Abstract
Background
Nonverbal communication deficits are characteristic of autism spectrum disorder (ASD) and have been reported in some later-born siblings of children with ASD (heightened-risk (HR) children). However, little work has investigated gesture as a function of language ability, which varies greatly in this population.
Aims
This longitudinal study characterizes gesture in HR children and examines differences related to diagnostic outcome (ASD, language delay, no diagnosis) and age.
Methods & Procedures
We coded communicative gesture use for 29 HR children at ages 2 and 3 years during interactions with a caregiver at home.
Outcomes & Results
Children in the ASD group produced fewer gestures than their HR peers at 2 years, though large individual differences were observed within each subgroup at both ages. In addition, reliance on particular types of gestures varied with age and outcome. Both ASD and language delay children exhibited a pattern of reduced pointing relative to their no diagnosis peers.
Conclusions & Implications
Similarities and differences exist between communication in HR infants with language delay and their HR peers, reinforcing our understanding of links between verbal and nonverbal communication in populations at risk for language delay.
Keywords: gesture, autism spectrum disorders (ASD), language
Introduction
Deficits and delays in gestural communication are a central component in the assessment and diagnosis of autism spectrum disorder (ASD) (American Psychiatric Association 2013, Lord et al. 2000). Delays in gesture have also been observed in the later-born siblings of children with ASD, a population that is at heightened biological risk (HR) for a subsequent ASD diagnosis (recurrence risk estimate of 18.7%; Ozonoff et al. 2011) and for communication and language delays (e.g., Iverson and Wozniak 2007). While nonverbal communication delays have been reported for some HR siblings both with and without ASD, there is also large variability within this population (Rogers 2009). In the present study, we characterize gesture use and development in HR toddlers, with a focus on exploring differences among subgroups of children with varying language and diagnostic outcomes. As will be discussed below, reported links between gesture and language in atypical populations (Capone and McGregor 2004) lead to the prediction that we may observe differences among HR infants based on presence of language delay (LD) in addition to differences based on ASD diagnosis.
Early gesture development in typically developing (TD) children
TD children begin to gesture around 9 to 13 months, with early gestures including deictic gestures that refer to concrete entities such as objects, people and locations (e.g., pointing to a dog to indicate, ‘dog’). These gestures include GIVING,1 RITUALIZED REQUESTS and SHOWING, with POINTING emerging later in this time period (Bates et al. 1979). In the second year, children increase their use of some of these types of gestures (e.g., POINTING), while stabilizing or decreasing their production of others (e.g., RITUALIZED REQUESTS) (Iverson et al. 1994, Ozcaliskan and Goldin-Meadow 2005).
Developmental changes among TD children in the second and third years also tend to be in the types of gestures that are produced. For instance, children begin to produce representational gestures including ICONICS (e.g., flapping arms to convey ‘bird’) and CONVENTIONAL gestures (e.g., nodding head to convey, ‘yes’). In these cases, the form of the gesture conveys the meaning. Developmental changes also occur in the ways in which they coordinate gestures with other behaviours (McNeill 1992, Ozcaliskan and Goldin-Meadow 2005). By the time they begin school, children’s gestural repertoires contain a variety of gestures (e.g., including adult-like ICONIC gestures and pragmatic discourse marking gestures) and have adult-like semantic and temporal integration with the speech they produce (McNeill 1992).
Gesture and ASD
ASD is a neurodevelopmental disorder characterized by impairments in social communication and interaction, as well as the presence of restricted interests and/or repetitive behaviours (American Psychiatric Association 2013). Gesture atypicalities and deficits are a well-established manifestation of the characteristic impairments in social interaction and communication (e.g., Lord et al. 2009). They tend to appear in a variety of ways, including reductions in gesture frequency, diversity of form and function, and integration with verbal and nonverbal context (see the review by Landa 2007).
Reliable ASD diagnosis does not typically occur until the third year (Woolfenden et al. 2012). However, prospective studies using parent report have reported gesture delays as early as the first year (Zwaigenbaum et al. 2005). Currently, there is a lack of prospective research on spontaneous gesturing in naturalistic situations in toddlerhood. However, research using semi-structured methods indicates that lower gesture frequency is evident in toddlers with ASD relative to comparison children with developmental delays (Stone et al. 1997). Delays in POINTING, in particular, are among some of the earliest indications of emerging ASD and remain prominent in characterizations of gesture use in ASD (Filipek et al. 1999, Wetherby et al. 2004).
Gesture in the later-born siblings of children with autism
In studies of the later-born siblings of children with ASD (heightened risk—HR), nonverbal communication delays have been observed as early as 13 months and are not limited to HR infants who receive a subsequent ASD diagnosis (Landa 2007, Mitchell et al. 2006, Winder et al. 2013). However, extensive variability is also characteristic of HR infant development, with some HR infants exhibiting early delays and others indistinguishable from peers with no family history of ASD (low-risk—LR) (Rogers 2009). Most research that has examined this variability within HR samples has focused on comparisons between HR infants with a subsequent ASD diagnosis (HR-ASD) and those with no such diagnosis since these children can serve as a valuable comparison group for ASD (Zwaigenbaum et al. 2007). However, research with TD children and children at risk for developmental delays suggests that variability in HR infants may also be attributed to differences in language outcome (Capone and McGregor 2004).
HR infants without ASD
Studies of gesture development in HR children with no ASD diagnosis have largely focused on the second year of life and have found that these children are delayed relative to TD children on both semi-structured experimenter administered assessments of communication (e.g., Early Social Communication Scales—ESCS) and parent report measures (e.g., MacArthur–Bates Communicative Development Inventory—CDI; Mitchell et al. 2006). When directly comparing HR-ASD children with HR children with no ASD diagnosis, results are mixed. Some studies have found lower gesture scores for HR-ASD children in the second year and as early as 12 months on both semi-structured and parent report measures (Rozga et al. 2011, Zwaigenbaum et al. 2005). However, Goldberg et al. (2005) failed to find any group differences on a semi-structured measure, while Mitchell et al. (2006) found differences on a parent report measure early in the second year but not later in the second year. Further, other studies using semi-structured measures of nonverbal communication have reported overlapping distributions of performance on nonverbal communication measures between HR-ASD children and HR children with no ASD diagnosis (Goldberg et al. 2005).
One potential complicating factor in this research is that HR infants without ASD are not a homogeneous group (Rogers 2009) and there may be differences in gesture development within this population. Of particular interest for us is whether there may be differences in gesture between those who have language delay (LD) and those who do not (no diagnosis—ND). This possibility is suggested by several findings. First, a study by Landa and Garrett-Mayer (2006) that compared LD and ND groups found evidence of differences in development in a variety of domains, though they did not look at gesture. Second, HR infants (with and without ASD) are at increased risk for language delay (Rogers 2009), and previous research with non-ASD children with language delays has found links between gesture and language development (Thal and Tobias 1992). In combination, this suggests that gesture in HR infants may also vary in relation to language levels.
Current study
In this study, we examine gesture production within a group of HR toddlers who vary in diagnostic outcome at age 3 (ASD, language delay, no diagnosis), which allows us to add to the current literature in several ways. We explore previously unexamined differences in toddlers’ gesture as a function of language outcome and whether similarities exist between LD and ASD groups or whether unique gesture profiles are associated with ASD. This enhances understanding of the nature of delays within the HR sample and the robustness of the gesture–language relations described above. Further, in contrast to previous research that has assessed communication and gesture use using semi-structured, experimenter-administered tasks carried out in the laboratory or parent report measures, we observed children’s gesture in naturalistic, semi-structured play with toys and a primary caregiver at home. Examining communication in different contexts is important in profiling children’s communication skills and development (see e.g., Tager-Flusberg et al. 2009 regarding verbal communication), particularly in light of recent findings of systematic differences between experimenter-assessed and naturalistically observed nonverbal communication in young children (Parlade 2012).
Second, we examine the development of different types of gestures such as POINTING, SHOWING, CONVENTIONAL, ICONIC and FUNCTIONAL ACTS (i.e., GIVE an object to an interlocutor, RITUALIZED REACHES). In typical development, different gesture types exhibit different developmental trajectories and varying relations with other aspects of cognitive development (e.g., Bates et al. 1979). However, studies of ASD and HR infants differ widely in the types of gestures included for study, with some focusing exclusively on a subset of gestures (e.g., POINTING) and others not discriminating between gesture types. Stone et al. (1997) directly examined the use of different gesture types in toddlers with ASD using a semi-structured paradigm. Compared to children with developmental and/or language delays, children with ASD produced fewer POINT and SHOW gestures, but no significant differences were found for GIVE and RITUALIZED REACH gestures. This result suggests that investigating production of gesture types may be useful in examining differences within the HR population and may add to the literature by resolving some of the conflicting findings in the HR literature. In particular, it can address the importance of pointing gestures in the communication profiles in the context of ASD, LD without ASD, and ND.
In sum, the present study was designed to address the limitations of prior research by examining the frequencies and types of gestures produced by HR toddlers during semi-structured play with a familiar caregiver in the home. We will (1) characterize the use of different types of gesture in HR toddlers as a function of diagnostic outcome group (ASD, LD and ND); and (2) examine their development from 2 to 3 years of age.
Methods
Participants
Two- and 3-year olds with HR (n = 29; 15 male) participated in this research as part of an ongoing, longitudinal study of HR infants from 5 to 36 months (details below). The diagnostic status of each HR child’s older sibling was independently confirmed prior to study enrolment using the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2009) and clinical judgment using DSM-IV criteria. The ADOS is an experimenter-administered, structured observation assessment of ASD widely used in research and clinical practice. All participants came from full-term, uncomplicated pregnancies and monolingual English-speaking households. Race and ethnicity for the sample was largely Caucasian (97%) and non-Hispanic (90%) and most households had a maternal education level of college degree or beyond (27.6% high school or some college or trade school, 34.5% bachelor’s degree, 37.9% post-bachelor’s education). Participants were recruited through a University Autism Research Center, parent groups, and local agencies and schools serving families of children with ASD.
Children were selected for this study from the larger longitudinal sample if they participated in a semi-structured caregiver–child play segment (details below) at both the 24- and 36-month sessions. In addition, we required that children in this study had completed the longitudinal study so that we would have outcome data on both ASD status and language abilities at 36 months. Because the larger study is ongoing, some participating children have not yet reached 36 months of age and are, thus, not included in this study.
Materials
The Mullen Scales of Early Learning was administered at 18, 24, and 36 months as part of the larger study from which participants were drawn (MSEL; Mullen 1995). The MSEL is an experimenter-administered standardized observation assessment comprising five subscales: Gross Motor and four cognitive subscales (Visual Reception, Receptive Language, Expressive Language and Fine Motor). A raw score and a standardized T-score are available for each subscale (T-score mean = 50, SD = 10). In addition, all four cognitive scales can be combined into an Early Learning Composite (ELC) for which standard scores are available (mean = 100, SD = 15). Scores for the Receptive Language Subscale, Expressive Language Subscale and Early Learning Composite 24 and 36 months are provided in table 1.
Table 1a.
Participant characteristics for each subgroup at 2 years
| Measure | Median | AD | Range | Mean | SD |
|---|---|---|---|---|---|
| No diagnosis (ND) | |||||
| CDI-II Percentile | 45 | 15.63 | 0–65 | 43.13 | 21.37 |
| 2 year MSEL ELC Standard Score | 100.5 | 8 | 92–117 | 103 | 9.52 |
| 2 year MSEL Receptive Language T-score | 54 | 6.75 | 40–66 | 54.25 | 7.72 |
| 2 year MSEL Expressive Language T-score | 49.5 | 5.25 | 38–63 | 50.25 | 7.27 |
| Language delay (LD) | |||||
| CDI-II Percentile | 20 | 20.83 | 0–85 | 30.77 | 25.32 |
| 2 year MSEL ELC Standard Score | 103 | 13.36 | 68–138 | 101.31 | 18.14 |
| 2 year MSEL Receptive Language T-score | 56 | 12.65 | 20–77 | 50.85 | 16.90 |
| 2 year MSEL Expressive Language T-score | 48 | 5.86 | 28–57 | 47.77 | 8.19 |
| Autism spectrum disorder (ASD) | |||||
| CDI-II Percentile | 5 | 7.78 | 0–25 | 8.33 | 9.83 |
| 2 year MSEL ELC Standard Score | 64 | 8.25 | 51–74 | 63.25 | 10.28 |
| 2 year MSEL Receptive Language T-score | 20 | 4.32 | 20–34 | 23.60 | 6.07 |
| 2 year MSEL Expressive Language T-score | 28 | 7.06 | 20–45 | 28.14 | 9.53 |
The MacArthur–Bates Communicative Development Inventory (CDI) was also administered at 18, 24 and 36 months as part of the larger study from which participants were drawn. The CDI is a parent-report measure of child communication and language development for which percentile scores are available. It is widely used to examine individual differences in language ability and has been validated with observational data (Fenson et al. 2007). Different versions of the CDI are to be used based on the child’s age. The CDI-II was administered at 18 and 24 months and the CDI-III was administered at 36 months. In addition, the CDI-I (infant form) was administered to one child with ASD at 18 months due to limited language ability. The CDI-II Words Produced section contains all words that appear on the CDI-I in addition to more advanced words. The one child with ASD produced very few words (11) on the CDI-I Vocabulary checklist. Thus, we included information for this child since ceiling effects are unlikely. Percentile scores on the Words Produced portion of the CDI-II at 24 months and the CDI-III at 36 months are provided in table 1. Standardized scores on the CDI were significantly related to standardized scores on the MSEL Expressive Language subscale (24 months: rs = .788, p < .001; 36 months: rs = .401, p = .042).
Diagnostic outcome
As part of the longitudinal study, children were classified into one of three mutually exclusive outcome categories following the 36-month visit: ASD, LD or ND. Classification procedures and criteria were as follows. HR children came to the University for an ASD outcome classification session at 36 months. Classification was made by a clinician who was trained in the administration of the ADOS and blind to all previous study data. The clinician administered the ADOS and evaluated whether the child met DSM-IV criteria for an ASD. Children scoring above ASD threshold on the ADOS and with clinical judgment of ASD were classified as ASD.
Children who did not receive an ASD diagnosis were then classified as LD or ND based on standardized language assessments administered over the course of the study. Categorization of HR children into these groups was done for research purposes only and does not imply presence/absence of a clinical diagnosis of language delay. HR children without ASD were classified as LD if they met one of two criteria: (1) standardized score at or below the 10th percentile on the CDI at more than one time point between 18 and 36 months (e.g., Weismer and Evans 2002, Heilmann et al. 2005); and/or (2) standardized score on the 36-month CDI at or below the 10th percentile and 36-month MSEL expressive or receptive language subscale scores > 1.5 SD below the mean (e.g., Landa and Garrett-Mayer 2006, Ozonoff et al. 2010). Children who did not meet either criterion were classified as ND. Based on these outcome classification procedures, our sample of 29 toddlers included: seven ASD (four male), 13 LD (six male) and nine ND (five male) children.2
Procedure
As part of the larger longitudinal study, infants and primary caregivers were videotaped at home for approximately 45 min each month between ages 5 and 14 months. In-home follow-up visits were conducted at 18, 24 and 36 months. Each session consisted of naturalistic observation, semi-structured play and standardized assessments. All study procedures received approval from the institutional review board. Informed consent was obtained for each infant prior to the first observation. In the present study, we focus on data collected in the families’ homes at the 24- and 36-month sessions. We observed caregiver–child dyadic play during a 13-min semi-structured play segment.3 During this time, parents were asked to play with experimenter-provided toys (e.g., teddy bear, spoon/bowl, barn set) in an attempt to keep play contexts as similar as possible across participants. However, parents were given no instructions for interaction other than to play with their child as they normally would.
Coding
We coded all communicative gestures produced by children during the caregiver–child play segment. Any communicative hand movement that did not involve manipulation of objects (e.g., twisting a jar open) or a ritualized game (e.g., patty cake) was considered a gesture (Ozcaliskan and Goldin-Meadow 2005). There were two exceptions to this rule: we coded SHOW and GIVE gestures, which are produced while holding an object. Head nods and shakes were also considered gestures. Gestures were considered to be communicative if they were directed toward another individual as determined by contextual factors such as body orientation, eye contact, vocalization, and other aspects of the verbal and nonverbal interaction (Iverson et al. 1994). Any of these contextual factors could be taken into account in determining whether a behaviour was communicative and no one particular factor was required to be present.
Each gesture was then classified into one of six mutually exclusive gesture type categories: CONVENTIONAL, POINT, SHOW, ICONIC, SIGN or FUNCTIONAL ACT. CONVENTIONAL gestures were those whose forms and meanings are prescribed by the culture (e.g., nodding the head ‘yes’). Deictic gestures were those that indicated concrete objects, people, or locations. We divided deictic gestures into two kinds: POINT (e.g., pointing to a dog) and SHOW (e.g., holding a doll up in the air). ICONIC gestures depicted the attributes or actions of an object via hand or body movements (e.g., moving the index finger in circles to convey a ball rolling). SIGNS were symbolic hand movements taken or modified from a formal or informal sign language system (e.g., touching finger-tips at midline to convey ‘more’). Lastly, GIVE gestures (e.g., extending a cup towards another individual to convey to the individual to take the cup) and RITUALIZED REQUEST (e.g., extending an arm towards a cup with an open/close hand movement to convey requesting that cup; Bates et al. 1979) were grouped together as FUNCTIONAL ACT gestures. We chose to include FUNCTIONAL ACTS because they are represented in communication assessments commonly used with infants with HR and children with ASD and are prominent in the ASD literature (e.g., ESCS; Mundy et al. 1986). In addition, they are common in the early gestural repertoires of very young children (Bates et al. 1979) and we wanted to capture the full developmental spectrum of gesture use. None of the children were learning a sign language formally, but may have been exposed to baby sign language.
Given the extensive focus on pointing gestures in ASD literature, we also further classified POINT gestures into one of four mutually exclusive pointing forms: Index, Palm, Touch and Object. Index (extended index finger hand shape) and Palm (open palm hand shape) points were empty-handed and did not make contact with the referent of the point. In contrast, for Touch points, finger(s) touched the object/person/location that was the referent of the gesture. Object points were produced while the child held an object (e.g., child was holding a cup while pointing to a dog). These forms can be viewed as ranging from more to less cognitively advanced and may shed light on the nature of any pointing differences we may observe (Bates et al. 1979, Werner and Kaplan 1963).
Two individuals, one of whom was blind to group membership, were responsible for all gesture coding. They began coding independently when they reached 80% agreement on two consecutive videos. Subsequently, reliability was assessed at regular intervals; videos were randomly selected for double coding such that a total of 10% of the videotaped sessions were double-coded and utilized for reliability calculations. Following this procedure, inter-coder agreement was 77.46% for identifying gestures and 96.23% for classifying gestures by type (e.g., POINT, FUNCTIONAL ACT, etc.) and pointing form (e.g., Touch point, Index point).
Data analysis
Prior to conducting data analyses, we examined normality of score distribution. The distribution of scores for several variables was skewed toward the low end of the range (e.g., number of Index points for the ASD group; see tables 2–5). Because of this skew we also examined the number of ‘0’ scores for each variable and report and account for this when appropriate (see the Results). In light of the skewed nature of the distributions, potential for outlier scores, and unequal sample sizes for between group analyses, non-parametric statistics were employed for all analyses with alpha set at .05.
Table 2.
Gesture types produced by each HR group at 2 years
| Behaviour | ND
|
LD
|
ASD
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | AD | Range | % | Median | AD | Range | % | Median | AD | Range | % | |
| Conventional | 4 | 2.30 | 1–10 | 89 | 4 | 3.47 | 0–16 | 77 | 2 | 1.43 | 0–5 | 71 |
| Deictic—Point | 3 | 7.19 | 0–29 | 89 | 1 | 1.41 | 0–6 | 77 | 0 | 0.82 | 0–3 | 29 |
| Deictic—Show | 1 | 1.41 | 0–6 | 67 | 1 | 1.92 | 0–6 | 54 | 0 | 0.82 | 0–2 | 29 |
| Iconic | 0 | 0 | 0 | 0 | 0 | 0.14 | 0–1 | 8 | 0 | 0 | 0 | 0 |
| Sign | 0 | 0.20 | 0–1 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Functional Act | 1 | 1.28 | 1–5 | 100 | 0 | 1.99 | 0–10 | 46 | 0 | 1.55 | 0–4 | 43 |
Note: The table includes descriptive statistics for each behaviour at two years: AD, average deviation, and % = percentage of children producing each behaviour.
Table 5.
Pointing forms produced by each HR group at 3 years
| Behaviour | ND
|
LD
|
ASD
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | AD | Range | % | Median | AD | Range | % | Median | AD | Range | % | |
| Index | 1 | 2.00 | 0–8 | 78 | 0 | 1.61 | 0–4 | 62 | 0 | 1.02 | 0–3 | 29 |
| Touch | 2 | 1.78 | 0–9 | 89 | 1 | 2.20 | 0–8 | 69 | 0 | 2.16 | 0–9 | 29 |
| Palm | 0 | 1.11 | 0–3 | 44 | 0 | 0.36 | 0–1 | 15 | 0 | 0 | 0 | 0 |
| Object | 0 | 0.62 | 0–2 | 44 | 0 | 0.66 | 0–3 | 23 | 0 | 0.82 | 0–3 | 0 |
Note: The table includes descriptive statistics for each behaviour at three years: AD, average deviation; and % = percentage of children producing each behaviour.
Results
Our study had two aims: (1) to characterize gesture use in HR toddlers; and (2) to examine the development of gesture in HR toddlers. For each of these aims, we examined differences between HR toddlers based on outcome status: HR toddlers with ASD, HR toddlers with LD and HR toddlers with ND.
Gesture in HR toddlers at 2 and 3 years of age
We first examined overall frequency of gesture use at each age. This is followed with descriptive data on production of gesture types and of different pointing gesture forms.
Overall gesture use
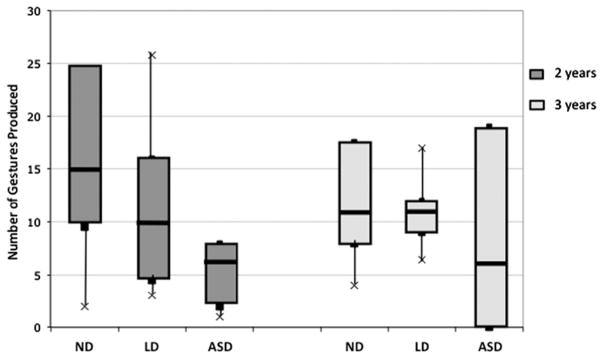
There were large individual differences in gesture production within each group at both 2 and 3 years (figure 1). There also appeared to be differences between groups, which we examined separately at each age using the Kruskal–Wallis test. Group differences were significant at 2 years (K = 8.47, p = .015), but not at 3 years (K = 2.43, p = .297).4 Examination of the numbers of children producing at least one gesture revealed that all children produced at least one gesture at 2 years. At 3 years, all children in the ND and LD groups produced at least one gesture, while five of the seven ASD children gestured.
Figure 1.
Gesture frequency for each HR group at 2 and 3 years. Boxes represent the interquartile range for each group; the line in the middle of each box represents the median and the crosses represent the 10th and 90th percentiles.
To examine predicted group differences, we conducted pairwise comparisons using the Mann–Whitney test.5 There were no significant differences between the ND and LD groups in overall gesture use at either age (p > .160). However, at 2 years, the ASD group differed from both HR comparison groups without ASD (ASD × ND: U = 2.66, p = .008; ASD × LD: U = 2.27, p = .023). At 3 years the differences were no longer statistically significant (ASD × ND: U = 1.28, p = .202; ASD × LD: U = 1.40, p = .163).
Types of gestures
Tables 2 and 3 contain descriptive information on the frequency of production of each gesture type at 24 and 36 months, respectively. As can be seen, there were differences between groups as well as individual differences in the types of gestures produced within each group. We did not include ICONIC gestures and SIGNS in our statistical analyses due to their low frequencies of occurrence.
Table 3.
Gesture types produced by each HR group at 3 years
| Behaviour | ND
|
LD
|
ASD
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | AD | Range | % | Median | AD | Range | % | Median | AD | Range | % | |
| Conventional | 4 | 1.78 | 0–7 | 89 | 3 | 1.75 | 1–9 | 100 | 2 | 1.80 | 0–6 | 71 |
| Deictic—Point | 4 | 1.70 | 0–10 | 89 | 1 | 1.94 | 0–7 | 69 | 0 | 1.84 | 0–6 | 29 |
| Deictic—Show | 1 | 1.48 | 0–5 | 56 | 2 | 1.82 | 0–9 | 69 | 2 | 1.22 | 0–3 | 57 |
| Iconic | 0 | 0.20 | 0–1 | 11 | 0 | 0.14 | 0–1 | 8 | 0 | 0 | 0 | 0 |
| Sign | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.84 | 0–7 | 29 |
| Functional Act | 0 | 0.86 | 0–3 | 22 | 0 | 1.17 | 0–6 | 31 | 0 | 0.24 | 0–1 | 14 |
Note: The table includes descriptive statistics for each behaviour at three years: AD, average deviation; and % = percentage of children producing each behaviour.
With regard to between group differences, at 2 years there were no significant differences for FUNCTIONAL ACT (K = 2.46, p = .292), CONVENTIONAL (K = 2.47, p = .291), and SHOW (K = 2.25, p = .325) gestures. However, the groups did differ on number of POINT gestures (K = 8.24, p = .016). As is apparent in table 2, children with ASD tended to produce fewer POINT gestures than the other HR groups. Pairwise comparisons revealed that this difference was significant for the ND group (p = .012) and nearly significant for the LD group (p = .061). Interestingly, there was also a marginally significant difference in POINT gestures between the LD and ND groups (p = .072), with LD children tending to produce fewer POINT gestures than ND peers.
By 3 years, there were no significant differences between groups for number of FUNCTIONAL ACT (K = 0.87, p = .648), CONVENTIONAL (K = 1.76, p = .415), or SHOW (K = 0.68, p = .712) gestures. There was a marginally significant difference between groups for number of POINT gestures (K = 5.21, p = .074). As at 2 years, toddlers in the ASD and LD groups tended to produce fewer POINT gestures than toddlers in the ND group (table 3). Pairwise comparisons revealed that while the ASD group did not differ from the LD group (p = .210), it did differ significantly from the ND group (p = .048). The difference between LD and ND groups was not significant (p = .102). Thus, at 2 years of age, children in the LD and ASD groups exhibited similar patterns of reduced pointing relative to children in the ND group, and this pattern persisted for the ASD group at 3 years.
We next examined profiles of gesture types within each HR group (tables 2 and 3) to determine whether relative production of different gesture types varied among the three groups. For example, Stone et al. (1997) found that relative to children with other developmental delays, POINT and SHOW gestures accounted for a smaller percentage of the total gestures produced by young children with ASD. We might therefore expect differences within the ASD group, such that children with ASD produce POINT gestures (and perhaps SHOW gestures) less frequently than other gesture types. In contrast, if HR children without ASD more closely resemble TD children, we would predict greater use of POINT gestures relative to other gesture types (Ozcaliskan and Goldin-Meadow 2005). To address these possibilities, separate analyses were conducted for each group using Friedman’s tests to examine whether there were differences in the frequencies with which the different gesture types were produced.
At 2 years, there were significant differences between gesture types within the ND group (Fr = 8.64, p = .035), marginally significant differences within the LD group (Fr = 7.12, p = .068) and no significant differences within the ASD group (Fr = 3.37, p = .338). Within the ND group, children tended to rely primarily on CONVENTIONAL and POINT gestures and produced the remaining gesture types infrequently (table 2). Differences between POINT gestures and the other gesture types were significant for FUNCTIONAL ACT gestures (p < .05) and marginally significant for SHOW gestures (p = .075). Within the LD group, children tended to rely on CONVENTIONAL gestures and produced relatively few POINT gestures (table 2). Pairwise comparisons confirmed this pattern; the difference was significant only for CONVENTIONAL gestures (p = .025). Although there was no main effect for the ASD group, we performed pairwise comparisons in light of our prediction above. None of the comparisons approached significance. However, it is important to note that at 2 years children with ASD produced very few gestures overall (figure 1), limiting our ability to investigate relative patterns of gesture type frequency.
At 3 years, there were significant differences between gesture types in all three groups (ND: Fr = 14.73, p = .002; LD: Fr = 14.82, p = .002; ASD: Fr = 9.64, p = .022). However, as seen in table 3, profiles of gesture use varied by group. Within the ND group, children exhibited an identical profile to that seen at 2 years: CONVENTIONAL and POINT gestures were produced more frequently than the other gesture types. Pairwise comparisons between POINT gestures and the other gesture types revealed that these differences were significant for FUNCTIONAL ACTS (p <.05) and marginally significant for SHOW gestures (p = .061). Within the LD group, children tended to rely on CONVENTIONAL gestures, as they had at 2 years, and produced POINT gestures relatively infrequently, though the pairwise comparison between POINT gestures and CONVENTIONAL gestures was no longer statistically significant. Like children with LD, children in the ASD group predominantly produced CONVENTIONAL gestures and relatively few POINT gestures. However, pairwise comparisons did not reveal any significant differences between POINT gestures and the other gesture types. In summary, an overall pattern emerged whereby the ND group exhibited a pattern previously reported for TD children, with heavy reliance on pointing gestures. In contrast, this pattern was not observed for the LD and ASD groups, who produced pointing gestures relatively infrequently.
Pointing gestures
Lastly, because of the prominence of POINT gestures in ASD assessment and the extensive attention they have received in the literature on ASD, we looked in detail at the different forms of POINT gestures produced by the three groups. As noted above, children in the ASD group produced fewer POINT gestures than the ND group at both 2 and 3 years, and there was also a non-significant trend whereby they produced fewer POINT gestures than the LD group at 2 years. Were these differences specific to particular POINT forms (e.g., Index points)? The relevant data are presented in tables 4 and 5. Because several POINT forms were produced by too few children and too infrequently in each group, we were unable to carry out analyses of differences in frequency. Thus, Fisher’s exact tests were performed on the numbers of children producing each gesture form in each group. This allowed us to investigate whether the likelihood of producing at least one of that form differed between the ASD group and each HR comparison group.
Table 4.
Pointing forms produced by each HR group at 2 years
| Behaviour | ND
|
LD
|
ASD
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | AD | Range | % | Median | AD | Range | % | Median | AD | Range | % | |
| Index | 2 | 4.86 | 0–23 | 67 | 1 | 1.30 | 0–6 | 39 | 0 | 0.82 | 0–3 | 29 |
| Touch | 4 | 3.85 | 0–15 | 78 | 1 | 1.44 | 0–6 | 54 | 0 | 0.61 | 0–2 | 29 |
| Palm | 0 | 0.86 | 0–3 | 44 | 0 | 0.26 | 0–1 | 23 | 0 | 0 | 0 | 0 |
| Object | 0 | 0.86 | 0–4 | 22 | 0 | 0.36 | 0–1 | 39 | 0 | 0 | 0 | 29 |
Note: The table includes descriptive statistics for each behaviour at two years: AD, average deviation; and % = percentage of children producing each behaviour.
At 2 years, the only difference that approached significance was between ASD and ND groups for Palm points (p = .088), with children with ASD less likely than ND children to produce Palm points. There were no differences between ASD and LD children for any of the pointing forms. At 3 years, ASD children were less likely to produce Touch points (p = .035) and Palm points (p = .088) than ND children, though only the difference for Touch points was significant. There were no differences between ASD and LD children for any of the pointing forms at either age. The only difference between the LD and ND groups that approached significance was for Index points (p = .099).
Gesture development from 2 to 3 years
Our next set of analyses examined the development of gesture use within each HR group. We focused first on developmental change in gesture use and then investigated whether there was stability in individual differences from 2 to 3 years.
Overall gesture use
Wilcoxon signed rank tests revealed no significant age differences in overall gesture use for any of the groups (ND: Z = 1.19, p = .236; LD: Z = 0.32, p = .753; ASD: Z = 1.02, p = .306; see figure 1). Descriptively, there was large within-group variability at each age. Variability within the ND and LD groups appeared to decrease from 2 to 3 years. In contrast, variability within the ASD group appeared to increase, with some children at 3 years producing no gestures and some producing as many or more than other HR children. We performed Spearman correlations to test whether there was stability within individuals for overall gesture use from 2 to 3 years. There were no significant correlations between gesture use at 2 and 3 years (rhos = .085–.385, p > .39).
Types of gestures
We next examined developmental change among gesture types (tables 2 and 3). Descriptively, production of FUNCTIONAL ACT gestures appeared to decrease over time for all groups. This difference was significant for the ND group (Z = 1.98, p = .048), but not for the other groups. There were no age differences that were statistically significant for any of the other gesture types for any of the groups. There was also no evidence of stability in use of the different gesture types from 2 to 3 years for the any of the groups (Spearman correlations: rhos = .054–.569, p > .097).
Pointing gestures
We performed statistical analyses of pointing forms for each group only when more than half of the group produced at least one instance of that gesture form (see percent of children producing in tables 4 and 5). Thus, we did not perform any statistical analyses for the ASD group. For the LD and ND groups, there were no differences across ages in use of Touch and Index points (all p > .796). There was also no significant relationship between 2 and 3 years of age in use of Touch and Index finger points respectively for either group (Spearman correlations: rhos = .130–.444, p > .186).
Discussion
Large individual differences were apparent in HR toddlers’ gestural communication, with profiles of gesture use varying both between HR groups and with age. Consistent with prior studies, at 2 years of age children with ASD gestured less frequently and made less extensive use of pointing gestures relative to HR children without ASD. However, we found a similar profile in HR children who did not have ASD, but did have LD. For instance, although pointing gestures were produced with greater frequency than other gestures by the ND group, this was not the case for the LD and ASD groups. Children in the LD group also exhibited less frequent pointing relative to ND children, a pattern that approached significance at 2 years. Taken together, these findings extend prior literature by highlighting the existence of relations between language and gesture in HR children who are at risk for delays in both domains. They also in indicate that examination of gesture use among subgroups of HR children who do not have ASD may yield valuable information on developmental trajectories and potential indicators of later delay. Each of these points will be addressed in turn.
Differences in gesture use between HR groups
We found delays in gesture production in the ASD group relative to both the ND and LD groups at 2 years of age. Previous research with 12–24-month-olds using semi-structured assessments and parent report measures have reported conflicting findings of differences between HR infants with and without ASD. Our observation of differences between ASD and both HR comparison groups in a naturalistic context provides additional information to be incorporated into the evolving picture of gesture development in this age range.
Interestingly, however, these differences were not apparent at 3 years of age. One interpretation of this finding is that gesture delays reported in toddlerhood begin to resolve for children with ASD at 3 years (relative to their HR peers). However, it is also possible that the absence of group differences at 3 years reflects changes in gesture use among a subset of the ND and LD children, such that some of these children may begin compensating for their language difficulties through gesture. Research with 2–6-year-old children with language difficulties (but not ASD) provides some support for this possibility (Iverson and Braddock 2011, Thal and Tobias 1992). These studies suggest that children may compensate for language difficulties via gesture, such that, to varying degrees, they produce more gestures than their TD peers. However, it is important to note that the large individual differences within the ASD group at 3 years make interpretation of the null results difficult; gesture delays may be resolving for some children, but not others. Future research with larger samples is clearly warranted to explore individual differences within groups. In addition, future work can examine differences in relatively more advanced aspects of gestural communication, including the use of gesture to regulate interaction and the temporal and semantic synchrony between gesture and accompanying speech (Ozcaliskan and Goldin-Meadow 2005, de Marchena and Eigsti 2010).
As predicted, we also found differences in gesture use in children with LD. We found a pattern of reduced use of pointing gestures for children with LD relative to other gesture types at 2 years. Children in the LD group also pointed less frequently than children in the ND group at 2 years, a finding that approached significance. Thus, there were some similarities in the profiles of gesture use for children with LD and children with ASD relative to their ND peers. This suggests that some of the observed gesture delays in the HR population may be attributed to language delay more generally in addition to unique aspects of the ASD phenotype. One commonality between the LD and ASD groups is the presence of language delays, with five of the seven children in the ASD group also meeting our study criteria for Language Delay. Further support for this possibility comes from studies of other populations of children who do not have ASD, but do have LD (Thal and Tobias 1992).
Why did we find particular delays in pointing gestures? It is possible that a unique relation exists between pointing and language (Kita 2003) and the similar profiles observed for LD and ASD groups are reflective of this relation. For instance, it has been argued that pointing is linked to cognitive and social developments that are intricately tied to early language development (Werner and Kaplan 1963) and a similar relationship may continue to exist at the later ages examined in this study. Along these lines, lack of pointing late in the second year appears to discriminate children with ASD from TD children, but not from children with other developmental delays (Wetherby et al. 2004).
Although there were group differences in overall number of POINT gestures produced, no such differences were evident when we looked at specific pointing forms. For instance, we did not find evidence of differences in index finger pointing that would support the hypothesis that there were particular deficits in pointing forms viewed as more cognitively advanced than others (Bates et al. 1979, Werner and Kaplan 1963). However, it should be noted that some forms were produced at very low rates across all groups and some children produced no POINT gestures at all.
Additional work is clearly needed to examine whether the gesture profiles observed in the LD and ASD groups reflect a relation between pointing and language development more generally. Previous research has shown that developments in gesture are tightly linked to developments in speech in the first few years in both typical and atypical development (e.g., Rowe and Goldin-Meadow 2009, Sauer et al. 2010). Future work with HR toddlers can directly examine relations between particular types of gestures, such as pointing, and various aspects of expressive language development (e.g., lexical development) (e.g., Bates and Dick 2002).
Taken as a whole, our findings have clinical implications for early identification of language difficulties. Due to the increased risk for communication delays in HR children, even among those who do not develop ASD, developmental monitoring of this sample can be valuable for early identification and intervention (Ozonoff et al. 2011). Our findings of differences in pointing at 2 years among HR children suggest that pointing may be a behaviour to monitor. In addition, our findings parallel those with previous samples of children at risk for language delay (e.g., Thal and Tobias 1992) and provide further support for the utility of examining gesture in profiling communication skills in toddlers at risk for delay with potential implications for diagnosis and identification of intervention targets (Crais and Watson 2009).
Gesture development
We did not find evidence of stability in gesture use or in developmental differences in gesture production from 2 to 3 years, though it is unclear from the literature whether we would have expected such findings at these ages. While research with TD children reports short-term stability in gesture between 9 and 16 months (Bates et al. 1979), it is unclear whether such correlations would be expected to extend to the toddler period examined in this study or to atypical populations. Further, the nature of gesture and its role in communication evolves substantially between 9 and 36 months, suggesting that we cannot assume that these early relations would also be observed in toddler years (McNeill 1992).
Regarding developmental changes in gesture, there is again little research in this age range. For TD children in the second year, pointing frequency increases, but frequency of gesturing overall and frequency of producing other gesture types stabilizes or decreases (Iverson et al. 1994, Ozcaliskan and Goldin-Meadow 2005). If we assume a similar process in the third year, the lack of age-related changes in overall gesturing may be reflective of typical developmental patterns. Further, although we did not find an increase in POINT gestures for the ND group, we did find a decrease in the use of earlier-appearing FUNCTIONAL ACT gestures. This suggests that the ND group may be following typical developmental patterns in the types of gestures they produce.
Limitations and future directions
Future work can address some of the limitations present in the current study, particularly regarding the size and characteristics of our study groups (ASD, LD, ND). First, large individual differences existed within subgroups at both ages on the majority of our gesture measures and samples size for each of the study groups was relatively small, limiting power to detect statistically significant effects. In combination, this limits the conclusions we can draw from some analyses, particularly results that approached but did not reach statistical significance. Nonetheless, the findings are suggestive, and studies with larger samples may clarify some of our findings by exploring these individual differences. In addition, consistent with previous research, children in the ASD group also had lower scores on the MSEL ELC than their peers without ASD (e.g., Landa and Garrett-Mayer 2006). This raises the possibility that differences between the ASD group and the ND and LD groups may be influenced by the presence of generally low cognitive performance. Future work with larger samples will afford use of statistical analyses that can examine the potential role of ELC in our study findings.
One strength of this study is that it utilized data collected in the naturalistic context of the home and with the child’s primary caregiver as opposed to an unfamiliar examiner. However, parent behaviour was not standardized, raising questions about bidirectional influences on children’s gesturing. Future work examining caregiver’s behaviour in these interactions will provide further information about the mutually influential roles of child and environment on gestural communication.
Finally, the MSEL language measures at 36 months did not distinguish the LD and ND groups, though the CDI at 36 months did. That parent reported expressive language (CDI) and observed (MSEL) expressive language were positively and significantly correlated suggests that there is some agreement between the parent reported (CDI) measure and observational measure (MSEL), and it is possible that the MSEL may be a less sensitive measure of language abilities at this age. Future studies with additional and more precise language measures can help tease apart relations between gesture and language (see below).
Taken together, our findings provide a starting point for future research. In addition to the directions noted above, one goal of our study was to investigate differences within the HR population, but future work can examine differences between the subgroups examined here and other populations. Our finding of relatively reduced pointing at 2 years in the LD group suggests that relations between gesture and language abilities exist within the HR infant population. A question of interest is whether ASD and LD groups exhibit gesture profiles that are similar to other atypically developing children without ASD, including those with language difficulties. There are both similarities and differences in gesture use across a variety of populations exhibiting atypical development (Capone and McGregor 2004) and comparison with children in our HR subgroups may further our understanding of the nature of gesture development in both typical and atypical development.
Table 1b.
Participant characteristics for each subgroup at 3 years
| Measure | Median | AD | Range | Mean | SD |
|---|---|---|---|---|---|
| No diagnosis (ND) | |||||
| CDI-III Percentile | 20 | 12.66 | 15–50 | 28.125 | 14.38 |
| 3 year MSEL ELC Standard Score | 117 | 12 | 84–122 | 111.33 | 15.96 |
| 3 year MSEL Receptive Language T-score | 53 | 7.83 | 35–70 | 54.44 | 10.25 |
| 3 year MSEL Expressive Language T-score | 61 | 5.63 | 47–68 | 59.11 | 7.13 |
| Language delay (LD) | |||||
| CDI-III Percentile | 5 | 3.55 | 0–10 | 3.85 | 4.16 |
| 3 year MSEL ELC Standard Score | 94 | 18.51 | 61–129 | 99.62 | 22.41 |
| 3 year MSEL Receptive Language T-score | 49 | 8.95 | 33–58 | 47.69 | 11.4 |
| 3 year MSEL Expressive Language T-score | 54 | 8.66 | 31–65 | 51.46 | 10.67 |
| Autism spectrum disorder (ASD) | |||||
| CDI-III Percentile | 0 | 5.56 | 0–20 | 3.33 | 8.16 |
| 3 year MSEL ELC Standard Score | 55 | 14.08 | 49–97 | 64.4 | 19.39 |
| 3 year MSEL Receptive Language T-score | 20 | 6.67 | 20–44 | 24 | 9.8 |
| 3 year MSEL Expressive Language T-score | 30.5 | 8.33 | 20–52 | 31.5 | 11.9 |
Note: Both tables include descriptive statistics at 2 and 3 years of age for the MSEL Early Learning Composite (ELC) Standard Score, Expressive Language T-score, Receptive Language T-score, and CDI Percentile. AD, average deviation; SD, standard deviation. CDI scores are unavailable for one child with ASD at 36 months and unavailable for one child with ND and one child with ASD at 24 months. MSEL scores are unavailable for one child with ASD at 36 months. MSEL scores are unavailable for one child with ND at 24 months; for two children with ASD on the Receptive Language subscale at 24 months; and for three children with ASD on the ELC Standard Score.
What this paper adds?
What is known about the subject?
Gesture deficits are characteristic of ASDs and siblings of children with ASD are at increased risk for ASD associated delays. Gesture delays in early childhood have been reported in a variety of populations exhibiting language delays. It is unclear whether gesture differences in toddlers with a sibling with ASD exist as a function of diagnostic status: (1) ASD; (2) no ASD but a presence of language delay; and (3) no delay.
What this paper adds?
As a result of this study we now know that in toddlers with a sibling with ASD gesture differences exist as a function of diagnostic status. In addition to relatively infrequent gesturing in the ASD group, a pattern of reduced pointing was observed in both (1) the ASD group and (2) the language delay group relative to (3) the no delay group. Our results reinforce links between verbal and nonverbal communication in populations at risk for language delay and potential indicators of delay and targets for early intervention.
Acknowledgments
This work was supported by grants from Autism Speaks and R01 HD054979 and R01 HD41607 to Jana M. Iverson. We thank D. Williams, N. Minshew, and the NICHD-funded University of Pittsburgh-Carnegie Mellon Collaborative Program of Excellence in Research (HD35469 and HD055748 to N. Minshew) for supporting as Sessments and assistance with participant recruitment; J. Hetherington for administrative assistance; C. Rush and K. Schuessler for assistance with coding and reliability; and members of the Infant Communication Lab for assistance with data collection. We offer special thanks to the families and infants who enthusiastically participated in the research.
Footnotes
Gesture types will be indicated by CAPITALS throughout the text.
The relatively high rate of LD is consistent with previous studies with HR infants that have found increased risk for language delays in HR infants without ASD (Rogers 2009).
A similar time period has been used by other studies examining communication in parent–child play (e.g., Bates et al. 1979).
Results of group differences in overall gesture use are unchanged when we excluded FUNCTIONAL ACT gestures. When we later examined development in overall gesture use, results were again unchanged when excluding FUNCTIONAL ACT gestures. As will be presented below, FUNCTIONAL ACTS accounted for few of the gestures produced at each age.
For all analyses, we use the Mann–Whitney test for between-subjects pairwise comparisons; we use the Wilcoxon Signed Rank test for within-subjects pairwise comparisons.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publ; 2013. [Google Scholar]
- Bates E, Benigni L, Bretherton I, Camaioni L, Volterra V. The Emergence of Symbols. New York, NY: Academic Press; 1979. [Google Scholar]
- Bates E, Dick F. Language, gesture, and the developing brain. Developmental Psychobiology. 2002;40:293–310. doi: 10.1002/dev.10034. [DOI] [PubMed] [Google Scholar]
- Capone N, McGregor K. Gesture development: a review for clinical and research practices. Journal of Speech, Language, and Hearing Research. 2004;47:173–186. doi: 10.1044/1092-4388(2004/015). [DOI] [PubMed] [Google Scholar]
- Crais ER, Watson LR, Baranek GT. Use of gesture development in profiling children’s prelinguistic communication skills. American Journal of Speech-Language Pathology. 2009;18:95–108. doi: 10.1044/1058-0360(2008/07-0041). [DOI] [PubMed] [Google Scholar]
- De Marchena A, Eigsti IM. Conversational gestures in autism spectrum disorders: asynchrony but not decreased frequency. Autism Research. 2010;3:311–322. doi: 10.1002/aur.159. [DOI] [PubMed] [Google Scholar]
- Fenson L, Marchman VA, Thal DJ, Dale PS, Reznick JS, Bates E. The MacArthur Communicative Development Inventories: User’s Guide and Technical Manual. 2. Baltimore, MD: Brookes; 2007. [Google Scholar]
- Filipek PA, Accardo PJ, Baranek GT, Cook EH, Jr, Dawson G, Gordon B, … Volkmar FR. The screening and diagnosis of autism spectrum disorders. Journal of Autism and Developmental Disorders. 1999;29:439–484. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- Goldberg WA, Jarvis KL, Osann K, Laulhere TM, Straub C, Thomas E, Filipek P, Spence MA. Brief Report: Early social communication behaviors in the younger siblings of children with autism. Journal of Autism and Developmental Disorders. 2005;35:657–664. doi: 10.1007/s10803-005-0009-6. [DOI] [PubMed] [Google Scholar]
- Heilmann J, Weismer SE, Evans J, Hollar C. Utility of the MacArthur–Bates Communicative Development Inventory in identifying language abilities of late-talking and typically developing toddlers. American Journal of Speech–Language Pathology. 2005;14:40–51. doi: 10.1044/1058-0360(2005/006). [DOI] [PubMed] [Google Scholar]
- Iverson J, Capirci O, Caselli MC. From communication to language in two modalities. Cognitive Development. 1994;9:23–43. [Google Scholar]
- Iverson JM, Braddock BA. Gesture and motor skill in relation to language in children with language impairment. Journal of Speech, Language, and Hearing Research. 2011;54:72–86. doi: 10.1044/1092-4388(2010/08-0197). [DOI] [PubMed] [Google Scholar]
- Iverson JM, Wozniak RH. Variation in vocal-motor development in infant siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37:158–170. doi: 10.1007/s10803-006-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita S. Pointing: Where Language, Culture, and Cognition Meet. Hillsdale, NJ: Erlbaum; 2003. [Google Scholar]
- Landa R. Early communication development and intervention for children with autism. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:16–25. doi: 10.1002/mrdd.20134. [DOI] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. Journal of Child Psychology and Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, … Rutter M. The Autism Diagnostic Observation Schedule—Generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- McNeill D. Hand and Mind. Chicago, IL: University of Chicago Press; 1992. [Google Scholar]
- Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, Bryson S. Early language and communication development of infants later diagnosed with autism spectrum disorder. Developmental and Behavioral Pediatrics. 2006;27:S69–S78. doi: 10.1097/00004703-200604002-00004. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. AGS Edn. [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining social deficits of autism: the contribution of non-verbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27:657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Ozcaliskan S, Goldin-Meadow S. Do parents lead their children by the hand? Journal of Child Language. 2005;32:481–505. doi: 10.1017/s0305000905007002. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, … Young GS. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:256–266. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Stone WL. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlade MV. Doctoral dissertation. University of Pittsburgh; 2012. The development of multimodal social communication in infants at high risk for autism spectrum disorders. [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M, Goldin-Meadow S. Early gesture selectively predicts later language learning. Developmental Science. 2009;12:182–187. doi: 10.1111/j.1467-7687.2008.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, Sigman M. Behavioral profiles of affected and unaffected siblings of children with autism: contributions of measures of mother–infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders. 2011;41:287–301. doi: 10.1007/s10803-010-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer E, Levine SC, Goldin-Meadow S. Early gesture predicts language delay in children with pre- or perinatal brain lesions. Child Development. 2010;81:528–539. doi: 10.1111/j.1467-8624.2009.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WL, Ousley OY, Yoder PJ, Hogan KL, Hepburn SL. Nonverbal communication in two-and three-year-old children with autism. Journal of Autism and Developmental Disorders. 1997;27:677–695. doi: 10.1023/a:1025854816091. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Rogers S, Cooper J, Landa R, Lord C, Paul R, … Yoder P. Defining spoken language benchmarks and selecting measures of expressive language development for young children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research. 2009;52:643–652. doi: 10.1044/1092-4388(2009/08-0136). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal D, Tobias S. Communicative gestures in children with delayed onset of oral expressive vocabulary. Journal of Speech and Hearing Research. 1992;35:1281–1289. doi: 10.1044/jshr.3506.1289. [DOI] [PubMed] [Google Scholar]
- Weismer SE, Evans JL. The role of processing limitations in early identification of specific language impairment. Topics in Language Disorders. 2002;22:15–29. [Google Scholar]
- Werner H, Kaplan B. Symbol Formation: An Organismic-Developmental Approach to Language and the Expression of Thought. New York, NY: Wiley; 1963. [Google Scholar]
- Wetherby AM, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the second year of life. Journal of Autism and Developmental Disorders. 2004;34:473–493. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- Winder BM, Wozniak RH, Parlade MV, Iverson JM. Spontaneous initiation of communication in infants at low and heightened risk for autism spectrum disorders. Developmental Psychology. 2013;49:1931–1942. doi: 10.1037/a0031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfenden S, Sarkozy V, Ridley G, Williams K. A systematic review of the diagnostic stability of autism spectrum disorder. Research in Autism Spectrum Disorders. 2012;6:345–354. [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, … Sigman M. Studying the emergence of autism spectrum disorders in high-risk infants: methodological and practical issues. Journal of Autism and Developmental Disorders. 2007;37:466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]