Abstract
Objectives
To examine the impact of sleep duration on lung function and asthma symptoms in adolescents.
Methods
Ten adolescents with asthma (60% female, 60% Caucasian, mean age=13.7 years, range 12-17) completed a three-week randomized, cross-over sleep manipulation protocol. Following a week of self-selected sleep duration, adolescents were randomized to a 5 night deficient sleep opportunity (6.5 hours in bed) or a healthy sleep opportunity (10 hours in bed), obtained by systematically changing bedtimes. Wake time remained consistent across all three weeks (including weekends). Daily reports of sleep patterns and asthma symptoms, actigraphy, and daily peak expiratory flow rates (PEFR), as well as weekly spirometry and exhaled nitric oxide were collected.
Results
Participants averaged 3.2 hours less sleep (p<0.001) per night in the short sleep condition vs. the long sleep condition. Further, they had an 8.4% decrease overnight in PEFR (p=0.007), and reported more asthma symptoms interfering with activities in the past 24 hours (p=0.02) in the short sleep condition than the long sleep condition. No significant differences between experimental weeks were found for weekly spirometry or exhaled nitric oxide.
Conclusions
This pilot study demonstrated the feasibility of a crossover sleep manipulation protocol in adolescents with asthma. Since overnight decrease in PEFR is a marker of nocturnal asthma, and has been associated with the severity of daytime airflow limitation, these early-stage results suggest that shortened sleep duration may exacerbate adolescent asthma and associated functional impairments.
Keywords: Adolescence, pediatrics, sleep deprivation, sleep extension, teenagers
INTRODUCTION
Asthma impacts nearly 10% of youth.1 While medications, practice guidelines, and better management have reduced asthma morbidity over the past 20 years, asthma prevalence among youth continues to rise, placing a significant burden on society.2 In 2008, asthma accounted for an estimated 14.4 million lost school days.2,3 Thus, there is a need to identify modifiable health behaviors that may reduce the frequency and severity of asthma exacerbations and their effect on key functional asthma outcomes.4,5
One such health behavior that may be important is sleep duration. Growing evidence has shown the impact of chronic partial sleep restriction on molecular, immune, and neural changes that contribute to disease development (e.g., cardiovascular disease, diabetes, obesity).6 For example, controlled studies that manipulate sleep duration have found changes to markers of inflammation, including IL-1β, TNF-α, IL-6, and hsCRP.7 In other words, deficient sleep promotes low-level systemic inflammation, even for individuals without a chronic illness.8 However, no studies have considered the role of chronic partial sleep restriction (routinely sleeping less than required for optimal functioning) on asthma.
While sleep quantity and quality may be disrupted by nocturnal asthma symptoms or primary sleep disorders (e.g., obstructive sleep apnea), for many adolescents chronic partial sleep restriction is behaviorally induced. Adolescents may occasionally “pull an all-nighter” (i.e., acute total sleep deprivation), but more typically they experience chronically short sleep. Homework, extracurricular activities, and social interactions, combined with early rise times for school, has resulted in a nation of chronically sleep restricted youth. Adolescents are known to have a biological sleep need of >9 hours,9 yet 46-69% of adolescents in the United States obtain less than 8 hours of sleep per night during the school week, with a national average of only 7.6 hours of sleep per night for 6th to 12th graders.10,11 Further, a recent study found that more adolescents with asthma report insufficient sleep compared to healthy adolescents.12
Thus the goals of this pilot study were to demonstrate the feasibility of a multi-night at home sleep manipulation protocol in adolescents with asthma and to examine the impact of sleep duration on lung function and asthma symptoms in adolescents. We hypothesized that the sleep manipulation protocol would result in significant differences to sleep duration, as well as changes in objective and subjective assessments of asthma severity and functional outcomes.
METHODS
This study was approved by the Institutional Review Board at National Jewish Health. Informed parental consent and adolescent assent were obtained for all participants.
Sleep Manipulation Protocol
The study protocol was similar to that used with healthy adolescents by Beebe and colleagues.13 Adolescents participated in a randomized, cross-over sleep manipulation protocol that included 1 week of sleep stabilization (baseline), 1 week of a deficient sleep opportunity (6.5 hours in bed per night), and 1 week of a healthy sleep opportunity (10 hours in bed per night). Participants were asked to select a wake time that would allow them to attend an 8:30 a.m. appointment. This wake time was maintained across all three study weeks, including weekends.
For the baseline week, adolescents were allowed to self-select their bedtimes. If participants were adherent to all study requirements (i.e., sleep schedule, daily measures of sleep and asthma, no napping, limited caffeine use), at the end of the baseline week they were randomly assigned to one of the two experimental weeks. Bedtimes were set based on the selected wake time. For the deficient sleep opportunity week, time in bed with lights out was limited to 6.5 hours. For the healthy sleep opportunity week, time in bed with lights out was extended to 10 hours. Study weeks ran from Sunday through Thursday nights to mimic a typical school week, with study visits conducted on Friday mornings. Weekends were used as a wash-out period between experimental weeks, with bedtimes self-selected, but wake times consistent with the weekday wake time. Previous studies have shown that two nights of recovery sleep is sufficient to normalize neurobehavioral functioning, daytime sleepiness, and other physiological outcomes.14-17
During the weekly study visits, actigraphy and peak flow meter data were downloaded and reviewed, participants completed eNO and spirometry measurements, and reminders about the following week’s sleep schedule and other study requirements were reviewed.
Subjects
Adolescents (12-17 years) with asthma were recruited from the outpatient clinics at a tertiary medical center, as well as from a database of youth who had participated in previous research studies at the institution. Inclusion criteria included evidence of active asthma in the past year (i.e., presence of recurrent daytime or nighttime symptoms; and/or an exacerbation that required an urgent care or ED visit or the use of systemic corticosteroids; and/or the regular use of inhaled or oral medications for asthma) and an average self-reported sleep duration of less than 10 hours. Asthma diagnoses were confirmed by medical record review (including objective diagnostics) and a physical exam by a member of the medical team. Exclusion criteria included an asthma exacerbation in the 30 days prior to the baseline study week; the presence of another significant chronic illness, sleep disorder, or developmental disorder that may interfere with sleep; BMI > 98th percentile; use of a medication known to impact sleep or cause daytime sleepiness; obligations that would require a bedtime later than 10:00 p.m. or a wake time prior to 6:00 a.m. during the study period; obligations that would require driving, using heavy machinery, or engaging in other dangerous activities during the deficient sleep opportunity week; daily consumption of more than 1 coffee or “energy drink” or more than 2 caffeinated sodas; obesity; pregnancy; or symptoms of significant sleep disorder breathing (>8 of 22 symptoms on the Pediatric Sleep Questionnaire18) or insomnia (15 or above on the Insomnia Severity Index19).
Measures
Actigraphy
Participants wore the Motionlogger Sleep Watch (Ambulatory-Monitory Inc., Ardsley, NY) on the non-dominant wrist (removed only for bathing, swimming, or if the watch could get damaged). Data were collected in 1-minute epochs using the Zero Crossing Mode and the Sadeh algorithm for scoring. Reported bedtime and wake time were scored based on the sleep diary and event marker data. These times were used to calculate time in bed (TIB). Actigraphic sleep outcome measures included sleep onset time (time participant fell asleep), sleep offset time (time participant woke to start the day), sleep onset latency, wake after sleep onset, and total sleep time (TST). Sleep efficiency was calculated (TIB/TST*100).
Daily Sleep and Asthma Diary
Participants completed an online daily diary that queried sleep patterns, napping and caffeine use, as well as daily asthma symptoms (coughing, wheezing, shortness of breath, or chest tightness, asked both for the day and night), as well as a single question asking how much asthma interfered with activities in the previous 24 hours. In addition, adolescents verified that they completed their peak flow measurement. Participants were also asked about albuterol use during the day and overnight, however, due to the infrequent use of albuterol by participants during the study (daytime use once each in 3 subjects [two during deficient sleep week and one during the healthy sleep week], nocturnal use once each for 2 subjects [one during deficient sleep week, one during healthy sleep week]) these data were not included in the analyses. To ensure adherence to the study protocol, the diary was checked daily by study staff and if the adolescent had not completed that day’s entry, a staff member called to remind the participant to complete all study requirements.
Peak Expiratory Flow Meter
Daily lung function assessments were completed in the morning and at night using an electronic peak flow meter (PiKo, nSpire, Longmont, CO). Participants were taught how to use the meter to ensure accurate data collection. Outcome measures were peak expiratory flow (PEF) and FEV1 (forced expiratory volume – 1 second).
Spirometry
Weekly assessments of spirometry were used to examine lung function at the end of each study week using the American Thoracic Society Standardization of Spirometry 1994 Guidelines. Measures of FVC, FEV1, FEV1/FVC, FEF 25-75, and PEF were obtained.
Exhaled Nitric Oxide (eNO)
eNO is a non-invasive measure of airway inflammation that correlates with clinical asthma severity, nocturnal symptoms, level of airway hyperresponsiveness, and beta-agonist reversibility. eNO measurement was obtained using ATS standardization guidelines on a NIOX MINO® machine in the Clinical Research Unit at National Jewish Health.
Data Analysis
As we expected to find changes to clinical outcomes based on changes to sleep duration, subjects were included in analyses only if they (a) showed both true sleep restriction and true sleep extension during the sleep manipulation, defined as sleep duration in the short sleep condition being less than during the baseline week, and sleep duration in the long sleep condition being greater than baseline week, and (b) showed reasonable sleep quality, operationalized as actigraphic sleep efficiency ≥ 80%.
Sleep duration (total sleep time), secondary sleep outcomes (time in bed, sleep onset latency, etc.) and asthma outcomes (FEV1, PEF, etc.) were assessed using linear mixed models. For dichotomous asthma outcomes, generalized linear models were used (binary distribution, logit link). Right skewed outcome variables were natural log transformed before analysis. For sleep outcome models, the predictor of interest was treatment (baseline, short or long sleep). For asthma outcome models, the predictors of interest were treatment (short or long sleep), period (week 1 or week 2 of treatment), and sequence (short sleep or long sleep week first). Daily asthma outcome models included a random intercept. Weekly asthma outcomes did not include random intercepts due to either no improved model fit, or inability for a model solution to converge. All models accounted for repeated measures with the spatial power covariance structure. All asthma outcome models were adjusted for Tanner score, age, gender and average asthma outcome value during the baseline week unless otherwise stated. In order to determine if treatment modified the relationship between spirometry and dichotomous asthma outcomes, a generalized linear model was used with the following predictors of interest: spirometry, experimental condition, and the spirometry by condition interaction.
Day within each treatment week was considered in all models, along with the interaction of treatment with period and treatment with sequence. These model terms were found to be insignificant or prevented the models from converging and were removed. All p-values were based on 2-tailed tests and a p-value of less than 0.05 was deemed significant. Unless otherwise stated results are presented as mean (SD). All analyses were completed using SAS version 9.4.
RESULTS
Study Participants
Figure 1 presents a CONSORT flow diagram of participant flow. Fifty-five adolescents were recruited, however 27 failed to meet inclusion criteria (asthma too mild, n=12; BMI >98th percentile, n=8; medical factors [recent concussion, chronic migraines]; psychiatric/developmental issues [ADD, Asperger’s, bipolar, developmental delay]; employment (e.g., lifeguarding) where working would be unsafe during the deficient sleep week), 11 had scheduling conflicts that prevented participating in the full three-week protocol, and 1 stated he was “not interested.” Following the baseline week, one subject dropped out of the study due to a death in the family and one subject was excluded from continuing in the study due to the development of a respiratory illness. Although they completed the full three-week study protocol, an additional 4 subjects were excluded from analyses (3 failed to demonstrate true sleep restriction, and 1 had a very low sleep efficiency of 71%). Of the remaining 10 adolescents, 60% were female, with a mean age of 13.7 years (SD=1.6). Parent-identified race of the participants included White (n=6), Black (n=1), Pacific Islander (n=2), and more than one race (n=1). Seventy percent of participants resided with two parents, and all mothers reported at least some college education (e.g., associate’s degree or higher).
Figure 1.
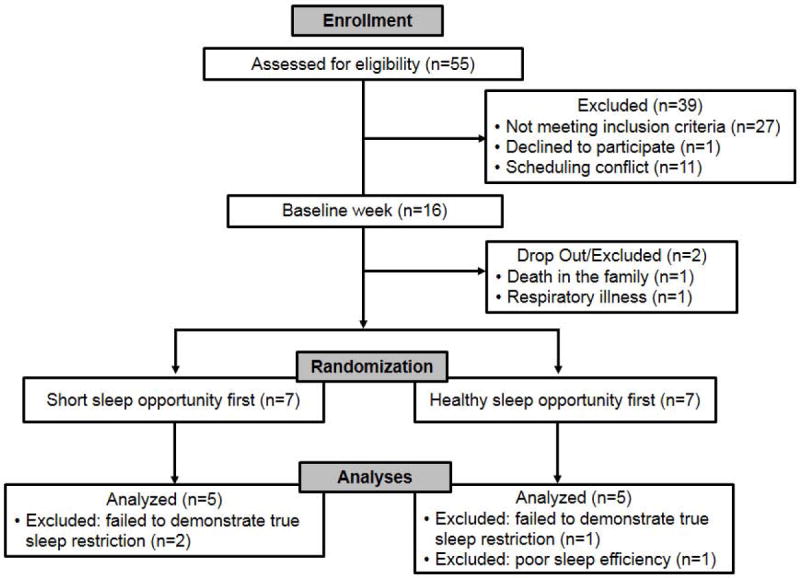
CONSORT diagram of participant recruitment, randomization, and analysis.
Study Feasibility
All of the final sample of 10 adolescents were adherent to the sleep manipulation protocol across the three study weeks. In particular, time in bed for the two experimental weeks significantly differed by 3.2 hours [deficient sleep opportunity = 6.8 (0.3) hours; healthy sleep opportunity = 10.0 (0.1) hours], and both experimental weeks were significantly different than baseline time in bed [8.4 (0.5) hours, p’s<0.001) (Figure 2). Significant differences were also found for reported bedtimes and actigraphic sleep duration (TST, Table 1).
Figure 2.
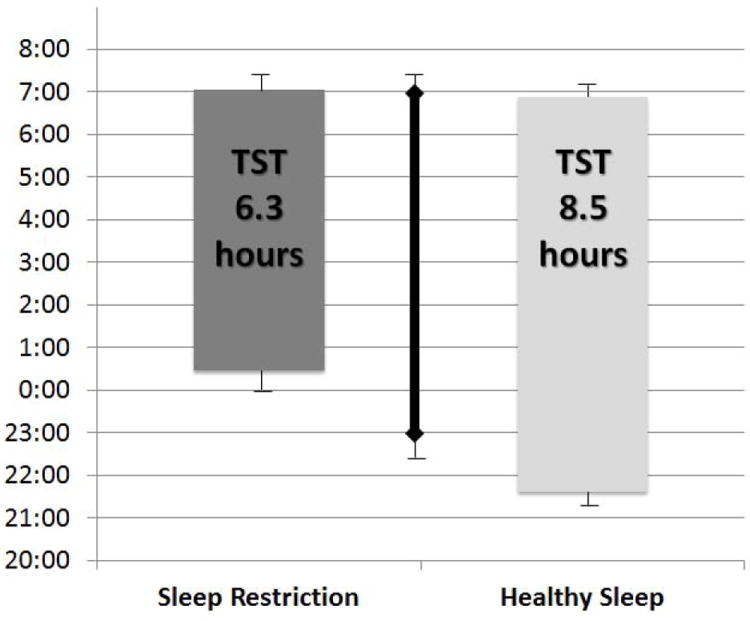
Sleep schedules and sleep duration for three sleep conditions. Plotted means with SD bars first averaged within subject, then across subject; values are unadjusted and not from model fit. Significant differences in sleep onset were found across all three sleep conditions based on a linear mixed model (p<0.001).
Bottom of each bar reflects actigraphic sleep onset
Top of each bar reflects actigraphic sleep offset/waking
Solid line reflects baseline sleep duration /schedule
Three weeks significantly different, p<.001
Table 1.
Sleep variable means (standard deviations) for the three sleep conditions.
| Baseline | Short Sleep Opportunity | Healthy Sleep Opportunity | pa | |
|---|---|---|---|---|
| Reported Bedtime | 22:39 (0:27) | 00:18 (0:28) | 21:04 (0:27) | <0.001c |
| Actigraphic Sleep Onset Time | 22:59 (0:32) | 00:28 (0:31) | 21:36 (0:24) | <0.001c |
| Wake Time | 7:04 (0:31) | 7:07 (0:25) | 6:59 (0:26) | 0.58 |
| Actigraphic Sleep Offset Time | 6:59 (0:30) | 7:03 (0:25) | 6:53 (0:25) | 0.67 |
| Time in Bed (TIB) – min | 505.4 (29.0) | 408.6 (16.1) | 596.7 (8.1) | <0.001c |
| Total Sleep Time (TST) – min | 449.2 (38.8) | 377.7 (24.5) | 512.1 (26.1) | <0.001c |
| Sleep Onset Latencyb – min | 13.6 (1.6) | 8.1 (1.4) | 25.3 (1.8) | 0.11 |
| Wake After Sleep Onsetb – min | 26.3 (1.7) | 16.3 (1.6) | 41.3 (1.7) | 0.02d |
| Sleep Efficiency (TST/TIB*100) - % | 88.9 (4.4) | 92.5 (3.6) | 85.8 (4.4) | 0.07 |
Note. Time variables expressed by 24-hour clock, SD expressed in minutes
p-value from Type 3 Test for overall difference in three sleep conditions from mixed model with random intercept. Repeated measures accounted for with a Spatial Power covariance structure; means (SDs) are unadjusted and not from model fit.
Variable was natural log transformed for mixed model. Geometric means (standard deviation) reported. P-value corresponds to log transformed values.
Significant difference across all sleep conditions
Significant difference between baseline and short sleep conditions, as well as short and long sleep conditions.
All subjects maintained a consistent wake time across all three study weeks (Table 1). As would be expected from the extended time in bed, wake after sleep onset was significantly greater in the healthy sleep opportunity week. Further, although non-significant, sleep onset latency was also longer during the healthy sleep opportunity week. Together this resulted in a lower sleep efficiency. Notably, all participants fell asleep earlier during the healthy sleep opportunity week than their baseline bedtime [healthy sleep opportunity sleep onset = 21:35 (0:23); baseline bedtime = 22:38 (0:27), p<0.001].
Sleep Duration and Asthma Outcomes
Significant differences between the experimental study weeks were found for the peak expiratory flow meter (Table 2), with morning FEV1 14% lower (p=0.03) and morning PEF 6% lower (p=0.05) in the deficient sleep opportunity week compared to the healthy sleep opportunity week. Overnight changes in PEFR were also greater in the deficient sleep opportunity week (p=0.007) (Figure 3). No significant differences were found between the experimental study weeks for spirometry or exhaled nitric oxide (Table 3).
Table 2.
Daily means (standard errors) for the deficient sleep and healthy sleep opportunity conditions (n=10).
| Short Sleep Opportunity | Healthy Sleep Opportunity | pa | |
|---|---|---|---|
| AM FEV1 | 2.29 (0.19) | 2.61 (0.19) | 0.03 |
| PM FEV1 | 2.40 (0.08) | 2.42 (0.08) | 0.83 |
| AM PEF | 376.72 (29.00) | 398.40 (28.98) | 0.05 |
| PM PEF | 386.64 (25.39) | 383.81 (25.46) | 0.76 |
| % relative change FEV1 | |||
| Morning to evening | 8.91 (4.17) | 1.28 (4.18) | 0.01 |
| Evening to morning after | -7.06 (6.33) | 1.36 (6.39) | 0.06 |
| % relative change PEF | |||
| Morning to evening | 5.51 (4.82) | -1.41 (4.93) | 0.04 |
| Evening to morning after | -8.38 (2.73) | -0.29 (2.70) | 0.007 |
p-value from linear mixed model with repeated measures (1 measure per day within sleep condition); least squares means (SE) correspond to a 14 year old male subject with a Tanner score of 3.3. All models adjusted for baseline level of outcome.
Figure 3.
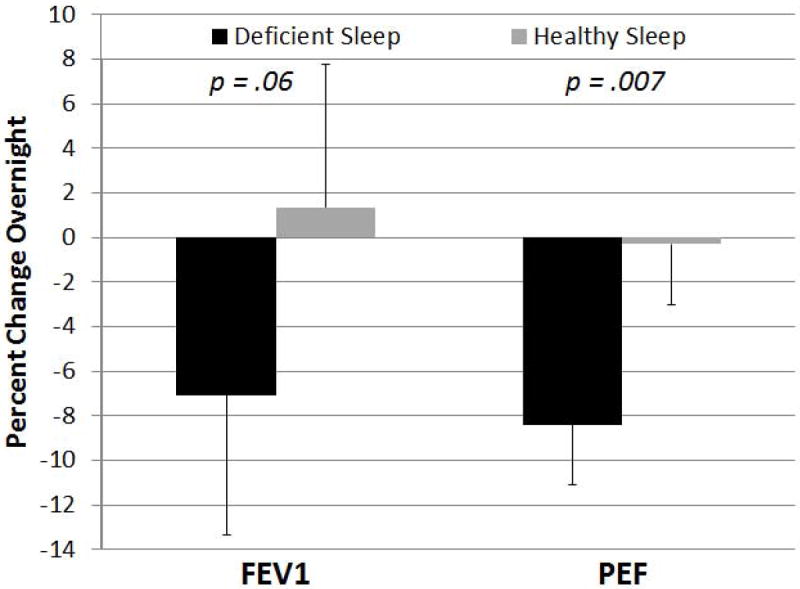
Comparison of overnight change in peak expiratory flow rate (as measured by daily peak flow assessment) for the deficient sleep and healthy sleep opportunities. Plotted values are mean estimates with SE bars obtained from linear mixed model. P-value is comparing deficient sleep to healthy sleep opportunity.
Table 3.
Weekly spirometry and exhaled nitric oxide means (standard errors) for the deficient sleep and healthy sleep opportunity conditions (n=10).
| Short Sleep Opportunity | Healthy Sleep Opportunity | pa | |
|---|---|---|---|
| Spirometry | |||
| % Predicted Pre FEV1 | 91.43 (1.63) | 93.13 (1.63) | 0.28 |
| Pre FEV1/FVC | 81.23 (1.32) | 81.33 (1.32) | 0.93 |
| % Change FEV1 | 4.70 (2.25) | 5.50 (2.25) | 0.67 |
| Exhaled Nitric Oxideb | 27.77 (1.42) | 28.00 (1.42) | 0.81 |
p-value from linear mixed model with repeated measures (1 measure per sleep condition); least squares means (SE) correspond to subject with Tanner score of 3.3 for spirometry outcome models, and to a 14 year old male subject with Tanner Score of 3.3 for exhaled nitric oxide model. All models adjusted for baseline level of outcome.
Natural Log transformed, geometric means (SE) reported. P-value corresponds to log transformed values.
Subjectively, adolescents reported that asthma symptoms interfered with their daily activities 3.4 fold times more often during the deficient sleep opportunity week than the healthy sleep opportunity week (p=0.02). Regression models showed that for each 1% drop in overnight FEV1, subjects were 6.4 times more likely to report asthma interference in the deficient sleep opportunity week than in the healthy sleep opportunity week (p=0.04).
DISCUSSION
The results from this pilot study demonstrate that it is feasible to conduct an experimental sleep manipulation in this population. However, doing so is not necessarily easy, as a number of children failed to meet strict criteria for participation or inclusion in the analyses. Among those who did meet the strict criteria we found that, similar to Beebe and colleagues,13,20 participants were able to adhere to the required sleep schedules, with notable changes in sleep duration. In addition, despite concerns about increasing sleep duration due to adolescent changes to the circadian rhythm, our participants were able to fall asleep earlier during the healthy sleep opportunity week than during their self-selected bedtime during the baseline week.
In terms of asthma-related outcomes, we found significant changes to both objective and subjective measures of daily lung function. These results are the first to suggest that deficient sleep in adolescents may contribute to these negative asthma outcomes. There are a number of possible mechanisms that may have played a role in our findings. For example, deficient sleep has been shown to cause changes in levels of markers of systemic inflammation (e.g., IL-1β, TNF-α, IL-6, and hsCRP).7,8 Further, emerging evidence suggests that IL-6 may mediate a relationship between asthma and lung function, identifying a potential pathway or association between deficient sleep and asthma.21 Alternatively, lower daily peak flow measurements may be a result of decreased effort due to adolescent sleepiness during the deficient sleep opportunity week. While clinically adolescents are monitored and encouraged to increase effort during spirometry, that is not the case when using home peak flow meters. Importantly, subjective reports of asthma interfering with daily activities was also worse during the deficient sleep week, suggesting that inadequate sleep can cause or contribute to notable changes in subjective functional outcomes in participants. Future studies are needed to elucidate the potential mechanisms. Despite this, these results are important in identifying clinical care issues, as many adolescents with asthma are chronically sleep deprived.12
Significant changes in weekly measures of spirometry and exhaled nitric oxide were not found. For spirometry this may further highlight that daily peak flow changes may have been due to lack of effort, as adolescents likely provide greater effort when supervised by a clinician. For exhaled nitric oxide, it is likely that 5 nights of sleep duration changes were not sufficient to trigger noticeable changes in airway inflammation, in particular, eosinophilic inflammation. It is important to note that as a pilot and feasibility study the sample size was selected to examine changes to sleep routines and sleep duration, but was likely underpowered to detect changes to these clinical outcomes.
Total sleep deprivation protocols (1-2 nights of total sleep loss) have demonstrated the circadian rhythm of pulmonary functioning (e.g., bronchoconstriction).22,23 However, these studies do not represent the actual day-to-day lives of adolescents, who regularly experience chronic partial sleep restriction (averaging 7.6 hours of sleep per night despite a biological need of >9 hours/night). While some aspects of sleep duration are not under an adolescent’s control (e.g., sleep loss due to nocturnal worsening of asthma, early school start time), a recent intervention study demonstrated the ability to increase adolescent sleep duration (including an earlier bedtime) during the school year.24 Thus it is important to examine the role of sleep duration as a modifiable health behavior in adolescents with asthma.
Correlational studies have shown an association between asthma and sleep disruptions in youth, but that approach ultimately cannot establish a cause-effect relationship or the direction of causation. In contrast, experimentally manipulated sleep protocols provide an opportunity to examine causal changes to outcomes based on sleep duration. In healthy adolescents an at-home multi-night sleep manipulation protocol has been used to demonstrate changes to attention, learning, mood, behavior, and dietary behaviors following sleep restriction.13,20,25,26 To our knowledge this is the first study to have applied this sleep manipulation protocol to adolescents with asthma, and examine the impact of sleep duration on asthma outcomes.
Given the small sample size, there are a number of qualifications in this study. A primary goal was to determine feasibility and this is now supported by the findings. Nevertheless, while the strict inclusion criteria and requirements for the sleep manipulation protocol enhanced the experimental nature of the results, it contributed to the small sample size, which may have limited power to detect changes in clinical outcomes. Further, 4 subjects were excluded after data collection. Despite having an inclusion criteria requiring an average typical sleep duration of less than 10 hours, we did not a priori require an average typical sleep duration of greater than 6.5 hours or sleep efficiency of >80% (by actigraphy). However, as a pilot feasibility study we determined it was important to demonstrate that the clinical outcomes were due to changes in sleep duration (the main study hypothesis), which would not have been possible had we retained the subjects in the analyses who failed to show changes in sleep duration.
Other study limitations included a required wake time on weekends, but not a required bedtime. This minimized inadvertent shifts in circadian phase, but had the potential to limit the washout period between conditions and prevented examination of the impact of the circadian phase shift commonly seen in adolescents (i.e., staying up late and sleeping in on weekends). In addition, participants were not blinded to experimental conditions, which may have resulted in biased subjective reports of asthma symptoms. With this in mind, it is reassuring that the subjective reports were consistent with the daily peak flow measurements. Participants were also not screened by polysomnography to rule out sleep disorders including obstructive sleep apnea, relying instead of a parent-report screening measure. Finally, actigraphy is a strong but imperfect predictor of PSG-measured sleep. Even so, actigraphy represents the most feasible objective sleep measure for nightly use over a three-week period, and the within-subjects crossover design minimized the impact of measurement bias on results.
To address these limitations, future studies should include a larger sample of adolescents. In addition, in order to control for potential issues related to weekend schedules additional studies should include a set bedtime and wake time on weekends. Further, it would be valuable to examine the relationship of sleep quality (including sleep efficiency and wake after sleep) with asthma outcomes, as well as to include validated self-report measures of asthma control and quality of life. Finally, to ensure there are no significant underlying sleep disruptors, all participants should be screened by polysomnography prior to enrolling in the sleep manipulation.
These limitations notwithstanding, this study provides an important first step to understanding the impact of adolescent sleep duration on asthma. By demonstrating the feasibility of the sleep manipulation protocol in this population, this methodology provides a controlled approach to examine the role of sleep duration on lung function and functional outcomes in adolescents with asthma. Because it is common for adolescents to have a significant sleep debt, it is critical to understand more about the role of deficient sleep on asthma and functional outcomes.
Acknowledgments
The authors thank the participants and their families for their time and effort; Ronina Covar, M.D. and J. Tod Olin, M.D. for referring patients to participate in the study; and Devon Ambler, D Sundstrom, and Michael White for their assistance with data collection. The study was supported by NIH/NCATS Colorado CTSI Grant UL1 TR001082.
The research was conducted at National Jewish Health.
This study was supported by NIH/NCATS Colorado CTSI Grant UL1 TR001082.
Footnotes
This paper was presented at the Sleep 2014, the 28th Annual Meeting of the Associated Professional Sleep Societies, Minneapolis, MN.
The authors have no conflicts of interest.
References
- 1.World Health Organization. Asthma Fact Sheet. 2011 Report No.: N307. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education - United States 2001-2009. Morb Mortal Wkly Rep Surveill Summ. 2011;60:546–552. [PubMed] [Google Scholar]
- 3.American Lung Association. Trends in asthma morbidity and mortality. 2012:28. [Google Scholar]
- 4.Szefler SJ. Advancing asthma care: the glass is only half full! J Allergy Clin Immunol. 2011;128:485–494. doi: 10.1016/j.jaci.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holgate ST. Pathophysiology of asthma: what has our current understanding taught us about new therapeutic approaches? J Allergy Clin Immunol. 2011;128:495–505. doi: 10.1016/j.jaci.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 6.Luyster FS, Strollo PJ, Jr, Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35:727–734. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24:775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faraut B, Boudjeltia K, Vanhamme L, Kerhofts M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2011 doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Carskadon MA, Orav EJ, Dement WC. Evolution of sleep and daytime sleepiness in adolescents. In: Guilleminault C, Lugaresi E, editors. Sleep/wake disorders: natural history, epidemiology, and long-term evolution. New York: Raven Press; 1983. pp. 201–216. [Google Scholar]
- 10.National Sleep Foundation. Sleep in America Poll. Retrieved March 31, 2006 from http://wwwsleepfoundationorg/
- 11.Eaton DK, McNight-Eily LR, Lowry R, Perry GS, Presley-Cantrell L, Crift JB. Prevalence of insufficient, borderline, and optimal hours of sleep among high school students - United States, 2007. J Adolesc Health. 2010;46:399–401. doi: 10.1016/j.jadohealth.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Meltzer LJ, Ullrich M, Szefler SJ. Sleep duration, sleep hygiene, and insomnia in adolescents with asthma. Journal of Allergy and Clinical Immunology: In Practice. 2014;2:562–569. doi: 10.1016/j.jaip.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beebe DW, Fallone G, Godiwala N, et al. Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. J Child Psychol Psychiatry. 2008;49:915–923. doi: 10.1111/j.1469-7610.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- 14.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–1939. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jay SM, Lamond N, Ferguson SA, Dorrian J, Jones CB, Dawson D. The characteristics of recovery sleep when recovery opportunity is restricted. Sleep. 2007;30:353–360. doi: 10.1093/sleep/30.3.353. [DOI] [PubMed] [Google Scholar]
- 16.Lamond N, Jay SM, Dorrian J, Ferguson SA, Jones C, Dawson D. The dynamics of neurobehavioural recovery following sleep loss. J Sleep Res. 2007;16:33–41. doi: 10.1111/j.1365-2869.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 17.Pejovic S, Basta M, Vgontzas AN, et al. Effects of recovery sleep after one work week of mild sleep restriction on interleukin-6 and cortisol secretion and daytime sleepiness and performance. Am J Physiol Endocrinol Metab. 2013;305:E890–E896. doi: 10.1152/ajpendo.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 19.Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 20.Beebe DW, Simon S, Summer S, Hemmer S, Strotman D, Dolan LM. Dietary intake following experimentally restricted sleep in adolescents. Sleep. 2013;36:827–834. doi: 10.5665/sleep.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neveu WA, Allard JL, Raymond DM, et al. Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir Res. 2010;11:28. doi: 10.1186/1465-9921-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catterall JR, Rhind GB, Stewart IC, Whyte KF, Shapiro CM, Douglas NJ. Effect of sleep deprivation on overnight bronchoconstriction in nocturnal asthma. Thorax. 1986;41:676–680. doi: 10.1136/thx.41.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballard RD, Tan WC, Kelly PL, Pak J, Pandey R, Martin RJ. Effect of sleep and sleep deprivation on ventilatory response to bronchoconstriction. J Appl Physiol. 1990;69:490–497. doi: 10.1152/jappl.1990.69.2.490. [DOI] [PubMed] [Google Scholar]
- 24.Dewald-Kaufmann JF, Oort FJ, Meijer AM. The effects of sleep extension and sleep hygiene advice on sleep and depressive symptoms in adolescents: a randomized controlled trial. J Child Psychol Psychiatry. 2014;55:273–283. doi: 10.1111/jcpp.12157. [DOI] [PubMed] [Google Scholar]
- 25.Beebe DW, Difrancesco MW, Tlustos SJ, McNally KA, Holland SK. Preliminary fMRI findings in experimentally sleep-restricted adolescents engaged in a working memory task. Behav Brain Funct. 2009;5:9. doi: 10.1186/1744-9081-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beebe DW, Rose D, Amin R. Attention, learning, and arousal of experimentally sleep-restricted adolescents in a simulated classroom. J Adolesc Health. 2010;47:523–525. doi: 10.1016/j.jadohealth.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


