Abstract
Objective
Noise-induced hearing loss (NIHL) is a worldwide health problem and a growing concern among young people. Although some people appear to be more susceptible to NIHL, genetic association studies lack a specific phenotype. We tested the feasibility of a bilateral 4000–6000 Hz audiometric notch as a phenotype for identifying genetic contributions to hearing loss in young adults.
Design
A case-control-control study was conducted to examine selected SNPs in 52 genes previously associated with hearing loss and/or expressed in the cochlea. A notch was defined as a minimum of a 15-dB drop at 4000–6000 Hz from the previous best threshold with a 5-dB ‘recovery’ at 8000 Hz.
Study sample
Participants were 252 individuals of European descent taken from a population of 640 young adults who are students of classical music. Participants were grouped as No-notch (NN), Unilateral Notch (UN), or Bilateral Notch (BN).
Results
The strongest evidence of a genetic association with the 4000–6000 Hz notch was a nonsynonymous SNP variant in the ESRR? gene (rs61742642:C>T, P386S). Carriers of the minor allele accounted for 26% of all bilateral losses.
Conclusion
This study indicates that the 4000–6000 Hz bilateral notch is a feasible phenotype for identifying genetic susceptibility to hearing loss.
Keywords: Hearing conservation, syndromes/genetics, noise, medical audiology
Introduction
Noise-induced hearing loss (NIHL) is the most prevalent form of hearing loss among young adults and is the most frequently occurring disability among current combat veterans (National Institute on Deafness and other Communication Disorders, 2010). The World Health Organization has named NIHL an important public health priority (WHO, 1997), and the Centers for Disease Control has included NIHL awareness in their publications on Adolescent and Child Health (CSD, 2011). As NIHL progresses through adulthood, it can negatively affect an individual’s ability to pursue a career which requires good hearing acuity, as well as their personal security and relationships.
Some individuals exposed to loud sound do not develop hearing loss. Alternately, Hsu et al. (2013) found notches that were not associated with any type of noise exposure, and concluded that another factor was involved. This clinical heterogeneity may be explained if we can consider a 4000–6000 Hz notch to be a phenotype resulting from a genetic susceptibility, possibly interacting with acoustic overload, in some people. An important challenge in associating 4000–6000 Hz notches with specific genetic polymorphisms, as in other gene-environment interactions, lies in the precision with which the phenotype and potential endophenotypes can be defined (Schulze & McMahon, 2004).
Acoustic exposure causes many physiological changes in the inner ear, and acoustic overexposure has been shown to cause changes to the stereocilia bundle, outer hair cells, pillar cells, afferent synaptic junctions and the stria vascularis in animals (Nordmann, et al., 2000; Wang, et al., 2006; Scheidt, et al., 2010). Changes triggered by acoustic overexposure can lead to temporary threshold shifts (TTS) in auditory sensitivity. Repeated exposures can cause a chronic increase in oxidative stress leading to the loss of outer hair cells (Henderson, et al., 2006), and a permanent threshold shift in the auditory region of 3000–6000 Hz. In early stages, this is distinct from high-frequency hearing loss, which includes a loss at 8000 Hz, and may, for instance, accompany physiologic changes seen in aging.
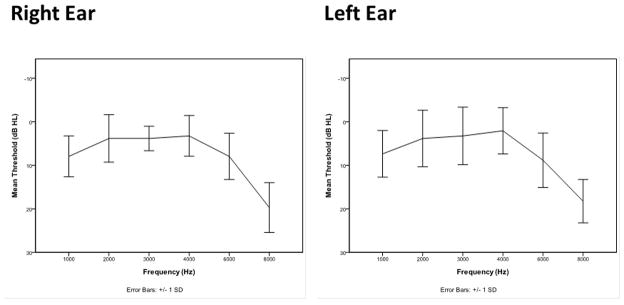
NIHL traditionally has been clinically defined as an audiometric “notch” at frequencies between 3000–6000 Hz, that is, a hearing threshold that is at least 15 dB higher between 4000 and 6000 Hz than thresholds at lower frequencies with a recovery of at least 5 dB at 8000 Hz, accompanied by a history of chronic or traumatic noise exposure (Fig. 1; Niskar, et al., 2001; Flamme et al., 2014). Consistent with a genetically predisposing condition requiring an environmental trigger, the incidence of audiometric notching in one or both ears steadily increases from 8.5% in children aged 6–11 years in the general NHANES population (Niskar, et al, 2001) to 17% for 16–19 year-olds in the NHANES data (Henderson et al., 2011). Twardella et al. (2013) reported a prevalence of 2.4% in German 15–16 year-olds. In an adult population aged 20–69 years, Mahboubi et al. (2013) found a prevalence of 12.8% with NIHL in one or both ears in the NHANES 1999–2004 population. In industrially exposed adult populations, reports range from 26 to 60% (Hong, 2005; Rachiotis, 2007). Among children, 1.8% of younger children and 3% of older ones show notches in both ears (Niskar, et al, 2001). Phillips, et al. (2010) reported that 11.5% of college-age classical music students had notches in both ears, while 35.5% had a notch in one ear and 53% showed no evidence of the “notched” audiogram configuration that typifies NIHL. These data suggest that the extreme exposures found in industrial environments are not necessary to cause notches in susceptible individuals. Similarly, Wilson (2011) found that among 3430 veterans (ages 20–89), 15.4% had bilateral notches and 40.6% of participants had notches in one ear.
Fig. 1.
Audiometric phenotype: Mean thresholds at 1–8 kHz for participants with normal hearing (solid line) vs. a bilateral 6 kHz “notch” (dashed line). Error bars indicate standard deviation.
The analysis of audiometric profiles over a range of frequencies to describe phenotypic definitions may lead to the identification of underlying genotypes associated with a subset of cochlear genes (Hildebrand et al., 2009). Notched configurations are not included in their current Audiogene model (http://audiogene.eng.uiowa.edu/), nor in the European Union GENDEAF recommendations.
NIHL has been associated with several single nucleotide polymorphisms (SNPs) lying within genes encoding proteins involved in sound processing. These include a variety of metabolic enzymes and heat shock proteins tied to redox regulation (Yang, et al, 2006; Konings, et al. 2007; Lin, et al., 2010; Liu, et al, 2010a, b; Abreu-Silva, et al., 2011), ion channels and ion transport proteins, and structural and gap junction proteins (Van Laer, et al., 2006; Konings, et al., 2007; Slwinska-Kowalska, et al., 2008; Pawelczyk, et al., 2009)., These findings have been replicated for catalase (CAT) the heat shock protein HSP70, potassium ion channels (KCNQ4, KCNE1), myosin 14 (MYO14) and protocadherin 15 (PCDH15).
The studies revealing these NIHL associations have been conducted primarily on adult subjects exposed to substantial and chronically high levels of sound in industrial settings. In most cases, the study designs have compared SNP genotypes and haplotypes for the 10% of individuals with the greatest hearing loss compared to the 10% showing the least amount of hearing loss rather than using the standard notch at 3000–6000 Hz (Van Laer, et al., 2006; Konings, et al., 2007; Slwinska-Kowalska, et al., 2008; Pawelczyk, et al., 2009; Liu, et al., 2010). These studies include older adults who may have other conditions contributing to hearing loss, and also do not differentiate between the notched configuration of NIHL and high-frequency hearing loss. Studies with subjects exposed to chronic and high sound levels may not allow for the identification of differences in people carrying genetic variants that cause a susceptibility to NIHL at more modest sound exposure levels.
Other methods of grouping participants have led to the identification of genes encoding proteins tied to redox regulation. When participants were categorized as those with audiograms suggestive of NIHL (loss at 4000,6000, and/or 8000 Hz), those with other forms of hearing loss, and those with no loss, associations of the NIHL group with glutathione S-transferase mu 1(GSTM1), glutathione S-transferase theta 1(GSTT1) and mitochondrial haplotype 1 were observed (Abreu-Silva, et al, 2011). Examination of industrial workers, sorted by the amount of temporary threshold shift they exhibited after a day of work, revealed associations with GSTM1, GSTT1 and glutathione S-transferase pi (GSTP; Lin et al, 2010). Factory workers grouped as those with NIHL (broadly defined as thresholds >25 dB HL in low or high frequencies) and those without resulted in an association with specific haplotypes of heat shock protein (HSP70; Yang et al, 2006).
Although audiometric configuration is often helpful for the clinical diagnosis of NIHL, previous genetic association studies have not uniformly defined an audiometric phenotype and indeed, there is some uncertainty about the definition of a notch (Nondahl et al., 2009). Some studies have utilized case-control groups with broad definitions of NIHL, while others have focused on subjects whose hearing loss lies at the extreme of a continuum. This may have resulted in associations that are related to high frequency hearing loss rather than 4000–6000 Hz notches, particularly since the populations included older adults.
Examining young adults in a genetic association study facilitates identification of a potential notch phenotype by avoiding the complications caused by age-related hearing loss or loss brought upon by long-term exposure to sound or other risk factors; relatively early onset is often indicative of a genetic component for an adult condition or disorder (Hauser et al, 2003). While young student musicians are exposed to measured intensity levels in practice and rehearsal rooms that exceed the National Institute of Occupational Safety and Health (NIOSH) recommendations for safe listening, their exposure does not typically extend through an entire workday (Phillips & Mace, 2008; Walter, 2009).
The question, therefore, is whether the clinical presentation of an audiometric notch can be viewed as a phenotype for genetic association studies. The current feasibility study examines the validity of the 4000–6000 Hz notch as a phenotype with utility for genetic association analysis in young adults. We selected single nucleotide polymorphisms of genes that are expressed in the ear and code proteins involved in processing sound.
Previous data from this population suggest that 4000–6000 Hz notches exist despite the lack of unusual exposures (Phillips, et al., 2010). For this feasibility study of potential genetic associations, college-age music students were tested for hearing acuity and grouped by notch status. Our previous study indicated that while almost half of young musicians show a notch in one ear, about 10–15% have a notch in both ears (Phillips et al, 2010). Therefore, we hypothesized that while severe environmental exposure could often lead to a notch in one ear, individuals, and particularly young adults, showing a 4000–6000 Hz notch in both ears are likely to be genetically predisposed to such notching, and may show notches even if their exposure to sound was neither extreme nor long in duration.
Materials and methods
Participants
All data collection procedures were approved by the University Institutional Review Boards at all collection sites. Participants (N=640) for this study were students of classical or jazz music (18–25 years of age) at one of five universities in North Carolina. Recruitment occurred during the academic years of 2010–11 and 2011–12. All participants were majoring in music with daily exposure that included individual practice and ensemble practice. Participants were also asked to complete an online survey and their hearing acuity was measured.
To investigate the hypothesis that individuals with bilateral notches are most likely to be genetically susceptible, we defined a case-control set within the larger cohort. All 84 participants of European extraction with bilateral notches were included in the case group. The two control groups included participants with unilateral notches and those with no notches, who were matched individually with case subjects for European extraction, gender and instrument played. Subjects were asked to report exposures or risk factors and none were excluded from the study based on this information.
Assessment of hearing status
An otoscopic exam was performed with all participants. Audiometric thresholds were obtained at 1000 Hz, 2000 Hz, 3000 Hz, 4000 Hz, 6000 Hz and 8000 Hz (Interacoustics AC-40), using the modified Hughson-Westlake procedure, after ensuring middle ear health through tympanometry (Maico MI 24). Data on all campuses were collected following a scripted standard operating procedure. The phenotype was defined by the appearance of a notch at 4000 or 6000 Hz. We took into consideration that normal hearing for young, healthy adults is a range of −10 to 15 dB HL. Our definition of a notch was a drop in hearing sensitivity of at least 15 dB from the self-referenced previous best threshold in a linear progression of frequencies, with a recovery of at least 5 dB after the notch (Figure 1). The 15 dB criterion was used because a 10 dB change in threshold is considered to be clinically significant, and we allowed for a 5-dB test-retest variability (Osieh-Lah, & Yeo, 2010; Wilson, 2011). A 15-dB criterion is also proposed by Flamme et al. (2014) to avoid false positive identification of notches.
The normal or no-notch participants (NN) were defined as having no threshold below 15 dB HL nor a 15 dB drop at 8000 Hz from a previous best threshold. Those subjects showing a notch in one ear were classified as unilateral notch (UN), and those showing a notch in both ears were classified as bilateral notch (BN). The BN category served as the case group for determining a potential genetic association, and each subject in this group was matched by gender with a NN and UN subject from the same university, and if possible, by playing the same or similar instrument. A potential second phenotypic configuration was identified as a 15+ dB drop in auditory sensitivity in the high frequencies (4000–8000 Hz) and not showing a notched configuration.
Survey
The survey included demographic data, personal and family medical history, prescription and over-the-counter medication use, and music, noise and chemical exposure throughout life (Supplementary Material). Music exposure questions asked about current as well as high school, middle school and elementary school exposures. Participants were asked about exposure to cell phones, personal listening devices, firearms, hunting, loud vehicles, clubs/rallies/races and power tools. Chemical exposure questions inquired about exposure to spray adhesives, house paints, fingernail polish/remover, carpet laying, furniture refinishing, silk screening/printing, permanent markers, auto refinishing, aerosol paints, tub/tile cleaners, pesticides/fungicides/herbicides and smoking. The analyses are restricted to music and noise exposure questions.
Genotyping
Buccal cell samples (Isohelix: Boca Raton, FL) were collected in duplicate at the time of audiometric testing and refrigerated prior to DNA extraction (Qiagen BioRobot). Genomic DNA was extracted from the de-identified buccal samples in the Molecular/Cellular Biology Core Laboratory at UNCG and outsourced for initial SNP genotyping and validation (GeneSeek; Lincoln, NE) on the Sequenom MassARRAY iPLEX platform.
As a screen of the candidate variants, all of the study cases and controls (N=252) were genotyped for 266 single nucleotide polymorphisms (SNPs) from 59 candidate genes. Candidate genes were selected if they had variants previously associated with NIHL or deafness or if they are expressed in the inner ear (Supplementary Table 1). SNPs within each of these candidate genes were selected if: (1) they had been implicated in earlier published NIHL studies, (2) they were directly implicated as causal for other health conditions, (3) they had been shown to affect expression of the candidate gene or (4) they affected the amino acid sequence of the gene’s protein product (i.e. nonsynonymous SNPs) and occurred at a population frequency between ~0.5% and 20%. This range was selected because SNPs occurring at a lower frequency would not be detected in a sufficient number of participants to allow for meaningful analysis, and those occurring at a higher frequency were likely to be incompletely penetrant, that is, a relatively low proportion of SNP carriers would exhibit the bilateral notched phenotype that affects 10–15% of the population.
SNPs that were most highly associated were also verified for the case-control subjects using Applied Biosystems’ customized TaqMan probes run on an AB 7500 real-time thermocycler utilizing the manufacturer’s protocols and software. In order to test the possibility of a haplotype association with the 4000–6000 Hz notch, a follow-up round of SNP testing was performed which tested tagging SNPs that were obtained from a NIH database (http://snpinfo.niehs.nih.gov/snpinfo/snptag.htm). Subsequently, all participants (beyond the case-controls) were genotyped for SNPs showing a possible association with bilateral notching.
Statistical analysis
The allele and genotype frequencies of candidate SNPs were analyzed in the case group and the two control groups. Frequencies for the bilateral group were compared to those of the non-bilateral (unilateral and no-notch groups combined) group and the no-notch group alone by first forming 2×2 tables of the case-control group by allele, and then computing odds ratios. P-values were calculated to assess evidence of association between allele frequency and group. We note that a conservative Bonferroni correction for 205 SNPs would require a p-value of 0.00024 or less to declare an association statistically significant at the overall 5% level. The data were analyzed using the STAT and GENETICS products of SAS Version 9.3. Relevant SNPs were also subjected to haplotype analysis and tested to determine whether the genotypes within the case-control population were consistent with Hardy-Weinberg equilibrium (HWE). SNP genotypes that did not fit a HWE model were not analyzed further. In summary, from the initial list of 266 SNPs, 205 were validated by GeneSeek and by testing for Hardy-Weinberg equilibrium. Of the 205, 99 SNP variants were found in the case-control population and analyzed for a possible association with bilateral notches.
Adjusted odds ratios were also calculated using logistic regression models to account for other possible risk factors, including gender, smoking status, and instrument and ensemble exposure levels. Instrument and ensemble intensity was ranked on a 1–10 scale, with 10 being very high exposure (trombone, concert band) and 1 being very low exposure (classical guitar, classical guitar ensemble). The scale was based upon exposure measurements made using doseBadge noise dosimeters (Cirrus Research) in practice rooms and ensembles during day-long data collection.
Exposure level to noise other than music (gunfire, hunting, power tools, loud vehicles, loud recreation venues, loud music venues) was also considered and used to compute adjusted odds ratios for comparing case and control groups. For these analyses, 0–1 types of exposure outside music studies was considered “low exposure,” exposure to 2–4 types was considered “medium exposure” and 5–6 types of exposure was considered as “high exposure.”
Results
Overall results
A total of 640 college-age music majors were tested for hearing acuity. Among the entire study population, 54.9% showed no notching (NN), 30.3% showed unilateral notching (UN), and 14.8% showed bilateral notching (BN). Four participants were excluded because their thresholds indicated a hearing loss that was not a 4000–6000 Hz notch. These included participants with the following audiometric results: one unilateral flat loss with thresholds between 55–65 dB HL, one steeply sloping loss above 2000 Hz to a profound loss at 4000–8000 Hz, one asymmetric loss that was steeply sloping to profound levels above 2000 Hz in the right ear and dropping somewhat steeply to moderate levels in the left, and one asymmetric loss with a 6000 Hz notch in both ears but a mild-to-moderate loss from 1000–4000 Hz. Mean threshold data showing the phenotype are seen in Figure 1. Overall, surveys were completed by 481 individuals, 66 of whom did not complete the hearing examination and buccal sample. Of the 640 total participants with hearing tests and buccal samples, 108 did not complete the survey. The case control sample (N=252, 84 BN cases and 168 controls) was comprised of 116 females and 136 males self-identified as more than 50% European extraction (Tang, et al., 2005), with a mean age for all participants of 20.23 years. Surveys were completed by 223 of the 252 case-control participants (90%).
Phenotype-genotype associations
In the initial analysis of BN vs. No-BN groups, a variant in the estrogen-related receptor beta (ESRRβ), rs61742642 (C→T), which changes an evolutionarily conserved proline residue in the ligand-binding portion of this nuclear receptor to a serine (P386S), was the one most strongly associated with BN. As seen in Table 1, of the 84 BN subjects in the case-control study, 20 (23.8%) carried the CT heterozygous genotype of rs61742642 compared to 16 (9.5%) of the combined UN and NN controls (OR = 2.7, CI: 1.36–5.37; p=0.0061). Other SNPs lying in the transcribed region near rs61742642 were tested in the case-control groups to other ESRRβ variants contributed to the occurrence or severity of notches (see Supplementary Table 2). This analysis showed that only rs61742642 was associated with an elevated frequency of bilateral notches. A quantile-quantile plot did not reveal evidence of population stratification or genotyping error, and the only SNP that stands out as having a lower p-value relative to the others is rs61742642 (Supplementary Figure 1). Odds ratios, confidence intervals and p-values for SNPs previously associated with notches can be seen in Supplementary Table 3.
Table 1.
ESRRβ variant numbers and proportions for case-control participants.
| Notch Group | CC | CT | Total |
|---|---|---|---|
| No notch | 76 (.905) | 8 (.095) | 84 |
| Unilateral notch | 76 (.905) | 8 (.095) | 84 |
| Bilateral notch | 64 (.762) | 20 (.238) | 84 |
| Total | 216 (.857) | 36 (.143) | 252 |
Other audiometric profiles
We also examined the inclusion of individuals with 4000 Hz notches on the case-control group. Two individuals had a bilateral 4000 Hz notch, and 14 bilaterally notched participants had a 4000 Hz notch in one ear and a 6000 Hz notch in the other ear. Odds ratios for BN among all SNP associations were calculated for a smaller case-control set (N=52 per group) exhibiting 6 kHz notches only. In this case, the odds ratio was similar to that of the previous phenotype definition for BN vs. non-bilateral (OR = 2.62, p=0.005). Previous data collected from this population have suggested that the 4000 Hz notch is associated with exposure to other noise, such as the use of power tools, which has a center frequency that is lower than that of music (Phillips, et al., 2010). These results support the suggestion that the frequency of the notch is dependent on the central frequency of the noise but involves the same underlying genetic susceptibility to notches in the 4000 Hz–6000 Hz range.
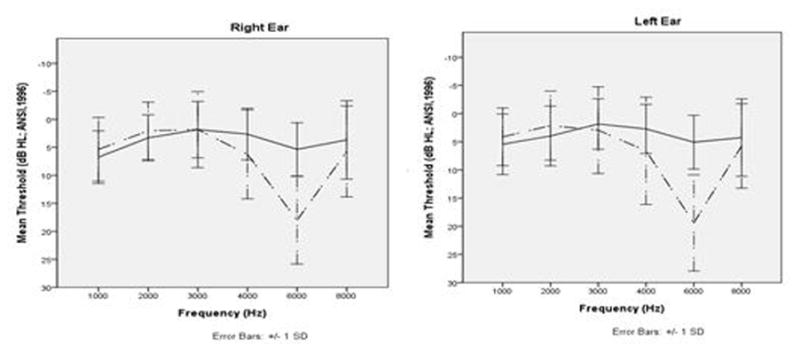
Another hearing loss configuration identified was a high frequency drop in sensitivity of at least 15 dB and minimally a 15 dB HL threshold that included 8000 Hz. There were 14 bilaterally affected participants showing this high-frequency loss, who were matched with unilaterally affected or unaffected individuals. Mean thresholds for these participants are shown in Figure 2. There was no evidence that rs61742642 CT genotype was associated with these high frequency drops, although the 8000 Hz case sample was too small to draw any conclusions about possible genotypic associations.
Fig. 2.
Audiometric profile for high frequency drop: Mean thresholds at 1–8 kHz for participants with a non-notch high frequency drop. Error bars indicate standard deviation.
Other variables
Gender, smoking status, instrument intensity, ensemble intensity, and the number of different types of exposure were investigated for possible association with BN, but tests for association of these factors with BN did not reveal any statistical associations. Further, adjusted odds ratios for comparing the bilateral group to the non-bilateral group for different genotypes of ESRRβ were computed using logistic regression, and were essentially identical to the unadjusted odds ratio reported earlier. We also examined several possible sources and characteristics of outside noise exposure from our survey results. We found no compelling evidence that these exposure variables affected the frequency of BN. As with the previous factors, adjusted odds ratios for comparing the bilateral group to the non-bilateral group for different levels of the ESRRβ genotype did not differ substantially from the unadjusted odds ratio. Additionally, there is no evidence that the use of hearing protection, or the reported frequency and duration of exposures affected the rate of notching or were associated with differences in hearing between genetically predisposed and other participants. This may be the consequence of limitations in survey self-reporting, and/or the relatively low power of analyzing a small population.
Discussion
The findings demonstrate that a 4000–6000 Hz notched audiogram can be found in some relatively young musicians who do not report unusual exposures. The presence of audiometric bilateral notches in these subjects was found in over twice as many subjects who carried a specific SNP (rs61742642) in a gene expressed in the inner ear that is required for normal hearing (ESRRβ), but the association was not significant after applying a Bonferroni correction for the 205 SNPs that were initially screened. Nevertheless, the findings indicate that the bilateral notch is a feasible test phenotype for larger scale gene association studies in the future because the magnitude of the observed odds ratio compared with the magnitude in typical studies of disease associations (>2) was high, and a larger study is likely to yield a statistically significant association. Also, the frequency of CT carriers in the population exceeds 10% (NCBI), pointing to the potential clinical importance of a phenotype-genotype association.
Comparisons with previous studies
Although few earlier reports include proportions of unilateral and bilateral notches, overall proportions of no notch, unilateral, and bilateral notches in this study resemble those reported by Wilson (2011) for veterans. Therefore, the consistency of bilateral notch frequency in the very large veteran population and the young adult musicians seen here further suggests the feasibility of examining this possible phenotype in a larger study. The measured bilateral notch frequency follows and build upon those previously reported earlier for student musicians (Phillips et al., 2010). As before, bilateral notches affect a relatively small proportion of young musicians, and while a possible association with rs61742642 must be tested further to reach a more conclusive outcome, the frequency of the rs61742642 genotype was about the same in both unilaterally notched and no-notch subjects. In other words, there is no evidence that unilateral notching is phenotypic as it was associated with any genotype in this study.
Familial mutations of ESRRβ cause deafness (Collin et al., 2008; Broskova et al., 2012). More generally, there is evidence that SNP variants of other genes associated with profound hearing loss also impose vulnerability for late onset and environmentally-induced loss. Slwinska-Kowalska, et al. (2008) found an association between a polymorphism in the cadherin-related 23 gene (CDH23) and NIHL, and other mutations in CDH23 have been associated with the DFNB12 form of deafness. Pawelczyk, et al. (2009) reported an association of a polymorphism (rs3751385) in GBJ2 with NIHL which could not be replicated in a second population, whereas numerous other mutations in GJB2 have been associated with DFNB1 deafness.
Physiology of the notch phenotype
The potential association found for the nonsynonymous SNP rs61742642 suggests that this variant is responsible for changes in protein structure that directly underlie the vulnerability to cochlear damage caused by acoustic overload. We postulate that for susceptible individuals, damage from exposure occurs at lower intensity levels or at a shorter duration of exposure than for non-susceptible individuals. Based on the specificity of the BN phenotype to the 4000–6000 Hz frequency range, the current results further suggest that the mechanistic processing of sounds in this frequency range is not equivalent to the processing found in the flanking frequency ranges. Therefore, it follows that a given genetic variant may not have the same impact on hearing acuity across the frequency range. In fact, a preliminary case-control analysis of subjects in this study who showed UN and BN at 8000 Hz (but no notches) provided no evidence that the SNPs described here involve a vulnerability to high-frequency hearing loss.
The audiometric assessment was done at one time point and thus it is unclear whether these bilateral notches are temporary or permanent and the whether the associations we observed would be different for temporary or permanent notches. Nordmann, et al. (2000) reported that the structural changes found in temporary threshold shifts in animals were different than those found with permanent threshold shifts. In a recent investigation, Bhatt et al. (2013) reported that carriers of the ESRRβ CT allele demonstrated greater temporary threshold shift post-narrow-band noise exposure than carriers of the CC allele. Repeated testing of participants may determine reversibility (i.e., TTS) vs. non-reversibility (i.e., PTS).
Genetic associations with strictly defined phenotypes are valuable for determining the mechanisms underlying the associated disorder. In this case, the potential associations are found within genes whose proteins are involved in redox homeostasis (ESRRβ). ESRRβ is expressed in the spiral ganglion, the supporting cells of the outer and inner hair cells, Reissner’s membrane and in the stria vascularis and spiral ligament of the cochlea (Collin, et al., 2008). ESRRβ is a nuclear receptor that is responsible for regulating the transcription of other genes. Unlike other nuclear receptors such as the estrogen receptor and vitamin D receptor, no activating ligand has been associated with ESRRβ, though its function is necessary for normal cochlear development and the proper expression of ion channel proteins (Chen & Nathan, 2007).
Limitations
As with any pilot study, there are limitations to these findings. The first is that the small sample size did not produce statistically significant findings after applying a Bonferroni correction, precluding conclusions regarding associations, especially for rarer SNPs. Secondly, participants in this study may not be representative of this age group in general. This possibility, without replication in a separate population, limits the generalizability of the results from the current study.
Another possible limitation is the strictness of the notch phenotype. As a check on our results, we re-examined our case-controls using a more strict 10 dB return of sensitivity at 8000 Hz. This reduced the number of participants with bilateral notches to 63, and the proportion of those carrying the CT variant of rs61742642 remained at 23.8%. In the revised case-control groups, the odds ratio continued to be ≥2
Future directions
In addition to replication with a larger sample, repeat hearing tests of participants as part of a longitudinal study are necessary to determine whether the relatively high frequency of BN seen in some participants is the result of a vulnerability to temporary threshold shifts or represents a permanent shift. Obtaining sound exposure measurements on all participants would allow for the examination of equivalent exposure and susceptibility to notches. If these associations are replicable, further exploration of the mechanisms involved in ESRRβ can be accomplished using cell lines of the stria vascularis or mouse studies.
Conclusions
We have tested the efficacy of using a strictly defined BN phenotype to examine genetic susceptibility to bilateral 4000–6000 Hz notches in a group of participants who are young, in relatively good health, with good hearing acuity and significant but not extreme exposure to sound. Cases with BN were more likely to carry the rs61742642 SNP T-allele in ESRRβ than controls. Among those who carried the 4000–6000 Hz notch phenotype bilaterally, 31% carried one or more of the associated minor alleles.
This is occurring at a young age which precedes the age at which the maximal incidence of NIHL is seen in the population. Our conclusion is that some of these young participants may be vulnerable to 4000–6000 Hz notches, despite the available evidence from this and other studies that their exposures do not vary substantially from those who do not experience notching, and do not have as high an exposure as participants in previous industrial population studies. The strict BN phenotype used in this study is shown to be an efficient and productive methodology for finding genetic associations.
Supplementary Material
Acknowledgments
This work is supported by the National Institutes of Deafness and other Communication Disorders grant R21DC009296-01. We also thank the Music Research Institute and the Center for Biotechnology, Genomics and Health Research for their support. We also acknowledge the contributions of Leslie Simmons and Sara Hunt for assistance with data collection, Jenna Callendar, Renuka Shivaji and Rohini Patel for technical assistance with the preparation and processing of DNA samples, and Jeong Sep Sihm for assistance with survey database management and analysis.
Abbreviations
- BN
Bilateral notch
- NIHL
Noise-induced hearing loss
- NN
Normal or no-notch
- SNP
Single nucleotide polymorphisms
- UN
Unilateral notch
References
- Abreu-Silva RS, Rincon D, Horimoto ARVR, Sguillar AP, Ricardo LAC, et al. The search of a genetic basis for noise-induced hearing loss (NIHL) Ann Hum Biol. 2011;38:210–218. doi: 10.3109/03014460.2010.513774. [DOI] [PubMed] [Google Scholar]
- Bhatt IS, Phillips SL, Richter SJ, Morehouse RC, Tucker DA, et al. A polymorphism in human estrogen-related receptor beta (ESRRB) associated with physiologic measures of noise-induced hearing loss. Poster presentation at ASHG; Boston. 2013. [Google Scholar]
- Broskova DS, Lastuvkova J, Machalova E, Lisonova J, Trkova M, Seeman P. DFNB35 due to a novel mutation in the ESRRB gene in a Czech consanguineous family. Intl J Ped Otorhinolaryng. 2012;76:1681–1684. doi: 10.1016/j.ijporl.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Adolescent and school health: About hearing loss. 2011 http://www.cdc.gov/healthyyouth/noise/signs.htm. Retrieved 8/8/2012.
- Chang T, Liu C, Huang K, Chen R, Lai J, Bao B. High-frequency hearing loss, occupational noise exposure and hypertension: a cross-sectional study in male workers. Env Health. 2011;10:1–8. doi: 10.1186/1476-069X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Nathan J. Estrogen-related receptor β/NR3B2 controls epithelial cell fate and endolymph production by the stria vascularis. Dev Cell. 2007;13:325–337. doi: 10.1016/j.devcel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Collin RWJ, Kalay E, Tariq M, Peters T, van der Zwaag B, et al. Mutations of ESRRB encoding estrogen-related receptor beta cause autosomal- recessive nonsyndromic hearing impairment DFNB35. Am J Hum Gen. 2008;82:125–138. doi: 10.1016/j.ajhg.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerich E, Rudel L, Richter F. Is the audiologic status of professional musicians a reflection of the noise exposure in classical orchestra music? Eur Arch Otorhinolaryng. 2008;265:753–758. doi: 10.1007/s00405-007-0538-z. [DOI] [PubMed] [Google Scholar]
- Flamme GA, Stephenson MR, Deiters KK, Hessenauer A, VanGessel DK, Geda K, Wyllys K, McGregor KD. Short-term variability of pure-tone thresholds with TDH-39 earphones. Int J Audiol. 2014;53:S5–S15. doi: 10.3109/14992027.2013.857435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser ER, Mooser V, Crossman DC, Haines JL, Jones CH, et al. Design of the genetics of early onset cardiovascular disease (GENCARD) study. Am Heart J. 2003;145:602–13. doi: 10.1067/mhj.2003.13. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- Hildebrand MS, DeLuca AP, Taylor KR, Hoskinson DP, Hur IA, et al. A contemporary review of AudioGene audioprofiling: a machine-based candidate gene prediction tool for autosomal dominant nonsyndromic hearing loss. Laryng. 2009;119:2211–2215. doi: 10.1002/lary.20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand MS, DeLuca AP, Taylor KR, Hoskinson DP, Hur IA, et al. A contemporary review of AudioGene audioprofiling: A machine-based candidate gene prediction tool for autosomal dominant nonsyndromic hearing loss. Laryng. 2009;119:2211–2215. doi: 10.1002/lary.20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong O. Hearing loss among operating engineers in American construction industry. Int Arch Occup Environ Health. 2005;78:565–574. doi: 10.1007/s00420-005-0623-9. [DOI] [PubMed] [Google Scholar]
- Hsu T, Wu C, Chang J, Lee S, Hsu C. Determinants of bilateral audiometric notches in noise-induced hearing loss. Laryng. 2013;123:1005–1010. doi: 10.1002/lary.23686. [DOI] [PubMed] [Google Scholar]
- Huygen PLM, Pennings RJE, Cremers CWRJ. Characterizing and distinguishing progressive phenotypes in nonsyndromic autosomal dominant hearing impairment. Audiol Med. 2003;1:37–46. [Google Scholar]
- Jansen EJM, Helleman HW, Dreschler WA, de Laat JA. Noise induced hearing loss and other hearing complaints among musicians of symphony orchestras. Int Arch Occup Environ Health. 2009;82:153–164. doi: 10.1007/s00420-008-0317-1. [DOI] [PubMed] [Google Scholar]
- Konings A, Van Laer L, Wiktorek-Smagur A, Rajkowska E, Pawelczyk M, et al. Candidate gene association study for noise-induced hearing loss in two independent noise-exposed populations. Ann Hum Genet. 2009;73:215–224. doi: 10.1111/j.1469-1809.2008.00499.x. [DOI] [PubMed] [Google Scholar]
- Lin CY, Wu JL, Shih T, Tsai P, Sun Y, et al. Glutathione S-transferase M1, T1, and P1 polymorphisms as susceptibility factors for noise-induced temporary threshold shift. Hear Res. 2010;269:42–47. doi: 10.1016/j.heares.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li X, Guo X, Liu B, Lin A, Rao S. Association between polymorphisms in SOD1 and noise-induced hearing loss in Chinese workers. Acta Oto-Laryngol. 2010;130:477–486. doi: 10.3109/00016480903253587. [DOI] [PubMed] [Google Scholar]
- Mahboubi H, Zardouz S, Oliaei S, Pan D, Bazargen M, Djalilian HR. Noise-induced threshold shift among U.S. adults and implications for noise-induced hearing loss: National Health and Nutrition Examination Survey. Eur Arch Otorhinolaryngol. 2013;270:461–467. doi: 10.1007/s00405-012-1979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoli M, Van Camp G, Newton V, et al. Recommendations for the description of genetic and Audiological data for families with nonsyndromic hereditary hearing impairment. Hereditary Hearing Loss Homepage. http://hereditaryhearingloss.org.
- National Institute on Deafness and other Communication Disorders. [Accessed 4/3/2012];Quick Statistics. 2010 http://www.nidcd.nih.gov/health/statistics/Pages/quick.aspx.
- Niskar AS, Kieszak SM, Holmes A, Esteban E, Rubin C, Brody DJ. Estimated Prevalence of Noise-Induced Hearing Threshold Shifts Among Children 6 to 19 Years of Age: The Third National Health and Nutrition Examination Survey, 1988–1994, United States. Pediatrics. 2001;108:40–43. doi: 10.1542/peds.108.1.40. [DOI] [PubMed] [Google Scholar]
- Nondahl DM, Shi X, Cruickshanks KJ, Dalton DS, Tweed TS, Wiley TL, Carmichael LL. Notched audiolgrams and noise exposure history in older adults. Ear Hear. 2009;30:696–703. doi: 10.1097/AUD.0b013e3181b1d418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann AS, Bohne BA, Harding GW. Histopathological differences between temporary and permanent threshold shift. Hear Res. 2000;139:13–30. doi: 10.1016/s0378-5955(99)00163-x. [DOI] [PubMed] [Google Scholar]
- Osieh-Lah V, Yeo LH. High frequency audiometric notch: An outpatient clinic survey. Int J Audiol. 2010;49:95–98. doi: 10.3109/14992020903300423. [DOI] [PubMed] [Google Scholar]
- Pawelczyk M, Van Laer L, Fransen E, Rajkowska E, Konings A, et al. Analysis of gene polymorphisms associated with K+ ion circulation in the inner ear of patients susceptible and resistant to noise-induced hearing loss. Ann Hum Gen. 2009;73:411–421. doi: 10.1111/j.1469-1809.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- Phillips SL, Mace ST. Sound level measurements in music practice rooms. Mus Perf Res. 2008;2:36–47. [Google Scholar]
- Phillips SL, Henrich VC, Mace ST. Prevalence of noise-induced hearing loss in student musicians. Int J Aud. 2010;49:309–31. doi: 10.3109/14992020903470809. [DOI] [PubMed] [Google Scholar]
- Rachiotis G, Alexopoulos C, Drivas S. Occupational exposure to noise, and hearing function among electro production workers. Auris Nasus Larynx. 2006;33:381–385. doi: 10.1016/j.anl.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Robinson PN. Deep phenotyping for precision medicine. Hum Mut. 2010;33:777–780. doi: 10.1002/humu.22080. [DOI] [PubMed] [Google Scholar]
- SAS/STAT and SAS/Genetics, Version 9.3. SAS Institute Inc; Cary, NC: 2010. [Google Scholar]
- Scheidt RE, Kale S, Heinz MG. Noise-induced hearing loss alters the temporal dynamics of auditory-nerve responses. Hear Res. 2010;269(1–2):23–33. doi: 10.1016/j.heares.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TG, McMahon FJ. Defining the phenotype in human genetic studies: Forward genetics and reverse phenotyping. Hum Hered. 2004;58:131–138. doi: 10.1159/000083539. [DOI] [PubMed] [Google Scholar]
- Sliwinska-Kowalsa M, Noben-Trauth K, Pawelczyk M, Kowalski TJ. Single nucleotide polymorphisms in the Cadherin 23 (CDH23) gene in polish workers exposed to industrial noise. Am J Hum Biol. 2008;20:481–483. doi: 10.1002/ajhb.20744. [DOI] [PubMed] [Google Scholar]
- Tang H, Quertermous T, Rodriguez B, Kardia SLR, Zhu X, et al. Genetic structure, self-identified race/ethnicity, and confounding in case-control studies. Am J Hum Gen. 2005;76:268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardella D, Perez-Alvarez C, Steffans T, Bolte G, Fromme H, Verdugo-Raab U. The prevalence of audiometric notches in adolescents in Germany: The Okhran-study. Noise & Health. 2013;15(67):412–419. doi: 10.4103/1463-1741.121241. [DOI] [PubMed] [Google Scholar]
- Van Laer L, Carlsson P, Ottschytsch N, Bondeson ML, Konings A, et al. The contribution of genes involved in potassium-recycling in the inner ear to noise-induced hearing loss. Hum Mut. 2006;27:786–795. doi: 10.1002/humu.20360. [DOI] [PubMed] [Google Scholar]
- Walter J. Sound exposure levels experienced by university wind band members. Med Prob Perf Art. 2009;24:63–70. [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. Some observations on the nature of the audiometric 4000 Hz notch: Data from 3430 veterans. J Am Acad Aud. 2011;22:23–33. doi: 10.3766/jaaa.22.1.4. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Prevention of noise-induced hearing loss: Strategies for prevention of deafness and hearing impairment 1997 [Google Scholar]
- Yang M, Tan H, Yang Q, Wang F, Yao H, et al. Association of hsp70 polymorphisms with risk of noise-induced hearing loss in Chinese automobile workers. Cell Stress Chaperones. 2006;11:233–239. doi: 10.1379/CSC-192R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.