Abstract
During development, sensory axons compete for limiting neurotrophic support, and local neurotrophin insufficiency triggers caspase-dependent axon degeneration. The signaling driving axon degeneration upon local deprivation is proposed to reside within axons. Our results instead support a model in which, despite the apoptotic machinery being present in axons, the cell body is an active participant in gating axonal caspase activation and axon degeneration. Loss of trophic support in axons initiates retrograde activation of a somatic pro-apoptotic pathway, which in turn is required for distal axon degeneration via an anterograde pro-degenerative factor. At a molecular level, the cell body is a convergence point of two signaling pathways whose integrated action drives upregulation of pro-apoptotic Puma, which, unexpectedly, is confined to the cell body. Puma then overcomes inhibition by pro-survival Bcl-xL and Bcl-w and initiates the anterograde pro-degenerative program, highlighting the role of the cell body as an arbiter of large-scale axon removal.
Introduction
Nervous system development involves a generative phase during which neurons are born and extend axons, which then branch extensively. This is followed by a regressive phase involving stereotyped pruning events that vary in scale from remodeling of individual synapses to the removal of entire axon branches or even the loss of entire neurons through degeneration. Axon pruning events can be triggered in response to local pro-degenerative cues from neighboring cells or by loss of neurotrophic support (Schuldiner and Yaron, 2015). Aberrant axon degeneration and neuronal cell death are also widely observed in a number of neurodegenerative diseases, highlighting the need to define factors that govern axon degeneration as potential therapeutic targets (Luo and O'Leary, 2005).
Developing sensory neurons of the mouse dorsal root ganglion (DRG) are produced in excess and culled along with their axons during an extensive remodeling process triggered by declining levels of target-derived neurotrophic support. Trophic deprivation (TD) triggers a Bax-dependent apoptotic pathway involving effector Caspases-3 and -6, which culminates in Calpain-dependent axon fragmentation (Cusack et al., 2013; Nikolaev et al., 2009; Schoenmann et al., 2010; Simon et al., 2012; Unsain et al., 2013). Caspase-dependent axon pruning also contributes to the stereotyped pruning of retinocollicular axons during development, a process that is not overtly trophic dependent (Nikolaev et al., 2009; Simon et al., 2012) and is paralleled by caspase-dependent pruning of dendrites (Kuo et al., 2006; Williams et al., 2006). Evidence for Caspase-dependent axon degeneration has also been obtained in neurodegenerative disease and stroke (Wang et al., 2015).
Although several effectors of axon degeneration have been defined, how these effectors come to be activated, and where in the neuron the degenerative program is initiated remain unanswered. Current models of axon degeneration posit that distal trophic deprivation of axons (with maintained trophic support of cell bodies) directly and locally initiates a signaling pathway at the level of the deprived axon that triggers caspase-dependent distal axon degeneration independent of somatic signaling events. (Ghosh et al., 2011). A study in zebrafish using an indirect reporter of caspase activation further pointed to activation of caspases at axon branch points as evidence for localized, branch-specific pro-degenerative signaling (Campbell and Okamoto, 2013).
While local activation of a pro-degenerative program within axons would seem a natural way to control distal axon degeneration, a role for the cell body in affecting this process has been indicated by the findings that pre-exposure of cell bodies to a transcriptional inhibitor, inhibition of somatic GSK3β, or total severing of axons from cell bodies blocked subsequent degeneration of axons triggered by trophic deprivation (Chen et al., 2012; Gerdts et al., 2013). These findings imply that local events are not sufficient to drive axon degeneration and that cell-wide signals are also necessary.
Here we have addressed in detail the role of the cell body through a comprehensive dissection of the signaling events that couple distal TD to axon degeneration. We show that although the apoptotic machinery is present and functional in axons, it is not activated directly by distal TD. Rather, the initiating signal for degeneration comes from the cell body, after being activated by convergent retrograde signals (loss of Akt signaling and activation of JNK signaling) from the distally-deprived axon. Further, we define the key regulated step in this process as transcriptional upregulation by Foxo3a and c-Jun of the pro-apoptotic protein Puma, whose antagonistic relationship to anti-apoptotic Bcl-2 family members Bcl-xL and Bcl-w governs axon degeneration. Puma is a key regulator, since loss of Puma potently protects distal axons from degeneration following TD, yet, quite unexpectedly, Puma expression is confined to the cell body. Our results also imply that rising Puma levels overcome inhibition by Bcl-xL and Bcl-w, then trigger axon degeneration by an additional somatically-derived factor(s) distinct from Puma that moves from the cell body to the axon.
Collectively, our data define the cell body as a key check-point that integrates pro-degenerative JNK and pro-survival Akt signaling pathways and gates the induction of axon degeneration via Foxo3a/c-Jun control of cell body-localized Puma. The stringent control by the cell body of axon degeneration in response to local deprivation allows for integration of pro-survival and pro-death signals from the periphery prior to axon removal, with implications for the development and refinement of neural circuits.
Results
Apoptotic machinery is present in axons but requires the cell body for activation by TD
NGF-dependent sensory axons undergo Caspase-dependent degeneration when deprived of neurotrophic (NGF) support. This degeneration is seen both when the entire neuron is deprived and when the manipulation is restricted to the axon through use of compartmented Campenot chambers that allow for independent manipulation of axons and cell bodies via fluid isolation of these compartments (Campenot, 1977).
Underlying TD-induced axon degeneration is a caspase cascade beginning with Bax activation and resulting in the ordered activation of Caspases-9, -3, and -6 (Figure 1A) (Cusack et al., 2013; Nikolaev et al., 2009; Simon et al., 2012). Bax-dependent activation of Caspase-9 typically results from cytochrome c release from the mitochondria that is bound by Apaf1 within the Caspase-9-containing “apoptosome” (Yuan and Akey, 2013). A recent study using an indirect measure of axon degeneration (‘maintained axon length’ of axons cultured in microfluidic chambers) argued against a role for Apaf1 in sympathetic axons, despite confirming a role for Bax and Caspase-9 (Cusack et al., 2013). We therefore re-visited the role Apaf1 in our system by directly examining axon degeneration in Apaf1 knockout sensory neurons, and found potent axon protection (Figure S1A,B), thereby establishing Apaf1 as a key component of the axon degeneration pathway per se (Figure 1A).
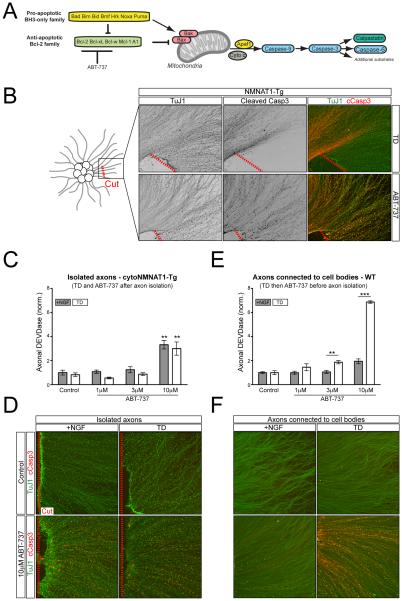
Figure 1. Axon degeneration after TD requires the cell body.
A) The mitochondrial apoptotic pathway is regulated by pro- and anti-apoptotic members of the Bcl-2 family. The balance of these factors regulates Bax/Bak oligimerization and activation of downstream Caspases.
B) Following 2 days in vitro (DIV), a portion of the axons in the explants from cytoNMNAT1-Tg mice were physically severed from their cell bodies (indicated with a dashed red line) followed immediately by TD (top row) or the addition of 10µM ABT-737 (bottom row) for 9hr and 4hr, respectively.
C and D) Axons from 2 DIV cytoNMNAT1-Tg dissociated and re-aggregated cultures were severed from their cell bodies (dashed red line) and treated with ABT-737 for 4hr in the presence of NGF or following 2hr TD (for a total of 6hr TD) as indicated. DEVDase activity of isolated axons was subsequently measured by the Caspase-Glo-3/7 assay (C) or with immunostaining (D). n=4 for all conditions except 1µM and 10µM ABT-737 plus TD where n=3. Values are normalized to the average value of the +NGF control condition. Statistical significance for each 10µM dose is determined relative its respective Control condition.
E and F) Intact DRG cultures were treated with ABT-737 in the presence of NGF or following 2hr TD (for a total of 6hr) as indicated. Following treatment, either cell bodies were removed and axonal DEVDase activity was measured as above (E) or cleaved Caspase-3 was visualized with immunostaining (F). n=4 for all conditions except 10µM ABT-737 plus TD where n=3. Values are normalized to the average value of the +NGF control condition.
Here and throughout, values are presented as mean ± SEM; *p<.05; **p<.01; ***p<.001
To begin to explore the role of the cell body in regulating local degeneration, we severed axons from their cell bodies and subjected the isolated axons to TD. To exclude confounding effects of degeneration induced by the injury (Wallerian degeneration), we performed these experiments using sensory neurons from a transgenic mouse expressing cytoplasmic NMNAT1, which protects axons from Wallerian degeneration (Vohra et al., 2010) but does not interfere with the processing of Caspase-3 in response to TD in intact neurons (Figure S1C). Protection of axons downstream of Caspase-3 likely implies that NMNAT1 expression interferes with activation of Calpains, the terminal enzymes in the axon degeneration pathway (Yang et al., 2013). Indeed, while the Caspase-3 substrate αII spectrin is cleaved, NMNAT1 expression blocks cleavage of the Calpain substrates Neurofilament-M and α I (Figure S1C). Separation of axons from their cell bodies completely blocked appearance of cleaved Caspase-3 immunoreactivity 9 hrs following TD, in contrast with strong immunoreactivity in intact axons (Figure 1B). This result confirmed that the cell body is required for caspase-dependent degeneration in response to TD (Gerdts et al., 2013). Similar results were obtained in Sarm1 knockout axons and in wild-type axons treated with methyl pyruvate (Figure S1D), two independent manipulations that protect axons from Wallerian degeneration (Gerdts et al., 2013; Osterloh et al., 2012; Wang et al., 2005).
A possible explanation for cell body dependence would be if the cell body is continually required to replenish components of the apoptotic pathway. To test whether the apoptotic machinery is present and functional in axons that have been disconnected from their cell bodies, we exposed isolated axons to ABT-737, a selective antagonist of the three pro-survival factors Bcl-2, Bcl-xL and Bcl-w (Oltersdorf et al., 2005) (Figure 1A), which causes Bax- and Caspase-3-dependent axon degeneration when applied directly to axons in Campenot chambers, even in the presence of NGF (Simon et al., 2012). We found that the response to ABT-737 is also Caspase-9-dependent, can be blocked by Bcl-xL overexpression, and is unaffected by transcriptional inhibition (Figure S1E-G), further indicating its specificity. 10 μM ABT-737 triggered robust Caspase-3 cleavage in isolated NMNAT1-Tg axons, Sarm1 mutant axons and methyl pyruvate-treated wild type axons, as assessed by immunohistochemistry (Figure 1B, S1D). To quantify this effect, we lysed isolated NMNAT1-Tg axons in a buffer containing a luciferase based probe for Caspase-3/7-like enzymatic activity (hereafter referred to as ‘DEVDase’ activity, as the Caspase target sequence in the probe is DEVD). Consistent with the results of immunohistochemistry, application of 10 μM ABT-737 increased DEVDase activity in isolated axons but TD did not (Figure 1C). TD also did not increase Caspase activity triggered by ABT-737, as assessed by measuring DEVDase activity (Figure 1C) and cleaved Caspase-3 immunoreactivity (Figure 1D).
We also applied ABT-737 to intact neurons, and observed a similar degree of DEVDase activation at 6hrs in their axons (which were isolated for DEVDase measurement at that time) as we had seen in isolated axons that were treated with ABT-737 only after being severed from their cell bodies (compare Figures 1C and 1E). Interestingly, in these intact neurons, a brief period of TD that was itself insufficient to cause a detectible increase in DEVDase activity dramatically potentiated the response of their axons to ABT-737 (Figure 1E,F). Thus, TD triggers an early cell body-dependent increase in the pro-apoptotic state of axons.
Collectively, our results indicate that axons contain all necessary apoptotic machinery required to initiate Caspase-3 activation, but local TD is incapable of activating this machinery directly in isolated axons.
Akt signaling controls axon survival and requires the cell body
To illuminate the role of the cell body, we set out to map the pathway of degeneration. We first examined how local removal of NGF initiates the degenerative response. One possibility is that the process is initiated by loss of pro-survival signaling mediated by the kinase activity of TrkA, the NGF receptor. It has, however, been proposed alternatively that TrkA is a “dependence receptor”, which, when unliganded, sends a pro-apoptotic signal that is independent of its kinase activity (Nikoletopoulou et al., 2010). To distinguish these possibilities, we interfered specifically with kinase-dependent signaling using sensory neurons from the TrkA F592A knock-in mouse in which TrkA is engineered to be sensitive to acute inhibition of its kinase activity by the small molecule 1NMPP1, which does not inhibit wild-type kinases (Chen et al., 2005). Application of 1NMPP1 specifically to axons induced axon degeneration (Figure 2A), consistent with loss of kinase activity being the driver of degeneration, rather than gain of a pro-apoptotic kinase-independent signal. 1NMPP1 induced cleavage of axonal Caspase-3 in intact axons, but not in NMNAT1-expressing axons disconnected from their cell bodies, further supporting a role for the cell body in axonal Caspase-3 activation downstream of TrkA inhibition (Figure S2A).
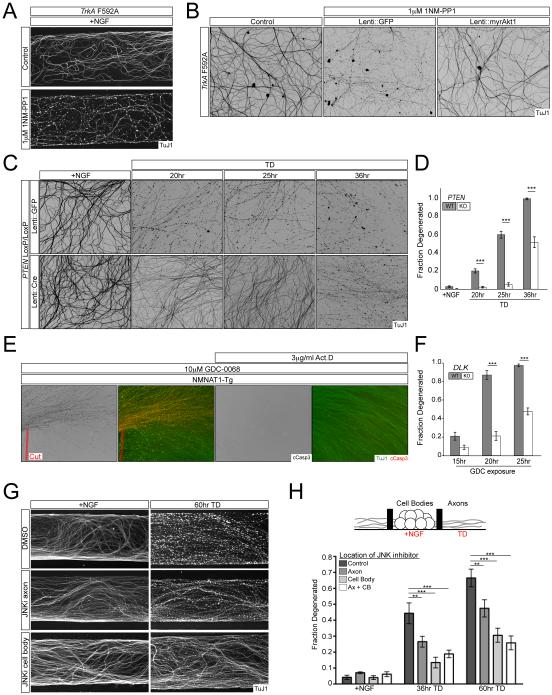
Figure 2. Akt and JNK signaling regulate axon degeneration via the cell body.
A) TrkA F592A knock-in DRGs were cultured in Campenot chambers and the axonal compartment was treated with vehicle or 1µM 1NM-PP1 for 24hr.
B) TrkA F592A dissociated DRG cultures were transduced with lentivirus expressing myristoylated-Akt1, Bcl-xL, or GFP and treated with 1µM 1NM-PP1 for 24hr. Lentiviral Bcl-xL overexpression also provided similar axonal protection (data not shown).
C and D) PTENloxP/loxP dissociated and reaggregated DRG cultures were transduced with lentivirus expressing CRE or GFP and axon degeneration following TD was visualized (C) and quantified (D). n=5 for Lenti::GFP conditions and n=6 for Lenti::Cre conditions except 25hr TD where n=7
E) Axons from cytoNMNAT1-Tg explants were severed from their cell bodies (dashed red line) or pre-treated with 3µg/ml Actinomycin D for 1hr prior to addition of 10µM GDC-0068 for 12hrs.
F) DLKloxP/loxP DRG cultures were transduced with lentivirus expressing GFP or Cre and subjected to treatment with 10µM GDC-0068 and axon degeneration was quantified over time. For Lenti::GFP n=5 (15hr), n=7 (20hr), and n=4 (25hr). For Lenti::Cre n=8 for all conditions. Representative images are presented in Figure S2F.
G and H) DRGs were cultured in Campenot chambers where cell bodies and axons are separated by a grease barrier that allows for fluidic isolation (see illustration). Cultures were treated with 10µM JNK inhibitor VIII or vehicle in either or both the cell body and axonal compartments, followed by TD as indicated. Degeneration was visualized (G) and quantified (H). n=4 for each condition.
A major pro-survival pathway downstream of TrkA kinase is Akt signaling. The degeneration induced by TrkA inhibition by 1NMPP1 was blocked by lentiviral expression of a constitutively active, myristoylated form of Akt1 (myrAkt1) (Figure 2B). MyrAkt1 also potently protected axons from degeneration following TD (Figure S2B, C). Phosphoinositide PIP3-dependent activation of Akt is antagonized by the tumor suppressor phosphatase and tensin homolog (PTEN), so genetic deletion of PTEN represents an alternative strategy to activate Akt signaling. Sensory neuron cultures were therefore established from a conditional mutant of PTEN and infected with lentivirus expressing either GFP or Cre recombinase. Deletion of PTEN similarly protected axons from degeneration induced by TD, though the duration of protection was shorter than for myrAkt1 expression (Figure 2C,D), presumably because it is less effective at activating downstream Akt signaling.
That Akt signaling regulates axon survival downstream of TrkA suggested acute pharmacological inhibition of Akt as a useful tool to probe the role of the cell body during TD. Inhibition of Akt in the axonal compartment of Campenot chambers using the selective pan-Akt inhibitor GDC-0068 induces axon degeneration (Figure S2E). Activation of axonal Caspase-3 induced by Akt inhibition was eliminated in Caspase-9 knockout cultures, indicating a specific activation of the apoptotic pathway by this treatment (Figure S2D). Further mirroring the dependence on cell body seen with local TD (Figure 1B), we found axonal Caspase-3 activation by GDC-0068 was prevented by axotomy in cultures derived from the NMNAT1 transgenic or by co-incubation with the transcriptional inhibitor Actinomycin D (Figure 2E). These results further support a requirement for ongoing somatic transcriptional activity in mediating axonal Caspase-3 activation.
Pro-degenerative JNK signaling is activated by loss of Akt signaling and requires the cell body
Previous studies have implicated the MAPKKK DLK as a JNK pathway activator in the regulation of axon degeneration in response to TD (Ghosh et al., 2011; Huntwork-Rodriguez et al., 2013). We therefore used the Akt inhibitor to examine a possible connection between loss of Akt signaling and the JNK pathway. Degeneration caused by acute Akt inhibition was significantly blunted in cultures of neurons in which DLK was genetically deleted (Figure 2F, S2F), placing DLK activation downstream of TD-associated loss of Akt signaling. Akt can directly phosphorylate DLK and inhibit its kinase activity (Wu et al., 2015), suggesting that DLK activation in response to local TD may reflect a disinhibition by removal of Akt signaling, although this remains to be established in our system.
It has been suggested that DLK directly promotes degeneration through local activation of the apoptotic pathway in axons (Ghosh et al., 2011). We cultured wild-type sensory neurons in Campenot chambers and subjected their axons to TD in the presence of a small molecule JNK inhibitor in either the axonal or cell body compartment. We found that axonal inhibition of JNK signaling provides modest protection of axons, consistent with previous findings (Chen et al., 2012; Ghosh et al., 2011), but also found that inhibition of JNK activity exclusively in the cell body compartment protected axons to a much greater degree (Figure 2G,H). This result was surprising because a previous study discounted the JNK target c-Jun as a significant regulator of axon degeneration (as opposed to cell body degeneration) (Ghosh et al., 2011). Here we revisited those findings and observed instead that, in addition to protecting against somatic activation of Caspase-3, genetic deletion of c-Jun also promotes significant axon survival in dissociated sensory neuron cultures (Figure S2G-I). The discrepancy in findings is likely due to inefficient excision of c-Jun in the prior study, in which Cre recombinase was driven by the Nestin promoter. Indeed, we found that lentiviral delivery of Cre to c-Jun conditional knockout cultures, as done here, provides a much more complete deletion of the mature c-Jun protein (Figure S2J). Thus, our results argue that activation of DLK regulates cell body JNK/c-Jun signaling that is essential for axon degeneration, but is not sufficient to directly activate degeneration locally in axons.
TD activates a Foxo3a-dependent pro-degenerative program
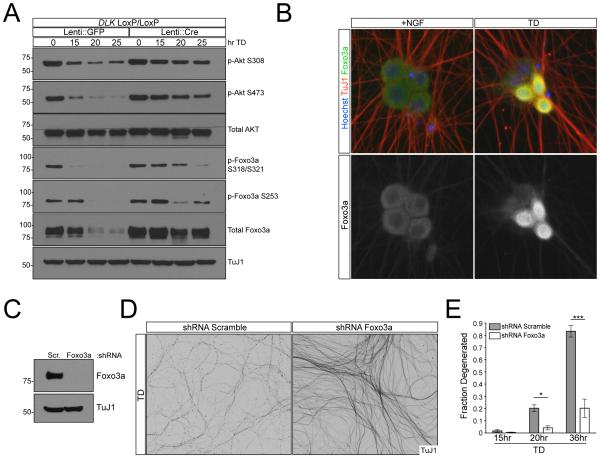
The observations that axon degeneration requires somatic JNK/c-Jun activity (Figure 2G,H) and axon degeneration following Akt inhibition requires transcription (Figure 2E) point to a pro-degenerative role of the cell body. We took a candidate approach and searched for transcription factors known to be downstream of Akt and JNK. Specifically, we focused on the transcription factor Foxo3a, whose pro-apoptotic activity is inhibited by Akt-mediated phosphorylation (Brunet et al., 1999). Following TD, we observed concurrent decreases in phosphorylation of Akt and of Foxo3a (Figure 3A), consistent with transcriptional activation of Foxo3a. Interestingly, in DLK knockout cultures the decreases in Akt phosphorylation and Foxo3a phosphorylation following TD occurred significantly more slowly, suggesting that DLK might participate in a feedback loop that enhances Akt inactivation following TD. Nuclear import to enable transcriptional activation normally follows de-phosphorylation of Foxo3a, and indeed we observed a nuclear migration of Foxo3a within several hours of TD (Figure 3B). Importantly, shRNA-mediated knockdown of Foxo3a strongly protected axons from degeneration following TD (Figure 3D,E), thus identifying Foxo3a as a key regulator of axon degeneration.
Figure 3. Foxo3a functions downstream of Akt during TD.
A) DLKloxP/loxP DRG cultures were transduced with lentivirus expressing GFP or Cre and lysed at indicated time points after TD. Phosphorylation of Akt and Foxo3a was assessed by immunoblot.
B) 7DIV WT dissociated DRGs were subjected to TD for 9hr. Foxo3a localization was visualized with immunostaining.
C) Knockdown of Foxo3a was confirmed by immunoblot.
D and E) WT dissociated and reaggregated DRG cultures were subjected to lentiviral-shRNA knockdown of Foxo3a or Scrambled control. Axon degeneration after TD was visualized (D) and quantified (E).
Pro-apoptotic Puma regulates axon degeneration
Axon degeneration after TD is Bax dependent (Nikolaev et al., 2009) so we reasoned that these upstream processes may converge on the pro-apoptotic ‘BH3-only’ family of Bcl-2 proteins, which includes 7 members (Figure 1A). We screened a panel of knockout mice deficient in members of this family for axonal protection in sensory neuron explant cultures after TD. Axons were not protected in Bad or Hrk single mutants or in Bid;Bim double mutants (Figure 4A, S3A, data not shown). Furthermore, shRNA-mediated knockdown of Noxa provided no obvious axonal protection (data not shown). Notably however, we observed potent axonal protection after genetic deletion of Puma (Figure 4A,B). Loss of Puma also completely blocked the appearance of axonal cleaved Caspase-3 in response to acute Akt inhibition (Figure S3B). We also cultured Puma knockout neurons in Campenot chambers and similarly observed potent axonal protection following TD in the axonal compartment (Figure 4C,D). Near complete protection was seen at early time points in Puma knockout cultures (e.g. only 10% of axons had degenerated by 60 hr after TD in Campenot chambers), indicating that Puma is a key regulator of degeneration. It should be noted, however, that this protection is slightly less complete than seen in Bax and Caspase-3 and -9 knockout cultures (Nikolaev et al., 2009; Simon et al., 2012), suggesting the presence of an additional Bax-activating factor(s), which remains to be defined, that functions in parallel to Puma and provides the residual protection. Indeed, other genes transcriptionally upregulated after TD appear to contribute modestly to axon degeneration (Chen et al., 2012), and may do so by collaborating with Puma.
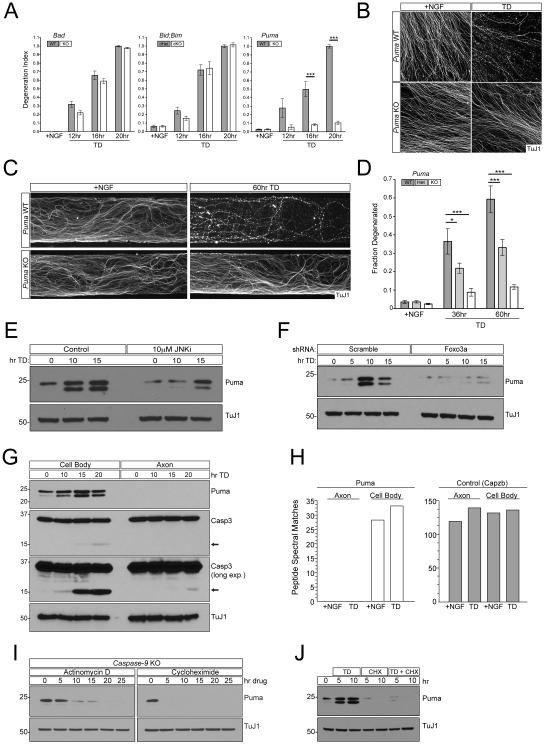
Figure 4. Puma promotes axon degeneration downstream of Foxo3a.
A and B) 2 DIV DRG cultures from the indicated genotypes were subjected to TD for indicated times. Axon degeneration was visualized with immunostaining and quantified (A). Representative images shown for Puma KO compared to WT(B). n=4 for Bad KO and WT. n=3 for Bid;Bim dKO and dHet. n=4 KO and 7 WT for Puma. Representative images for Bad and Bid;Bim cultures are presented in Figure S3A.
C and D) Puma WT, Het, or KO DRGs were cultured in Campenot chambers and the axonal compartment was subject to TD for the indicated times. Degeneration was visualized (C) and quantified (D). n=6 +NGF, n=6 36hr TD, and n=5 60hr TD for WT. n=6 +NGF, n=6 36hr TD, and n=3 60hr TD for Het. n=8 TD, n=7 36hr TD, and n=3 60hr TD for KO.
E) WT DRG cultures were treated with 10µM JNK inhibitor VIII or vehicle and protein was harvested at indicated time points after TD. Puma expression was assayed by immunoblot.
F) WT dissociated and reaggregated DRG cultures were subjected to lentiviral-shRNA knockdown of Foxo3a or Scrambled control. Protein was harvested at indicated time points after TD. Puma expression was assayed by immunoblot.
G) Separate cell body and axonal preparations were collected from WT DRG cultures at indicated time points after TD and subjected to immunoblot analysis.
H) At 12DIV Large-scale cultures made from WT embryonic DRGs were subjected to TD for 24hr. Separate cell body and axonal samples were collected and analyzed by tandem mass spectrometry. The number of peptide spectral matches are shown for each sample for either Bbc3 (Puma) or the loading control Capzb (capping protein actin filament z-line). An additional loading control and the spectra corresponding to each identified peptide are presented in Figure S3D-F.
I) Caspase-9 KO DRG cultures in the presence of NGF were treated with Actinomycin D or cycloheximide, harvested at the indicated time points and subjected to immunoblot analysis.
J) WT DRG cultures were subjected to TD, cycloheximide, or both treatments concurrently as indicated. Puma levels were analyzed by immunoblot.
Puma is classically described as a transcriptionally regulated gene induced by cellular stress (Yu and Zhang, 2008). Interestingly, we found that Puma protein is basally expressed in sensory neuron cultures in the presence of NGF, and its levels are potentiated in response to TD (Figure 4E). We similarly observed a significant rise in Puma mRNA levels following TD (data not shown), implicating transcriptional regulation. The rise is Puma is also accompanied by the de novo appearance of a second Puma band at a slightly lower molecular weight (Figure 4E), whose molecular identity and physiological significance remain to be determined. The increase in Puma expression and appearance of the second band occurred to a similar extent in Caspase-9 knockout cultures (Figure S3C), which are protected from degeneration (Simon et al., 2012), indicating that both the rise of overall Puma levels and the appearance of the second band are not simply a secondary consequence of the degenerative process. Pharmacological inhibition of JNK prevented this TD-induced rise in Puma (Figure 4E), consistent with a known role of c-Jun in promoting Puma expression (Akhter et al., 2015) and providing a mechanism for the somatic requirement for JNK signaling that we observed (Figure 2G,H). Puma has also been identified as a transcriptional target of Foxo3a (You et al., 2006), and accordingly we found that shRNA-mediated knockdown of Foxo3a in sensory neurons largely blocked the rise in Puma levels that accompanies TD (Figure 4F). Thus, activation Puma appears to be a point of convergent action of Akt/Foxo3a and JNK/c-Jun signaling that is required for axon degeneration following distal TD.
Puma functions in the cell body to promote axon degeneration
Puma can directly regulate Bax/Caspase activation, and since Caspase activation is observed in axons and deletion of Puma protects axons in Campenot chambers, we expected Puma to be present in axons as well. We were therefore very surprised not to detect any Puma protein in axons. Sensory neuron cultures were subjected to a time course of TD followed by clear separation and specific lysis of axons and cell bodies using methods that we have previously established (Yang et al., 2013). TD was accompanied by a rise in Puma protein levels in cell bodies, but there was no detectible signal in axons as assessed by immunoblotting (Figure 4G). To confirm this finding, we used mass spectrometry. We identified a number of stable Puma peptides, and used these as a basis to specifically probe for Puma in extracts of axons and cell bodies before and during TD. As an internal control for similar protein content between samples, we found that two separate proteins with similar mass to Puma (Capzb and 14-3-3γ) are present at equivalent levels in cell body and axon samples. Using this approach we confirmed the presence of Puma in the cell body, but again did not detect Puma in the axon under any condition (Figure 4H, S3D-F). We cannot of course exclude the presence of very low, undetectable levels of Puma in axons, however such expression would not be physiologically relevant as the strong expression of the Puma antagonists Bcl-xL and Bcl-w that we observe in axons (see below) would be expected to inhibit the activity of any low-level of Puma that is present.
To explore the molecular basis of this somatic restriction we exposed sensory neuron cultures to either the transcriptional inhibitor Actinomycin D or the translation inhibitor cycloheximide. Caspase-9 knockout cultures were used to prevent an apoptotic response due to drug-induced cellular stress (as mentioned, Puma levels change to the same extent following TD in these cultures as in wild-type cultures). Interestingly, following 20hr of Actinomycin D or 5hr of cycloheximide treatment, Puma protein became undetectable (Figure 4I), suggesting that Puma has an extremely short half-life in healthy cells and is subject to an active gene expression program. Puma levels are similarly sensitive to cycloheximide during TD (Figure 4J), indicating that de novo expression rather than stabilization of existing Puma protein underlies its rise during TD. This rapid turnover might in principle contribute to Puma’s exclusion from sensory axons, though it is possible that there are active mechanisms that exclude it as well. Importantly, the dependence of axon degeneration on Puma, together with the exclusion of Puma from the axon, imply the existence of a somatically-derived triggering factor distinct from Puma that moves to the axon after TD as a consequence of Puma’s somatic activity.
Bcl-xL and Bcl-w regulate the survival of sensory axons
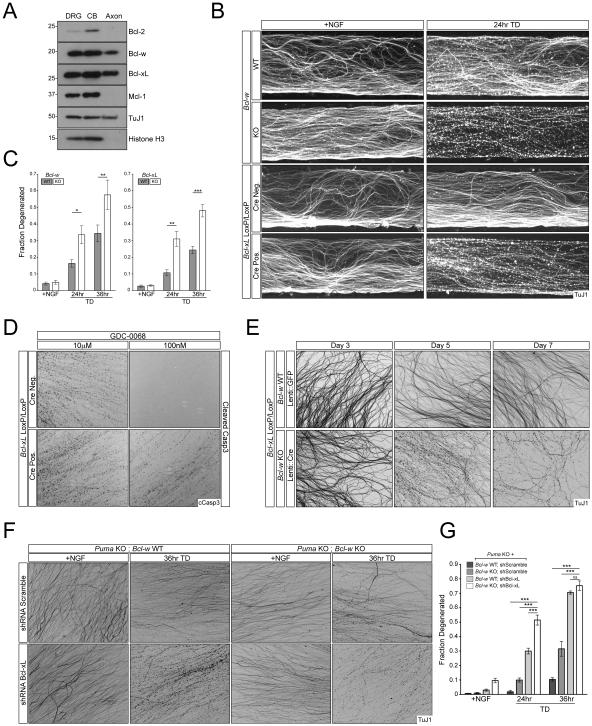
Puma’s pro-apoptotic activity is antagonized by its binding to each of the five known anti-apoptotic Bcl-2 family members, three of which are the targets of ABT-737 (Figure 1A). We therefore examined the expression of the five anti-apoptotic factors in axons, cell bodies, and whole DRGs by immunoblotting (Figure 5A). We were unable to detect A1 protein in either axons or cells bodies; each of the four additional family members, Bcl-2, Bcl-w, Bcl-xL, and Mcl-1 were expressed in sensory cell bodies, but only Bcl-xL and Bcl-w were detected in axons (Figure 5A). We focused our analysis on Bcl-xL and Bcl-w for several reasons: they are the only ones detected in axons; Mcl-1 is not targeted by ABT-737; the Bcl-2-specific inhibitor ABT-199 (Souers et al., 2013) did not induce axon degeneration in our assays (data not shown); and Bcl-w has already been implicated in sensory axon survival (Courchesne et al., 2011). Consistent with the latter study, loss of Bcl-w accelerated axon degeneration in response to TD in both whole sensory explant cultures and in Campenot chambers (Figure 5B,C, S4A,B). Unexpectedly, loss of Bcl-xL, which has not previously been studied in this context, resulted in a comparable phenotype, suggesting a shared role in regulation of axon survival (Figure 5B,C, S4A,B). Genetic loss of Bcl-xL similarly sensitized axons to Akt inhibition (Figure 5D), as did genetic loss of Bcl-w (data not shown). Thus, Bcl-xL and Bcl-w each individually antagonize pro-apoptotic signaling driving TD-induced axon degeneration.
Figure 5. Bcl-xL and Bcl-w regulate axon survival.
A) Separate cell body and axon preparations were harvested from WT DRG cultures for immunoblot analysis.
B and C) DRGs from the indicated genotypes were cultured in Campenot chambers and the axonal compartment was subjected to TD for the indicated time points. Axon degeneration was visualized (B) and quantified (C). n=3 for all time points for Bcl-xLloxP/loxP. n=6 +NGF, n=5 24hr TD, and n=6 36hr TD for Bcl-xLloxP/loxP; Nestin::Cre. n=9 +NGF, n=8 24hr TD, and n=9 36hr TD for Bcl-w WT. n=9 +NGF, n=8 24hr TD, and n=9 36hr TD for Bcl-w WT. n=6 for all time points for Bcl-w KO.
D) 7 DIV Embryonic DRG cultures from Bcl-xLloxP/loxP and Bcl-xLloxP/loxP; Nestin::Cre embryos were treated with the indicated concentrations of Akt inhibitor for 12hr.
E) Dissociated and reaggregated DRG cultures from indicated genotypes were transduced with lentivirus expressing GFP or Cre. Axon degeneration was visualized at 3, 5, and 7 days post-infection.
F and G) Puma KO or Puma KO; Bcl-w KO dissociated and reaggregated DRG cultures were subjected to lentiviral-shRNA knockdown of Bcl-xL or Scrambled control. Axon degeneration was visualized (F) and quantified (G). n=2 for all Puma KO conditions and n=4 for all Puma KO; Bcl-w KO conditions.
Even more dramatically, combined genetic deletion of both Bcl-xL and Bcl-w caused gradual axon degeneration even in the presence of NGF (Figure 5E), such that after 7 days in culture axons no longer survive. What causes this degeneration? The basal expression of Puma in sensory neurons in the presence of NGF distinguishes these neurons from many other cell types where Puma is absent until it is induced by cellular stress (Yu and Zhang, 2008). We therefore tested whether Puma was responsible for the degeneration. Consistent with this possibility, we found that the progressive degeneration observed in presence of NGF following combined loss of Bcl-xL and Bcl-w is suppressed by further genetic deletion of Puma (Figure 6A,B) (in this experiment, Puma and Bcl-w were deleted genetically, and Bcl-xL was knocked down by shRNA, because of the difficulty of obtaining triple knockout mice). That Puma is epistatic to Bcl-xL and Bcl-w in the presence of NGF indicates both that basally expressed Puma is functional, and that a physiological role of the anti-apoptotic proteins in this context is to suppress basal Puma activity. This finding also supports a role for Puma as an apoptotic activator even in the presence of NGF (albeit one that is normally inhibited by Bcl-xL/w).
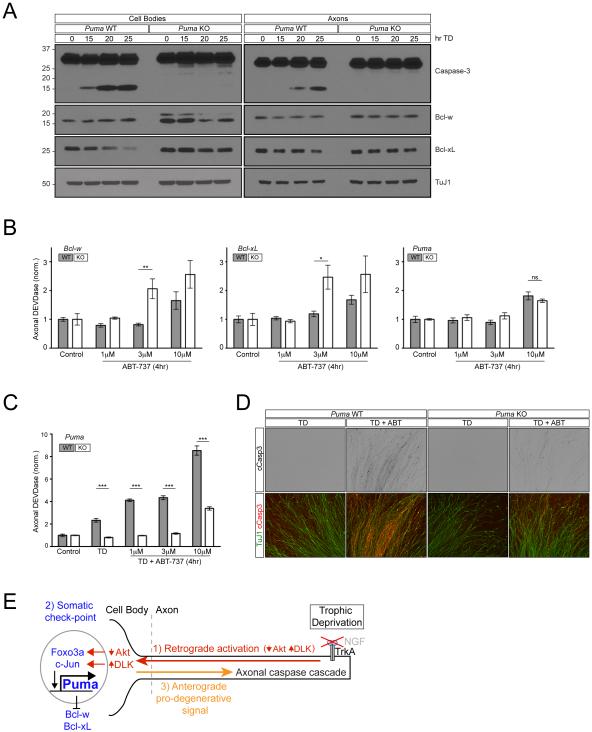
Figure 6. Puma regulates an anterograde pro-degenerative signal via the cell body.
A) 7 DIV Puma WT and KO dissociated and reaggregated DRG cultures were subjected to TD for the indicated times. Separate cell body and axon preparations were subjected to immunoblot analysis.
B) DRG cultures from the indicated genotypes were treated with ABT-737 for 4hr as indicated. Cell bodies were removed at the end of the treatment and axonal DEVDase activity was measured using the Caspase-Glo-3/7 assay. n=4 for all conditions except 10µM in Bcl-xL WT, 3µM in Bcl-xL KO, and 10µM in Bcl-w WT where n=3. Values are normalized to the average value of the control condition for each respective genotype.
C and D) Puma WT and KO DRG cultures were treated with ABT-737 for 4hr following 2hr TD (for a total of 6hr TD) as indicated. Following treatment, either cell bodies were removed and axonal DEVDase activity was measured with the Caspase-Glo-3/7 reagent
(C) or Caspase-3 activation was visualized by immunostaining (D). n=4. Values are normalized to the average value of the control condition for each respective genotype.
E) Model for the role of the cell body in the initiation of axon degeneration.
Finally, we examined the effect of TD on neurons with combined loss of Bcl-xL, Bcl-w and Puma (we could not study the effect of TD on Bcl-xL;Bcl-w double knockouts because they degenerated spontaneously: Figure 5E). Interestingly, degeneration was still observed after TD (Figure 6A, B); this degeneration was largely inhibited by the JNK antagonist (data not shown). A likely possibility is that this residual degeneration reflects the action of the parallel Bax-activating factor that provides residual pro-degenerative activity in the absence of Puma (see above), and which we therefore infer is also inhibited by Bcl-w and Bcl-xL (since it is revealed in their absence).
Puma activates a somatically-derived pro-degenerative signal
The picture that emerges from these studies is that basal Puma activity in the cell body is antagonized by Bcl-xL and Bcl-w, and that TD leads to a rise in Puma (and appearance of a second band) that overcomes this inhibition to activate an anterograde pro-degenerative signal that travels down the axon. We argued above that this signal was not likely to be Puma itself because it is not detectable in axons (Figure 4G,H). Several additional lines of evidence reinforced this conclusion.
First, axons express abundant Bcl-xL and Bcl-w that could inhibit any low levels of Puma that might be present (Figure 5A), and although this expression declined after TD, the decrease was very modest, an effect that was not altered by Puma deletion (Figure 6A), so that even after TD both remain abundant and presumably able to block any low level of Puma in axons.
Second, we used ABT-737 as a probe of the balance of pro- and anti-apoptotic Bcl-2 family members in the axon. In these experiments, we monitored short-term responses to ABT-737 (applied for 4 hrs). If Bcl-xL and Bcl-w function to inhibit the apoptotic pathway within axons, we would predict that genetic deletion of either factor should sensitize the axons to direct activation of this pathway by ABT-737. Likewise, if Puma functions to promote the apoptotic pathway within axons, we would predict that loss of Puma would make the axons less sensitive to ABT-737. We found that axons lacking either Bcl-xL or Bcl-w were indeed more sensitive to Caspase activation by ABT-737, as assessed by DEVDase activity, whereas deletion of Puma did not alter axonal sensitivity (Figure 6C). These results thus support a direct role for Bcl-xL and Bcl-w within axons, and also support a lack of direct role for Puma in axons.
Finally, we returned to potentiation of the axonal response to ABT-737 by short-term TD (Figure 1E,F). Since this potentiation is dependent on the cell body, we predict that it should require Puma activity. Indeed, genetic deletion of Puma abolished the potentiating effect of TD on the axonal response to ABT-737 (Figure 6C,D). This finding reinforces that Puma is capable of regulating axonal sensitivity but appears to do so at the level of the cell body.
Collectively, these results further support the view that Puma functions in the cell body to activate an anterograde pro-degenerative signal that is distinct from Puma itself (Figure 6F).
Discussion
A common view has been that axon degeneration is a local event confined to the specific axon that loses neurotrophic support, a view supported indirectly by observations that axon degeneration precedes overt cell loss in a range of neurodegenerative conditions. Instead, in line with prior observations (Chen et al., 2012; Gerdts et al., 2013) our findings on developmental sensory axon death in response to distal trophic deprivation support a model in which signaling via the cell body is an integral component of the molecular pathway that initiates axon degeneration. We find that the apoptotic machinery is present in the axon but that its activity is gated by an active process in the cell body that can be broken down into three discrete steps following distal TD: 1) retrograde signaling to the cell body; 2) the key regulated step of Foxo3a/c-Jun-mediated upregulation of Puma in the cell body, enabling it to overcome inhibition by Bcl-xL and Bcl-w, and initiate the degenerative program, and 3) the ultimate triggering of axon degeneration by an as yet unidentified somatically-derived factor distinct from Puma that moves down the axon to activate the Bax/Caspase cascade (Figure 6F). By integrating distinct signaling pathways that originate with loss of trophic support in the axon, the cell body functions as a key check-point to initiate axon degeneration.
Retrograde signaling to the cell body following TD is coordinated by loss of axonal Akt signaling. Akt has two principal roles that support axonal survival. The first is to inhibit the DLK/JNK/c-Jun pathway and thereby suppress pro-apoptotic MAPK signaling and activation of c-Jun. The second is to suppress Foxo3a-dependent Puma expression. The inhibition of Akt that accompanies TD therefore promotes axon degeneration through dis-inhibition of DLK/JNK/c-Jun and Foxo3a, which we show converge to regulate Puma expression in the cell body. A similar role for the JNK/c-Jun and/or the Akt/Foxo3a pathways in Puma induction has been described in the context of the apoptotic cell body death of cortical neurons following DNA damage (Wyttenbach and Tolkovsky, 2006) and of cerebellar granule neurons deprived of extracellular potassium (Ambacher et al., 2012). GSK3β, which is directly inhibited by Akt phosphorylation (Cross et al., 1995), has also been implicated as part of the Akt/Foxo3a pathway in promoting Puma expression in cerebellar neurons (Ambacher et al., 2012). Interestingly, in sensory neurons GSK3β is dephosphorylated after TD and its activity in the cell body is required for axon degeneration (Chen et al., 2012), perhaps by contributing to the induction of Puma as part of the Akt/Foxo3a pathway in those cells as well.
Upregulation of Puma defines the key regulated step in the initiation of axon degeneration. Puma is expressed in healthy neurons even in the presence of trophic support (unlike in many other cell types where it is absent until induced by stress) but its pro-degenerative activity is blocked by pro-survival Bcl-xL and Bcl-w – the latter already implicated in sensory axon survival (Courchesne et al., 2011). Following TD, the rise in Puma levels overcomes their effects, leading to activation of an anterograde pro-degenerative signal that travels down the axon. Our results imply further that this signal is distinct from Puma itself, as Puma is only detectable in the cell body even after TD, as assessed using two independent techniques. While it remains possible that a small amount of Puma below the threshold of detection is present in axons, it would likely be neutralized by the abundant Bcl-xL and Bcl-w present there, so any Puma within axons is unlikely to account for the large degenerative phenotypes documented here.
The identity of the anterograde pro-degenerative signal remains to be determined. Somatic induction of Puma also leads to activation of Caspase-3 in cell bodies, so it is intriguing to speculate that generation of the anterograde-pro-degenerative signal may be dependent on activation of the mitochondrial apoptotic pathway in the soma. For example, it is conceivable that somatically-released cytochrome c, an activated Caspase, or a Caspase-cleavage product could induce the axonal apoptotic pathway as part of a traveling feed-forward loop that promotes degeneration down the axon. The fact that the Caspase-3 antagonist DEVD-Fmk can block cell body death but not axon degeneration (which is only blocked by more complete Caspase-3 inhibition than is achieved by the inhibitor) (Nikolaev et al., 2009; Simon et al., 2012) appears to imply that cell body death per se is not required for generation of the anterograde signal, but this does not exclude that residual somatic Caspase-3 activity may still be required, a possibility that requires further exploration. Finally, our results also imply that to activate the Caspase pathway the anterograde signal must overcome the antagonistic effects of Bcl-xL and Bcl-w within the axons.
Beyond defining the molecular steps leading from distal trophic deprivation to axon degeneration, our findings and prior results (Chen et al., 2012; Gerdts et al., 2013) highlight the pivotal, active role of the cell body, which functions as a locus of control in axon degeneration in response to distal deprivation. Within the axon, there appears to be a remarkable biochemical compartmentalization of the upstream activator and downstream effector pathways for control of degeneration. At a functional level, this compartmentalization and stringent control of distal axon degeneration by the cell body provides an essential buffer, ensuring that transient local changes in trophic support do not trigger irreversible local degeneration events, and instead allowing the cell body to integrate pro-degenerative and pro-survival signals to execute axon degeneration in a global and a coordinated fashion.
Methods
Cell culture
Sensory neuron cultures were harvested from E12.5 embryos and grown in Neurobasal/B27 media supplemented with 50ng/mL NGF as previously described (Simon et al., 2012).
Western blotting
Axons and cell bodies were independently harvested from sensory neurons cultured as re-aggregated spots of 20,000 dissociated DRGs per spot in 24-well plates using either a biopsy punch needle or scalpel as previously described (Yang et al., 2013). Axon or cell body material from between 5 and 10 spots were pooled for each condition and blotted using standard techniques.
Immunohistochemistry
Cultures were fixed in 4% Paraformaldehyde containing 10% sucrose for 20 minutes at room temperature and blocked for at least one hour at room temperature in 3-6% donkey serum in PBS containing 0.1% Triton-X-100 (PBS-X). Slides were incubated in primary antibody overnight in 3% donkey serum in PBS-X at room temperature. Secondary antibodies coupled to Alexa dyes were added at 1:500 in 3% donkey serum in PBS-X.
Measurement of axonal DEVDase activity
Sensory neurons were cultured either for 2 days as DRG explants in 48 well plates or for 7 days as dissociated and re-aggregated spots (2x10^4 cells/spot) in 24 well plates. In both cases mitotic inhibitor was added the day after plating to remove proliferating cells. Following the indicated treatments, the cell body region was removed as previously described and outlined above (Yang et al., 2013). Immediately after removal of the cell bodies, half of the media was removed and replaced with an equal volume of Caspase-Glo 3/7 reagent (Promega). Following a one-hour lysis at room temperature, the contents of each well was transferred to an opaque 96-well plate and luminescence was determined using a plate reader. For axon specific measurements in neurons expressing cytoplasmic NMNAT1, axons were severed then subjected to TD, followed by lysis. In Figure 1C, statistical significance was assessed using one-way ANOVA with Bonferroni post test using Prism version 5.0 (GraphPad). In all other figures for this assay, statistical significance was assessed using two-way ANOVA with Bonferroni post test.
Supplementary Material
Acknowledgements
The authors wish to thank the members of the Tessier-Lavigne laboratory for insightful discussions. A. Ding, A.Strasser, M.Merad, N.Danial, E.Wagner, J.Stoller, A.DiAntonio, J. Milbrandt and D. Ginty generously provided mice. Research was supported The Rockefeller University and the NIH (1R01NS089786). DJS was supported in part by a fellowship from the Leon Levy Foundation. NTH was supported by a fellowship from the Helen Hay Whitney Foundation. YY was supported by the Japan Society for the Promotion of Science and the International Human Frontier Science Program Organization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
D.J.S, J.P, and M.T-L designed experiments and wrote paper. D.J.S and J.P performed experiments. N.T.H, O.O, M.T.M, and H.M performed and analyzed mass spectroscopy experiments. J.Y contributed data for Figure S1. Y.Y contributed unpublished data.
References
- Akhter R, Sanphui P, Das H, Saha P, Biswas SC. The regulation of p53 up-regulated modulator of apoptosis by JNK/c-Jun pathway in beta-amyloid-induced neuron death. Journal of neurochemistry. 2015;134:1091–1103. doi: 10.1111/jnc.13128. [DOI] [PubMed] [Google Scholar]
- Ambacher KK, Pitzul KB, Karajgikar M, Hamilton A, Ferguson SS, Cregan SP. The JNK- and AKT/GSK3beta-signaling pathways converge to regulate Puma induction and neuronal apoptosis induced by trophic factor deprivation. PloS one. 2012;7:e46885. doi: 10.1371/journal.pone.0046885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Campbell DS, Okamoto H. Local caspase activation interacts with Slit-Robo signaling to restrict axonal arborization. The Journal of cell biology. 2013;203:657–672. doi: 10.1083/jcb.201303072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Maloney JA, Kallop DY, Atwal JK, Tam SJ, Baer K, Kissel H, Kaminker JS, Lewcock JW, Weimer RM, et al. Spatially coordinated kinase signaling regulates local axon degeneration. J Neurosci. 2012;32:13439–13453. doi: 10.1523/JNEUROSCI.2039-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Courchesne SL, Karch C, Pazyra-Murphy MF, Segal RA. Sensory neuropathy attributable to loss of Bcl-w. J Neurosci. 2011;31:1624–1634. doi: 10.1523/JNEUROSCI.3347-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Cusack CL, Swahari V, Hampton Henley W, Michael Ramsey J, Deshmukh M. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nature communications. 2013;4:1876. doi: 10.1038/ncomms2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Sasaki Y, DiAntonio A, Milbrandt J. Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J Neurosci. 2013;33:13569–13580. doi: 10.1523/JNEUROSCI.1197-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AS, Wang B, Pozniak CD, Chen M, Watts RJ, Lewcock JW. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. The Journal of cell biology. 2011;194:751–764. doi: 10.1083/jcb.201103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntwork-Rodriguez S, Wang B, Watkins T, Ghosh AS, Pozniak CD, Bustos D, Newton K, Kirkpatrick DS, Lewcock JW. JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. The Journal of cell biology. 2013;202:747–763. doi: 10.1083/jcb.201303066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annual review of neuroscience. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nikoletopoulou V, Lickert H, Frade JM, Rencurel C, Giallonardo P, Zhang L, Bibel M, Barde YA. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature. 2010;467:59–63. doi: 10.1038/nature09336. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science (New York, NY. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmann Z, Assa-Kunik E, Tiomny S, Minis A, Haklai-Topper L, Arama E, Yaron A. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J Neurosci. 2010;30:6375–6386. doi: 10.1523/JNEUROSCI.0922-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner O, Yaron A. Mechanisms of developmental neurite pruning. Cell Mol Life Sci. 2015;72:101–119. doi: 10.1007/s00018-014-1729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, Weimer RM, McLaughlin T, Kallop D, Stanger K, Yang J, O'Leary DD, Hannoush RN, Tessier-Lavigne M. A caspase cascade regulating developmental axon degeneration. J Neurosci. 2012;32:17540–17553. doi: 10.1523/JNEUROSCI.3012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature medicine. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Unsain N, Higgins JM, Parker KN, Johnstone AD, Barker PA. XIAP regulates caspase activity in degenerating axons. Cell reports. 2013;4:751–763. doi: 10.1016/j.celrep.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Vohra BP, Sasaki Y, Miller BR, Chang J, DiAntonio A, Milbrandt J. Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J Neurosci. 2010;30:13729–13738. doi: 10.1523/JNEUROSCI.2939-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z. A local mechanism mediates NAD-dependent protection of axon degeneration. The Journal of cell biology. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Cao Q, Zhang Y, Su XD. Activation and regulation of caspase-6 and its role in neurodegenerative diseases. Annual review of pharmacology and toxicology. 2015;55:553–572. doi: 10.1146/annurev-pharmtox-010814-124414. [DOI] [PubMed] [Google Scholar]
- Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. Local caspase activity directs engulfment of dendrites during pruning. Nature neuroscience. 2006;9:1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- Wu CC, Wu HJ, Wang CH, Lin CH, Hsu SC, Chen YR, Hsiao M, Schuyler SC, Lu FL, Ma N, et al. Akt suppresses DLK for maintaining self-renewal of mouse embryonic stem cells. Cell Cycle. 2015;14:1207–1217. doi: 10.1080/15384101.2015.1014144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyttenbach A, Tolkovsky AM. The BH3-only protein Puma is both necessary and sufficient for neuronal apoptosis induced by DNA damage in sympathetic neurons. Journal of neurochemistry. 2006;96:1213–1226. doi: 10.1111/j.1471-4159.2005.03676.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Weimer RM, Kallop D, Olsen O, Wu Z, Renier N, Uryu K, Tessier-Lavigne M. Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron. 2013;80:1175–1189. doi: 10.1016/j.neuron.2013.08.034. [DOI] [PubMed] [Google Scholar]
- You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. The Journal of experimental medicine. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27(Suppl 1):S71–83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Akey CW. Apoptosome structure, assembly, and procaspase activation. Structure. 2013;21:501–515. doi: 10.1016/j.str.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.