Abstract
Ebola hemorrhagic fever is one of the most fatal viral diseases worldwide affecting humans and nonhuman primates. Although infections only occur frequently in Central Africa, the virus has the potential to spread globally and is classified as a category A pathogen that could be misused as a bioterrorism agent. As of today there is no vaccine or treatment licensed to counteract Ebola virus infections. DNA, subunit and several viral vector approaches, replicating and non-replicating, have been tested as potential vaccine platforms and their protective efficacy has been evaluated in nonhuman primate models for Ebola virus infections, which closely resemble disease progression in humans. Though these vaccine platforms seem to confer protection through different mechanisms, several of them are efficacious against lethal disease in nonhuman primates attesting that vaccination against Ebola virus infections is feasible.
Keywords: animal model, Ebola virus, filovirus, prophylaxis, vaccine
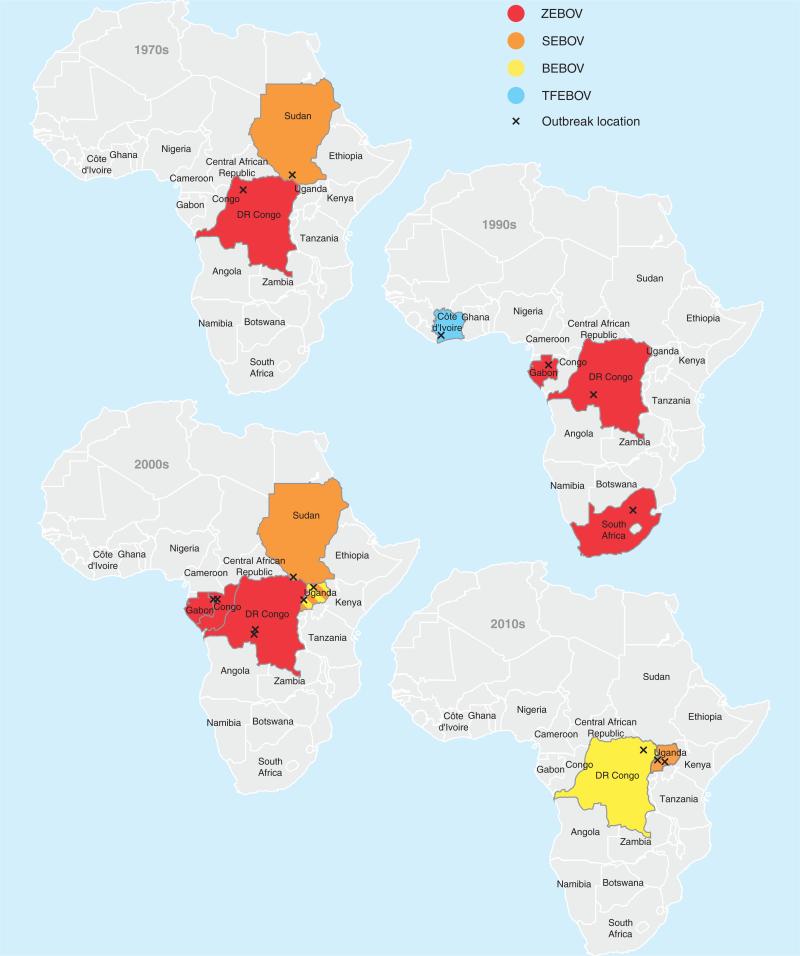
Filoviruses are negative strand RNA viruses within the order of Mononegavirales [1]. Both members of the Filoviridae family, Ebola virus [EBOV; species Zaire ebolavirus (ZEBOV), Sudan ebolavirus (SEBOV), Taï Forest ebolavirus and Bundibugyo ebolavirus (BEBOV)] and Marburg virus (MARV; species Lake Victoria marburgvirus), cause a severe form of viral hemorrhagic fever in humans with case fatality rates up to 90% [1]. MARV originates from Africa but was discovered in 1967 during an outbreak in Marburg, Germany. EBOV was identified 9 years later in 1976 in Central Africa with two simultaneous outbreaks in Sudan and Zaire (now Democratic Republic of the Congo) (Figure 1). In the past two decades, EBOV hemorrhagic fever (EHF) epidemics were frequently reported from Central Africa and continue to emerge/re-emerge; for example, in 2012 dozens of EBOV cases were identified in Uganda and in the Democratic Republic of the Congo (Figure 1) [2,3]. Noteworthy, since the beginning of the millennium, EHF outbreak locations have moved from the border region of Gabon and the Republic of Congo (ZEBOV outbreaks) to the east (BEBOV and SEBOV outbreaks) accompanied by a change in the EBOV species causing the infections (Figure 1). Although EBOV pathogenesis has been well characterized in animal disease models, particularly the macaque models, and the limited data available from human cases, there is still no licensed vaccine or treatment available. EBOV is a category A pathogen and can only be handled in maximum containment laboratories.
Figure 1. Ebola hemorrhagic fever outbreaks in Africa since discovery in 1976.
Maps depicting documented outbreaks of Ebola hemorrhagic fever in Africa for the last five decades. 1970s – five documented outbreaks; 1980s – no reported outbreaks; 1990s – six confirmed outbreaks; 2000s – 10 documented outbreaks; 2010s – so far five confirmed outbreaks.
BEBOV: Bundibugyo ebolavirus; SEBOV: Sudan ebolavirus; TFEBOV: Taï forest ebolavirus; X: outbreak location; ZEBOV: Zaire ebolavirus.
The ‘gold standard’ animal disease models for EBOV are the rhesus and cynomolgus macaque, which can be infected with non-adapted virus strains. Immunocompetent rodent models are not commonly available for EBOV species with the exception of ZEBOV. For ZEBOV mouse, hamster and guinea pig disease models have been established using either mouse-adapted (MA-ZEBOV) or guinea pig-adapted (GPA-ZEBOV) challenge strains [4,5]. While the rodent disease models show limitations in representing certain aspects of EHF in humans, the macaque model is considered the surrogate EHF model displaying all critical parameters of disease [6]. For highly pathogenic viruses, such as EBOV, for which efficacy of therapeutics, antivirals and vaccines cannot be tested in humans, the US Food and Drug Administration has implemented the animal rule allowing licensing based on efficacy data in animal models recapitulating human disease in combination with safety and immunogenicity studies in humans.
The development of vaccines and treatment options for EHF has been a priority of research laboratories and a few pharmaceutical companies resulting in ongoing testing of different approaches. The vaccine platforms currently available for experimental analyses range from DNA and subunit vaccines to nonreplicating and replication-competent viral vectors and are mostly specific for the species ZEBOV. In the more recent past, efforts have been made to shed light on the mechanism of protection for EBOV vaccines. Several different studies resulted in similar conclusions clearly pointing toward a critical role of antibodies for protection. In this review, we compare the available viral vector-based vaccine platforms and some other immunization strategies in regard to their protective efficacy mainly by evaluating the immune responses elicited by each vaccine candidate (Tables 1 & 2).
Table 1.
Comparison of immune responses induced in rodents.
| Vaccine candidate | Rodent species |
ZEBOV antigen(s) |
Vaccine doses (n) |
Time to challenge (days) |
Survival rate (%) |
T cell response |
Humoral response |
Ref. |
|---|---|---|---|---|---|---|---|---|
| Inactivated virus | Guinea pig | Whole virus | 1 | 21 | 100 | n.a. | n.a. | [7] |
| Virus-like particles | Mouse | GP, VP40 | 3 | 84 | 100 | ++ | ++ | [10–12] |
| Virus-like particles | Mouse | GP, NP, VP40 | 2 | 49 | 100 | ++ | ++ | [15] |
| Alphavirus replicon | Mouse | NP | 2 | 56 | 80 | +++ | ++ | [19] |
| Alphavirus replicon | Mouse | GP, NP | 2 | 56 | 100 | n.a. | ++ | [17] |
| Alphavirus replicon | Guinea pig | GP, NP | 3 | 160 | 100 | n.a. | ++ | [17] |
| Flavivirus replicon | Guinea pig | GP | 2 | 40 | 86 | n.a. | ++ | [22] |
| DNA | Mouse | GP | 2 | 70 | 100 | n.a. | ++ | [27] |
| DNA | Mouse | GP | 1 | 28 | 100 | ++ | ++ | [28] |
| DNA | Guinea pig | GP, NP | 3 | 147 | 67 | n.a. | ++ | [25] |
| DNA | Guinea pig | GP | 2 | 58 | 100 | n.a. | ++ | [28] |
| rec. Adenovirus | Mouse | GP | 1 | 28 | 100 | - | +++ | [47] |
| rec. Adenovirus | Guinea pig | GP | 3 | 132 | 100 | + | +++ | [29] |
| rec. Adenovirus | Guinea pig | GP | 1 | 28 | 82 | n.a. | ++ | [47] |
| rec. Ebola virus ΔVP30 | Mouse | Whole virus | 2 | 42 | 100 | + | +++ | [50] |
| rec. Ebola virus ΔVP30 | Guinea pig | Whole virus | 2 | 63 | 100 | n.a. | n.a. | [50] |
| rec. Vaccina virus | Guinea pig | GP | 1 | 30 | 60 | n.a. | ++ | [52] |
| rec. Cytomegalovirus | Mouse | NP epitope | 2 | 70 | 100 | +++ | +/− | [53] |
| rec. Paramyxovirus | Guinea pig | GP | 1 | 25 | 100 | n.a. | ++ | [57] |
| rec. Vesicular stomatitis virus | Mouse | GP | 1 | 14 | 100 | - | +++ | [65] |
| rec. Vesicular stomatitis virus | Hamster | GP | 1 | 3 | 100 | n.a. | ++ | [67] |
| rec. Vesicular stomatitis virus | Guinea pig | GP | 1 | 21 | 100 | n.a. | ++ | [66] |
| rec. Rabiesvirus | Mouse | GP | 1 | 77 | 100 | ++ | ++ | [74] |
+: Contributing to protection; ++: Important for protection; +++: Critical for protection; +/−: Not conclusive; n.a.: Not analyzed.
Table 2.
Comparison of immune responses induced in NHPs.
| Vaccine candidate | ZEBOV antigen(s) | Vaccine doses (n) | Time to challenge (days) | Survival rate (%) | T cell response | Humoral response | Ref. |
|---|---|---|---|---|---|---|---|
| Inactivated virus | Whole virus | 3 | 78 | 25 | n.a. | + | [8] |
| Virus-like particles | GP, NP, VP40 | 3 | 126 | 100 | + | +++ | [13] |
| Alphavirus replicon | GP | 1 | 28 | 100 | n.a. | ++ | [21] |
| DNA + rec. Adenovirus | GP | 4 | 224 | 100 | ++ | ++ | [29] |
| rec. Adenovirus | GP | 1 | 28 | 100 | +++ | ++ | [46] |
| rec. Vaccina virus | GP | 3 | 98 | 0 | n.a. | + | [8] |
| rec. Paramyxovirus | GP | 2 | 67 | 100 | + | ++ | [56] |
| rec. Vesicular stomatitis virus | GP | 1 | 28 | 100 | +/− | +++ | [72] |
| rec. Rabiesvirus | GP | 1 | 75 | 100 | +/− | +++ | [77] |
+: Contributing to protection; ++: Important for protection; +++: Critical for protection; +/−: Not conclusive; n.a.: Not analyzed.
Inactivated virus & subunit vaccines
Shortly after the discovery of EBOV in 1976, attempts for vaccine development were initiated using inactivated virus. Although inactivated ZEBOV was efficacious in guinea pigs infected with ZEBOV [7], nonhuman primates (NHPs) were not protected from lethal disease when given inactivated virus with or without liposome [8]. A few decades later, attempts were made using classical subunit vaccines such as recombinant expressed purified viral proteins. However, these strategies were only shown to be partially protective in rodent models for ZEBOV infection (reviewed in [9]), and further improvement of immunogenicity is needed in order to justify efficacy studies in the macaque model.
A more complex protein-based vaccine approach is the platform using virus-like particles (VLPs). VLPs consist of the ZEBOV matrix protein VP40 and glycoprotein (GP). In some cases, the nucleoprotein (NP) is also present in these preparations. VP40 expression in cells leads to ZEBOV-like particle formation and budding from the cells. Coexpression of GP and/or NP leads to incorporation of these proteins in the VLPs. Efficacy studies in rodents have resulted in 100% protection from lethal ZEBOV infection with VLPs consisting of VP40 and GP [10–12]. When NHPs were vaccinated three-times with VLPs containing GP, NP and VP40 and the RIBI adjuvant, the animals elicited immune responses that were protective against lethal ZEBOV challenge [13]. The VLP platform is considered a safe vaccine approach in comparison to some of the replication-competent platforms, which will be discussed in the following chapters. Furthermore, VLPs are highly immunogenic, and vaccination has been shown to induce innate, humoral and cellular immune responses [14]. In order to be able to increase VLP production, which was done in limited quantities in 293T cells, researchers switched to a baculovirus-based expression system using insect cells. VLPs produced in these insect cells are immunogenic and have proven to be efficacious with QS-21 adjuvant in mice [15]; however, their efficacy to protect NHPs against lethal ZEBOV challenge remains to be evaluated.
This platform has also been developed for MARV, and cross-protection against ZEBOV and MARV was evaluated in guinea pigs using chimeric VLPs (ZEBOV-GP/MARVVP40 and vice versa) as well as a blend of ZEBOV- and MARV-like particles. The results demonstrated that protection is dependent on GP, and the blended VLPs show better efficacy compared with the chimeric VLPs [10]. However, the mechanism of protection for the VLP platform has not yet been defined, but most certainly humoral immune responses are more significant as shown for other protein-based vaccines like the human papilloma vaccine [16]. The incorporation of NP into the VLP preparation may add further targets for T cell-mediated immunity, but these immune responses have not yet been investigated.
Nonreplicating vaccine vectors
Alphavirus & flavivirus replicons
Venezuelan equine encephalitis virus (VEEV) is an alphavirus and was used early on in the EBOV vaccine development as a potential platform. In order to generate the VEE replicon vaccines, the structural genes of an attenuated VEEV strain were replaced with ZEBOV-GP, -NP, -VP24, -VP30, -VP35 or -VP40 and expressed from an RNA expression vector; particle formation was achieved by providing the structural VEEV genes in trans [17,18]. Although all the vectors were immunogenic in mice, only the one expressing ZEBOV-NP conferred 100% protection in the mouse model; furthermore, a combination of the vectors expressing ZEBOV-GP and ZEBOV-NP resulted in 100% survival in mice [17,18]. It was demonstrated that ZEBOV-NP elicited a strong cytotoxic T lymphocyte (CTL) response in mice, and adoptive transfer of T cells from vaccinated into naïve mice was protective [19]. In contrast, transfer of serum antibodies did not protect naïve mice from lethal MA-ZEBOV infection [17]. Protective efficacy of these two promising VEEV/ZEBOV vaccine vectors was further investigated using strain 13 guinea pigs. The result differed from the data obtained in mice showing that the VEEV/ZEBOV-GP vector alone or in combination with the vector-expressing ZEBOV-NP showed 100% protection [17]. Passive transfer of serum from vaccinated animals into naïve strain 13 guinea pigs resulted in no protection from lethal infection [17] hinting toward a critical role of CTL responses for this vaccine in rodents. Ultimately, the promising VEEV-based vaccine vectors were tested in cynomolgus macaques. Groups of three animals were immunized with three doses of VEEV/ZEBOV-GP or VEEV/ZEBOV-NP or a combination of both vectors. After infection with 1000 pfu ZEBOV, all animals developed viremia and needed to be euthanized 6 or 7 days after challenge [8]. This vaccine approach was further developed for biodefense purposes into a multiagent platform [20]. Recently, the improvement of the manufacturing process for VEEV/ZEBOV-GP and VEEV/SEBOV-GP enabled vaccination with a dose of 1010 particles, 1000-times higher than previously administered [8,21]. For both vaccines, one dose was fully protective in NHPs against homologous challenge, but cross-species protection was only partially observed. The authors could demonstrate that for protection against aerosol infection with SEBOV, two vaccine doses were required; one dose was not sufficient [21]. Only humoral immune responses following vaccination were analyzed, no data exist for postchallenge humoral and T cell responses. This improved vaccine approach is promising, but the fact that a very high vaccine dose is needed for immunization, vaccine production could be a potential caveat. Furthermore, more effort needs to be made to understand the mechanism of protection.
Recently, Reynard et al. developed a vaccine replicon based on Kunjin virus, an Australian subtype of West Nile virus in the Flaviviridae family, expressing different versions of ZEBOV-GP [22]. The protective efficacy of this platform was evaluated in guinea pigs resulting in partial protection with up to 86% survival. All the animals responded to the vaccine, the antigen-specific antibody responses were analyzed and shown to be variable between the animals; T cell immunity was not evaluated. This platform needs further improvement in regard to vaccination dose and time in order to justify efficacy studies in NHPs.
DNA vaccines
DNA vaccination has been developed over the last two decades for a number of viruses including ZEBOV. Particularly in regard to emerging and re-emerging pathogens, DNA vaccines have the advantage to be rapidly adapted as pathogens evolve and that the plasmids are noninfectious and easy to produce in large quantities. Furthermore, as pre-existing immunity is not relevant, this approach is reusable. DNA vaccines have been shown to induce cellular as well as humoral immune responses, but regularly require administration of several doses to achieve the desired immunity [23]. For ZEBOV, the first successful immunization strategy using DNA was described in 1998 showing that 100% of the vaccinated mice can be protected from lethal disease when given four doses of plasmid DNA encoding either ZEBOV-GP or ZEBOV-NP [24]. Partial protective efficacy with three doses of plasmid DNA was later reported for strain 13 guinea pigs. Notably, 50% of the surviving animals developed viremia [25]. For NHPs, there are no data for DNA vaccination alone, but DNA combined with immunization by recombinant Adenovirus 5 (rAd5)-based vectors was effective and is discussed below. Subsequently, a Phase I clinical trial was initiated showing that three doses of a DNA vaccine-encoding ZEBOV-GP, -NP and SEBOV-GP are immunogenic in humans. The 20 participants in this study showed no adverse effects to the immunizations and all developed specific antibodies as well as CD4+ T cell responses to at least one of the vaccine components. In addition, vaccine-specific CD8+ T cells were detected in eight individuals [26].
In the past year, a DNA vaccination strategy for filoviruses was further improved by using plasmids expressing codon-optimized antigen and intramuscular electroporation allowing for administration of larger quantities of DNA. Groups of mice were vaccinated with two or three doses of a combination of plasmids encoding ZEBOV-GP, SEBOV-GP and MARV-GP or each plasmid alone, and animals were subsequently challenged with a lethal dose of MA-ZEBOV. All animals that received the ZEBOV-GP DNA vaccine were protected [27]. Antigen-specific IgG responses were detected by ELISA. It could be shown that intramuscular electroporation was most efficient, but the vaccine dose, 1, 5 or 20 μg DNA, did not significantly influence the level of the humoral response [27]. T cell immunity was not analyzed in this study. The authors were very encouraged by these data and hope to carry the project forward to evaluate the protective efficacy in NHPs.
More recently, a prime/boost approach of a mix of plasmids encoding optimized ZEBOV-GP, SEBOV-GP and MARV-GP has been shown to protect guinea pigs from lethal GPA-ZEBOV challenge [28]. A very robust antibody response was measured and it was suggested that these antibodies might have contributed to protection. Furthermore, the authors investigated immune responses after only one vaccination in the mouse model and found that antibodies as well as T cell responses of the Th1-type seem to be important for protection. A single immunization protected 100% of vaccinated mice from lethal MA-ZEBOV challenge [28]. This is a very promising vaccine that can be produced relatively inexpensively and rapidly, but in order to carry this further, NHP efficacy testing is required.
Recombinant adenovirus-based vectors
The use of recombinant Adenovirus 5 (rAd5)-based vectors expressing EBOV antigens as a vaccine was first described in 2000 by Sullivan et al [29]. For this purpose, EBOV-GP or -NP was introduced into the rAd5 full-length plasmid, and expression in infected cells was confirmed as described previously [30]. Initially, the most promising rAd5-based vector, the one expressing ZEBOV-GP as an antigen, was used to boost a DNA prime vaccination. The immunization strategy took 6 months to be completed, but was the first one to be 100% protective in NHPs against lethal ZEBOV challenge [29]. Vaccination with a single dose rAd5-containing ZEBOV-GP as the antigen [rAd5/ZEBOV-GP; 1010 infectious units (IFU)] was later evaluated and shown to be efficacious in NHPs against lethal ZEBOV infection [31]. A new rAd5 vector expressing a codon-optimized ZEBOV-GP was tested in rodents and shown to be protective at lower doses [32]. Recently, a dose of 1010 IFU of this improved vaccine was tested in NHPs in combination with 109 IFU rAd5-expressing interferon α (rAd5/IFNα) resulting in 100% survival of the immunized animals [33]. Although the vaccine doses are quite high in titer, the rAd5 platform is a non-replicating vaccine approach and therefore considered safe. In contrast, the production of this high-titered vaccine could be problematic.
A significant problem for the use of this vaccine platform in humans is pre-existing immunity to Ad5 varying between 60 and 90% in certain populations [34]. In experimental infections of rodents and NHPs, it was demonstrated that pre-existing immunity significantly lowered protective efficacy of Ad5-based vaccines [33,35–39], and scientist have started to improve the immunization route or the vaccine vector itself to overcome this problem. It could be demonstrated that delivery of the vaccine via the oral, nasal or intratracheal route can circumvent pre-existing immunity without affecting the protective efficacy against lethal challenge; in addition, the stimulation of T cell responses was significantly improved [33,36,37,39]. Furthermore, administration of a boosting vaccine dose has been shown to overcome the presence of rAd5-specific immunity in NHPs [40]. In addition, effort has been made to change the rAd5 vector to a different adenovirus serotype-based backbone with less or no pre-existing immunity in humans, like Ad26 and Ad35 [38], or to primate-specific adenoviruses, such as chimpanzee Ad and simian Ad21 [35,41]. Recently, rAd26 and rAd35 have been employed to develop a pan-filovirus vaccine approach and, although no challenge data were provided, it was shown that antigen-specific antibodies and T cell responses could be induced [42]. This was not the first study demonstrating immunity against multiple filoviruses using this platform. A complex/blended Ad-based vaccine containing several filovirus antigens was reported to protect NHPs against lethal challenge with the individual homologous viruses [40,43]. In addition to the complex/blended vaccine approach, cross-protection against BEBOV was achieved using rAD5-expressing ZEBOV-GP and SEBOV-GP [44]. These results demonstrate that it should be possible to achieve cross-protective immunity against several filovirus species.
The rAd5 vaccine-expressing ZEBOV-GP has been tested in a Phase I clinical trial and was found to be safe and immunogenic [45]. The individuals developed dose-dependent antigen-specific T cell responses, although the quality of the immune responses were likely insufficient for protection. In an earlier study, NHP protection from the rAd5 vaccine-expressing ZEBOV-GP correlated with CD8+ T cell responses [46]. More recently, however, Wong et al. analyzed ZEBOV-GP-specific T cell immunity and antibody responses in rodents and NHPs immunized with rAd5 vaccines and found that the critical component for protection was ZEBOV-GP-specific IgG and not T cell immunity [47].
Recombinant ZEBOVΔVP30
The development of reverse genetics systems for RNA viruses including ZEBOV enabled the research community to generate genetically diverse variants of these viruses. Halfmann and colleagues described in 2008 the establishment of a recombinant biologically contained ZEBOV [48]. To this end, an essential gene in the ZEBOV genome, VP30 (a virus-specific transcription activator) was deleted and the resulting recombinant virus, rZEBOVΔVP30, lost its ability to replicate. Propagation of rZEBOVΔVP30 needs provision of VP30 in trans, otherwise no progeny virus can be produced and the virus cannot spread [48]. The safety of this recombinant virus was evaluated in STAT1−/− mice, which are highly susceptible to ZEBOV infection [49]. rZEBOVΔVP30 infection of these animals did not cause any signs of disease, viremia or death and the authors concluded that rZEBOVΔVP30 might be an interesting vaccine candidate [50]. Inoculation of Balb/c mice with rZEBOVΔVP30 resulted in robust ZEBOV-GP antibody and ZEBOV-NP CTL responses and all the animals survived a lethal challenge with MA-ZEBOV. Furthermore, this vaccine was able to confer 100% protection in the guinea pig model for ZEBOV [50]. Data on the efficacy of this vector in the NHP model is currently not available.
rZEBOVΔVP30 is a noteworthy vaccine approach as the vector resembles wild-type (wt) ZEBOV very closely and contains all but one protein to elicit ZEBOV-specific immune responses. Nevertheless, concerns remain with the fact that this virus is 95% identical to wtZEBOV, lacking only one essential gene. Currently, there is no evidence for recombination events during EBOV replication that could lead to re-integration of VP30 into the viral genome. This safety concern has been addressed experimentally by serial passaging of rZEBOVΔVP30 in Vero cells expressing VP30 with no evidence for recombination over seven passages.
Replication-competent vaccine vectors
Recombinant vaccinia virus-based vaccine vectors
One of the first platforms engaged to develop an EBOV vaccine was based on vaccinia virus (VV). VV belongs to the orthopoxviruses in the Poxviridae family and is an enveloped virus with a single, linear, double-stranded DNA genome of 130–300 kb [51]. VV-based vaccines were generated by homologous DNA recombination and, in case of EBOV, several ZEBOV genes were chosen as single antigens: GP, solube GP (sGP), NP, the polymerase co-factor VP35 and VP40. When strain 13 guinea pigs were vaccinated with each single vector, only VV expressing GP showed partial protection (three of five animals survived), the other vectors did not confer any protection [52]. The surviving guinea pigs did not develop viremia, and the study was followed up with a NHP experiment. Three cynomolgus macaques were vaccinated subcutaneously with three doses of the VV vector expressing GP [8]. Although the animals developed an antibody response to the vaccine, they became viremic and needed to be euthanized on days 6 and 7 after challenge with 1000 pfu ZEBOV [8]. This vaccine approach has since not been further developed for ZEBOV.
Recombinant cytomegalovirus-based vaccine vectors
Another DNA virus-based vaccine platform has been recently developed with particular interest in immunizing African wildlife. In a ‘proof-of-concept,’ a recombinant murine Cytomegalovirus (CMV), family Herpesviridae, was genetically engineered to express a CTL epitope located on ZEBOV-NP (amino acid 43–54) by fusing it to the ie2 gene. CTL responses to the ZEBOV-NP epitope were easily detected after a single immunization in mice [53]. Subsequently, C57BL/6 mice were vaccinated with two doses of recombinant murine CMV/ZEBOV-NP and challenged with a lethal dose of MA-ZEBOV. The vaccinated mice survived the challenge, but were not protected from MA-ZEBOV replication [53]. These data point toward a role of CTL responses in protection from lethal ZEBOV infection, but clearly need to be verified in more animal models using species-specific CMVs (e.g., macaques).
The CMV platform represents a disseminating vaccine platform based on spread of infected/vaccinated individuals to contact animals and persistence of the virus in the host. This is a desirable feature for a wildlife vaccine, and because this virus is highly species-specific, spread into humans or other wildlife populations with a gorilla- or chimpanzee-specific CMVEBOV vaccine would be unlikely. If wildlife (e.g., great apes) in ZEBOV endemic areas could be successfully vaccinated, outbreaks could likely be prevented since hunting and preparation of bush meat for consumption is one of the sources for ZEBOV infections in humans.
Recombinant paramyxovirus-based vectors
In addition to DNA virus-based vaccines, several approaches have been undertaken using negative-stranded RNA virus vectors. One of these platforms is based on human parainfluenza virus 3 (HPIV3). This virus is a common respiratory pathogen, and a recombinant HPIV3 (rHPIV3) has been investigated as a dual vaccine approach against HPIV3 and measles infections in infants [54]. For vaccination against ZEBOV, rHPIV3 was modified to express the ZEBOV-GP and/or ZEBOV-NP [55]. A single dose of each vector, rHPIV3/ZEBOV-GP or rHPIV3/ZEBOV-GP/NP, given intranasally to guinea pigs was sufficient to protect all the animals from lethal disease [55]. In rhesus macaques, two vaccine doses were required to achieve 100% protection when given via the respiratory tract [56]. As with the rAd5 vaccines, pre-existing immunity against HPIV3 may influence the efficacy of this vaccine platform. In order to circumvent these reactions, the vaccine vector was improved by deleting the HPIV3 F and HN genes, which are the main targets for the HPIV3-specific humoral immune response [57]. The resulting vector expressed ZEBOV-GP more efficiently and was attenuated in comparison to the previous construct. Guinea pigs vaccinated with this new rHPIV3ΔFΔHN/ZEBOV-GP construct were protected from lethal challenge with GPA-ZEBOV [57]. Up to date, there are no data available in regard to the protective efficacy of this second-generation HPIV3-based vaccine in NHPs.
One of the main advantages of the HPIV3-based vector platform is the potential for needle-free administration [58], the vaccine is relatively easy to produce in large quantities, and it induces a systemic as well as local immunity in the lungs likely advantageous against aerosol infection. Similar to other vaccine approaches, the induced antibody response seems to correlate with survival, although cellular immunity has not been investigated. However, the main obstacle of pre-existing immunity in humans remains. Therefore, Bukreyev and colleagues developed a new vector based on Newcastle disease virus (NDV), an avian paramyxovirus, with no detectable pre-existing immunity in humans. Rhesus macaques were vaccinated with two doses of this new recombinant vector-expressing ZEBOV-GP (rNDV/ZEBOV-GP) and immune responses were evaluated. The antigen-specific IgG response did not reach the same level compared with vaccination with the original rHPIV3/ZEBOV-GP construct although ZEBOV-GP-specific IgA titers in the lungs were the same for both vaccines as were neutralizing antibody titers [59]. Overall, first data suggest that the rNDV/ZEBOV-GP vector might be less immunogenic than the HPIV3-based vaccine. Protection studies in NHPs immunized with the new vector against lethal ZEBOV challenge have not been performed.
Recombinant vesicular stomatitis virus-based vectors
Vesicular stomatitis virus (VSV) is the prototype member of the family Rhabdoviridae, and a very promising vaccine platform for EBOV is based on the reverse genetics system developed for this virus [60]. The current vector used for the EBOV vaccine lacks the VSV glycoprotein (G), the viral determinant for neurotropism and pathogenicity and resembles an attenuated version of VSV serotype Indiana [61,62]. Pre-existing immunity is very limited in the general population, and if present directed toward VSV-G, which is not existent in this vector [63,64]. The rVSV/ZEBOV vector encodes the ZEBOV-GP as the immunogen in place of VSV-G; this vaccine virus is attenuated but still can be easily propagated, is highly immunogenic and immunized individuals only experience transient vector viremia [61].
The protective potential of the rVSV vaccine against ZEBOV infection has been demonstrated extensively in rodents [65–67] and also in NHPs showing that a single dose can protect animals from lethal disease 4 weeks post-immunzation [68]. The animals were completely protected from disease and did not develop detectable ZEBOV viremia. NHPs were further protected when immunized with a single dose orally [69] or when challenged by the aerosol route [70]. This platform was since then expanded for all known EBOV species by constructing vectors encoding the corresponding GPs [66]. In order to confer protection against multiple species of EBOV and MARV, the single rVSV vectors were blended and administered to NHPs. The animals were protected against lethal challenge with three species of EBOV and MARV, showing that cross-protective immunity can be achieved [71]. For the future, the platform will likely be developed into a single vector conferring protection against several filoviruses.
More current studies are targeted toward the mechanism of protection of the rVSV vaccine platform against ZEBOV challenge. Wong and colleagues analyzed serum samples from vaccinated rodents and NHPs and found that serum IgG levels specific to ZEBOV-GP correlate with survival [47]. Marzi and colleagues depleted NHPs of CD4+ T cells during vaccination with rVSV/ZEBOV-GP resulting in the lack of antigen-specific antibodies and lack of protection in the depleted animals [72]. For both studies, T cell responses were analyzed and found to be sporadic; their contribution to protection is currently unknown. These data indicated that ZEBOV-GP-specific IgG clearly play a critical role and future vaccines should be improved toward prompting antibody responses.
Recombinant rabies virus-based vectors
In 2011, Blaney and colleagues published the first report on another rhabdovirus-based vector, a dual vaccine against Rabies virus (RABV) and ZEBOV for potential use in at-risk populations in Africa. The vector BNPSP333 is based on the reverse genetics system for the RABV vaccine strain SAD B19, which is currently used for wildlife immunizations. This vector contains a mutation at amino acid 333 in the RABV glycoprotein (RABV-G), resulting in decreased neurovirulence in adult mice [73]. First, immunogenicity and vaccine safety of different recombinant RABV (rRABV) vaccine vectors expressing ZEBOV-GP were evaluated, showing that all vectors and an inactivated vaccine are highly attenuated in mice, but confer 100% protection against challenge with RABV and MA-ZEBOV [74,75]. T cell responses did not significantly differ among the vaccine vectors; while humoral immune responses against RABV were comparable, ZEBOV-GP-specific antibodies were significantly elevated when two doses of the inactivated rRABV vaccine were administered [76]. Furthermore, preexisting immunity against RABV did not impact immunogenicity of these vaccines in mice; however, challenge data are not available [76]. This novel vaccine approach for ZEBOV has recently been evaluated in the rhesus macaque model demonstrating that one dose of the rRABV/ZEBOV-GP vector was 100% protective, whereas a single dose of the two other rRABV vaccines resulted in only 50% protection [77]. Adaptive immune responses were carefully investigated concluding that survival from ZEBOV infection was largely dependent on the quality of ZEBOV-GP-specific antibodies [77]. These data are very encouraging towards the development of a dual vaccine for RABV and ZEBOV, which might be more acceptable in an African target population and again support the notion that effective vaccination against ZEBOV seems to depend on induction of strong humoral immune responses.
Expert commentary
The major impediment for EBOV vaccine platforms to move forward at this point appears to be funding for GMP/GLP vaccine production and execution of Phase I and/or Phase II clinical trials. With outbreaks occurring sporadic affecting usually a small number of people in Central Africa, there is no real commercial market for EBOV vaccines fading the interest of larger industry in vaccine development. The biothreat potential of EBOV has been the driving force for the development of EBOV countermeasures such as immunization and treatment strategies. Small biotech companies funded through government contracts seem to be the reasonable choice to move the individual approaches forward. Vaccine evaluation has been performed extensively in the NHP model, and several of the vaccine platforms are ready for clinical trials, the most promising ones being rAd5 and rVSV. Researchers of biocontainment facilities are continuously at risk for exposure and present a study/target group for intervention strategies; thus, vaccination should be considered for this group. Immunization of medical personnel, aid workers and military personnel with deployment orders into EBOV endemic areas or outbreak regions appears feasible as well. Yet, vaccination of at-risk populations in endemic areas will be more difficult requiring ideally a single-dose approach and may not even be justified. During outbreaks (and in case of a bioterrorism event), ring vaccination and protection of local medical personnel and other high-risk exposure groups, such as family members, might be the most appropriate strategy to go forward. This, however, will require immunization strategies with a short time to immunity, single delivery and easy administration. It is further dependent on the release of larger amounts of vaccine doses from industry or federal stockpiles without proper compensation as endemic areas are in regions with poor income and infrastructure. For now, more classical approaches of disease prevention, such as education, avoiding exposure and proper patient isolation, should be considered as alternative interim strategies as they can be immediately implemented. Definite identification of the reservoir species of EBOV might provide opportunities for more effective intervention strategies at an early level in the transmission cycle, preventing introduction of the virus into the human population. Intervention on the level of potential intermediate/interim host species or other end host species such as the great apes will reduce spread among animal species and transmission to humans. Therefore, vaccine approaches targeting wildlife are of interest and importance for animal and public health.
Five-year view
The goal over the next 5 years should be that at least one of the current EBOV vaccine approaches will be moved through licensing for stockpiling in case of emergencies such as outbreaks, case importations or intentional release of this pathogen. This vaccine should then be first considered for immediate immunization of at-risk groups such as researchers in high-containment facilities. Although some light has been shed on the mechanisms of protection for a few EBOV vaccine platforms, further studies into defining the mechanisms of protection mediated by EBOV vaccines need to follow. Interestingly, the level of total IgG antibodies seems to be a common correlate of protection in response to different EBOV vaccine approaches; this important read-out for effective vaccination needs to be verified and further defined in future studies. Furthermore, it will be important to better define the reservoir(s) and potential interim hosts for the different EBOV species to educate people effectively in preventing EBOV transmissions into the human population. Here, hunting and consumption of bush meat are major concerns. In this regard and from a conservation standpoint, it is reasonable to consider wildlife vaccination in Africa, most importantly the great ape populations. The strategy of bivalent (multi-valent) vaccine approaches, simultaneously targeting pathogens with higher animal and/or public health impact (i.e. rabies virus, malaria), might be helpful in increasing interest by industry and compliance in the population.
Key issues.
Ebola viruses cause sporadic outbreaks of hemorrhagic fever in Central Africa with increasing frequency and high case fatality rates.
There are no approved vaccines or treatment strategies available, making efforts toward effective prophylaxis and therapeutics urgent.
Several experimental vaccine approaches have shown promising protective efficacy in nonhuman primate models of Ebola hemorrhagic fever [rec. vesicular stomatitis virus (rVSV), rec Adenovirus 5 (rAd5), virus-like particles, rec. rabies virus (rRABV)], but development has not progressed past Phase I Clinical Trials.
While antibodies have been shown to be a mechanism of protection for the rhabdovirus-based vaccine vectors (rRABV and rVSV), protective efficacy with the rAd5 platform seems to be dependent on CD8+ T cell and antibody responses.
The development of various vaccine platforms is encouraged as they have their advantages/disadvantages for distinct application approaches.
Acknowledgements
The authors would like to thank Austin Athman (Rocky Mountain Laboratories, NIAID) for assistance with production of Figure 1.
Ebola virus research is supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH. H Feldmann claims intellectual property regarding the vesicular stomatitis virus-based filovirus vaccines.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley PM, editors. Fields virology. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2006. pp. 1409–48. [Google Scholar]
- 2.Outbreak news Ebola haemorrhagic fever, Uganda – update. Releve epidemiologique hebdomadaire/ Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record/Health Section of the Secretariat of the League of Nations. 2012;87:493. [Google Scholar]
- 3.Outbreak news Ebola haemorrhagic fever, Democratic Republic of the Congo. Releve epidemiologique hebdomadaire/ Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record/Health Section of the Secretariat of the League of Nations. 2012;87:338–9. [PubMed] [Google Scholar]
- 4.Bente D, Gren J, Strong JE, Feldmann H. Disease modeling for Ebola and Marburg viruses. Dis Model Mech. 2009;2:12–17. doi: 10.1242/dmm.000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebihara H, Zivcec M, Gardner D, et al. A Syrian golden hamster model recapitulating ebola hemorrhagic fever. J Infect Dis. 2013;207:306–18. doi: 10.1093/infdis/jis626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safronetz D, Geisbert TW, Feldmann H. Animal models for highly pathogenic emerging viruses. Curr Opin Virol. 2013;3:205–9. doi: 10.1016/j.coviro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lupton HW, Lambert RD, Bumgardner DL, et al. Inactivated vaccine for Ebola virus efficacious in guineapig model. Lancet. 1980;2:1294–5. doi: 10.1016/s0140-6736(80)92352-1. [DOI] [PubMed] [Google Scholar]
- 8.Geisbert TW, Pushko P, Anderson K, et al. Evaluation in nonhuman primates of vaccines against Ebola virus. Emerg Infect Dis. 2002;8:503–7. doi: 10.3201/eid0805.010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoenen T, Groseth A, Feldmann H. Current ebola vaccines. Expert Opin Biol Ther. 2012;12:859–72. doi: 10.1517/14712598.2012.685152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swenson DL, Warfield KL, Negley DL, et al. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine. 2005;23:3033–42. doi: 10.1016/j.vaccine.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 11.Warfield KL, Bosio CM, Welcher BC, et al. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci USA. 2003;100:15889–94. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Warfield KL, Olinger G, Deal EM, et al. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J Immunol. 2005;175:1184–91. doi: 10.4049/jimmunol.175.2.1184. [Proposes the mechanism of protection for the VLP platform.] [DOI] [PubMed] [Google Scholar]
- 13.Warfield KL, Swenson DL, Olinger GG, et al. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196(Suppl 2):S430–7. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 14.Warfield KL, Aman MJ. Advances in virus-like particle vaccines for filoviruses. J Infect Dis. 2011;204(Suppl 3):S1053–9. doi: 10.1093/infdis/jir346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warfield KL, Posten NA, Swenson DL, et al. Filovirus-like particles produced in insect cells: immunogenicity and protection in rodents. J Infect Dis. 2007;196(Suppl 2):S421–9. doi: 10.1086/520612. [DOI] [PubMed] [Google Scholar]
- 16.Stanley M, Pinto LA, Trimble C. Human papillomavirus vaccines–immune responses. Vaccine. 2012;30(Suppl 5):F83–7. doi: 10.1016/j.vaccine.2012.04.106. [DOI] [PubMed] [Google Scholar]
- 17.Pushko P, Bray M, Ludwig GV, et al. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine. 2000;19:142–53. doi: 10.1016/s0264-410x(00)00113-4. [DOI] [PubMed] [Google Scholar]
- 18.Wilson JA, Bray M, Bakken R, Hart MK. Vaccine potential of Ebola virus VP24, VP30, VP35, and VP40 proteins. Virology. 2001;286:384–90. doi: 10.1006/viro.2001.1012. [DOI] [PubMed] [Google Scholar]
- 19.Wilson JA, Hart MK. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J Virol. 2001;75:2660–4. doi: 10.1128/JVI.75.6.2660-2664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JS, Groebner JL, Hadjipanayis AG, et al. Multiagent vaccines vectored by Venezuelan equine encephalitis virus replicon elicits immune responses to Marburg virus and protection against anthrax and botulinum neurotoxin in mice. Vaccine. 2006;24:6886–92. doi: 10.1016/j.vaccine.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Herbert AS, Kuehne AI, Barth JF, et al. Venezuelan equine encephalitis replicon particle vaccine protects nonhuman primates from ebolavirus intramuscular and aerosol challenge. J Virol. 2013;87(9):4952–64. doi: 10.1128/JVI.03361-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynard O, Mokhonov V, Mokhonova E, et al. Kunjin virus replicon-based vaccines expressing Ebola virus glycoprotein GP protect the guinea pig against lethal Ebola virus infection. J Infect Dis. 2011;204(Suppl 3):S1060–5. doi: 10.1093/infdis/jir347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu S, Wang S, Grimes-Serrano JM. Current progress of DNA vaccine studies in humans. Expert Rev Vaccines. 2008;7:175–91. doi: 10.1586/14760584.7.2.175. [DOI] [PubMed] [Google Scholar]
- 24.Vanderzanden L, Bray M, Fuller D, et al. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology. 1998;246:134–44. doi: 10.1006/viro.1998.9176. [DOI] [PubMed] [Google Scholar]
- 25.Riemenschneider J, Garrison A, Geisbert J, et al. Comparison of individual and combination DNA vaccines for B. anthracis, Ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine. 2003;21:4071–80. doi: 10.1016/s0264-410x(03)00362-1. [DOI] [PubMed] [Google Scholar]
- 26.Martin JE, Sullivan NJ, Enama ME, et al. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccine Immunol. 2006;13:1267–77. doi: 10.1128/CVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant-Klein RJ, Van Deusen NM, Badger CV, et al. A multiagent filovirus DNA vaccine delivered by intramuscular electroporation completely protects mice from ebola and Marburg virus challenge. Hum Vaccin Immunother. 2012;8(11):1703–6. doi: 10.4161/hv.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shedlock DJ, Aviles J, Talbott KT, et al. Induction of broad cytotoxic T cells by protective DNA vaccination against Marburg and Ebola. Mol Ther. 2013;21:1432–44. doi: 10.1038/mt.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan NJ, Sanchez A, Rollin PE, et al. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–9. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 30.Aoki K, Barker C, Danthinne X, et al. Efficient generation of recombinant adenoviral vectors by Cre-lox recombination in vitro. Mol Med. 1999;5:224–31. [PMC free article] [PubMed] [Google Scholar]
- 31•.Sullivan NJ, Geisbert TW, Geisbert JB, et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–4. doi: 10.1038/nature01876. [Introduces the first vaccine platform with efficacy against EBOV in nonhuman primates.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson JS, Yao MK, Tran KN, et al. Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine. PLoS One. 2009;4:e5308. doi: 10.1371/journal.pone.0005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson JS, Pillet S, Bello AJ, Kobinger GP. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. J Virol. 2013;87(7):3668–77. doi: 10.1128/JVI.02864-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mast TC, Kierstead L, Gupta SB, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–7. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 35.Kobinger GP, Feldmann H, Zhi Y, et al. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology. 2006;346:394–401. doi: 10.1016/j.virol.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 36.Croyle MA, Patel A, Tran KN, et al. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS One. 2008;3:e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson JS, Abou MC, Tran KN, et al. Impact of systemic or mucosal immunity to adenovirus on Ad-based Ebola virus vaccine efficacy in guinea pigs. J Infect Dis. 2011;204(Suppl 3):S1032–42. doi: 10.1093/infdis/jir332. [DOI] [PubMed] [Google Scholar]
- 38.Geisbert TW, Bailey M, Hensley L, et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol. 2011;85:4222–33. doi: 10.1128/JVI.02407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi JH, Schafer SC, Zhang L. A single sublingual dose of an adenovirus-based vaccine protects against lethal Ebola challenge in mice and guinea pigs. Mol Pharm. 2012;9:156–67. doi: 10.1021/mp200392g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pratt WD, Wang D, Nichols DK, et al. Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clin Vaccine Immunol. 2010;17:572–81. doi: 10.1128/CVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy S, Zhi Y, Kobinger GP, et al. Generation of an adenoviral vaccine vector based on simian adenovirus 21. J Gen Virol. 2006;87:2477–85. doi: 10.1099/vir.0.81989-0. [DOI] [PubMed] [Google Scholar]
- 42.Zahn R, Gillisen G, Roos A, et al. Ad35 and ad26 vaccine vectors induce potent and cross-reactive antibody and T-cell responses to multiple filovirus species. PLoS One. 2012;7:e44115. doi: 10.1371/journal.pone.0044115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swenson DL, Wang D, Luo M, et al. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin Vaccine Immunol. 2008;15:460–7. doi: 10.1128/CVI.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hensley LE, Mulangu S, Asiedu C, et al. Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus Species. PLoS Pathog. 2010;6:e1000904. doi: 10.1371/journal.ppat.1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ledgerwood JE, Costner P, Desai N, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29:304–13. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 46•.Sullivan NJ, Hensley L, Asiedu C, et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med. 2011;17:1128–31. doi: 10.1038/nm.2447. [Demonstrates the mechanism of protection for the rAd5 platform against EBOV in nonhuman primates.] [DOI] [PubMed] [Google Scholar]
- 47.Wong G, Richardson JS, Pillet S, et al. Immune parameters correlate with protection against ebola virus infection in rodents and nonhuman primates. Sci Transl Med. 2012;4:158ra46. doi: 10.1126/scitranslmed.3004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halfmann P, Kim JH, Ebihara H, et al. Generation of biologically contained Ebola viruses. Proc Natl Acad Sci USA. 2008;105:1129–33. doi: 10.1073/pnas.0708057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raymond J, Bradfute S, Bray M. Filovirus infection of STAT-1 knockout mice. J Infect Dis. 2011;204(Suppl 3):S986–90. doi: 10.1093/infdis/jir335. [DOI] [PubMed] [Google Scholar]
- 50.Halfmann P, Ebihara H, Marzi A, et al. Replication-deficient ebolavirus as a vaccine candidate. J Virol. 2009;83:3810–15. doi: 10.1128/JVI.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moss B. Poxviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia PA, USA: 2006. pp. 2906–45. [Google Scholar]
- 52.Gilligan JK GJ, Jahrling PB, Anderson K. Assessment of protective immunity conferred by recombinant vaccinia virus to guinea pigs challenged with ebola virus. In: BD, Brown F, Doherty P, Mekalanos J, Norrby E, editors. Vaccines. cold spring harbor. Lanoratory Press; Cold Spring Habor, NY, USA: 1997. pp. 87–92. [Google Scholar]
- 53.Tsuda Y, Caposio P, Parkins CJ, et al. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis. 2011;5:e1275. doi: 10.1371/journal.pntd.0001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durbin AP, Skiadopoulos MH, McAuliffe JM, et al. Human parainfluenza virus type 3 (PIV3) expressing the hemagglutinin protein of measles virus provides a potential method for immunization against measles virus and PIV3 in early infancy. J Virol. 2000;74:6821–31. doi: 10.1128/jvi.74.15.6821-6831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bukreyev A, Yang L, Zaki SR, et al. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge. J Virol. 2006;80:2267–79. doi: 10.1128/JVI.80.5.2267-2279.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bukreyev A, Rollin PE, Tate MK, et al. Successful topical respiratory tract immunization of primates against Ebola virus. J Virol. 2007;81:6379–88. doi: 10.1128/JVI.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bukreyev A, Marzi A, Feldmann F, et al. Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge. Virology. 2009;383:348–61. doi: 10.1016/j.virol.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bukreyev AA, Dinapoli JM, Yang L, et al. Mucosal parainfluenza virus-vectored vaccine against Ebola virus replicates in the respiratory tract of vector-immune monkeys and is immunogenic. Virology. 2010;399:290–8. doi: 10.1016/j.virol.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DiNapoli JM, Yang L, Samal SK, et al. Respiratory tract immunization of non-human primates with a Newcastle disease virus-vectored vaccine candidate against Ebola virus elicits a neutralizing antibody response. Vaccine. 2010;29:17–25. doi: 10.1016/j.vaccine.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Scie U S A. 1995;92:4477–81. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marzi A, Feldmann H, Geisbert TW, Falzarano D. Vesicular stomatitis virus-based vaccines for prophylaxis and treatment of filovirus infections. J Bioterror Biodef. 2011:S1. doi: 10.4172/2157-2526.S1-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Mire CE, Miller AD, Carville A, et al. Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS Negl Trop Dis. 2012;6:e1567. doi: 10.1371/journal.pntd.0001567. [Study supporting the safety profile of rVSV vaccines in nonhuman primates.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rose NF, Marx PA, Luckay A, et al. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106:539–49. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 64.Garbutt M, Liebscher R, Wahl-Jensen V, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol. 2004;78:5458–65. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones SM, Stroher U, Fernando L, et al. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis. 2007;196(Suppl 2):S404–12. doi: 10.1086/520591. [DOI] [PubMed] [Google Scholar]
- 66.Marzi A, Ebihara H, Callison J, et al. Vesicular stomatitis virus-based Ebola vaccines with improved cross-protective efficacy. J Infect Dis. 2011;204(Suppl 3):S1066–74. doi: 10.1093/infdis/jir348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsuda Y, Safronetz D, Brown K, et al. Protective efficacy of a bivalent recombinant vesicular stomatitis virus vaccine in the Syrian hamster model of lethal Ebola virus infection. J Infect Dis. 2011;204(Suppl 3):S1090–7. doi: 10.1093/infdis/jir379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones SM, Feldmann H, Stroher U, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–90. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 69.Qiu X, Fernando L, Alimonti JB, et al. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PLoS One. 2009;4:e5547. doi: 10.1371/journal.pone.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, et al. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26:6894–900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Geisbert TW, Geisbert JB, Leung A, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J Virol. 2009;83:7296–304. doi: 10.1128/JVI.00561-09. [Presents that a single dose of a blended rVSV vaccine can protect against multiple species of EBOV and Marburg virus.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Marzi A, Engelmann F, Feldmann F, et al. Antibodies are necessary for rVSV/ZEBOVGP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci USA. 2013;110:1893–8. doi: 10.1073/pnas.1209591110. [First study to demonstrate the mechanism of protection for the rVSV/ZEBOV-GP platform in nonhuman primates.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGettigan JP, Pomerantz RJ, Siler CA, et al. Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 gag have greatly reduced pathogenicity but are highly immunogenic. J Virol. 2003;77:237–44. doi: 10.1128/JVI.77.1.237-244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blaney JE, Wirblich C, Papaneri AB, et al. Inactivated or live-attenuated bivalent vaccines that confer protection against rabies and Ebola viruses. J Virol. 2011;85:10605–16. doi: 10.1128/JVI.00558-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papaneri AB, Wirblich C, Cann JA, et al. A replication-deficient rabies virus vaccine expressing Ebola virus glycoprotein is highly attenuated for neurovirulence. Virology. 2012;434:18–26. doi: 10.1016/j.virol.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Papaneri AB, Wirblich C, Cooper K. Further characterization of the immune response in mice to inactivated and live rabies vaccines expressing Ebola virus glycoprotein. Vaccine. 2012;30:6136–41. doi: 10.1016/j.vaccine.2012.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77•.Blaney JE, Marzi A, Willet M, et al. Antibody quality and protection from lethal ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLoS Pathog. 2013;9:e1003389. doi: 10.1371/journal.ppat.1003389. [First study demonstrating efficacy of a dual vaccine vector in nonhuman primates.] [DOI] [PMC free article] [PubMed] [Google Scholar]