Abstract
Although there is considerable evidence that a subpopulation of regulatory CD4+CD25+ T cells can suppress the response of autoreactive T cells, the underlying molecular mechanism is not understood. We find that transmission of a suppressive signal by CD4CD25+ regulatory cells requires engagement of the B7 molecule expressed on target T cells. The response of T cells from B7-deficient mice is resistant to suppression in vitro, and these cells provoke a lethal wasting disease in lymphopenic mice despite the presence of regulatory T cells. Susceptibility of B7-deficient cells to suppression is restored by lentiviral-based expression of full-length, but not truncated, B7 lacking a transmembrane/cytoplasmic domain. Because expression of these B7 truncation mutants restores CD28-dependent costimulatory activity, these findings that indicate B7-based transmission of suppressive activity suggest new approaches to modifying autoimmune responses.
Approximately 10% of CD4 cells in healthy mice and people constitutively express the IL-2 receptor α-chain CD25. These cells include a regulatory subpopulation, termed T-reg, that also express OX40, 4-1BB, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), and glucocorticoid-induced tumor necrosis factor receptor family-related gene (1, 2), as well as the Foxp3 transcription factor (3, 4). T-reg efficiently suppress CD4 and CD8 cell expansion in lymphopenic hosts and B cell proliferation (5). Deficiency of T-reg has been associated with the development of organ-specific autoimmunity that includes thyroiditis, gastritis, oophoritis, orchitis and colitis (6), experimental autoimmune encephalomyelitis (7), Type I diabetes (8), and allergic reactions (9). Suppression by T-reg in vitro requires direct contact with target cells (10) and can be overcome by provision of costimulatory signals or a source of IL-2 (11). Although recent studies have provided insight into the development of T-reg (12), the molecular mechanism of T-reg-dependent suppression has not been clarified.
The B7 genes B7-1 and B7-2 encode type I transmembrane proteins that can interact with CD28 and CTLA-4 receptors on T cells. Most attention has been paid to the costimulatory activity provided by B7-dependent ligation of CD28 and the inhibitory impact after B7 ligation of CTLA-4. However, recent studies have suggested that B7 may participate in outside-in signaling, e.g., ligation of B7 may transmit suppressive signals after engagement by CTLA-4 (13, 14). Purified B7-deficient T cells can initiate enhanced graft-versus-host disease (15) that is down-regulated by expression of B7-2 on B7-deficient T cells. However, graft-versus-host activity did not depend on the absence of B7 expression on donor T cells, and the underlying mechanism, including the role of T-reg, remains unclear.
We have investigated the contribution of B7 to the inhibitory effects of T-reg on the development of tissue-specific autoimmune disease in lymphopenic mice. Our findings indicate that delivery of a suppressive signal by T-reg requires engagement of B7 on target T cells.
Materials and Methods
Mice. BALB/c, C57BL/6, CD28-deficient, recombination activation gene 2 (RAG-2)-deficient BALB/c, RAG-2-deficient C57BL/6, B7-deficient C57BL/6, B7-1-deficient C57BL/6, and B7-2-deficient C57BL/6 mice were purchased from Jackson Laboratories. B7-deficient BALB/c mice were generously provided by A. Sharpe (Harvard Medical School). All mice were used at 4–12 weeks of age and maintained under specific pathogen-free conditions in accordance with institutional guidelines for animal welfare.
Abs. Purified mAbs against CD3ε (145-2C11), CD28 (37.51), B7-1 (16-10A1), B7-2 (PO3), CD8 (53-6.7), CD4 (GK1.5), B220 (RA3-6B2), MAC-1 (M1/70), GR-1 (RB6-8C5), NK1.1 (PK136), biotin-conjugated anti-CD25 (PC61), anti-B7-1 (16-10A1), anti-B7-2 (GL1), phycoerythrin-(PE) or FITC-conjugated anti-CD4 (RM4-5), anti-CD8 (53-6.7), and streptavidin-FITC, streptavidin-PE, streptavidin-Cy, and mouse IL-2 were purchased from Pharmingen. CTLA-4IgΔFc and CTLA-4IgFc fusion proteins were purchased from Chimerigen (Allston, MA).
Isolation of Lymphocytes. CD4 and CD8 T cells were purified by negative selection in 1× PBS/2% FBS. Single-cell suspensions were prepared from spleen and lymph nodes (mandibular, cervical, axillary, superficial inguinal, and mesenteric) and incubated for 30 min with rat anti-mouse CD8 (53-6.7) or CD4 (GK1.5), B220 (RA3-6B2), MAC-1 (M1/70), GR-1 (RB6-8C5), and NK1.1 (PK136) (Pharmingen). After being washed, cells were incubated for 30 min with magnetic sheep anti-rat Ab-coated beads (Dynal, Great Neck, NY), and Ab-bound cells were removed by magnetic separation to isolate CD4 or CD8 T cells, the purity of which was checked by fluorescence-activated cell sorter (FACS) analysis with a Beckman-Coulter Epics XL. CD4+CD25+ cells were isolated by positive selection from purified CD4 cells by using biotin–anti-CD25 (7D4) and anti-biotin beads (Miltenyi Biotec, Lake Success, NY). Both the flow-through and the eluate were collected. The flow-through was reanalyzed by FACS analysis and contained CD4CD25– cells (≥97% pure), whereas eluted cells were CD4CD25+ (≥99% pure). Spleen cells were depleted of T cells by using Dynabeads mouse pan T (Thy1.2) according to the manufacturer's protocol (Dynal Biotech).
Proliferation Assays. T cells were cultured in RPMI-1640 medium supplemented with 10% FBS/50 μM 2-mercaptoethanol/10 mM Hepes/1 mM sodium pyruvate (Sigma)/2 mM l-glutamine/50 units/ml penicillin/50 μg/ml streptomycin (GIBCO/BRL). Spleens were depleted of T cells with anti-Thy1.2 magnetic beads (Dynal) according to the manufacturer's protocol. The remaining cells were γ-irradiated (3,000 rads), washed, and resuspended in T cell media (see above). Dendritic cells were isolated from spleens by using anti-CD11c-beads (Miltenyi Biotech) according to the manufacturer's protocol and activated as described in ref. 16. Antigen-presenting cells (APC) and purified T cells were cultured in the presence of anti-CD3 mAb (145-2C11) or peptide in 96-well U-bottom plates for 66 h. [3H]thymidine (1 μCi per well; 1 Ci = 37 GBq) (NEN) was added for the last 18 h of this 66-h culture. Results are expressed as the mean of triplicate cultures ± SD.
FACS Analysis of B7-1 and B7-2 on CD4CD25– or CD8+ T Cells. T cells from B7 wild-type (wt) or B7-deficient mice were stimulated with anti-CD3 Ab 2C11 at 10 μg/ml on irradiated, T cell-depleted splenocytes. Splenocytes were depleted by using Dynabeads mouse pan T (Thy 1.2) according to the manufacturer's protocol (Dynal Biotech). Unlabeled mouse IgG was used to block B7-1 staining; B7-2 and CTLA-4Fc FACS stains were blocked with Fc (Pharmingen). Cells were stained with FITC or CyChrome-labeled anti-CD4, anti-CD8, anti-CD3, or anti-CD28; purified anti-B7-1 (16-10A1); and anti-B7-2 (PO3), followed by biotinylated secondary Ab for anti-B7-1 (goat anti-rat Ig) or anti-B7-2 (anti-hamster Ig mixture) and streptavidin-PE. CD8 cells were stained with CTLA-4 Fc/nonlytic (mouse) followed by PE-labeled goat anti-mouse Ig. Isotype control Abs were biotinylated hamster IgG, group 1κ, and biotinylated rat IgG2b, κ, followed by streptavidin-PE (Pharmingen). Secondary Ab–streptavidin-PE staining without addition of primary Ab resulted in staining <2%, and B7-deficient cells were used to demonstrate specificity of B7-1 and B7-2 staining.
Lentiviral Expression of B7. Full-length ORFs of B7-1 and B7-2 were amplified by RT-PCR from C57BL/6 spleen RNA, digested with BglII/XhoI, and cloned into pLenti6/V5 (Invitrogen) by using BamHI (compatible ends with BglII) and XhoI restriction sites. All constructs were confirmed by sequencing. Enhanced GFP alone was cloned into pLenti6/V5 as a negative control. Lentiviral stocks were generated by cotransfection of 293 T cells with the packaging plasmids pLP1, pLP2, and pLP/VSVG (Invitrogen) by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Viral supernatants were collected 72 h after transfection and the viral titer for all transfections was determined to be 107 plaque-forming units/ml. Naive B7-deficient CD4CD25– cells or dendritic cells were infected with at a multiplicity of infection of 5–10 for 3–6 hat37°C with 5% CO2 and either washed and used directly after a 3-h infection time or washed and rested for an additional 15 h at 37°C with 5% CO2. Expression levels as determined by FACS analysis were ≥95% for all lentivirally encoded B7 gene products (Fig. 6, which is published as supporting information on the PNAS web site.). (For primer sequences, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.)
Lentiviral Expression of Glycosylphosphatidylinositol (GPI)-Anchored B7-1 and B7-2 Extracellular Domains. GPI-anchored B7-1 and B7-2 extracellular domains were generated by PCR. Briefly, B7-1 and B7-2 cDNA was amplified with sense primers specific for the initiation codon of B7-1 or B7-2 and antisense primers complementary to the 3′ end of the extracellular domain. The antisense primers contain overhangs complimentary to the 5′ end of GPI-anchor signal sequence from CD16B. A second PCR was done with the same sense primers and a new antisense primer complimentary to the 3′ end of the first-round PCR products containing an overhang encoding for the residual GPI-signal sequence. Resulting PCR products were cloned into pLenti/V5 vector by using restriction sites introduced during amplification (BglII and XhoI). All constructs were confirmed by sequencing. Lentiviral particles were produced as described above. Expression levels as determined by FACS analysis were ≥95% for all lentivirally encoded B7 gene products (Fig. 6). (For primer sequences, see Supporting Materials and Methods.)
Histology. Colons, stomachs, and skin were fixed in Bouins' solution, and paraffin-embedded sections were prepared by the Harvard Rodent Pathology Core and stained with hematoxylin and eosin. Colitis and gastritis were graded in a blinded fashion according to the criteria published in refs. 17 and 18.
Results
T Cells Deficient in B7 Expression Are Resistant to Suppression. Expression of B7, also termed B7-1 (CD80) and B7-2 (CD86), is a hallmark of activated APC and provides an important source of APC-dependent costimulatory signals (19, 20). Expression of both B7-1 and B7-2 is up-regulated on CD4 and CD8 cells within 12–24 h after T cell receptor-linked activation (Fig. 1), albeit at lower levels than APC (21). We asked whether B7 expression on activated T cells might be necessary to target T-reg-suppressive activity using cells from B7-deficient mice (22). B7-deficient (CD4CD25–) T helper (Th) cells were fully resistant to suppression by concentrations of T-reg that exerted substantial suppressive effects against B7 wt Th cells in cultures containing either B7 wt or B7-deficient splenic APC (Fig. 2a, second panel versus fourth panel). Hence, genetic deficiency of B7 expression by Th cells is necessary and sufficient for resistance to suppression and independent of B7 availability on APC (Fig. 2a).
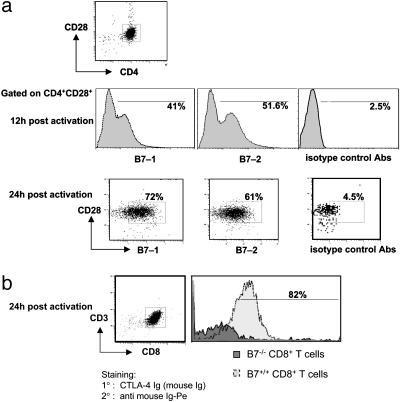
Fig. 1.
Expression of B7-1 and B7-2 on Th (CD4CD25–) or CD8+ T cells after activation. Th (CD4CD25–) (a) or CD8+ T cells (b) from B7 wt or B7-deficient mice were stimulated with anti-CD3 on T cell-depleted irradiated spleen for the indicated time. Live cells were isolated and washed, and CD4 cells were stained with the indicated combinations of fluorescent-labeled Abs to CD4, CD28, and B7-1 or B7-2 or isotype control. CD8 cells were stained with CD3 and CTLA-4 Fc/nonlytic (mouse) followed by PE-labeled goat anti-mouse Ig. Isotype control Abs were biotinylated hamster IgG, group 1κ, and biotinylated rat IgG2b, κ, followed by streptavidin-PE (Pharmingen). Secondary Ab–streptavidin-PE staining without addition of primary Ab resulted in staining <2% (data not shown), and B7-deficient cells were used to demonstrate specificity of B7-1 and B7-2 staining.
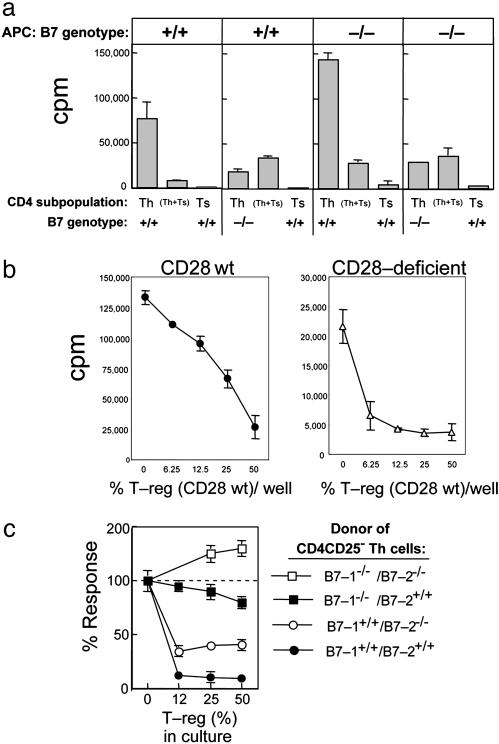
Fig. 2.
Susceptibility of Th (CD4CD25–) cells to suppression by T-reg (CD4CD25+). (a) Role of B7 expression on target Th cells. Purity of all cell isolations was ≥98%. Results shown are representative of three independent experiments. CD4CD25– cells (n = 2.5 × 104, first and second panels) or CD4CD25– T cells (n = 5 × 104, third and fourth panels) from B7 wt donors (first and third panels) or B7-deficient (second and fourth panels) and CD4CD25+ B7 wt T cells (n = 5 × 104, all panels) were stimulated by themselves or mixed on 1 × 105 irradiated B7 wt splenocytes (first and second panels) or 2 × 105 irradiated B7-deficient splenocytes (third and fourth panels) with anti-CD3 mAb 2C11 (10 μg/ml). (b) Role of CD28 expression on target Th cells. Purity of all cell isolations was ≥98%. Results shown are representative of three independent experiments. CD4CD25– T cells (n = 5 × 104) from CD28 wt donors (Left) or CD28-deficient donors (Right) and the indicated numbers of CD4CD25+ CD28 wt T cells were stimulated by themselves or admixed on 2 × 105 irradiated B7 wt splenocytes with anti-CD3 mAb 2C11 (10 μg/ml). (c) Role of B7-1 versus B7-2 expression on target Th cells. Purity of all cell isolations was ≥98%. B7 wt (•), B7-deficient (□), B7-1-deficient/B7-2 wt (▪), and B7-1 wt/B7-2-deficient (○) CD4CD25– Th cells (n = 2.5 × 104) were stimulated alone or in the presence of the indicated numbers of CD4CD25+ regulatory T cells on 1 × 105 irradiated B7 wt spleen with soluble anti-CD3 2C11 (10 μg/ml). Data are shown as percent response: 100% corresponds to ≈80,000 cpm for B7-1/B7-1 wt Th and ranged from 30,000 to 50,000 for singly and doubly deficient Th cells. Proliferation was determined by measuring incorporation of [3H]thymidine (1 μCi per well) during the last 18 h of a 66-h culture by using scintillation counting.
We next delineated the susceptibility of CD28-deficient CD4 cells to examine the role of B7 in the absence of B7-dependent costimulatory signals. Although CD28-deficient T cells displayed impaired proliferative responses similar to that of B7-deficient T cells (Fig. 1), CD28-deficient Th cells were susceptible to suppression by T-reg (Fig. 2b). To delineate the respective roles of B7-1 and B7-2 in this suppressive interaction, we compared the impact of isolated B7-1 or B7-2 deficiency on susceptibility to suppression by graded numbers of T-reg. B7 wt Th cells were efficiently suppressed, whereas T cells that were doubly B7-deficient (B7-1–/– and B7-2–/–) were completely resistant to suppression. T cells singly deficient in B7 showed intermediate levels of resistance, suggesting the following rank order of susceptibility: B7-1+B7-2+ > B7-1+B7-2– > B7-+–B7-2+ > B7-1–B7-2– (Fig. 2c).
Infusion of B7-Deficient CD4 Cells into RAG-2-Deficient Mice Results in Lethal Wasting and Organ-Specific Inflammatory Disease. To test the susceptibility of B-7-deficient CD4 cells to T-reg mediated suppression in vivo, we transferred B7-deficient or B7 wt CD4 cells into B7 wt RAG-2-deficient mice. Recipients of B7-deficient, but not B7 wt Th, cells (CD4+, 106 per mouse) appeared wasted and moribund 30 days later and harbored ≈20-fold more donor CD4 cells in their lymph nodes (Fig. 3a and Table 1). Moreover, although B7-deficient and B7 wt CD4CD25– cells induced autoimmune disease, disease induction by the latter but not the former cells was inhibited by inclusion of CD4CD25+ T-reg (Table 2). Recipients of B7-deficient CD4CD25– cells admixed with B7 wt CD4CD25+ T-reg developed a lethal wasting disease associated with T cell-dependent inflammation, lymphadenopathy, colitis, and gastritis (Table 2) (17, 18, 23).
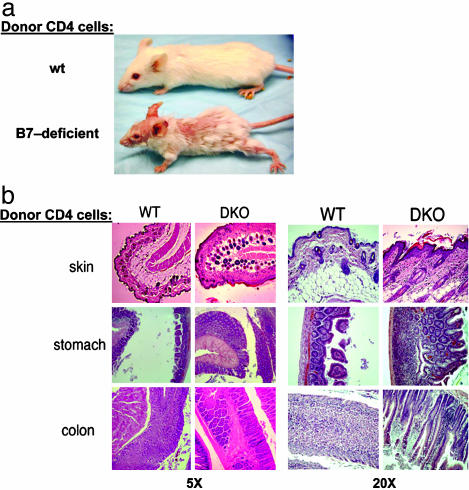
Fig. 3.
Development of lethal wasting disease in RAG-2-deficient recipients of B7-deficient Th cells. (a) CD4+ cells (n = 106) purified from B7 wt or B7-deficient donor mice were transferred i.v. into syngeneic RAG-2-deficient recipients (n = 5) before being killed 30 days after transfer and preserved in Bouins' solution. Complete necropsies and disease scoring for gastritis and colitis (17, 18) were performed blind by the Harvard University Rodent Pathology Core. (b) Dermatitis is marked by inflammatory cells in the subdermal layer interspersed with hyperplastic endocrine cells and hyalinization of subepithelial space; gastritis by lymphocyte clusters in the mucosa, severe destruction of the mucosal architecture, disappearance of parietal and chief cells, and hyperplasia of mucous and endocrine cells; and colitis by accumulation of inflammatory cells between glands, mucin depletion from goblet cells, and marked thickening of the glands. DKO, double knock out (B7-1–B7-2–).
Table 1. Lymphadenopathy in recipients of B7-deficient CD4+ T cells.
| Donor CD4 cells | LN cells,*n × 106 | CD4 cells, % | CD4 cells, n × 106 | Spleen cells,*n × 106 | CD4 cells, % | CD4 cells, n × 106 |
|---|---|---|---|---|---|---|
| B7-/- | 4.0 ± 0.85 | 51.5 | 2.0 ± 0.44 | 24.0 ± 3.39 | 21.5 | 5.16 ± 0.73 |
| B7+/+ | 0.4 ± 0.04 | 26.8 | 0.1 ± 0.01 | 27.6 ± 3.20 | 16.7 | 4.6 ± 0.53 |
CD4+ T cells purified (98% pure) from B7 wt (+/+) or B7-deficient (-/-) donor mice were transferred intravenously into syngeneic RAG-2-deficient recipients. Recipients of unseparated CD4+ T cells were killed 30 days after transfer. The mandibular, superficial cervical, axillary, superficial inguinal and mesenteric lymph nodes (LNs), and spleens of all recipients were isolated, and the total cell numbers were determined. The percentage of CD4+ T cells in lymph nodes and spleens were determined by FACS analysis by using anti-CD4 (Pharmingen RM4-5 PE) on a Beckman—Coulter. Five mice were analyzed per group. Data are given as mean ± SD.
Mandibular, cervical, axillary, inguinal, and mesenteric lymph nodes (LN).
Table 2. Gastritis/colitis in mice given CD4 cells deficient in B7 expression.
| B7 genotype of donor CD4+T cells
|
Mean gastritis score (grade 0.0-3.0)
|
Severe gastritis (grade 2.5-3.0)
|
Mean colitis score (grade 0.0-5.0)
|
Severe colitis (grade 3.0-5.0)
|
|
|---|---|---|---|---|---|
| CD4CD25- | CD4CD25+ | ||||
| -/- | None | 2.5 ± 0.8 | 6/8* | 4.0 ± 1.3 | 6/8 |
| -/- | -/- | 2.6 ± 0.4 | 4/5 | 4.2 ± 0.8 | 4/5 |
| -/- | +/+ | 2.3 ± 0.5 | 6/8* | 3.5 ± 1.5 | 6/8 |
| +/+ | None | 1.7 ± 1.2 | 3/5 | 4.7 ± 0.6 | 5/5 |
| +/+ | -/- | Not done | Not done | Not done | Not done |
| +/+ | +/+ | 0.0 + 0.0 | 0/5 | 0.0 + 0.0 | 0/5 |
Th (CD4CD25-) or T-reg (CD4CD25+) cells (n = 106) purified (98% pure) from B7 wt or B7-deficient donor mice were transferred i.v. into syngeneic RAG-2-deficient recipients. Recipients were killed at day 8. All mice were preserved in Bouins' solution. Histological analyses of skin, stomach, and colon and disease scoring were performed by the Harvard University Rodent Pathology Core. Five to 10 mice were analyzed per group.
n = 10. Two mice died of wasting before histological analysis.
Histological analysis of these mice revealed inflammatory infiltrates in the dermis and epidermis and lesions in stomach and colon that were consistent with gastritis and colitis (Fig. 3b). Dermatitis was marked by inflammatory cells in the subdermal layer interspersed with hyperplastic endocrine cells and hyalinization of subepithelial space; gastritis was associated with lymphocyte clusters in the mucosa, severe destruction of the mucosal architecture, disappearance of parietal and chief cells, and hyperplasia of mucous and endocrine cells. Colitis was characterized by accumulation of inflammatory cells between glands, mucin depletion from goblet cells, and marked thickening of the glands. These findings indicate that the unremarkable phenotype of B7-deficient mice (22) might reflect the sum of two opposing effects: diminished APC costimulatory activity that is unable to activate T cells on the one hand and diminished susceptibility of B7-deficient T cells to suppression by T-reg on the other.
Retroviral Expression of B7 Restores Susceptibility of B7-Deficient Th Cells to Suppression in Vitro and in Vivo. These data suggest an important role of B7 in the inhibitory interaction between T-effector cells and T-reg. An additional possibility is that T cells from B7-deficient mice undergo defective intrathymic selection leading to increased autoreactive T cells. According to this idea, wasting disease/gastritis/colitis caused by B7-deficient CD4 cells and resistance to suppression in vitro reflects B7-dependent developmental defects rather than deficiency of T-reg targeting molecules. We directly evaluated this explanation by using two distinct experimental approaches. In the first, we tested the susceptibility of B7-deficient CD4 cells that had developed in the thymus that expressed B7 on epithelial or hematopoietic/dendritic cells (24).
First, sublethally irradiated RAG-2-deficient mice reconstituted with either B7 wt or B7-deficient hematopoietic stem cell-enriched bone marrow (BM) and (B7+) RAG-2-deficient BM were used to generate CD4 cells for analysis of susceptibility to T-reg. Development and selection of these B7-deficient CD4 cells in a B7 wt environment (containing B7+ thymic epithelia plus hematopoietic cells) did not restore susceptibility of these CD4CD25– cells to suppression by T-reg: The response of these B7-deficient Th cells was not suppressed by concentrations of T-reg, which effectively suppressed B7+ CD4CD25– cells from RAG-2-deficient recipients of B7 wt hematopoietic stem cells (Fig. 4a).
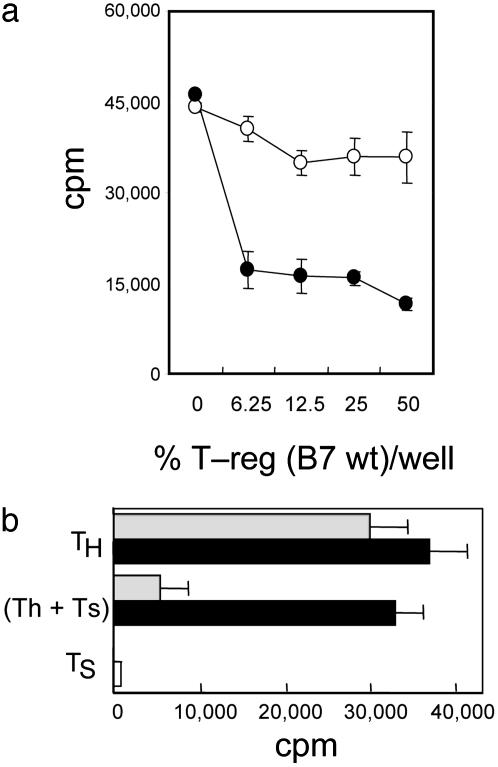
Fig. 4.
Absence of B7 from T cell targets, but not altered T cell development in B7-deficient mice, accounts for resistance to T-reg-mediated suppression. (a) BM chimera-derived B7 wt cells, but not B7-deficient Th cells, are susceptible to T-reg-mediated suppression. CD4CD25– cells (n = 2.5 × 104) from BM chimeric mice reconstituted with B7-deficient BM (○) or B7 wt BM (•) and the indicated numbers of B7 wt CD4CD25+ T-reg were stimulated by themselves or admixed on 1 × 105 irradiated B7 wt splenocytes with anti-CD3 mAb 2C11 (10 μg/ml). Purity of all cell isolations was ≥98%; results shown are representative of three independent experiments with a total of 10 mice per group. (b) Lentiviral expression of B7-1 reconstitutes susceptibility of B7-deficient Th cells to T-reg-mediated suppression. Empty vector pL6/V5 control transfectants (n = 2.5 × 104, black bars) or B7-1-transfected CD4CD25– T cell targets (n = 2.5 × 104, gray bars) were stimulated on 2 × 105 B7 wt irradiated splenocytes with soluble anti-CD3 (10 μg/ml) in the presence or absence of 2.5 × 104 B7 wt CD4CD25+ T-regs. Proliferation was determined by measuring incorporation of [3H]thymidine (1 μCi per well) during the last 18 h of a 66-h culture by using scintillation counting. Results are representative of two independent experiments.
A second indication that absence of B7 on Th cells, rather than altered T cell development, accounted for resistance to suppression came from the effects of B7 reexpression in vitro on susceptibility to suppression. Lentiviral-mediated expression of B7-1 in CD4CD25– cells from B7-deficient mice specifically restored susceptibility to T-reg activity (Fig. 4b).
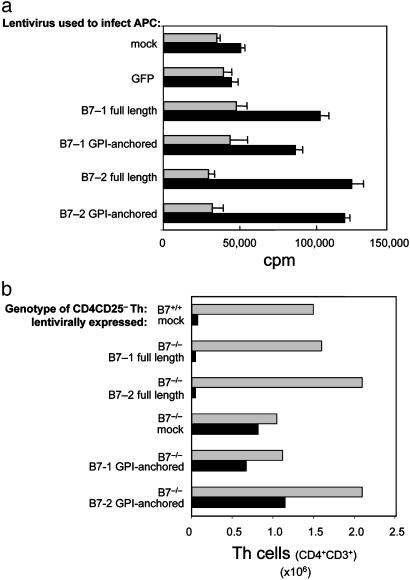
An important corollary to the hypothesis that signaling through B7 or B7-associated molecules transmits an inhibitory message is a requirement for the transmembrane/cytoplasmic domain. We therefore compared the ability of lentiviral-mediated expression of mutant B7 molecules to restore susceptibility to T-reg in vivo. Although improperly expressed B7 cytoplasmic deletion mutants fail to costimulate T cells (25), anchorage of the extracellular domain by a GPI signal sequence allows robust expression of costimulatory activity through the formation of pseudo-micelle-based anchorage sites (26). We therefore generated GPI-anchored B7-1 and B7-2 extracellular domains by using the GPI-signal 311–347 sequence from CD16B spliced to the extracellular domains of B7-1 (amino acids 1–247) and B7-2 (amino acids 1–275). GPI-linked expression of B7-1 or B7-2 external domains fully restored CD28-dependent costimulatory activity to B7-deficient dendritic cells (Fig. 5a).
Fig. 5.
Susceptibility of B7-deficient cells to suppression is restored by lentiviral-based expression of full-length, but not truncated, B7 lacking a transmembrane/cytoplasmic domain. (a) Examination of the costimulatory ability of cells infected with full-length or GPI-anchored B7-1 or B7-2. CD28 wt (black bars) or CD28–/– (gray bars) CD4CD25– T cells (n = 5 × 104) were incubated with 1 × 104 irradiated B7-deficient lentivirally infected dendritic cells in the presence of anti-CD3 (10 μg/ml). The ability of full-length or GPI-anchored B7-1 or B7-2 to provide costimulatory activity was determined from the CD28-dependent increase in proliferation. Proliferation was determined by measuring incorporation of [3H]thymidine (1 μCi per well) during the last 18 h of a 66-h culture by scintillation counting. Purity of all cell isolations was ≥98%. Results shown are representative of two independent experiments. (b) Lentiviral expression of B7-1 reconstitutes susceptibility of B7-deficient Th cells to T-reg-mediated suppression. Th (CD4CD25–) mock- or lentivirally infected B7-deficient or B7 wt T cells (n = 106) were transferred intravenously into B7 wt RAG-2-deficient recipients (n = 4 per group) in the presence (black bars) or absence (gray bars) of 0.5 × 106 CD4+CD25+ T cells. Recipients were killed 6 weeks after transfer, and the mandibular, cervical, axillary, inguinal, and mesenteric lymph nodes were then isolated. Total cell numbers were determined by trypan blue exclusion, and the percentage of CD4+ T cells was determined by FACS analysis. Transferred Th cells were ≥97% pure, and expression of lentivirally expressed B7 was ≥95% in all groups as confirmed by FACS analysis (Fig. 6). Data shown for each experimental group is the average of four pooled mice.
We then compared the susceptibility of lentiviral-transduced B7-deficient cells that expressed full-length or GPI-anchored B7-ectodomain to suppression after adoptive transfer into RAG-2-deficient mice. CD4CD25– cells expressing full-length B7 but not GPI-anchored B7-1 or B7-2 truncation mutants were susceptible to suppression by T-reg (Fig. 5b). The observed increase in cells recovered from recipients of B7 truncation mutants in the absence of T-reg possibly reflects increased costimulatory activity of B7+ T cells. The numbers of CD4 cells recovered from recipients of mixtures of CD4CD25– cells expressing a B7 truncation mutant and T-reg cells were indistinguishable from the numbers recovered from recipients of B7-deficient CD4CD25– T cells (alone or with T-reg). CD4CD25– cells expressing costimulatory-competent B7 truncation mutants and B7-deficient CD4CD25– T cells expanded in the presence of T-reg, whereas CD4CD25– recipients of full-length B7-1 or B7-2 CD4+ cells did not expand detectably in the presence of T-reg. These results indicate that expression of costimulationcompetent extracellular B7 domains by CD4CD25– cells does not suffice to restore susceptibility of B7-deficient Th cells to T-reg-dependent suppressive activity (Fig. 5b). Although these findings indicate that B7-dependent suppressive activity, but not costimulatory activity, is transmembrane/cytoplasmic domain-dependent, additional analysis is needed to more precisely dissect the molecular interactions responsible for this B7-based regulatory pathway.
Discussion
Although B7 is expressed by activated T cells, costimulatory activity produced by B7 expressed by APC has been the major focus of research. Recently, expression of B7 on APC has been implicated in immune regulation through tryptophan degradation (27). Outside-in signaling through B7 has been suggested to account for the observation that engagement of B7 on dendritic cells by CTLA-4 Ig or CTLA-4+ T-reg up-regulates tryptophan catabolism in a signal transducer and activator of transcription 1 (STAT-1)/IFN-γ-dependent manner (14, 27), leading to inhibition of T cell responses (28). The present study suggests that outside-in signaling through B7 on activated T cells can effectively inhibit expansion of these T cells in vivo. Because suppression did not depend on IFN-γ production or STAT-1 activation (data not shown), the B7-linked signaling pathway described in this report is distinct from the pathway detected in dendritic cells (Fig. 7, which is published as supporting information on the PNAS web site).
Both B7 receptors, CD28 and CTLA-4, are expressed on T-reg. The CD28 protein is unlikely to contribute to this inhibitory interaction, because T-reg from CD28-deficient donors mediate suppression after activation (29). It is relevant that anti-CTLA-4 Fab fragments, but not intact anti-CTLA-4 Ab, can abrogate T-reg-dependent suppression in vitro (29), and tailless CTLA-4 protein can rescue CTLA-4-deficient mice (13, 30). Disease development in CTLA-4-deficient mice requires costimulation of autoreactive T cells, because CTLA-4/B7 or CTLA-4/CD28 doubly deficient mice are free of inflammatory disease. The blunted phenotype of B7-deficient mice may resemble these doubly deficient animals. In this case, the costimulatory defect of B7-deficient mice may rescue these animals from lethal inflammatory disease associated with disruption of the T-reg suppressive pathway.
The apparent synergy of B7-1 and B7-2 expression for efficient transmission of suppression after engagement by T-reg may reflect complementary signaling roles of B7-1 and B7-2 and/or differential engagement of these molecules by counter-receptors on T-reg cells. Although CTLA-4/B7-1 and CD28/B7-2 pairing is favored in solution (31), this view has not fully explained the varying effects of reagents specific for B7-1 and/or B7-2 in modifying clinically relevant transplantation and autoimmune responses. Interpretation of these B7-based experimental approaches has assumed that therapeutic effects reflected costimulatory blockade of APC. Delineation of the signaling pathways that underlie B7-dependent suppressive signaling may provide new insight into these studies and improve the design of new trials. Further definition of the intracellular events associated with B7 engagement leading to the suppressed T cell phenotype should also allow new and more direct approaches to B7-based immunotherapy.
In sum, these studies delineate the role of B7 in the suppressive interaction between CD25+ and CD25– subpopulations of CD4 cells. Additional studies are needed to evaluate the role of B7-independent interactions among CD4 and CD8 subsets (32) in regulating the tissue-specific autoimmune diseases described here.
Supplementary Material
Abbreviations: T-reg, CD4+CD25+ regulatory T cell; FACS, fluorescence-activated cell sorter; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; GPI, glycosylphosphatidylinositol; wt, wild type; PE, phycoerythrin; Th, T helper; RAG-2, recombination activation gene 2; BM, bone marrow.
References
- 1.McHugh, R. S., Shevach, E. M. & Thornton, A. M. (2001) Microb. Infect. 3, 919–927. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. (2002) Nat. Immunol. 3, 135–142. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. (2003) Nat. Immunol. 4, 330–336. [DOI] [PubMed] [Google Scholar]
- 4.Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299, 1057–1061.12522256 [Google Scholar]
- 5.Bystry, R. S., Aluvihare, V., Welch, K. A., Kallikourdis, M. & Betz, A. G. (2001) Nat. Immunol. 2, 1126–1132. [DOI] [PubMed] [Google Scholar]
- 6.Wood, K. J. & Sakaguchi, S. (2003) Nat. Rev. Immunol. 3, 199–210. [DOI] [PubMed] [Google Scholar]
- 7.Furtado, G. C., Olivares-Villagomez, D., Curotto de Lafaille, M. A., Wensky, A. K., Latkowski, J. A. & Lafaille, J. J. (2001) Immunol. Rev. 182, 122–134. [DOI] [PubMed] [Google Scholar]
- 8.Bach, J.-F. & Chatenoud, L. (2001) Annu. Rev. Immunol. 19, 131–161. [DOI] [PubMed] [Google Scholar]
- 9.Singh, B., Read, S., Asseman, C., Malmstrom, V., Mottet, C., Stephens, L. A., Stepankova, R., Tlaskalova, H. & Powrie, F. (2001) Immunol. Rev. 182, 190–200. [DOI] [PubMed] [Google Scholar]
- 10.Ermann, J., Szanya, V., Ford, G. S., Paragas, V., Fathman, C. G. & Lejon, K. (2001) J. Immunol. 167, 4271–4275. [DOI] [PubMed] [Google Scholar]
- 11.Shevach, E. M. (2002) Nat. Rev. Immunol. 2, 389–400. [DOI] [PubMed] [Google Scholar]
- 12.Bluestone, J. A. & Abbas, A. K. (2003) Nat. Rev. Immunol. 3, 253–257. [DOI] [PubMed] [Google Scholar]
- 13.Tivol, E. A., Boyd, S. D., McKeon, S., Borriello, F., Nickerson, P., Strom, T. B. & Sharpe, A. H. (1997) J. Immunol. 158, 5091–5094. [PubMed] [Google Scholar]
- 14.Grohmann, U., Orabona, C., Fallarino, F., Vacca, C., Calcinaro, F., Falorni, A., Candeloro, P., Belladonna, M. L., Bianchi, R., Fioretti, M. C., et al. (2002) Nat. Immunol. 3, 1097–1101. [DOI] [PubMed] [Google Scholar]
- 15.Taylor, P. A., Lees, C. J., Fournier, S., Allison, J. P., Sharpe, A. H. & Blazar, B. R. (2004) J. Immunol. 172, 34–39. [DOI] [PubMed] [Google Scholar]
- 16.Ridge, J. P., Di Rosa, F. & Matzinger, P. (1998) Nature 393, 474–478. [DOI] [PubMed] [Google Scholar]
- 17.Suri-Payer, E. & Cantor, H. (2001) J. Autoimmun. 16, 115–123. [DOI] [PubMed] [Google Scholar]
- 18.Asseman, C., Mauze, S., Leach, M. W., Coffman, R. L. & Powrie, F. (1999) J. Exp. Med. 995, 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman, A. S., Freeman, G., Horowitz, J. C., Daley, J. & Nadler, L. M. (1987) J. Immunol. 139, 3260–3267. [PubMed] [Google Scholar]
- 20.Reiser, H., Freeman, G. J., Razi-Wolf, Z., Gimmi, C. D., Benacerraf, B. & Nadler, L. M. (1992) Proc. Natl. Acad. Sci. USA 89, 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenfield, E. A., Howard, E., Paradis, T., Nguyen, K., Benazzo, F., McLean, P., Hollsberg, P., Davis, G., Hafler, D. A., Sharpe, A. H., et al. (1997) J. Immunol. 158, 2025–2034. [PubMed] [Google Scholar]
- 22.Borriello, F., Sethna, M. P., Boyd, S. D., Schweitzer, A. N., Tivol, E. A., Jacoby, D., Strom, T. B., Simpson, E. M., Freeman, G. J. & Sharpe, A. H. (1997) Immunity 6, 303–313. [DOI] [PubMed] [Google Scholar]
- 23.Mottet, C., Uhlig, H. H. & Powrie, F. (2003) J. Immunol. 170, 3939–3943. [DOI] [PubMed] [Google Scholar]
- 24.Dull, T., Zufferey, R., Kelly, M., Mandel, R. J., Nguyen, M., Trono, D. & Naldini, L. (1998) J. Virol. 72, 8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doty, R. T. & Clark, E. A. (1996) J. Immunol. 157, 3270–3279. [PubMed] [Google Scholar]
- 26.Brunschwig, E. B., Levine, E., Trefzer, U. & Tykocinski, M. L. (1995) J. Immunol. 155, 5498–5505. [PubMed] [Google Scholar]
- 27.Fallarino, F., Grohmann, U., Hwang, K. W., Orabona, C., Vacca, C., Bianchi, R., Belladonna, M. L., Fioretti, M. C., Alegre, M. L. & Puccetti, P. (2003) Nat. Immunol. 4, 1206–1212. [DOI] [PubMed] [Google Scholar]
- 28.Munn, D. H., Sharma, M. D., Lee, J. R., Jhaver, K. G., Johnson, T. S., Keskin, D. B., Marshall, B., Chandler, P., Antonia, S. J., Burgess, R., et al. (2002) Science 297, 1867–1870. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi, T., Tagami, T., Yamazaki, S., Uede, T., Shimizu, J., Sakaguchi, N., Mak, T. W. & Sakaguchi, S. (2000) J. Exp. Med. 192, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson, C. B. & Allison, J. P. (1997) Immunity 7, 445–450. [DOI] [PubMed] [Google Scholar]
- 31.Collins, A. V., Brodie, D. W., Gilbert, R. J., Iaboni, A., Manso-Sancho, R., Walse, B., Stuart, D. I., van der Merwe, P. A. & Davis, S. J. (2002) Immunity 17, 201–210. [DOI] [PubMed] [Google Scholar]
- 32.Hu, D., Ikizawa, K., Lu, L., Sanchirico, M. E., Shinohara, M. L. & Cantor, H. (2004) Nat. Immunol. 5, 516–523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.