Abstract
Background
Various transmission routes contribute to spread of Carbapenem-resistant Klebsiella pneumoniae (CRKP) in hospitalized patients. Patients with readmissions during which CRKP is again isolated (“CRKP readmission”) potentially contribute to transmission of CRKP.
Objective
Evaluate CRKP readmissions in the Consortium on Resistance against Carbapenems in K. pneumoniae (CRaCKle).
Design
Cohort study from 12/24/2011 to 7/1/2013
Setting
CRaCKle is a multicenter consortium of acute care hospitals in the Great Lakes region.
Patients
All patients who were discharged alive during the study period were included. Each patient was included only once at the time of the first CRKP positive culture.
Methods
All readmissions within 90 days of discharge from the index hospitalization during which CRKP was again found were analyzed. Risk factors for CRKP readmission were evaluated in multivariable models.
Results
Twenty percent of patients who were discharged alive (56/287) had a CRKP readmission. A history of malignancy was associated with CRKP readmission (aOR 3.00, 95% CI 1.32-6.65, p<0.01). During the index hospitalization, 160 (56%) patients received antibiotic treatment targeted against CRKP. The choice of antibiotic regimen was associated with CRKP readmission (p=0.02). Receipt of tigecycline-based therapy (aOR 5.13, 95% CI 1.72-17.44, using aminoglycoside-based therapy as a reference in those treated with anti-CRKP antibiotics) was associated with CRKP readmission.
Conclusion
Hospitalized patients with CRKP – specifically those with a history of malignancy – are at high risk of readmission with recurrent CRKP infection or colonization, which may contribute to transmission of CRKP in healthcare systems. Treatment during the index hospitalization with a tigecycline-based regimen increases this risk.
Keywords: carbapenem resistant Enterobacteriaceae, Klebsiella pneumoniae, readmission, transmission, tigecycline
Introduction
In spite of aggressive treatment, carbapenem-resistant Klebsiella pneumoniae (CRKP) infections remain associated with high morbidity and mortality.1,2 Posing a global threat, CRKP are now endemic in areas worldwide including in the United States, Asia, India, Europe and South America.3 Klebsiella spp. expressing Klebsiella pneumoniae carbapenemases (KPC) are the most common carbapenem-resistant Enterobacteriaceae (CRE) in the United States and have increased rapidly in prevalence during the past two decades.4,5
The rising prevalence of CRKP impacts infection control policies within healthcare settings. KPC β-lactamases encoded by blaKPC may be acquired through both clonal and plasmid expansion, facilitating spread of carbapenem resistance among Enterobacteriaceae.6 Various routes of transmission were demonstrated in recent CRKP outbreaks.7-9 Patients residing in long-term care facilities (LTCF) who are subsequently admitted to acute care hospitals are thought to significantly contribute to the transmission of CRKP. A recent study confirms that patients who are admitted to acute care hospitals from high-acuity LTCF are more likely to be colonized with KPC-producing Enterobacteriaceae.10
As hospital readmissions of patients with persistent or recurrent CRKP may contribute to the accelerated spread of this pathogen, rates of and risk factors for readmission in patients with CRKP during which the organism is again isolated are important to delineate. In order to better understand the manner in which CRKP is disseminated in the Great Lakes region, we sought to determine how often patients infected or colonized with CRKP were readmitted with repeat positive cultures for CRKP and whether the choice of treatment regimen directed against CRKP influenced CRKP readmission rates.
Patients and Methods
Design
A nested cohort study was conducted within the Consortium on Resistance against Carbapenems in Klebsiella pneumoniae (CRaCKle) cohort, which was previously described 11. Briefly, CRaCKle is a multicenter, prospective, longitudinal, observational study of hospitalized patients with positive cultures for CRKP in the Great Lakes Region. The cohort consists of CRaCKle patients who survived their index hospitalization and whose index hospitalization started after 12/24/2011 and ended on or before July 1, 2013. Routine screening of asymptomatic patients for CRKP carriage was not performed at any of the study sites during the study period. The Institutional Review Boards of all sites involved approved the study.
Definitions
The primary outcome of this study was CRKP readmission, which was defined as a hospital readmission within 90 days of the index hospitalization during which CRKP was again cultured from the patient. The index hospitalization was defined as the first hospitalization within the study period during which CRKP was identified. Each patient was included only once at the time of the index hospitalization.
Standardized definitions of infection were used, as previously described.11 Treatment regimens effective against CRKP were defined as follows: receipt of an aminoglycoside, colistin, tigecycline, trimethoprim-sulfamethoxazole (TMP-SMX), or fosfomycin unless in vitro resistance was documented to that antimicrobial in the patient’s isolate. In all instances guidelines from the Clinical and Laboratory Standards Institute (aminoglycosides, TMP-SMX, and fosfomycin) and the European Committee on Antimicrobial Susceptibility Testing (colistin and tigecycline) were followed. For analysis purposes, the type of regimen was assigned as previously reported.12 Briefly, any regimen which contained an aminoglycoside was deemed “aminoglycoside-based”, then any regimen that contained colistin but not an aminoglycoside was designated “colistin-based”, followed by any regimen that contained tigecycline but not colistin or aminoglycoside, was regarded as “tigecycline-based”. All other regimens were classified as “other”. Chronic kidney disease (CKD) was defined as a serum creatinine >2 mg/dL upon admission. Critical illness was designated using a Pitt bacteremia score greater or equal to 4 points on the day of the index culture.13 Charlson comorbidity index was calculated as described.14
Microbiology
In our study CRKP are K. pneumoniae isolates with non-susceptibility per CLSI guidelines to the following carbapenems: meropenem, imipenem, or ertapenem.15 Bacterial identification and routine antimicrobial susceptibility testing was performed with MicroScan (Siemens Healthcare Diagnostics) or Vitek2 (BioMerieux), supplemented by GN4F Sensititre tray (Thermo Fisher) or Etest (bioMerieux), as indicated. In more than 90% of tested isolates, carbapenem resistance was mediated through blaKPC-2 or blaKPC-3, as previously described.11
Statistical Analysis
Differences between groups were analyzed using Wilcoxon Rank Sum for continuous variables. Fisher’s Exact, and Pearson testing were used for categorical variables where appropriate. All variables that were associated with CRKP readmission at the p<0.1 level were included in multivariable logistic models, and adjusted odds ratios (aOR) with associated confidence intervals (CI) were calculated. A Kaplan-Meier curve was constructed to compare time to readmission. A Cox proportional hazards model on time to 90-day readmission was used to calculate adjusted hazard ratios (aHR). All variables that were associated with CRKP readmission at the p<0.1 level were included in this model in addition to treatment variables. P values of ≤0.05 were considered statistically significant. JMP 10.0.1 software (SAS, Inc, Cary, NC) was used for all analyses.
Results
Patients
The demographic characteristics of the 287 patients who met inclusion criteria are summarized in Table 1. CRKP infection was present during index hospitalization in 109 (38%) patients, and the remaining 178 patients were classified as having CRKP colonization. During the index hospitalization, 192 (67%) patients had CRKP isolated from a urine sample, 32 (11%) patients had CRKP isolated from respiratory specimens, 30 (10%) patients had CRKP isolated from wounds, 24 (8%) patients had CRKP isolated from blood, and 9 (3%) patients had CRKP isolated from “other” sites, which included abdominal sources such as bile, ascites, and abdominal abscess.
Table 1. Clinical Characteristics.
| All | CRKP readmission |
No CRKP readmission |
p* | p† | |
|---|---|---|---|---|---|
| N | 287 | 56 (20) | 231 (80) | ||
| Age, median (IQR) | 70 (58-81) | 69 (56-83) | 70 (59-81) | 0.73 | |
| Female | 167 (58) | 28 (50) | 139 (60) | 0.18 | |
| Race/Ethnicity | |||||
| White | 150 (52) | 26 (46) | 124 (54) | 0.37 | |
| Black | 120 (42) | 30 (54) | 90 (39) | 0.051 | 0.06 |
| Hispanic | 8 (3) | 0 | 8 (3) | 0.36 | |
| Other | 9 (3) | 0 | 9 (4) | 0.21 | |
|
Charlson comorbidity index, median (IQR) |
3 (2-5) | 3 (2-6) | 3 (2-5) | 0.40 | |
| Diabetes mellitus | 153 (53) | 28 (50) | 125 (54) | 0.65 | |
| Renal failure ‡ | 63 (22) | 18 (32) | 45 (19) | 0.048 | 0.0922 |
| Heart disease | 160 (56) | 28 (50) | 132 (57) | 0.37 | |
| COPD | 77 (27) | 15 (27) | 62 (27) | 1.00 | |
| Malignancy | 23 (12) | 12 (21) | 22 (10) | 0.02 | <0.01 |
| Origin | 0.49 | ||||
| Skilled nursing facility | 159 (55) | 30 (53) | 129 (56) | ||
| Home | 84 (29) | 20 (36) | 64 (28) | ||
| Hospital transfer | 28 (10) | 3 (5) | 25 (11) | ||
| Long term acute care | 16 (6) | 3 (5) | 13 (6) | ||
|
Length of stay, days, median (IQR) |
9 (6-16) | 8 (6-16) | 10 (6-16) | 0.35 | |
| Critical illness § | 70 (24) | 13 (23) | 57 (25) | 0.86 | |
| Infection | 109 (38) | 22 (39) | 87 (38) | 0.88 | |
| Source | 0.46 | ||||
| Urine | 192 (67) | 41 (73) | 151 (65) | ||
| Respiratory | 32 (11) | 4 (7) | 28 (12) | ||
| Wound | 30 (10) | 7 (13) | 23 (10) | ||
| Blood | 24 (8) | 2 (4) | 22 (10) | ||
| Other | 9 (3) | 2 (4) | 7 (3) | ||
| Any treatment | 160 (56) | 30 (54) | 130 (56) | 0.77 | |
| Disposition | 0.51 | ||||
| Skilled nursing facility | 157 (55) | 28 (50) | 129 (56) | ||
| Home | 58 (20) | 14 (25) | 44 (19) | ||
| Hospital transfer | 9 (3) | 3 (5) | 6 (3) | ||
| Long term acute care | 63 (22) | 11 (20) | 52 (23) |
All data expressed as n (%), unless otherwise indicated. IQR, interquartile range; LOS, length of stay.
univariable relationship between variable of interest and CRKP readmission
multivariable model including black race, renal failure and malignancy
renal failure defined as creatinine >2 mg/dL upon admission
critical illness defined as Pitt bacteremia score ≥4 at the time of index culture
Fifty-six out of 287 (20%) patients had a readmission during which CRKP was again isolated (“CRKP readmission”) within 90 days. We evaluated 17 patients on whom we had paired isolates from index admission and readmission. In 16/17 (94%) of patients the same rep-PCR strain was identified upon readmission. During their readmission, 22 (39%) patients had CRKP infection while 34 (61%) patients had colonization of a site with CRKP (Table 1). In univariable analysis, CKD and history of malignancy were significantly associated with CRKP readmission within 90 days. Eighteen of 56 (32%) of patients with CRKP readmission had CKD as compared to 45/231 (19%) in patients without CRKP readmission (p=0.048). A history of malignancy was present in 12/56 (21%) of patients with CRKP readmission, as compared to 22/231 (10%) in others (p=0.02). In addition, a trend towards increased CRKP readmissions was seen in Black patients; thirty out of 56 patients with CRKP readmission (54%) were Black compared to 90/231 (39%) without CRKP readmission (p=0.051). In a multivariable model which included CKD, history of malignancy, and Black race, only a history of malignancy remained associated with CRKP readmission (OR 3.00, 95% CI 1.32-6.65, p<0.01). Gender, age, and CRKP colonization vs. infection status were not associated with CRKP readmission. In addition, a trend was seen towards more CRKP readmissions in patients with index isolates resistant to trimethoprim-sulfamethoxazole was observed (p=0.07). Other antimicrobial susceptibility testing results were also not significantly associated with CRKP readmission (Table 2).
Table 2. Antimicrobial susceptibilities.
| All | CRKP readmission |
No CRKP readmission |
|
|---|---|---|---|
| n | 287 | 56 (20) | 231 (80) |
| Amikacin* | |||
| not tested | 89 | 15 (17) | 74 (83) |
| susceptible | 151 | 31 (21) | 120 (79) |
| intermediate | 10 | 0 | 10 (100) |
| resistant | 37 | 10 (27) | 27 (73) |
| Gentamicin* | |||
| not tested | 2 | 0 | 2 (100) |
| susceptible | 114 | 22 (19) | 92 (81) |
| intermediate | 25 | 6 (24) | 19 (76) |
| resistant | 146 | 28 (19) | 118 (81) |
| Colistin† | |||
| not tested | 160 | 30 (19) | 130 (81) |
| susceptible | 117 | 25 (21) | 92 (79) |
| resistant | 10 | 1 (10) | 9 (90) |
| Tigecycline† | |||
| not tested | 84 | 16 (19) | 68 (81) |
| susceptible | 107 | 25 (23) | 82 (77) |
| intermediate | 60 | 10 (17) | 50 (83) |
| resistant | 36 | 5 (14) | 31 (86) |
| TMP/SMX* | |||
| not tested | 9 | 0 | 9 (100) |
| susceptible | 82 | 11 (13) | 71 (87) |
| resistant | 196 | 45 (23) | 151 (77) |
Based on Clinical and Laboratory Standards Institute guidelines
Based on European Committee on Antimicrobial Susceptibility Testing
Treatment
During the index hospitalization, 160 (56%) patients received antibiotics directed against CRKP within the first 7 days of the first positive culture for CRKP. Being treated with antibiotics with in vitro activity against CRKP was not associated with CRKP readmission; 30/56 (54%) of those with CRKP readmissions received some form of treatment, whereas 130/231 (56%) of those without CRKP readmissions were treated (p=0.77).
The impact of specific treatment choices on CRKP readmissions was then evaluated in patients who received antibiotics effective against CRKP during their index hospitalization (Table 3). In univariable analysis, patients who received >1 drug with in vitro activity against CRKP were more likely to have a CRKP readmission; 13/42 (31%) of patients treated with more than one drug were readmitted versus 17/118 (14%) of patients treated with a single agent (OR 2.66, 95% CI 1.16-6.12, p=0.02). Most patients were treated with either an aminoglycoside-based regimen (n=70, 44%) or a tigecycline-based regimen (n=49, 31%) during their index hospitalization. In patients with CRKP readmission, 14/30 (47%) were treated with a tigecycline-based regimen as compared to 35/130 (27%) in those without CRKP readmissions (OR 2.38, 95% CI 1.05- 5.37, p=0.047). When evaluating the receipt of tigecycline during the index hospitalization – regardless of other anti-CRKP antibiotics – a similar association between tigecycline use and CRKP readmission was observed (OR 2.64, 95% CI 1.15-6.09, p=0.03). Fosfomycin use during the index hospitalization occurred in a total of 17 patients (11%) and was also associated with CRKP readmission in univariable analysis (OR 3.65, 95% CI 1.26-10.58, p=0.02). In multivariable analysis (Table 4), regimen base remained significantly associated with CRKP readmission (p=0.02). Using patients who received aminoglycoside-based therapy as a reference group, the adjusted OR (aOR) of tigecycline-based therapy was 5.13 (95% CI 1.72-17.44). In addition, receipt of more than one anti-CRKP antibiotic during index hospitalization was strongly associated with CRKP readmission (aOR 5.14, 95% CI 1.78-16.41, p<0.01). When comparing patients who received more than one to those who received only one anti-CRKP antibiotic, no significant differences were found in age, Charlson comorbidity index, or Pitt bacteremia score.
Table 3. Treatment characteristics.
| All | CRKP readmission |
No CRKP readmission |
p | |
|---|---|---|---|---|
| n | 160 | 30 (19) | 130 (81) | |
|
Any in vitro active drug in
first 7 days |
||||
| aminoglycoside | 70 (44) | 11 (37) | 59 (45) | 0.42 |
| colistin | 27 (17) | 5 (17) | 22 (17) | 1.00 |
| tigecycline | 76 (48) | 20 (67) | 56 (43) | 0.03 |
| trimethoprim/sulfamethoxazole | 14 (9) | 1 (3) | 13 (10) | 0.47 |
| fosfomycin | 17 (11) | 7 (23) | 10 (8) | 0.02 |
| Base of regimen | ||||
| aminoglycoside | 70 (44) | 11 (37) | 59 (45) | 0.42 |
| colistin | 22 (14) | 3 (10) | 19 (15) | 0.77 |
| tigecycline | 49 (31) | 14 (47) | 35 (27) | 0.047 |
| other | 19 (12) | 2 (7) | 17 (13) | 0.53 |
|
>1 in vitro active drug in first
7 days |
42 (26) | 13 (43) | 29 (22) | 0.02 |
Table 4. Multivariable logistic regression for CRKP readmission in treated patients (n=160).
| OR | 95% CI | p | |
|---|---|---|---|
| Black race | 1.69 | 0.68-4.23 | 0.26 |
| History of malignancy | 4.07 | 1.14-14.45 | 0.03 |
| Renal failure | 1.10 | 0.37-3.05 | 0.86 |
| Base of regimen | 0.02 | ||
| aminoglycoside (ref.) | - | - | |
| colistin | 1.26 | 0.24-5.30 | |
| tigecycline | 5.13 | 1.72-17.44 | |
| other | 2.03 | 0.26-11.57 | |
| >1 in vitro active drug in first 7 days | 5.14 | 1.78-16.41 | <0.01 |
Time to CRKP readmission
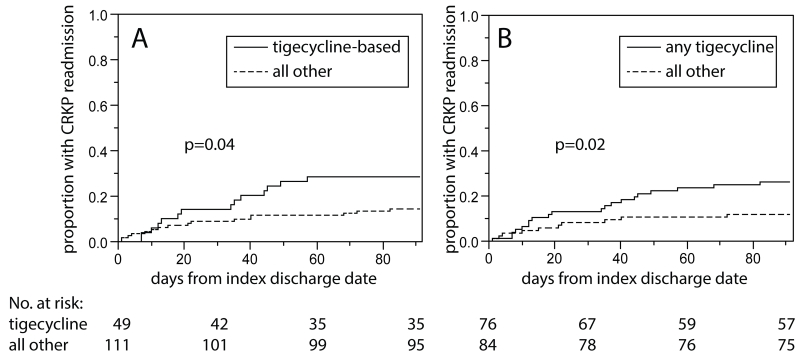
Tigecycline-based treatment was associated with a decreased time to 90-day CRKP readmission (p=0.04 by log-rank, Figure 1.A). Similarly, receiving any tigecycline – regardless of receipt of other antibiotics with in vitro activity against CRKP – was associated with decreased time to 90-day CRKP readmission (p=0.02 by log-rank, Figure 1.B). In Cox proportional hazards analysis (Table 5), treatment regimen base remained significantly associated with time to 90-day CRKP readmission (p=0.02). Using the patients who received aminoglycoside-based therapy as a reference group, the aHR for tigecycline-based therapy was 4.33 (95% CI 1.67-11.60). Of note, when urinary source was forced into the model as a confounding variable, the association between treatment regimen and time to CRKP readmission remained significant (data not shown). In addition, receipt of >1 in vitro active antibiotic in the first 7 days after the first positive CRKP culture during the index hospitalization was also associated with time to 90-day CRKP readmission (aHR 4.46, 95% CI 1.77-11.36; p<0.01). To determine if the association between tigecycline-based therapy and time to 90-day CRKP readmission was dependent on infection status during index hospitalization, a stratified analysis was performed. In both the CRKP colonization group as well as the CRKP infection group, treatment base was associated with time to 90-day CRKP readmission in Cox proportional hazard analysis (p=0.03 and p=0.04, respectively). The hazard ratios of tigecycline-based therapy, when using aminoglycoside-based therapy as a reference, were 3.99 (95% CI 1.20-14.31) and 6.56 (95% CI 1.39-34.81) for colonization and infection, respectively.
Figure 1.
Time-to-90-day CRKP readmission for patients who received anti-CRKP antibiotics (n=160) during their index hospitalization. Panel A: comparing patients who received tigecycline-based treatment during index hospitalization (n=49) vs. all others (n=111). Panel B: comparing patients who received any tigecycline during index hospitalization (n=76) vs. all others (n=84).
Table 5. Cox proportional hazards model on time to CRKP readmission within 90 days in treated patients (n=160).
| aHR | 95% CI | p | |
|---|---|---|---|
| Black race | 1.93 | 0.85-4.30 | 0.12 |
| Malignancy | 3.17 | 1.12-7.83 | 0.03 |
| Renal failure | 1.06 | 0.41-2.46 | 0.88 |
| Base of regimen | 0.02 | ||
| aminoglycoside (ref.) | - | - | |
| colistin | 1.28 | 0.29-4.15 | |
| tigecycline | 4.33 | 1.67-11.60 | |
| other | 1.87 | 0.26-8.80 | |
|
>1 in vitro active drug in first
7 days |
4.46 | 1.77-11.36 | <0.01 |
aHR, adjusted hazard ratio: CI, confidence interval.
Discussion
The present study evaluates readmission rates with CRKP and analysis of risk factors from a prospective multicenter cohort. We observed that it was common for hospitalized patients with CRKP infection or colonization to have a readmission during which CRKP was again isolated. Interestingly, this occurred in 20% of patients who survived their index hospitalization. This finding suggests that patients with CRKP – especially those patients treated with tigecycline and those with a history of malignancy – carry CRKP for prolonged periods of time and have frequent and recurrent healthcare exposures during which they are likely to interact with other vulnerable patients. In contrast, the presence of CRKP infection vs. CRKP colonization did not appear to have an impact on CRKP readmission rates.
The observation of CRKP readmission is an outcome that requires two related but distinct occurrences. Firstly, the patient needs to be readmitted, and secondly, during that readmission, CRKP must be cultured from a clinically important site. Thus, in the current study, we evaluated the overlapping risk factors for hospital readmission and prolonged CRKP carriage.
Hospital readmission rates are the subject of multiple studies to identify risk factors for readmission.16,17 Hospital reimbursement is increasingly being linked to readmission rates. In a recent report of 90-day readmissions following hospitalization for severe sepsis, the investigators noted a 42.6% readmission rate with 41.6% of these readmissions being for potentially preventable conditions such as heart failure exacerbation, pneumonia, and urinary tract infection.18 Potentially preventable readmissions occurred significantly more frequently in patients with severe sepsis compared to matched controls with other acute care diagnoses.
During a 90-day time period, a high rate of finding CRKP again during readmission was consistent with findings from studies of duration of CRE carriage. In one study, among patients who have CRE isolated during their index hospitalization, 78% of patients still had CRE carriage at 3 months while 39% still had detectable CRE carriage at 1 year.19 Those patients who were readmitted and in whom CRE was isolated in a clinical culture as opposed to a surveillance culture had significantly longer CRE carriage (641 days vs. 387 days).19 Another study identified risk factors for recurrent positive CRE screens during hospital encounters including prior fluoroquinolone use, admission from another hospital or healthcare facility, and hospital readmission within 3 months of initial positive CRE screen.20 In a case-control study of recurrence of CRE carriage from Israel, recurrence of CRE was common after presumed eradication at 6 months after last positive sample and associated with recurrent admissions after presumed eradication.21
The current study did not directly address the role of CRE decolonization as a means to reduce the risk of future infection and spread of CRE to other patients. Decolonization is another potential target for infection control measures in high risk patients. In experimental models, oral high-dose polymyxin therapy resulted in long-term elimination of CRE carriage.22 This principle was then evaluated in a pilot study of selective digestive decontamination for eradication of CRKP carriage. A double blind randomized control trial was conducted in 40 patients comparing oral gentamicin and polymixin E versus placebo.23 The investigators showed the CRKP isolation in rectal cultures was significantly reduced by 2 weeks, and this reduction was maintained through the 6 week time period of the study.
Patients with malignancies were found to be at increased risk for CRKP readmission. This is likely secondary to an increased overall readmission risk in this cohort. In addition, cancer has been linked to microbiome changes24. This may theoretically influence the duration of CRKP carriage.
The finding of an increased risk of CRKP readmission when patients are treated with tigecycline raises concern for a potential relationship between tigecycline use and risk for subsequent CRKP treatment failure whether demonstrated through recurrent infection or persistent colonization. A number of potential explanations for this observed association could be considered. As this is an observational study, our data may simply reflect confounding by indication. For some unmeasured reason, patients who are more likely to receive tigecycline may be the same patients who are more likely to get readmitted and have CRKP again isolated. Alternatively, this may represent a true association. Meta-analyses evaluating randomized controlled trials on the use of tigecycline in non-CRE infections are suggestive of inferior efficacy vs. comparators.25-28 Secondly, tigecycline is bacteriostatic rather than bacteriocidal and has a low urinary excretion. This antibiotic property likely plays an important role in this context; one wonders if the numbers of CRKP are not reduced sufficiently. Thirdly, current tigecycline dosing strategies (especially monotherapy) may not be optimal for treating multidrug-resistant bacteria such as CRKP.29 Lastly, tigecycline was also shown in murine models to promote the intestinal overgrowth of CRKP.22 In subgroup analysis, the association between tigecycline treatment and CRKP readmission was observed in both the group of patients with CRKP infection as well as the group with CRKP colonization.
Identifying risk factors for CRE isolation, infection, and readmission can potentially reduce patient-to-patient transmission of CRE within healthcare facilities. Multiple studies reported successful reduction in rates of epidemic CRE through targeted surveillance and infection control measures.30-33 Further study is needed to determine whether infection control measures such as routine culture screening, empiric contact isolation upon readmission, and standardized environmental cleaning protocols implemented in previous studies would be beneficial to patients at risk for CRE readmission.
Within the present study, receipt of more than one antibiotic with in vitro activity against CRKP in the first 7 days after a positive culture was also significantly associated with CRKP readmission. Our data cannot explain why this association was observed, but it is possibly related to reasons for adding or changing antibiotic regimens such as perceived failure of therapy or the occurrence of side effects. Alternatively, the use of several antibiotics may lead to increased disruption of the gut microbiome which in turn could lead to persistence of CRKP carriage. Similarly, antibiotic usage was linked to recurrence of CRE carriage in the case-control study by Bart et al.21
Limitations of this study include its observational nature; patients were not actively screened for CRKP on hospital admission, and antibiotic treatment was not randomized but based on clinical indication. As in all observational studies, we only can report on association rather than on causality. However, our study represents an inclusive cohort of consecutive patients admitted at various different hospitals being treated in a way that reflects current medical practice. Additionally, among patients who did not have a 90-day CRKP readmission, data was not collected beyond their index hospitalizations. Nonetheless, if patients were readmitted with CRKP during that time frame it is most likely that this readmission would have happened within the CRaCKle network and captured in our cohort, as the consortium covers most area hospitals.
In conclusion, we have established that in our population 20% of hospitalized patients with CRKP are readmitted within 90 days with repeat isolation from CRKP from clinical cultures. Interestingly, many of these isolates are the same strain as the index isolate. These patients contribute to the CRKP colonization pressure in acute care settings. Furthermore, we found that a history of malignancy and choice of treatment impact this risk. Further studies in patients with CRE are needed to better characterize relationships between treatment, subsequent risk for readmission, duration of CRE carriage and risk for subsequent CRE infection. Moreover, identifying patients at risk for CRKP treatment failure and readmission and intervening through infection control measures and choice of treatment regimens based on mechanism of action should be important future directions arising from this study.
Acknowledgements
none
Funding:
This work was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number UM1AI104681, and by funding to DVD and FP from the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. VGF was supported by Mid-Career Mentoring Award K24-AI093969 from NIH. In addition, this work was supported in part by the Veterans Affairs Merit Review Program (RAB), the National Institutes of Health (Grant AI072219-05 and AI063517-07 to RAB), and the Geriatric Research Education and Clinical Center VISN 10 (RAB), the Research Program Committees of the Cleveland Clinic (DVD), an unrestricted research grant from the STERIS Corporation (DVD). YD was supported by research awards R01AI104895 and R21AI107302 from the NIH. KSK is supported by the National Institute of Allergy and Infectious Diseases (Division of Microbiology and Infectious Diseases protocol 10-0065 and RO1 1R01AI119446-01)
S.S.R: Research support from bioMerieux, BD Diagnostics, BioFire, OpGen, Forest Laboratories, Achaogen, Nanosphere and Pocared. Honorarium from bioMerieux. Y.D.: Grant support: Merck, NIH. Consulting fee: Melinta. Advisory board: Shionogi. K.K: Forest Laboratories, Inc., Consultant, Grant Investigator and Speaker’s Bureau, Consulting fee, Grant recipient and Speaker honorarium. R.A.B.: AstraZeneca: Grant Investigator, Grant recipient, Merck: Grant Investigator, Grant recipient, Melinta: Grant Investigator, Grant recipient, Steris: Grant Investigator, Grant recipient, NIH: Grant Investigator, Grant recipient, VA Merit Review: Grant Investigator, Grant recipient. V.G.F.: Grant/ Research Support: Advanced Liquid Logic, Cubist, Cerexa, MedImmune, Merck, NIH, Novartis, Pfizer, Theravance. Paid Consultant: Affinium, Baxter, Cerexa, Cubist, Debiopharm, Durata, Merck, Novartis, NovaDigm, The Medicines Company, MedImmune, Pfizer, Theravance, Trius. Honoraria: Arpida, Astellas, Cubist, Inhibitex, Merck, Pfizer, Targanta, Theravance, Wyeth, Ortho-McNeil, Novartis, Vertex Pharmaceuticals. Membership: Merck Co-Chair V710 Vaccine. D.v.D.: Actavis, Tetraphase, Sanofi-Pasteur, Advisory Board. Steris Inc., Research funding. Scynexis Research funding
Footnotes
Preliminary results from this study were presented at IDWeek; October 8-12, 2014, Philadelphia, Pennsylvania, USA.
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest:
No potential conflicts: J.A.M, E.C., F.P., R.A.S., R.C.K., R.R.W., N.M.S., and S.E.
Potential conflicts of interest:
All other authors: no conflicts reported
References
- 1.Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30:972–976. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen M, Eschenauer GA, Bryan M, et al. Carbapenem-resistant Klebsiella pneumoniae bacteremia: factors correlated with clinical and microbiologic outcomes. Diagn Microbiol Infect Dis. 2010;67:180–184. doi: 10.1016/j.diagmicrobio.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013;13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease C, Prevention Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 5.Thaden JT, Lewis SS, Hazen KC, et al. Rising rates of carbapenem-resistant enterobacteriaceae in community hospitals: a mixed-methods review of epidemiology and microbiology practices in a network of community hospitals in the southeastern United States. Infect Control Hosp Epidemiol. 2014;35:978–983. doi: 10.1086/677157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold RS, Thom KA, Sharma S, Phillips M, Kristie Johnson J, Morgan DJ. Emergence of Klebsiella pneumoniae carbapenemase-producing bacteria. South Med J. 2011;104:40–45. doi: 10.1097/SMJ.0b013e3181fd7d5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benenson S, Warburg G, Hidalgo-Grass C, et al. Comparison of two carbapenem-resistant Klebsiella pneumoniae clones: from a contained outbreak in a paediatric population and from a national epidemic. J Antimicrob Chemother. 2012;67:1651–1654. doi: 10.1093/jac/dks115. [DOI] [PubMed] [Google Scholar]
- 8.Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med. 2005;165:1430–1435. doi: 10.1001/archinte.165.12.1430. [DOI] [PubMed] [Google Scholar]
- 9.Leavitt A, Navon-Venezia S, Chmelnitsky I, Schwaber MJ, Carmeli Y. Emergence of KPC-2 and KPC-3 in carbapenem-resistant Klebsiella pneumoniae strains in an Israeli hospital. Antimicrob Agents Chemother. 2007;51:3026–3029. doi: 10.1128/AAC.00299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prabaker K, Lin MY, McNally M, et al. Transfer from high-acuity long-term care facilities is associated with carriage of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae: a multihospital study. Infect Control Hosp Epidemiol. 2012;33:1193–1199. doi: 10.1086/668435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Duin D, Perez F, Rudin SD, et al. Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother. 2014;58:4035–4041. doi: 10.1128/AAC.02636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Duin D, Cober E, Richter SS, et al. Impact of therapy and strain type on outcomes in urinary tract infections caused by carbapenem-resistant Klebsiella pneumoniae. J. Antimicrob. Chemother. 2015;70:1203–1211. doi: 10.1093/jac/dku495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents. 1999;11:7–12. doi: 10.1016/s0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI document M100-S24. 2014:34. [Google Scholar]
- 16.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemkens LG, Contopoulos-Ioannidis DG, Ioannidis JP. Concordance of effects of medical interventions on hospital admission and readmission rates with effects on mortality. CMAJ. 2013;185:E827–837. doi: 10.1503/cmaj.130430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA. 2015;313:1055–1057. doi: 10.1001/jama.2015.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmerman FS, Assous MV, Bdolah-Abram T, Lachish T, Yinnon AM, Wiener-Well Y. Duration of carriage of carbapenem-resistant Enterobacteriaceae following hospital discharge. Am. J. Infect. Control. 2013;41:190–194. doi: 10.1016/j.ajic.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Schechner V, Kotlovsky T, Tarabeia J, et al. Predictors of rectal carriage of carbapenem-resistant Enterobacteriaceae (CRE) among patients with known CRE carriage at their next hospital encounter. Infect. Control Hosp. Epidemiol. 2011;32:497–503. doi: 10.1086/659762. [DOI] [PubMed] [Google Scholar]
- 21.Bart Y, Paul M, Eluk O, Geffen Y, Rabino G, Hussein K. Risk Factors for Recurrence of Carbapenem-Resistant Enterobacteriaceae Carriage: Case-Control Study. Infect. Control Hosp. Epidemiol. 2015;36:936–941. doi: 10.1017/ice.2015.82. [DOI] [PubMed] [Google Scholar]
- 22.Perez F, Pultz MJ, Endimiani A, Bonomo RA, Donskey CJ. Effect of antibiotic treatment on establishment and elimination of intestinal colonization by KPC-producing Klebsiella pneumoniae in mice. Antimicrob. Agents Chemother. 2011;55:2585–2589. doi: 10.1128/AAC.00891-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saidel-Odes L, Polachek H, Peled N, et al. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect. Control Hosp. Epidemiol. 2012;33:14–19. doi: 10.1086/663206. [DOI] [PubMed] [Google Scholar]
- 24.Garrett WS. Cancer and the microbiota. Science. 2015;348:80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect. Dis. 2011;11:834–844. doi: 10.1016/S1473-3099(11)70177-3. [DOI] [PubMed] [Google Scholar]
- 26.Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob. Agents Chemother. 2011;55:1162–1172. doi: 10.1128/AAC.01402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin. Infect. Dis. 2012;54:1699–1709. doi: 10.1093/cid/cis270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J. Antimicrob. Chemother. 2011;66:1963–1971. doi: 10.1093/jac/dkr242. [DOI] [PubMed] [Google Scholar]
- 29.Xie J, Wang T, Sun J, et al. Optimal tigecycline dosage regimen is urgently needed: results from a pharmacokinetic/pharmacodynamic analysis of tigecycline by Monte Carlo simulation. Int. J. Infect. Dis. 2014;18:62–67. doi: 10.1016/j.ijid.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Schwaber MJ, Lev B, Israeli A, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis. 2011;52:848–855. doi: 10.1093/cid/cir025. [DOI] [PubMed] [Google Scholar]
- 31.Ben-David D, Maor Y, Keller N, et al. Potential role of active surveillance in the control of a hospital-wide outbreak of carbapenem-resistant Klebsiella pneumoniae infection. Infect. Control Hosp. Epidemiol. 2010;31:620–626. doi: 10.1086/652528. [DOI] [PubMed] [Google Scholar]
- 32.Munoz-Price LS, Hayden MK, Lolans K, et al. Successful control of an outbreak of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae at a long-term acute care hospital. Infect. Control Hosp. Epidemiol. 2010;31:341–347. doi: 10.1086/651097. [DOI] [PubMed] [Google Scholar]
- 33.Kochar S, Sheard T, Sharma R, et al. Success of an infection control program to reduce the spread of carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2009;30:447–452. doi: 10.1086/596734. [DOI] [PubMed] [Google Scholar]