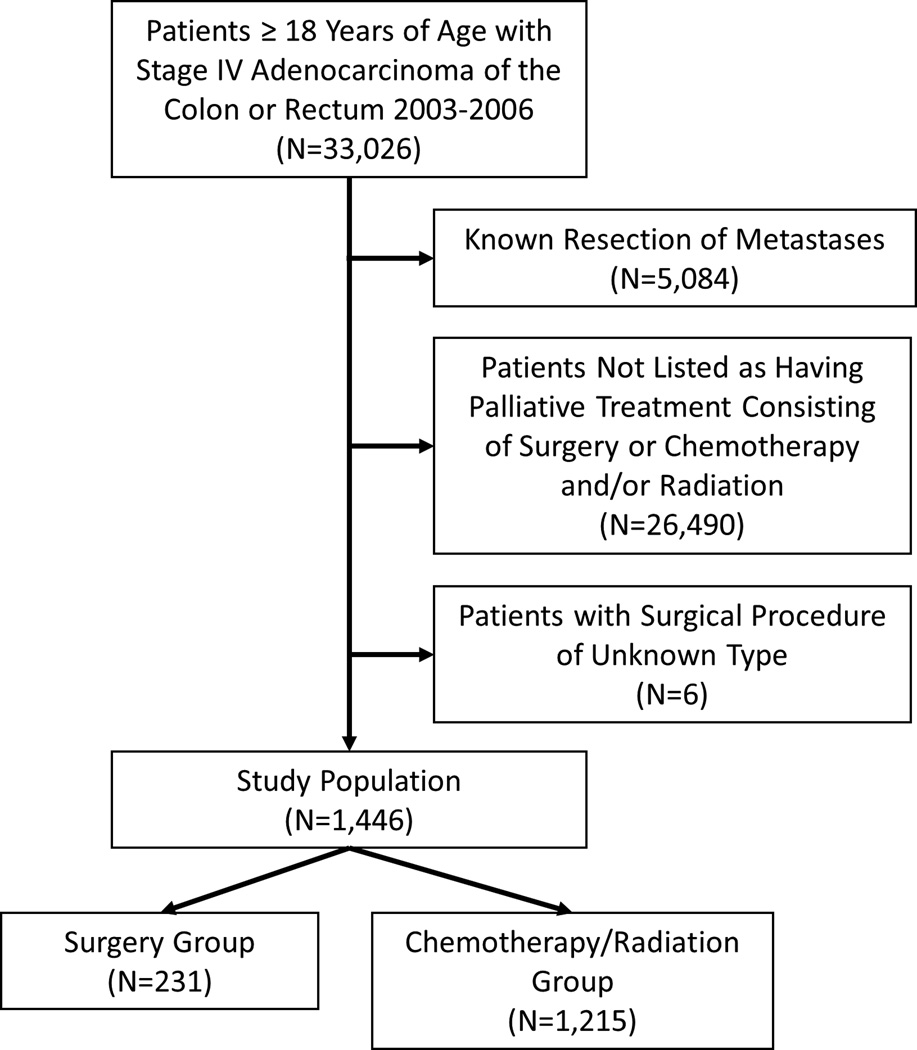
A total of 1,446 patients met study inclusion criteria, of which 231 (16%) underwent a colectomy or a rectal resection of their primary tumor without undergoing a metastectomy (Figure 1). Of the included patients, 586 (40.5%) had rectal tumors while 860 (59.5%) had colon tumors. The median time from disease diagnosis to surgery was 12 days (interquartile range [IQR]: 3, 29) among patients receiving a colorectal resection. Patients who underwent a colorectal resection were more likely to be female (50.6% vs 43.5%), and were more likely to be of higher socioeconomic status (38.1% of surgical patients had private insurance vs 30.4% of patients in the non-surgery group) than patients who were treated with palliative chemotherapy and/or radiation therapy alone. Patients who underwent surgical resection were also significantly less likely to have rectal tumors (8.2% vs 46.7%, p<0.001). All other baseline characteristics were similar between groups (Table 1).
Figure 1.
Consort diagram.
Table 1.
Baseline characteristics by group both before and after propensity matching.
| Before Propensity Match | After Propensity Match | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Chemotherapy/ Radiation |
Surgery | Standardized Difference (%) |
p-value | Chemotherapy/ Radiation |
Surgery | Standardized Difference (%) |
p-value |
| N | 1215 (84%) | 231 (16%) | 231 (50%) | 231 (50%) | ||||
| Age | 67 (57, 77) | 68 (57, 80) | −7.4 | 0.27 | 69 (58, 77.5) | 68 (57, 80) | 1.5 | 0.74 |
| Female | 529 (44%) | 117 (51%) | −14.3 | 0.06 | 113 (48.9%) | 117 (50.6%) | −3.5 | 0.78 |
| White Race | 987 (81%) | 192 (83%) | −4.9 | 0.56 | 190 (82.3%) | 192 (83.1%) | −2.3 | 0.90 |
| Private Insurance | 369 (30%) | 88 (38%) | −16.3 | 0.03 | 94 (40.7%) | 88 (38.1%) | 5.3 | 0.63 |
| Charlson/Deyo Comorbidity Index | 0.45 | 0.53 | ||||||
| 0 | 944 (78%) | 171 (74%) | 8.6 | 181 (78.4%) | 171 (74.0%) | 10.2 | ||
| 1 | 212 (17%) | 48 (21%) | −8.5 | 39 (16.9%) | 48 (20.8%) | −10.0 | ||
| 2 | 59 (4.9%) | 12 (5.2%) | −1.6 | 11 (4.8%) | 12 (5.2%) | −2.0 | ||
| Academic Center Status | 380 (31%) | 75 (33%) | −2.6 | 0.78 | 79 (34.2%) | 75 (32.5%) | 3.7 | 0.77 |
| Tumor Location (Rectal vs Colon) | 567 (46.7%) | 19 (8.2%) | 95.5 | <0.01 | 19 (8.2%) | 19 (8.2%) | 0.0 | 0.99 |
Continuous variables are presented as median (interquartile range) while categorical variables are presented as frequency (percentage).
Only 6.1% (n=14) of surgical patients received any radiation therapy as compared to 47.9% (n=582) of non-surgical patients (p<0.01). In contrast, 42.6% (n=98) of surgical patients received any form of chemotherapy as compared to 66.0% (n=801) of non-surgical patients (p<0.01). Median length of stay following surgical resection was 7 days (IQR: 5, 11), and 16.6% (n=37) of surgical patients were readmitted within 30 days of discharge. 30-day mortality was 15.2% (n=35) in the surgical group.
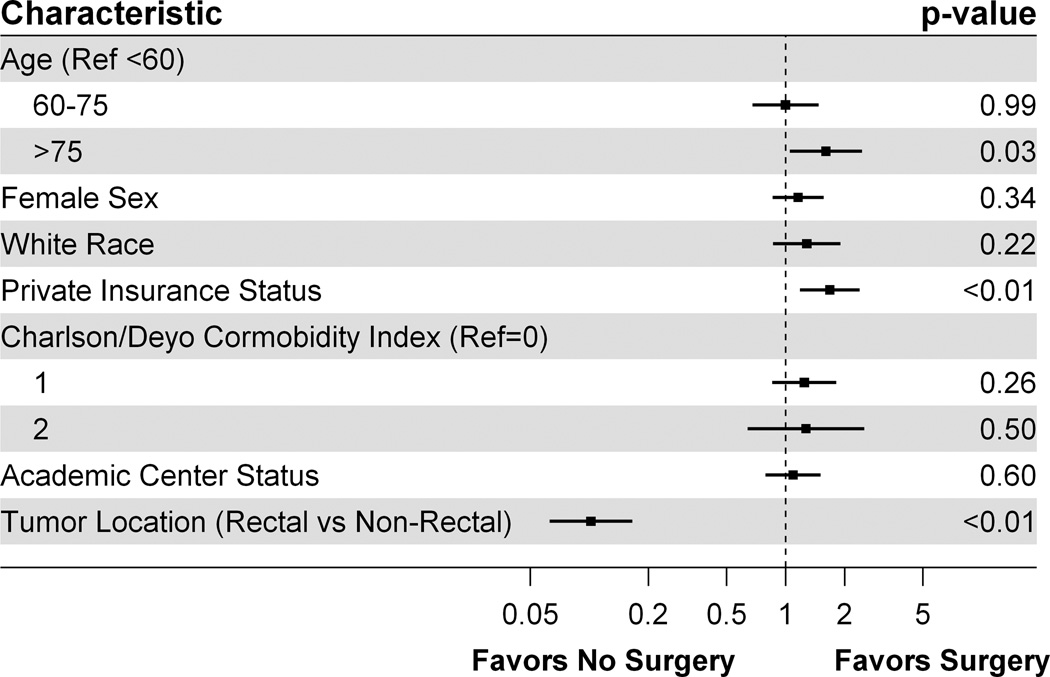
Using multivariable logistic regression to determine patient characteristics associated with surgical resection, we found that age greater than 75 years (adjusted odds ratio [AOR]: 1.60, 95% confidence interval [CI]: 1.05, 2.45) and private insurance status (AOR: 1.68, 95% CI: 1.18, 2.38) were both associated with a significantly increased odds of receiving a palliative surgical resection of the primary tumor (Figure 2). Conversely, having a rectal tumor as compared with a colon tumor was associated with a significantly reduced odds of receiving a palliative surgical resection of the primary tumor (AOR: 0.10, 95% CI: 0.06, 0.17).
Figure 2.
Factors associated with undergoing a surgical resection of the primary tumor in Stage IV colorectal cancer.
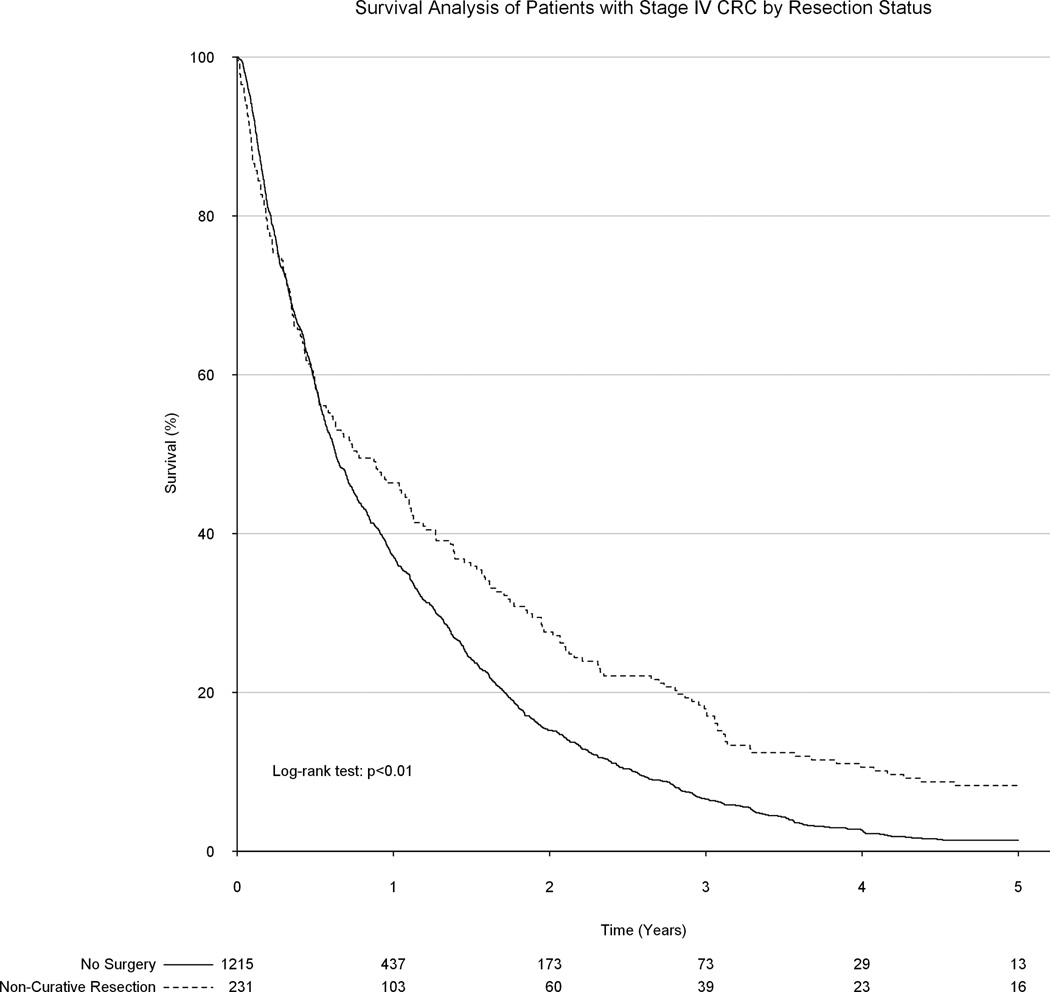
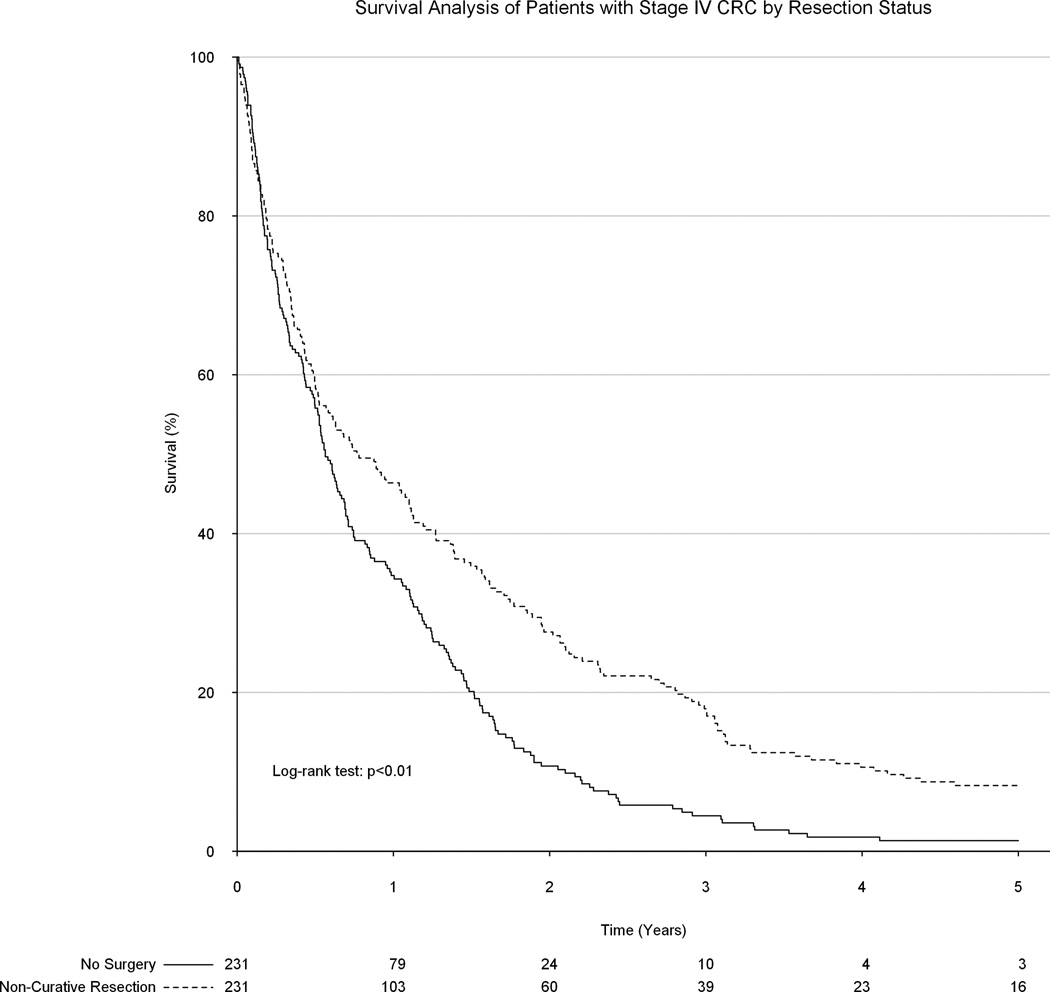
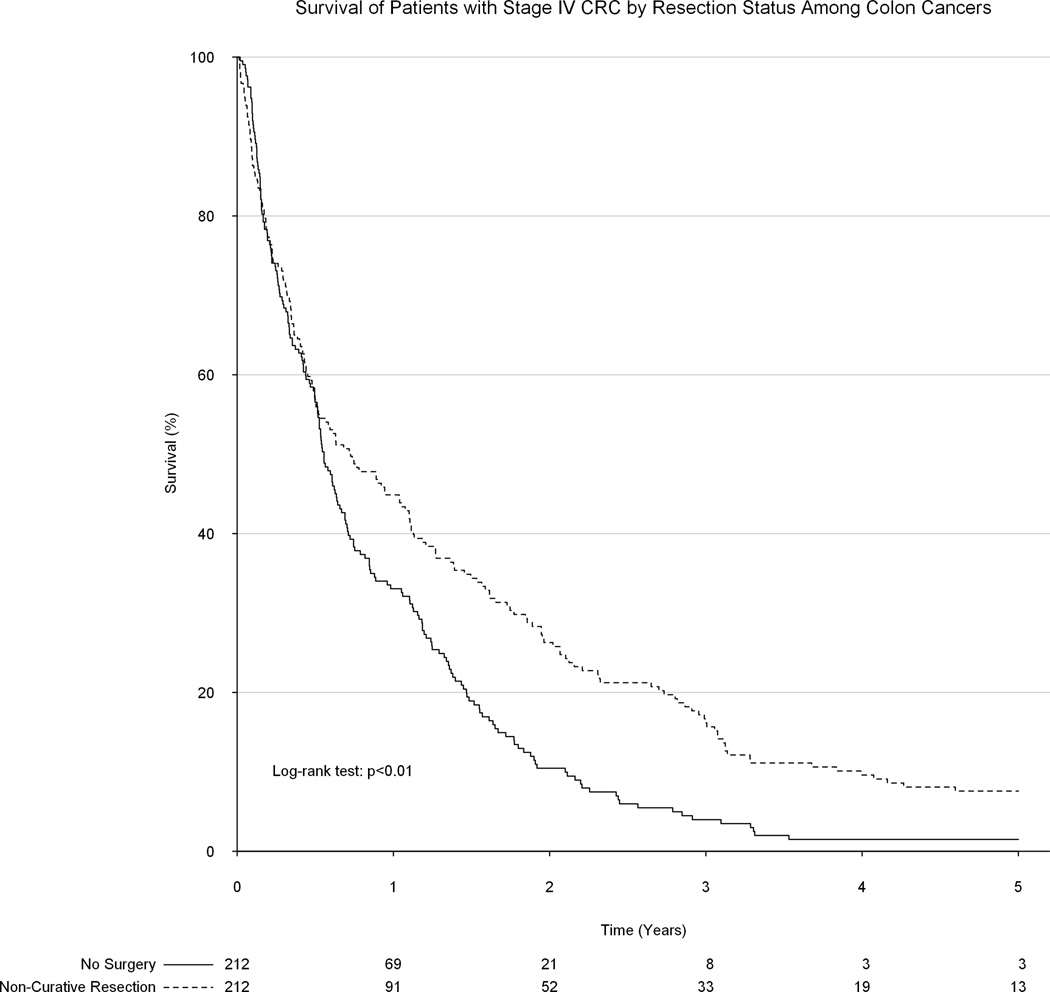
Upon unadjusted analysis, a non-curative formal colorectal resection was associated with significantly improved long-term survival as compared to receiving only palliative chemotherapy and/or radiation therapy (median survival 9.2 vs 7.6 months, p<0.01, Figure 3). Following propensity matching, all 231 patients in the surgical group were matched to 231 patients in the group which did not undergo a surgical resection. No substantial baseline differences remained between groups. However, surgical resection of the primary tumor remained significantly associated with improved survival (median survival: 9.2 vs 7.3 months, p<0.01, Figure 4). Among only patients with colon tumors, palliative surgical resection of the primary tumor was again associated with a significant improvement in overall survival (8.7 vs 6.7 months, p<0.01, Figure 5).
Figure 3.
Unadjusted association between surgical resection status and overall survival.
Figure 4.
Association between surgical resection status and overall survival following adjustment after propensity score matching.
Figure 5.
Association between surgical resection status and overall survival among only patients with colon tumors.
Discussion
Recent advancements in chemotherapeutic agents and more aggressive surgical resection of isolated metastases have helped extend survival for patients with Stage IV CRC. Nevertheless, overall prognosis remains poor with a five-year survival of only 8–20%.5,13,14 In this study, using strict study criteria in an attempt to reduce bias from possibly including patients who underwent a surgical resection with an intent to cure, we have found that overall survival is improved among patients with stage IV CRC who underwent a colorectal resection to resect their primary tumor. Furthermore, this association remained even after adjustment, as well as in a subanalysis of only patients with colon cancers.
Interestingly, our benefit of only 1–2 months is much smaller than that seen in other studies. For instance, Gresham and colleagues demonstrated an improvement in median survival from 7.9 months to 17.9 months, while Ruo and colleagues demonstrated an improvement in median survival from 9 months to 16 months.9,10 Tarantino and colleagues demonstrated an improvement in survival from between 3 to 7 months to 12 to 20 months depending on the year analyzed.8 Our reduced survival benefit is likely secondary to a patient cohort with more advanced disease, as we only selected patients listed as being treated with “palliative care.” On the other hand, some studies have demonstrated that surgical resection of the primary tumor in this patient population is not associated with a significant survival benefit, so it may be that the variation in study results is due to a wide distribution of treatment effects across different patient populations.15,16 Also, we demonstrated a very high 30-day mortality associated with surgery in this study, which may indicate that the majority of surgical procedures performed in our analysis was for symptomatic patients, which tend to have much worse post-operative survival than patients who have a primary resection for asymptomatic disease.17 Both of these points demonstrate the importance of careful patient selection when deciding to proceed with surgical resection of the primary tumor in these patients.
Even though we have demonstrated a survival benefit associated with the resection of the primary tumor in Stage IV CRC, we cannot determine the etiology of this association, especially as the NCDB does not contain data regarding cause of death. There are a number of theories that may explain this phenomenon. For instance, it is possible that surgical resection of the primary tumor prevents long-term, potentially fatal complications including obstruction and bleeding. Although rates vary, studies have demonstrated that patients with Stage IV CRC will eventually require emergent surgical intervention 7–20% of the time, and when these emergent interventions are required, they are associated with a substantially higher mortality than an elective resection.9,17,18 Our findings support this theory, as the survival benefit secondary to resection appears late, after roughly six months, which may be the time when complications occur requiring emergent surgery. Conversely, it may be that this is when the benefit of surgical resection finally outweighs the increased early mortality related to the operation itself.
Unfortunately, the NCDB also does not contain data related to the quality of life following surgical resection of the primary tumor. Theoretically, a patient is trading early time healing from their resection for additional survival at a later date, and therefore the initial decrease in quality of life is off-set by a late benefit in quality of life. This trade-off must be weighed on a patient-by-patient basis, and patients’ preferences should be taken into account when determining whether this procedure should be undertaken. Future research should focus on the effects of surgical resection of these tumors on quality of life.
The demonstration that patients receiving surgical resection were more likely to have private insurance demonstrates differences in treatment by socioeconomic status previously seen in studies of oncology patients.19 Furthermore, socioeconomic status has been found to be significantly associated with survival among oncology patients.20,21 Although a more extensive investigation into the etiology of this discrepancy is not possible due to the nature of the NCDB, this finding demonstrates that even in the absence of a known treatment effect, socioeconomic status is an important determinant of how a patient is treated.
There are a number of limitations to this study which should be mentioned. First, many of the previous studies on this topic have investigated either asymptomatic or symptomatic patients, as these are two very different populations of patients. Symptomatic patients are likely to have substantially increased peri-operative morbidity and mortality than asymptomatic patients. By not being able to differentiate these two groups, it is possible that our findings are less applicable to the overall population. Second, the NCDB has poor recording of tumor-specific characteristics such as tumor size and grade among patients who did not undergo a surgical resection, and therefore we could not adjust for these in our analysis. Symptomatic patients are more likely to have larger and more invasive tumors, and therefore the resection group may have a disproportionate number of these tumors. Conversely, patients who did not undergo surgical resection may have had more extensive metastatic disease, limiting their ability to undergo a surgical resection. Lastly, as with any retrospective analysis, there is the potential for unobserved confounding and bias which we could not adjust for in our analysis. For example, it is possible that surgeons were more likely to operate on patients with less disease burden, or who had a better functional status at the time of diagnosis, thus biasing our results. Nonetheless, this is a potential concern of any retrospective study, and we feel that these results still provide important data for clinical decision making while awaiting the results from prospective clinical trials.
Although treatment options for CRC have improved, survival for patients with distant unresectable metastatic disease is still poor.13,14 In this study, we have demonstrated that surgical resection of the primary tumor for palliative reasons in stage IV CRC is associated with significant improvements in survival. Future research is necessary to determine which patients may benefit from this intervention.
Acknowledgements
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in this study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Funding Sources
Institutional funding was used in order to support this project.
Footnotes
Meeting Information: This work was presented as an oral presentation (Presentation Number S6) at the annual scientific meeting of the American Society of Colon and Rectum Surgeons, Boston, MA, May 30-June 3, 2015.
Author Contributions
BC Gulack: Dr. Gulack contributed to the conception and design of the study, statistical analysis, manuscript writing and editing, and the critical revision of the manuscript.
DP Nussbaum: Dr Nussbaum contributed to the conception and design of the study, as well as the critical revision of the manuscript.
JE Keenan: Dr Keenan contributed to the conception and design of the study, as well as the critical revision of the manuscript.
AM Ganapathi: Dr Ganapathi contributed to the conception and design of the study, as well as the critical revision of the manuscript.
Z Sun: Dr Sun contributed to the conception and design of the study, as well as the critical revision of the manuscript.
M Worni: Dr Worni contributed to the data analysis, the conception and design of the study, and the critical revision of the manuscript.
J Migaly: Dr Migaly contributed to the conception and design of the study, as well as the critical revision of the manuscript.
CR Mantyh: Dr. Mantyh contributed to the conception and design of the study, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
Author Conflicts of Interest
BC Gulack: Dr. Gulack has no relevant disclosures to report.
DP Nussbaum: Dr Nussbaum has no relevant disclosures to report.
JE Keenan: Dr Keenan has no relevant disclosures to report.
AM Ganapathi: Dr Ganapathi has no relevant disclosures to report.
Z Sun: Dr Sun has no relevant disclosures to report.
M Worni: Dr Worni has no relevant disclosures to report.
J Migaly: Dr Migaly has no relevant disclosures to report.
CR Mantyh: Dr Mantyh has no relevant disclosures to report.
Conflicts of Interest: The authors have no conflicts of interest to report.
References
- 1.Cancer Facts & Figures 2015. [Accessed March 15, 2015]; www.cancer.org. [Google Scholar]
- 2.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 5.Castleberry AW, Guller U, Tarantino I, et al. Discrete improvement in racial disparity in survival among patients with stage IV colorectal cancer: a 21-year population-based analysis. J Gastrointest Surg. 2014 Jun;18:1194–1204. doi: 10.1007/s11605-014-2515-3. [DOI] [PubMed] [Google Scholar]
- 6.Saito Y, Omiya H, Kohno K, et al. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: a prognostic assessment. J Thorac Cardiovasc Surg. 2002;124:1007–1013. doi: 10.1067/mtc.2002.125165. [DOI] [PubMed] [Google Scholar]
- 7.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 8.Tarantino I, Warschkow R, Worni M, et al. Prognostic Relevance of Palliative Primary Tumor Removal in 37,793 Metastatic Colorectal Cancer Patients: A Population-Based, Propensity Score-Adjusted Trend Analysis. Ann Surg. 2014 Nov 4; doi: 10.1097/SLA.0000000000000860. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9.Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg. 2003 May;196:722–728. doi: 10.1016/S1072-7515(03)00136-4. [DOI] [PubMed] [Google Scholar]
- 10.Gresham G, Renouf DJ, Chan M, et al. Association between palliative resection of the primary tumor and overall survival in a population-based cohort of metastatic colorectal cancer patients. Ann Surg Oncol. 2014 Nov;21:3917–3923. doi: 10.1245/s10434-014-3797-0. [DOI] [PubMed] [Google Scholar]
- 11.The CoC/NCDB PUF Data Dictionary Items. [Accessed: April 7, 2015]; http://ncdbpuf.facs.org/?q=print-pdf-all. [Google Scholar]
- 12.American College of Surgeons. National Cancer Data Base. [Accessed: April 1, 2015]; http://www.facs.org/cancer/ncdb. [Google Scholar]
- 13.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 14.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scoggins CR, Meszoely IM, Blanke CD, Beauchamp RD, Leach SD. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol. 1999 Oct-Nov;6:651–657. doi: 10.1007/s10434-999-0651-x. [DOI] [PubMed] [Google Scholar]
- 16.Yun JA, Huh JW, Park YA, et al. The role of palliative resection for asymptomatic primary tumor in patients with unresectable stage IV colorectal cancer. Dis Colon Rectum. 2014 Sep;57:1049–1058. doi: 10.1097/DCR.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 17.Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379–3384. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCahill LE, Yothers G, Sharif S, et al. Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of NSABP trial C-10. J Clin Oncol. 2012 Sep 10;30:3223–3228. doi: 10.1200/JCO.2012.42.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byers TE, Wolf HJ, Bauer KR, et al. The impact of socioeconomic status on survival after cancer in the United States. Cancer. 2008;113:582–591. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 20.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 21.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]