Abstract
Background
Asthma causes significant morbidity in children, and studies have demonstrated that environmental allergies contribute to increased asthma morbidity.
Objective
We investigated the differences between allergen skin tests and specific IgE and the role of IgG in regards to allergen exposure levels, and asthma morbidity in inner-city children.
Methods
Five hundred and six serum samples from the National Cooperative Inner City Asthma Study (NCICAS) were evaluated for specific IgE to cockroach (Blattella germanica), dust mite (Dermatophagoides farinae) and Alternaria as well as specific IgG and IgG4 to cockroach (B. germanica) and total IgE levels. Associations between sensitization to these allergens, exposures, and asthma morbidity were determined.
Results
Sensitization to environmental allergens and total IgE correlated with increased healthcare and medication use, but not with wheeze symptoms. Sensitization with exposure to cockroach was associated with increased asthma morbidity, whereas dust mite sensitization was correlated with asthma morbidity independent of exposure. There was also a strong correlation between specific IgE levels and skin test results, but the tests did not always agree. The relationship between specific IgE and asthma morbidity is linear with no obvious cutoff value. Increased Bla g 1 in the home was a good predictor for sensitization; however this relationship was not demonstrated for Der f 1. Cockroach-specific IgG correlated with increased healthcare use, however, there was no modifying effect of specific IgG or IgG4 on the association between cockroach-specific IgE and asthma morbidity.
Conclusions
Specific IgE levels and prick skin test results to environmental allergens can serve as markers of severe asthma for inner-city children. Asthma morbidity increased in a linear manner with specific IgE levels. Cockroach-specific IgG was not an important predictor or modifier of asthma morbidity.
Keywords: Alternaria, asthma, cockroach, dust mite, sensitization
Introduction
Allergic sensitization to environmental allergens e.g. cockroach, dust mite and fungus, has long been implicated as an important contributor to asthma morbidity.1–6 Asthma and wheezing are consistently associated with sensitization to specific allergens, whether measured by allergen prick skin tests or specific IgE (SIgE).7–10 Few studies have compared the properties and relative effectiveness of allergen specific-IgE and skin test results for predicting asthma symptoms and exacerbations.
The relationships between sensitization, environmental allergen exposure and asthma are complex and specific to the allergen evaluated. Levels of cockroach and dust mite allergens in the home have been shown to be associated with allergic sensitization,11,12 while levels of cat allergen in the home have not.12,13 Looking at exposure and sensitization by prick skin testing, Rosenstreich et al.14 and Gruchalla et al.12 were able to demonstrate that the combination of sensitization and exposure to cockroach is related to asthma morbidity. However, this same association was not demonstrated for dust mites or cats.
While food-specific IgE levels are commonly used to predict clinical reactivity to food allergens15, the utility of specific IgE levels to aeroallergens as predictors of clinical reactivity has not been as widely investigated. Pastorello et al.16 found that a cutoff level of 10.7 kU/L for seasonal allergens and 8.4 kU/L for Dermatophagoides pteronyssinus distinguished patients with symptomatic allergy from those who were not symptomatic.
The “modified Th2” hypothesis has been proposed to explain the immunologic tolerance in a subset of individuals who have high exposures to cat allergens.13 Certain individuals with exposure to high levels of cat allergen develop low levels of IgE and high levels of IgG4. This combination appears to be associated with decreased levels of allergen prick skin test reactivity and allergic diseases such as asthma. Similar observations have been reported for rat allergen.17 In contrast, this phenomenon is not seen for non-mammalian allergens such as dust mites.13 High IgG4 to dust mite was associated with worse asthma in adults, and this effect was independent of IgE sensitization.18 Less work has been published regarding IgG and IgG4 to cockroach, another allergen that has been found to play role in asthma, though correlations have been seen with increased cockroach specific IgE and increased specific IgG.19
Data from the National Cooperative Inner City Asthma Study (NCICAS) offer the opportunity to explore the associations among allergen sensitization, allergen exposure, and asthma in a well characterized inner-city population. In this descriptive study, we compare the utility of specific IgE versus prick skin testing for determining sensitization to aeroallergens, and for predicting asthma morbidity, and we assessed the role of IgG and IgG4 antibodies with respect to cockroach allergy. We also sought to determine whether specific IgE levels to inhalant allergens are correlated with asthma morbidity and whether there are defined cutoffs that predict morbidity.
Methods
Study population
NCICAS included 1528 children 4–9 years of age with asthma who were recruited from emergency departments and clinics in inner-city areas in the United States (Bronx, New York; East Harlem, New York; St. Louis; Washington, D.C.; Baltimore; Chicago; Cleveland; and Detroit).20,21 The study was approved by the institutional review board at each site, and written informed consent was obtained from the parents or legal guardians of participants. At enrollment, participants were invited to provide a voluntary blood sample with the understanding that it would be stored for future analyses of markers of atopy (ie. specific and total IgE). Serum samples were obtained from 572 participants, and 506 with at least 4 aliquots of stored serum were selected to participate in this study.
Clinical data previously collected from the NCICAS study was utilized to determine asthma morbidity. Baseline assessments included demographics, health history, access to care, environmental factors, and medication use. Symptom data (e.g. wheeze, night waking) were collected over the 9-month follow-up using a 14-day recall period. Health care utilization data (e.g. unscheduled asthma clinic visits, emergency department (ED) visits, hospitalizations) were collected using a 3-month recall period.
Prick skin testing to 13 indoor and outdoor environmental allergens and histamine and diluent controls was performed using a Multi-Test device during enrollment in NCICAS. Extracts were obtained from Greer Laboratories (Lenoir, NC). Data from three of these, house dust mites (Dermatophagoides farinae and D. pteronyssinus), a mixture of German cockroach (Blattella germanica) and American cockroach, and Alternaria, were used in this study. A prick skin test wheal size of > 3 mm than the negative control indicated sensitization. All testers attended a central training where they were required to perform at least 4 histamine tests that produced at least a 5 mm wheal and that were within 1 mm of each other in order to be certified to do skin testing in the NCICAS study.
Home visits were performed in a subset of participants (N=199 of those with blood analyzed) and allergen levels for Dermatophagoides farinae (Der f 1), D. pteronyssinus (Der p 1), and Blattella germanica (Bla g 1) were measured from settled dust as described previously.14
Assays
The serum samples were evaluated for specific IgE (ImmunoCAP® system; Phadia; Uppsala, Sweden) to whole body cockroach (Blattella germanica), whole body culture of Dermatophagoides farinae, and Alternaria (spores and mycelium). A specific IgE (SIgE) level > 0.35 kU/L indicated sensitization. Specific IgG (SIgG) and specific IgG4 (SIgG4) levels were assayed for whole body cockroach (ImmunoCAP® system). The limits of detection for cockroach-specific IgG was 2 to 200 mgA/L and was 0.01 to 30 mgA/L for cockroach-specific IgG4. Total serum IgE also was measured. A level > 100 kU/L was considered to be elevated.,
Statistical analysis
The relationship between the dichotomous measure of specific or total IgE and continuous asthma morbidity measures (days of wheeze in the last 14 days and percent of missed school days in the last 3 months) was examined by analysis of variance. The dichotomous healthcare utilization measures were examined using a log-binomial regression model to estimate the relative risks (RR) and 95% confidence intervals.22 These analyses were repeated using the log transformed continuous measurements of specific and total IgE and no major discrepancy was apparent.
Immunoglobulins (IgE, IgG4 and IgG) values were logarithmically transformed as they followed a log-normal distribution. In particular IgG followed a censored log-normal distriburtion with values less than 2 mg/L representing the lower limit of detection. Values are given as geometric means (geometric standard error). The associations among the immunoglobulins were examined using the Pearson’s correlation coefficient.
The effect of immunoglobulin levels (IgE, IgG and IgG4) in response to cockroach allergen on morbidity outcomes was compared using the same modeling approach, but examining the standardized regression coefficient (one standard deviation increase) to account for IgE and IgG being measured in different units (kU/L vs mg/L).
The agreement between specific IgE to cockroach > 0.35 kU/L and skin test wheal size to cockroach > 3 mm was examined using the kappa statistic. This same relationship was examined in the continuous case using R2 as an overall measure of fit.
For all statistical tests a two-sided p-value < 0.05 was considered significant. Analyses were conducted using SAS (version 9.1, SAS Institute, Cary, North Carolina, USA) and the R system for statistical computing (version 2.7.0).23
Results
Of the 506 serum samples analyzed, 64% had elevated serum SIgE (> 0.35 kU/L) to at least 1 allergen and 11% had sensitization to all 3 allergens measured. Forty-four percent had elevated specific IgE to cockroach (Blattella germanica), 33% to dust mite (Dermatophagoides farinae), and 34% to Alternaria. Two thirds (67%) had elevated total IgE levels (>100 kU/L). The mean age of the children in these analyses was 6.3 years with 63.2% male. There were minor differences between the subset with available serum compared to the unselected NCICAS patient group in terms of racial distribution (more African-Americans and fewer Hispanics), atopy (i.e. skin test positivity) and asthma morbidity (Table 1).
Table 1.
Characteristics of NCICAS participants included and not included in the analysis.
| Specific IgE sample (N=506) |
Not under analysis (N=784) |
|
|---|---|---|
| Race Ethnicity * | ||
| African-American | 83.1% | 69.8% |
| Hispanic | 11.7% | 22.9% |
| White | 0.8% | 0.6% |
| Other | 4.4% | 6.7% |
| Sex (male) | 63.2% | 62.8% |
| Age at recruitment (years) * | 6.3 | 6.0 |
| Caretaker completed high school | 65.7% | 67.7% |
| ≥1 household member employed | 49.9% | 51.0% |
| Household income < US$15000 | 64.4% | 62.0% |
| Skin test sensitivity | ||
| Alternaria | 40.5% | 35.8% |
| Cockroach* | 31.2% | 37.4% |
| Der p mite | 30.7% | 30.5% |
| Penicillium* | 24.2% | 17.1% |
| Der f mite | 22.4% | 22.8% |
| Rat | 20.7% | 18.3% |
| Cat* | 20.3% | 26.5% |
| Dog | 16.8% | 15.8% |
| Mouse | 13.5% | 15.0% |
| Morbidity | ||
| Days of wheeze, chest tightness, or cough | 3.43 | 3.48 |
| Days of school missed * | 5.6% | 7.1% |
| Annual unscheduled asthma visits * | 1.56 | 1.91 |
| Annual hospitalizations | 0.26 | 0.24 |
| Using steroids at baseline | 29.8% | 30.3% |
Data are given as means, unless otherwise specified.
Significant difference between samples (p<0.05)
Comparison of cockroach IgE levels and skin tests
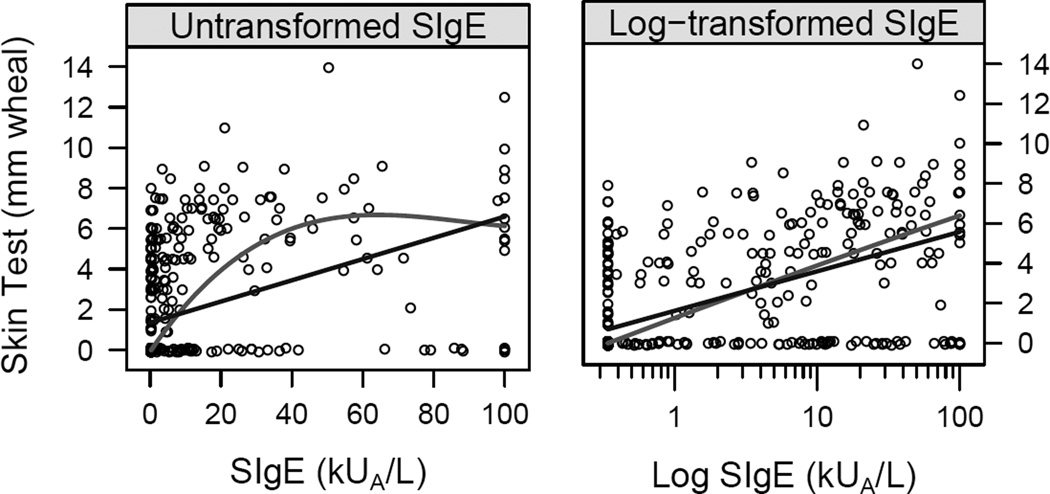
The concordance between SIgE levels to cockroach (B. germanica) and skin test positivity to cockroach (mix of American and German cockroach) was approximately 76% (Kappa=0.50, 95% CI: 0.42–0.58), with some patients having evidence of serum SIgE with negative skin tests and others having no detectable serum SIgE levels with positive skin tests. There was a correlation between skin test wheal size and serum SIgE at lower levels of allergen-specific IgE. At high levels of SIgE, the skin test wheal sizes plateau. A linear relationship was found between log transformed cockroach SIgE levels and prick skin test wheal sizes (Figure 1). The concordance between SIgE levels to D. farinae and skin testing positivity to house dust mites (Dermatophagoides farinae and D. pteronyssinus) was 78% (Kappa=0.45, 95% CI: 0.36–0.54) and for Alternaria was 83% (Kappa=0.64, 95% CI:0.57–0.71).
Figure 1.
Relationship between cockroach (Blattella germanica) SIgE level and prick skin test wheal size for cockroach. (mix of American and German cockroach). Also shown are a linear regression line (dark, R2=0.17) and smooth curve (light, R2=0.26) on the original scale and a linear (dark, R2=0.33) and smooth curve (light, R2=0.34) on the log10 transformed cockroach SIgE to illustrate the nonlinear relation on the original scale.
Effect of Total and Specific IgE on asthma morbidity
Children with cockroach sensitivity were twice as likely to be hospitalized during the observation year compared to children not sensitized (RR=1.96, 95% CI: 1.19–3.24). They were also somewhat more likely to have unscheduled asthma visits (ED or clinic), and were more likely to be using steroids at baseline (Table 2). Similarly, dust mite (D. farinae) sensitivity was associated with an increased risk of unscheduled visits, hospitalizations and medication use. Sensitization to Alternaria was associated only with a significant increase in the risk of hospitalization for asthma (RR=1.89, 95% CI: 1.16–3.06). Higher Total IgE was associated with increased hospitalization, and steroid use. There was no significant difference in asthma symptoms (ie. maximum symptom days and wheeze) with increased SIgE to cockroach, dust mite, and Alternaria or with high total IgE.
Table 2.
Asthma morbidity and healthcare utilization by specific and total IgE levels.
| Days of wheeze in past 14 days |
Percent missed school days in last 3 months |
Any Unscheduled asthma visits in year (%) |
Any Hospitalizations in year (%) |
Any steroid use in 3 months prior to baseline (%) |
|||
|---|---|---|---|---|---|---|---|
| N | Mean (SE) days |
N | Mean (SE) days |
||||
| Total IgE | |||||||
| < 100 kU/L | 153 | 3.38 (0.22) | 144 | 5.48 (0.51) | 42.5 | 4.6 | 16.4 |
| ≥ 100 kU/L | 318 | 3.46 (0.16) | 305 | 5.74 (0.35) | 48.7 | 15.7 | 36.2 |
| p-value | 0.77 | 0.67 | |||||
| RR (95% CI) | 1.15 (0.92–1.42) | 3.43 (1.60–7.40) | 2.21 (1.52–3.21) | ||||
| Cockroach (B.germanica) SIgE |
|||||||
| < 0.35 kU/L | 260 | 3.45 (0.17) | 246 | 5.65 (0.39) | 43.1 | 8.5 | 23.4 |
| ≥ 0.35 kU/L | 211 | 3.42 (0.19) | 203 | 5.67 (0.43) | 51.2 | 16.6 | 37.5 |
| p-value | 0.92 | 0.99 | |||||
| RR (95% CI) | 1.19 (0.98–1.44) | 1.96 (1.19–3.24) | 1.60 (1.22–2.10) | ||||
| Dust mite (D.farinae) SIgE |
|||||||
| < 0.35 kU/L | 312 | 3.34 (0.16) | 293 | 5.72 (0.36) | 43.3 | 8.6 | 24.7 |
| ≥ 0.35 kU/L | 159 | 3.63 (0.22) | 156 | 5.54 (0.49) | 53.5 | 18.9 | 39.4 |
| p-value | 0.28 | 0.76 | |||||
| RR (95% CI) | 1.24 (1.02–1.50) | 2.18 (1.34–3.54) | 1.60 (1.22–2.08) | ||||
| Alternaria SIgE | |||||||
| < 0.35 kU/L | 304 | 3.45 (0.16) | 284 | 6.01 (0.36) | 46.0 | 9.2 | 28.0 |
| ≥ 0.35 kU/L | 167 | 3.41 (0.22) | 165 | 5.06 (0.47) | 47.9 | 17.4 | 32.9 |
| p-value | 0.88 | 0.11 | |||||
| RR (95% CI) | 1.04 (0.85–1.27) | 1.89 (1.16–3.06) | 1.18 (0.90–1.55) | ||||
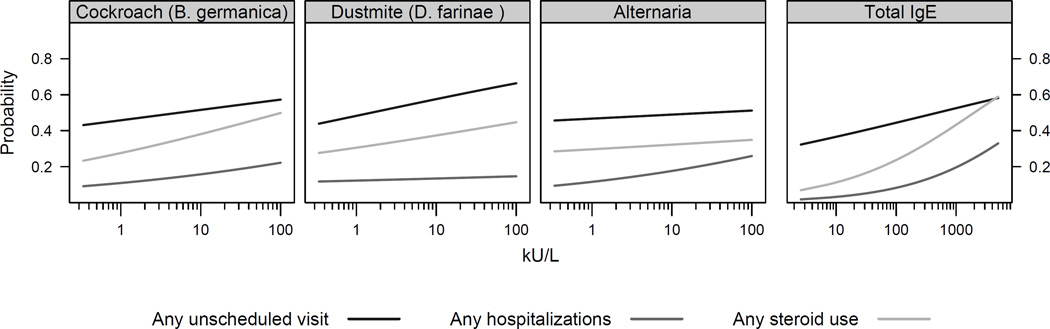
Figure 2 demonstrates that the relationship between SIgE or total IgE and the risk of unscheduled visits, hospitalizations and medication use is essentially linear. In this circumstance, any cutoff value for sensitization above 0.35 kU/L would be arbitrary. There is also no value at which the asthma outcome is greater than 90%, as is seen in food challenges. This outcome would not be possible for asthma since allergen challenges are not generally performed to prove that specific allergens will elicit asthmatic symptoms. Furthermore, unlike food allergy, there are multiple outcomes that can be measured for asthma which can give varying results.
Figure 2.
Linear dose-response relationship between SIgE and total IgE with asthma morbidity.
Comparison of Predictive ability of specific IgE and skin tests for asthma morbidity
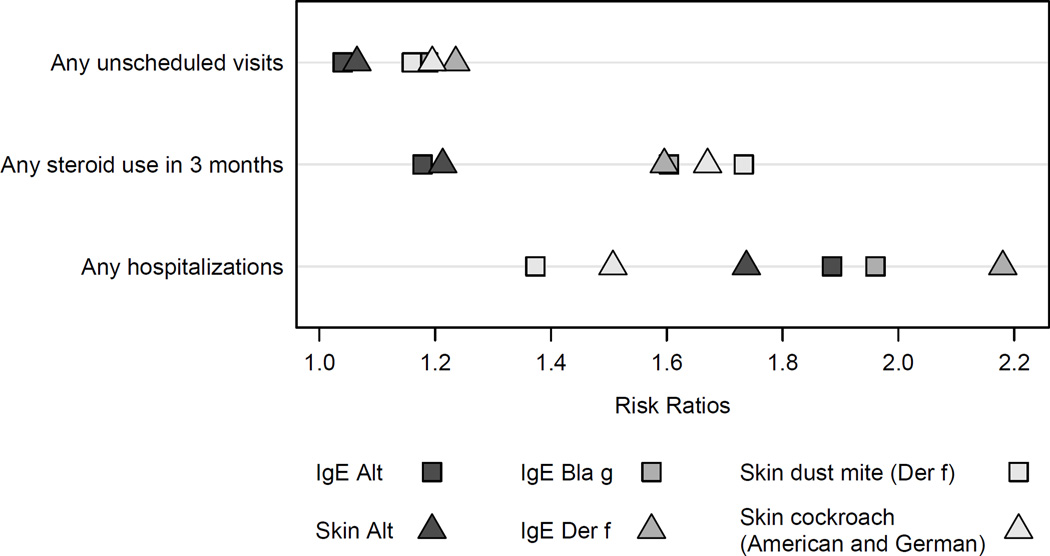
Neither SIgE levels nor skin test wheal sizes were consistently better at predicting asthma morbidity. Results varied depending on the allergen and asthma morbidity parameter (Figure 3). SIgE for dust mite was the best predictor for asthma hospitalizations, whereas dust mite skin test wheal size was a better predictor for steroid use. For unscheduled visits, skin test wheal size and SIgE to cockroach and dust mite have similar predictive values.
Figure 3.
Risk ratios for asthma morbidity parameters by allergen and type of allergy test (specific IgE and prick skin test).
Total IgE also correlated well with positive skin tests. A positive skin test to cockroach (German and American mix), dust mite (Dermatophagoides farinae and D. pteronyssinus), or Alternaria was associated with an increased risk of having total IgE above 100 kU/L (cockroach RR= 2.55; 95% CI: 1.74–3.74; dust mite RR=3.48; 95% CI: 2.05–5.88; Alternaria RR 2.01; 95% CI: 1.51–2.66).
The effect of allergen exposure on SIgE levels
The geometric mean for Bla g 1 exposure in the home were: 5.36 U/g in the bedroom (geometric standard error= 8.65), 54.02 U/g in the kitchen (std error = 13.31), and 7.30 U/g in the family room (std error 6.42). The geometric mean for dust mite (Der f 1 and Der p 1) exposure in the home were: 0.29 mcg/g in the bedroom (geometric standard error= 3.95), 0.12 mcg/g in the kitchen (std error = 1.75), and 0.24 mcg/g in the family room (std error 3.59). In this study, there was a significant difference in SIgE levels between patients who were determined to have high exposure to Bla g 1 as compared to those with low exposures. A high level of Bla g 1 allergen in the bedroom, kitchen or family room (> 8 U/gram) was significantly associated with SIgE level to cockroach (Table 3). In addition, having more rooms in the home with elevated Bla g 1 levels tended to be a good predictor for sensitization.
Table 3.
Mean cockroach-specific IgE, IgG4 and IgG level by home allergen exposure.
| IgE (kU/L) | IgG (mg/L) | IgG4 (mg/L) | |||||
|---|---|---|---|---|---|---|---|
| N | Geometric Mean (SE) |
Geometric Mean (SE) |
Geometric Mean (SE) |
||||
| Bla g 1 > 8 U/g in bedroom | Yes | 95 | 5.30 (0.92)*** | 4.43 (0.25)** | 0.30 (0.04) | ||
| No | 103 | 1.94 (0.41) | 3.63 (0.20) | 0.28 (0.03) | |||
| Bla g 1 > 8 U/g in kitchen | Yes | 149 | 3.68 (0.55)** | 4.09 (0.18) | 0.29 (0.03) | ||
| No | 47 | 1.81 (0.60) | 3.52 (0.29) | 0.28 (0.05) | |||
| Bla g 1 > 8 U/g in family Room |
Yes | 89 | 4.92 (0.88)*** | 4.21 (0.24)* | 0.30 (0.04) | ||
| No | 108 | 1.96 (0.40) | 3.68 (0.20) | 0.28 (0.03) | |||
| Number of rooms with Bla g 1 > 8 U/g† |
3 | 55 | 6.25 (1.38)*** | 4.70 (0.34)** | 0.32 (0.05) | ||
| 2 | 46 | 3.06 (0.84) | 3.59 (0.30) | 0.27 (0.05) | |||
| 1 | 48 | 1.81 (0.57) | 3.91 (0.31) | 0.24 (0.05) | |||
| 0 | 34 | 1.65 (0.64) | 3.38 (0.33) | 0.33 (0.06) | |||
p-value < 0.10
p-value < 0.05
p-value < 0.01
among families with information for all 3 rooms.
High bedroom exposure to Bla g 1 and increased number of rooms with high exposure also correlated with higher cockroach-specific IgG levels, and a borderline significant association was seen for high exposure in the family room and SIgG. Bla g 1 exposure was not associated with levels of cockroach-specific IgG4 (Table 3).
Overall, there was a low home exposure to Der f 1 in this study population14 and Der f 1 exposure did not correlate with SIgE levels (data not shown).
The effect of allergen exposure and sensitization on morbidity
We found that exposure in the home (Bla g 1 > 8 U/gram) and sensitization (SIgE > 0.35 kU/L) to cockroach are associated with increased asthma exacerbations (unscheduled visits RR=1.48, 95% CI 1.07–2.05; hospitalizations RR=3.55, 95% CI 1.60–7.90; and steroid use RR=1.63, 95% CI 1.06–2.50). However, the combined effect of exposure and sensitization did not impact the reported symptoms of wheeze.
A similar analysis was performed for dust mite, but no effect of exposure was seen. Dust mite sensitization has an effect on asthma exacerbations regardless of home exposure levels (Table 2). This is likely due to low exposure levels in the home environment for this study population or exposures from other sources outside the home that were not measured in the NCICAS study.
Correlation of cockroach-specific IgG and IgG4 levels with asthma morbidity
Cockroach-specific IgG was detected in 444 samples (88.7%), and IgG4 was detectable in 504 samples (99%). The presence of IgG antibody to cockroach was significantly associated with increased hospitalization for asthma (Table 4). IgG4 levels were not associated with morbidity. We examined the data for a mediating effect of cockroach-specific IgG on the relationship between SIgE and asthma morbidity, but this effect was not seen in our study (data not shown).
Table 4.
Comparison of effects of cockroach-specific IgE, IgG and IgG4 levels on asthma morbidity.
| IgE | IgG | IgG4 | ||||
|---|---|---|---|---|---|---|
| Outcome | Estimate* | p-value | Estimate* | p-value | Estimate* | p-value |
| Wheeze in past 14 days | 0.20 | 0.11 | 0.01 | 0.93 | 0.09 | 0.49 |
| Percent of missed school days in past 3 months |
0.35 | 0.22 | 0.12 | 0.67 | 0.23 | 0.44 |
| Any unscheduled asthma visits in year |
RR | p-value | RR | p-value | RR | p-value |
| 1.10 | 0.03 | 1.02 | 0.62 | 1.03 | 0.53 | |
| Any hospitalizations in year |
1.33 | 0.006 | 1.25 | 0.03 | 1.08 | 0.48 |
| Any steroid use in 3 months prior to baseline |
1.27 | <0.001 | 1.12 | 0.06 | 1.05 | 0.46 |
Change in morbidity for a 1 standard deviation increase.
Modification of the correlation of cockroach-specific IgG and IgG4 levels with SIgE by allergen exposure
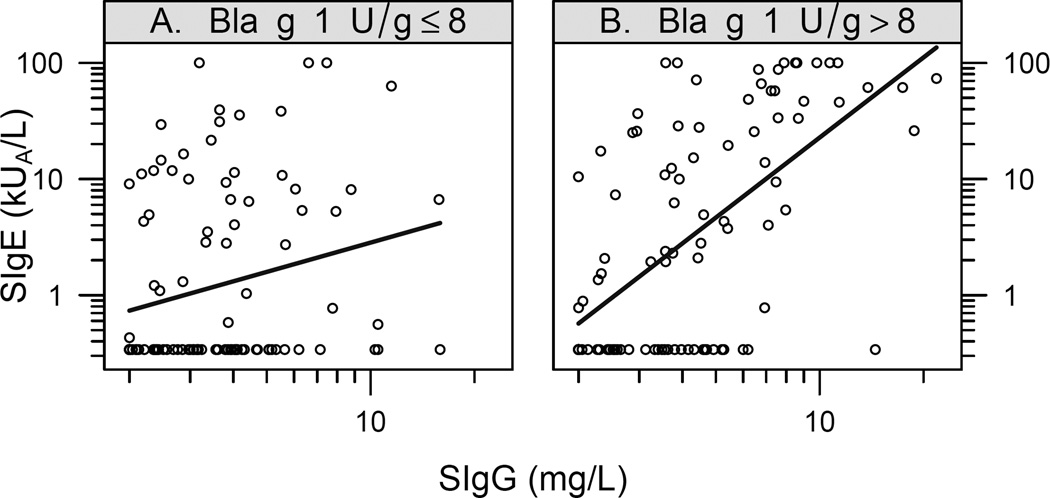
Cockroach-specific IgE and IgG levels were positively correlated, and the strength of correlation varied depending on the level of Bla g 1 exposure in the home (Figure 4). Specifically, at high levels of Bla g 1 allergen in the bedroom (> 8 U/gram), SIgE and SIgG levels (but not SIgG4) to cockroach were strongly correlated (0.60, 95% CI: 0.46, 0.72). Below 8 U/gram, there was a weaker association between SIgE and SIgG (0.23, 95% CI: 0.04, 0.41). These correlations are significantly different from one another (p<0.0001).
Figure 4.
Relationship between level of cockroach-specific IgE and IgG by Bla g 1 exposure. Also shown is the linear regression line. Panel A – Exposure level of <8 U/gram Bla g 1 in the bedroom. Panel B – Exposure level of >8 U/gram of Bla g 1 in the bedroom.
Discussion
There was a high prevalence of atopy (ie. skin test positivity) in this inner city asthmatic population, and almost 2/3 of the group had detectable serum specific IgE to at least one of the environmental allergens measured. The prevalence of IgE sensitization to allergens would likely be higher if we tested to a more extended panel. SIgE levels to environmental allergens can serve as predictors for asthma severity. There was a moderate correlation between allergen-specific IgE levels and skin testing, but each test identified some sensitive children that were not identified by the other test. In addition, there is no mediating effect of cockroach-specific IgG or IgG4 on IgE levels or asthma morbidity factors.
Our results demonstrate that allergen-specific IgE correlated well with skin test results, with 76–83% concordance. However, a significant portion of patients were negative in one, but not the other. This is due in part to the differences in allergens tested for cockroach and dust mite. Specific IgE testing was performed to whole body Blattella germanica and whole body culture of Dermatophagoides farinae whereas skin testing was performed using mixtures of cockroach (American and German) and dust mite (D. farinae and D. pteronyssinus). For Alternaria, the skin testing extract and specific IgE reagent were more comparable, thus providing a higher concordance rate. Repeating the study with allergen skin tests to the individual species of cockroach and dust mite may show a better concordance rate. In addition, both tests may be limited by their inherent sensitivity. The Multi-Test skin test device (Lincoln Diagnostics, Decatur, IL) is known to have intra- and inter-test variability,24 and can contribute to the differences in results. Another factor to consider for those with detectable IgE to cockroach, but negative skin testing is that the ImmunoCAP for whole body cockroach contains Bla g 7, a tropomyosin known to have cross reactivity with dust mites and crustaceans. The original NCICAS study did not include an assessment of food allergies, therefore, whether food allergies to shrimp or other shellfish resulted in positive cockroach-specific IgE for some is uncertain. Since there is a 76–83% concordance between skin testing with the Multi-Test device and serum SIgE, one should consider using the alternate test to exclude sensitization if clinically indicated.
Sensitization to environmental allergens has been demonstrated to be a risk factor for asthma. While sensitization does not always imply clinical reactivity, we found a linear dose-response relationship between SIgE to cockroach (Blattella germanica), dust mite (Dermatophagoides farinae) and Alternaria and asthma morbidity, including increased frequency of exacerbations and increased medication requirements.
Using relative risk analysis, neither allergen-specific prick skin tests nor IgE levels appear to be a more consistent predictor for asthma morbidity. Depending on which morbidity parameter is being tested, SIgE or prick skin tests for a given allergen may be a better indicator. For example, dust mite SIgE is a better predictor for hospitalizations, whereas prick skin test wheal size to dust mite is a better predictor for steroid use.
In contrast to prior studies, we examined both SIgE and prick skin testing cockroach, dust mite, and Alternaria to investigate the relationship between asthma morbidity and sensitization. Our data are consistent with Rosenstriech et al.14 who used prick skin testing alone to assess allergic sensitization. We found a strong correlation between exposure to environmental allergens and its sensitization determined by SIgE. Our data confirm previous findings that sensitization to cockroach, in conjunction with exposure, correlates with asthma exacerbations for inner-city children.12,14 This relation of cockroach allergic sensitization, cockroach allergen exposure and asthma morbidity is a phenomena that is found in children, but not in adults.25 We were unable to demonstrate such an association between SIgE and exposure to cockroach and asthma symptoms (i.e. wheeze), which is in contrast to Gruchalla et al.’s12 finding that sensitivity as defined by prick skin testing and exposure did significantly impact maximum symptom days for their cohort. We also failed to demonstrate a similar result for dust mites as our exposure levels were quite low.
Modifying allergen exposure may have beneficial effects for asthma management in these inner city asthmatic children. While there is an association between cockroach exposure and sensitization, the question remains as to whether exposure should be measured for each individual patient on clinical grounds. One should keep in mind that for environmental allergens, a true exposure level cannot be performed given the ubiquity of allergens outside of patients’ homes.
In current clinical practice, serum SIgE levels to environmental allergens have been used as a positive or negative marker for sensitization. In contrast, SIgE levels to food allergens provide the likelihood of reacting with exposures to specific foods. Different predictive values have been determined for each of the major food allergens.15 Unlike with food allergens, we found a linear dose-response relationship between SIgE to cockroach, dust mite, and Alternaria with asthma morbidity. We used an approach similar to Simpson et al.,10 who examined the association between SIgE only with asthmatic symptoms (i.e. wheezing) in an unselected population. In the NCICAS populations, SIgE to cockroach, dust mites and Alternaria have an impact on asthma morbidity even at low levels of sensitization. Levels > 0.35 kU/L were associated with increased exacerbations and medication use in our inner-city asthma population. However, we were unable to show a significant association between SIgE and asthma symptoms, in contrast to Simpson et al.10 The clinical significance of SIgE level cut-offs for these aeroallergens, however, is very different from those for food allergens. Perhaps no natural cutoff exists for B. germanica, D. farinae, and Alternaria because exposure to environmental allergens cannot be controlled in the same manner as exposure to foods. Furthermore, the clinical relevance of environmental sensitization is not as easily demonstrated as in food challenges.
In contrast, Pastorello et al.16 was able to establish cutoff values for aeroallergens as a predictor of the presence or absence of allergy symptoms based on participant reports. Their ROC analysis showed a cutoff of 8.4 kU/L for Dermatophagoides pteronyssinus, another dust mite species, to be associated with symptomatic allergy. However, that study included self-reported symptoms of rhinoconjunctivitis and asthma in recently diagnosed patients. Patients who suffered from asthma for over 2 years were excluded, and the study lacked objective measures of asthma morbidity such as the use of medications and ED/hospital visits. One can surmise that specific cutoff levels for different aeroallergens may be found when using different clinical outcomes. Nevertheless, our data strongly suggest an association between environmental allergen sensitization and asthma morbidity. Finally, the measure of total IgE correlated well with individual environmental allergen sensitization and with asthma morbidity.
In this inner-city asthmatic population, cockroach-specific IgG and IgG4 could be detected in the majority of our patients. Strong correlations between specific IgG and specific IgE were seen at high levels of Bla g 1 exposure in the bedroom, but this correlation was not seen for cockroach specific-IgG4. Furthermore, high cockroach specific IgG (but not IgG4) was associated with increased hospitalizations for asthma. This is in contrast to what was seen with the mammalian allergens where there is a strong IgG4 response with high exposures to cat and rat, and this is associated with less clinical disease.13,17 Jarvis et al.18- demonstrated that for dust mite allergens (Der p 1 and Der f 1), there was no evidence of an increase in house dust mite specific IgG and IgG4 with increasing exposure. They did not demonstrate that high IgG4 was associated with less clinical disease for dust mite allergens, in contrast, they showed that higher house dust mite specific IgG4 was associated with more wheeze and asthma independent of IgE sensitization. We did not examine dust mite specific IgG and IgG4 levels in our patient population, but our data indicate that the immune response to allergens can be very distinct for different allergens and the role of allergen-specific IgG and IgG4 may be more complicated.
We conclude that SIgE levels to environmental allergens can serve as predictors for asthma severity. There was also a strong correlation between SIgE levels and prick skin test results, but the tests did not always agree. We did not find a natural cutoff level for SIgE to cockroach, dust mite, or Alternaria that was highly predictive of clinical reactivity. Instead, SIgE to these allergens appear to have a linear dose-response relationship with asthma morbidity. Finally, there is no mediating effect of cockroach-specific IgG or IgG4 on SIgE levels or asthma morbidity. In fact, cockroach-specific IgG was associated with increased hospitalization for asthma. Although we did not examine dust mite-specific IgG in our study, Platts-Mills et al.13 found that presence of IgG to mite allergens was significantly associated with asthma as well. While sensitization does not automatically predict clinical allergy or reflect exposure, the awareness of new potential triggers and the modification of these environmental exposures may prove beneficial for a significant number of asthmatic patients.
Table ?
Geometric mean allergen-specific IgE levels and house dust allergen exposure.
| N | Geometric Mean | Geometric Std | |
|---|---|---|---|
| Total IgE (kU/L) | 511 | 181.21 | 4.60 |
| Cockroach (B.germanica) SIgE | 511 | 1.40 | 6.84 |
| Dust mite (D.farinae) SIgE | 511 | 0.69 | 4.11 |
| Alternaria SIgE | 511 | 0.97 | 5.38 |
| Bla g 1 (U/g) exposure | |||
| Bedroom | 199 | 5.36 | 8.65 |
| Kitchen | 197 | 54.02 | 13.31 |
| Family room | 198 | 7.30 | 6.42 |
| Dustmite (mcg/g) exposure | |||
| Bedroom | 199 | 0.29 | 3.95 |
| Kitchen | 198 | 0.12 | 1.75 |
| Family room | 198 | 0.24 | 3.59 |
Acknowledgments
We thank Phadia for kindly providing the reagents for specific IgE to cockroach (Blattella germanica), dust mite (Dermatophagoides farinae), and Alternaria and specific IgG and IgG4 to Blattella germanica.
Funding Source: The National Cooperative Inner-City Asthma Study was supported by grants UOI A1-30751, A1-30752, A1-30756, A1-30772, A1-30773-01, A1-30777, A1-30779, A1-30780, N01-A1-15105 from the National Institutes of Allergy and Infectious Disease (National Institutes of Health, Bethesda, MD).
The project described was supported in part by Grant Number M01-RR-00071 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Abbreviations
- NCICAS
National Cooperative Inner City Asthma Study
- IgE
Immunoglobulin E
- IgG
Immunoglobulin G
- Bla g 1
Blattella germanica 1
- Der f 1
Dermatophagoides farinae 1
- SIgE
Specific IgE
References
- 1.Zimmerman B, Feanny S, Reisman J, Hak H, Rashed N, McLaughlin FJ, et al. Allergy in asthma I. The dose relationship of allergy to severity of childhood asthma. J Allergy Clin Immunol. 1988;81:63–70. doi: 10.1016/0091-6749(88)90221-7. [DOI] [PubMed] [Google Scholar]
- 2.Inouye T, Tarlo S, Broder I, Corey P, Davies G, Leznoff A, et al. Severity of asthma in skin test-negative and skin test-positive patients. J Allergy Clin Immunol. 1985;75:313–319. doi: 10.1016/0091-6749(85)90063-6. [DOI] [PubMed] [Google Scholar]
- 3.Sarpong SB, Karrison T. Skin test reactivity to indoor allergens as a marker of asthma severity in children with asthma. Ann Allergy Asthma Immunol. 1998;80:303–308. doi: 10.1016/S1081-1206(10)62973-0. [DOI] [PubMed] [Google Scholar]
- 4.Gelber LE, Seltzer LH, Bouzoukis JK, Pollart SM, Chapman MD, Platts-Mills TA. Sensitization and exposure to indoor allergens as risk factors for asthma among patients presenting to hospital. Am Rev Respir Dis. 1993;147:573–578. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- 5.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I), the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 6.Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy. 2000;55:501–504. doi: 10.1034/j.1398-9995.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 7.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 8.Sears MR, Burrows B, Flannery EM, Herbison GP, Holdaway MD. Atopy in childhood I. Gender and allergen related risks for development of hayfever and asthma. Clin Exp Allergy. 1993;23:941–948. doi: 10.1111/j.1365-2222.1993.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 9.Kotaniemi-Syrjanen A, Reijonen TM, Romppanen J, Korhonen K, Savolainen K, Korppi M. Allergen-specific immunoglobulin E antibodies in wheezing infants: the risk for asthma in later childhood. Pediatrics. 2003;111:e255–e261. doi: 10.1542/peds.111.3.e255. [DOI] [PubMed] [Google Scholar]
- 10.Simpson A, Soderstrom L, Ahlstedt S, Murray CS, Woodcock A, Custovic A. IgE antibody quantification and the probability of wheeze in preschool children. J Allergy Clin Immunol. 2005;116:744–749. doi: 10.1016/j.jaci.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102:563–570. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 12.Gruchalla RS, Pongracic J, Plaut M, Evans R, 3rd, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Platts-Mills TAE, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–756. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 14.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 15.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 16.Pastorello EA, Incorvaia C, Ortolani C, Bonini S, Canonica GW, Romagnani S, et al. Studies on the relationship between the level of specific IgE antibodies and the clinical expression of allergy: I. Definition of levels distinguishing patients with symptomatic from patients with asymptomatic allergy to common aeroallergens. J Allergy Clin Immunol. 1995;96:580–587. doi: 10.1016/s0091-6749(95)70255-5. [DOI] [PubMed] [Google Scholar]
- 17.Jeal H, Draper A, Harris J, Taylor AN, Cullinan P, Jones M. Modified Th2 Responses to High-Dose Exposures to Allergen Using an Occupational Model. Am J Respir Crit Care Med. 2006;174:21–25. doi: 10.1164/rccm.200506-964OC. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis D, Zock JP, Heinrich J, Svanes C, Verlato G, Olivieri M, et al. Cat and dust mite allergen levels, specific IgG and IgG4, and respiratory symptoms in adults. J Allergy Clin Immunol. 2007;119:697–704. doi: 10.1016/j.jaci.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 19.Satinover SM, Reefer AJ, Pomes A, Chapman MD, Platts-Mills TAE, Woodfolk JA. Specific IgE and IgG antibody-binding patterns to recombinant cockroach allergens. doi: 10.1016/j.jaci.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Kattan M, Mitchell H, Eggleston P, Gergen P, Crain E, Redline S, et al. Characteristics of inner-city children with asthma: the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:253–262. doi: 10.1002/(sici)1099-0496(199710)24:4<253::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell H, Senturia Y, Gergen P, Baker D, Joseph C, McNiff-Mortimer K, et al. Design and methods of the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:237–252. doi: 10.1002/(sici)1099-0496(199710)24:4<237::aid-ppul3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Robbins AS, Chao SY, Fonseca VP. What’s the relative risk? A method to directly estimate risk ratios in cohort studies of common outcomes. Ann Epidemiol. 2002;12:452–454. doi: 10.1016/s1047-2797(01)00278-2. [DOI] [PubMed] [Google Scholar]
- 23.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2007 ISBN 3-900051-07-0 URL http://www.R-project.org.
- 24.Berkowitz RB, Tinkelman DG, Lutz C, Crammie A, Smith K. Evaluation of the Multi-Test device for immediate hypersensitivity skin testing. J Allergy Clin Immunol. 1992;90:979–985. doi: 10.1016/0091-6749(92)90471-d. [DOI] [PubMed] [Google Scholar]
- 25.Wisnivesky JP, Sampson HA, Berns S, Kattan M, Halm EA. Lack of association between indoor allergen sensitization and asthma morbidity in inner-city adults. J Allergy Clin Immunol. 2007;120:113–120. doi: 10.1016/j.jaci.2007.03.044. [DOI] [PubMed] [Google Scholar]