Abstract
Purpose
While actigraphy has gained popularity in pediatric sleep research, questions remain about the validity of actigraphy as an estimate of sleep-wake patterns. In particular, there is little consistency in the field in terms of scoring rules used to determine sleep onset latency. The purpose of this study was to evaluate different criteria of immobility as a measure of sleep onset latency in children and adolescents.
Methods
Ninety-five youth (ages 3-17 years, 46% male) wore both the Ambulatory-Monitoring Inc. Motionlogger Sleep Watch (AMI) and the Philips Respironics Mini-Mitter Actiwatch-2 (PRMM) during overnight polysomnography in a pediatric sleep lab. We examined different sleep onset latency scoring rules (3, 5, 10, 15, and 20 minutes of immobility) using different algorithms (Sadeh and Cole-Kripke) and sensitivity settings (Low, Medium, High) for the devices. Comparisons were also made across age groups (preschoolers, school-aged, adolescents) and sleep disordered breathing status (no obstructive sleep apnea [OSA], mild OSA, clinically significant OSA).
Results
For the AMI device, shorter scoring rules performed best for children and longer scoring rules were better for adolescents, with shorter scoring rules best across sleep disordered breathing groups. For the PRMM device, medium to longer scoring rules performed best across age and sleep disordered breathing groups.
Conclusions
Researchers are encouraged to determine the scoring rule that best fits their population of interest. Future studies are needed with larger samples of children and adolescents to further validate actigraphic immobility as a proxy for sleep onset latency.
Keywords: actigraphy, validation, sleep onset latency, pediatric, immobility
Introduction
Actigraphy has become more widely used over the past 20 years in both research and clinical settings [1]. In pediatric research alone, the number of published studies using actigraphy in 2010 (n=41) was greater than the total number of published pediatric studies using actigraphy from 1991-2001 (n=38) [2]. The most notable benefit of using actigraphy with pediatric populations is the ability to objectively estimate sleep-wake patterns over an extended period of time in the child's natural sleep setting.
In general, actigraphy has been found to have good sensitivity to detect sleep compared to polysomnography (PSG) for pediatric populations (83-97%) [2]. However, there remain important questions about the validity of actigraphy, in particular the poor specificity, or ability of actigraphy to detect wake after sleep onset compared to PSG, with 55% of pediatric validation studies reporting specificity values under 60% [2]. In other words, in more than half of all published pediatric validation studies, actigraphy correctly identified wake after sleep onset less than 60% of the time, resulting in an inflated estimate of sleep duration.
Because actigraphy relies on the absence of activity to estimate sleep without any corroborating physiological data (e.g., electroencephalography) or direct observation of behavior, it is inevitable that there will be differences between actigraphy and PSG. For example, even with a valid report of bedtime (which in and of itself can be challenging to obtain), motionless wakefulness can result in an underestimation of sleep onset latency (SOL), calling into question the utility of actigraphy to measure SOL in adults [1;3].
While adults can remain immobile for extended periods of time, younger children are less likely to lie still for prolonged periods of time. In addition, while older adolescents are more likely to have motionless wakefulness similar to adults, the age at which children develop this has not been examined.
One study [4] examined specific criterion for the measurement of SOL with actigraphy, reporting 5 minutes of immobility provided the most accurate estimate of SOL. However, this study only included adults wearing one type of actigraphy device, and all of the patients had sleep disordered breathing.
To our knowledge, different criteria for immobility as a measure of SOL in children and adolescents have not been directly compared to PSG. Thus the purpose of this study was to examine different immobility rules for the measurement of actigraphic sleep onset latency in both children and adolescents, with and without sleep disordered breathing, and using two different actigraphy devices. We hypothesized that shorter scoring rules would provide more accurate estimates for younger children, and longer scoring rules would provide more accurate estimates for adolescents. Our examination of scoring rules for children with and without sleep disordered breathing was exploratory and thus no hypotheses were generated.
Methods
Participants
Participants were 95 youth (ages 3-17) who participated in a larger study examining the validity of actigraphy compared to PSG [5]. Participants were scheduled for overnight PSG at a tertiary care children's hospital sleep laboratory (clinical study = 85, research protocol for youth with sickle cell disease = 10). The study was approved by the hospital's institutional review board and informed consent and assent (when appropriate) was obtained for all participants.
Procedure
Polysomnography (PSG)
Overnight PSG was performed with a Rembrandt polysomnography system (Embla, Broomfield, CO). Recorded parameters included: electroencephalography (F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1); left and right electrooculography; submental electromyography; bilateral tibial electromyography; electrocardiography; oronasal airflow; nasal pressure with pressure transducer; rib cage and abdominal wall motion via respiratory impedance plethysmography; end-tidal capnography; and arterial oxygen saturation with pulse waveform. Finally, digital video and audio were also recorded. Studies were scored based on American Academy of Sleep Medicine (AASM) 2007 pediatric criteria [6]. The sleep period was scored from “lights out” (time child attempted to fall asleep) to “lights on” (time child was awakened), with lights out scheduled as close as possible to the child's normal sleep schedule. Average “lights off” time was 21:17 and average “lights on” time was 06:04. All participants had at least 7.6 hours of PSG recording completed.
Actigraphy
Participants wore two different actigraph devices on the non-dominant wrist, the Ambulatory Monitoring Inc. Motionlogger Sleep Watch (AMI, Ardsley, NY) and the Philips Respironics Mini-Mitter Actiwatch-2 (PRMM, Bend, OR). For the youngest children, parents were asked which hand the child most often used for activities such as eating and coloring. Actigraphs were placed on the wrist of all participants by a member of the research team in the evening, and removed by a sleep technician in the morning. Random assignment was used to place the actigraphs in relation to the wrist (AMI-PRMM or PRMM-AMI).
The AMI device collected data in 1-minute epochs using the Zero-Crossing Mode, and was scored using AMI software (Action W-2 version 2.6.9905 software, Ardsley NY). The Sadeh algorithm and the Cole-Kripke algorithm were applied separately [2;7;8].
The PRMM device was set to record data in 1-minute epochs, with data scored using PRMM software (Actiware version 5.59.0015, Bend, OR). The medium (default) sensitivity threshold (40 counts per epoch), as well as the low (80 counts per epoch) and high (20 counts per epoch) wake sensitivity thresholds were applied separately.
Data Analyses
In order to compare exact intervals for “lights off” and “lights on,” the time was synchronized by initializing the actigraphs on the same computer used for PSG. The start and end points for actigraphy were identified by using the PSG “lights off” and “lights on” times. Thirty-second PSG epochs were combined to match the 1-minute actigraphy epochs, with each minute of PSG data scored as wake if either one or both 30-second epochs were scored as wake [7;9]. Thus one minute of sleep on PSG required both 30-second epochs to be scored as sleep. Only 4.7% of the combined 1-minute PSG epochs were scored as wake when one of the 30-second PSG epochs was scored as sleep (translating to a median of 23 minutes that included both sleep and wake). For a subset of data (n=20) we also examined the data by dividing each 1-minute actigraphy epoch into two 30-second epochs. However, this resulted in highly variable individual error, with a logistic regression model predicting more than 30% error for the full dataset.
PSG sleep onset was determined by the time of the first epoch of sleep as scored by PSG. Actigraphy sleep onset was examined using five different scoring rules identified in the literature [2]. These included the first minute of 3, 5, 10, 15, or 20 consecutive minutes of immobility (0 activity count), with one minute of activity allowed within the time period for the 10, 15, and 20 minute rules.
To examine immobility changes over development, participants were divided into three age groups: 3-5 years (n=31), 6-12 years (n=43), and 13-17 years (n=21). These age groups were chosen based on developmental changes in sleep reported in the literature [10;11] and interviews conducted with 10 pediatric sleep experts (Meltzer & Forrest, unpublished data). To examine immobility differences by sleep disordered breathing status, participants were divided into three groups: no sleep disordered breathing [SDB] (Apnea-Hypopnea Index [AHI] < 1.5 per hour), mild OSA (AHI 1.5 to 5 per hour), and clinically significant OSA (AHI >5 per hour) [12-16]. Preliminary analyses were run to determine if there were any demographic differences for the three age groups or the three SDB groups.
To determine the accuracy of actigraphic values in reference to PSG, repeated measures ANOVA was used to compare mean SOL for each age group and each SDB group for both the AMI device (Sadeh and Cole-Kripke algorithms) and the RMM device (medium [default], low and high thresholds). Post-hoc pairwise comparisons were then used to examine the difference between each scoring rule and PSG, with a more conservative p-value of ≤.001 used to control for the multiple pairwise comparisons. Results are presented graphically to highlight differences, with numerical differences found in the tables.
Results
Participants were 51 girls and 44 boys with a mean age 8.5 ± 4.2 years. Self-identified race was 46.3% Caucasian, 38.9% African-American, 3.2% Hispanic, and 11.6% Other. For SDB, 50.5% of participants had no OSA (AHI <1.5, mean = 0.4), 31.6% had mild OSA (AHI 1.5 to 5, mean = 2.6), and 17.9% had clinically significant OSA (AHI >5, mean = 11.5). Four participants (4.2%) had periodic limb movement disorder (PLM index >5, mean = 14.0, median = 8.2). There were no significant differences in sex, race, AHI, or PLM index for the three age groups, and there were no significant differences in sex, race, or age for the three SDB groups.
Sleep Onset Latency and Age Group
AMI (Figure 1 and Table 1)
Figure 1.
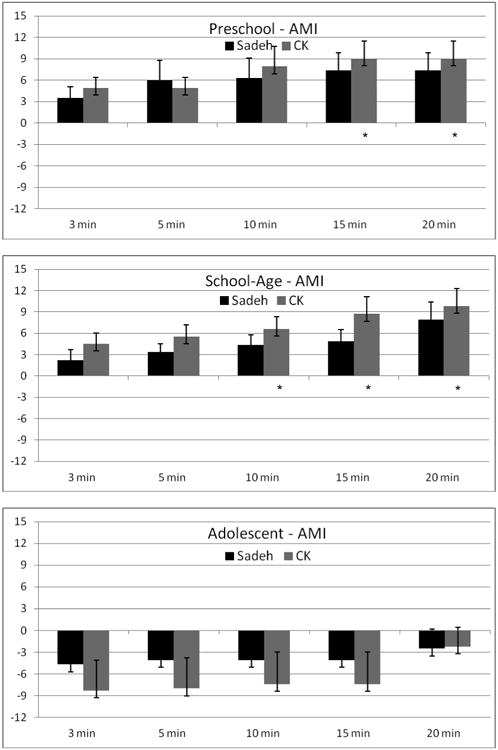
Difference in sleep onset latency (SOL) between AMI actigraphy and PSG by age group. PSG is indicated by the zero (0) axis. Positive values indicate overestimation of SOL by actigraphy, and negative values indicate underestimation of SOL by actigraphy. * indicates signficiant post-hoc comparison between actigraphy and PSG (p ≤ .001).
Table 1. Mean (SE) Values for Polysomnography and Over/Underestimation (SE) of Sleep Onset Latency for Actigraphy Settings by Developmental Age Group.
|
Preschool AMI n=28, PRMM n=29 |
AMI: Sadeha | AMI: Cole-Kripkea | PRMM: Mediumc | PRMM: Lowb | PRMM: Highb |
|---|---|---|---|---|---|
| PSG | 25.5 (3.2) | 25.5 (3.2) | 27.5 (3.2) | 27.5 (3.2) | 27.5 (3.2) |
| 3 minute rule | 3.5 (1.6) | 4.9 (1.5) | -11.3 (3.1)* | -7.3 (2.6) | -15.0 (2.9)* |
| 5 minute rule | 6.0 (2.8) | 4.9 (1.5) | -8.0 (2.7) | -6.0 (2.5) | -14.5 (2.9)* |
| 10 minute rule | 6.3 (2.8) | 7.9 (2.8) | -2.8 (2.1) | 0.0 (1.8) | -6.8 (2.3) |
| 15 minute rule | 7.4 (2.4) | 9.0 (2.5)* | -0.5 (1.5) | 2.0 (2.3) | -4.1 (1.8) |
| 20 minute rule | 7.4 (2.4) | 9.0 (2.5)* | 0.8 (2.0) | 6.6 (2.9) | -1.9 (2.2) |
|
| |||||
|
School-Age AMI n=40, PRMM n=40 |
AMI: Sadehc | AMI: Cole-Kripkeb | PRMM: Mediumb | PRMM: Lowb | PRMM: Highb |
|
| |||||
| PSG | 23.2 (2.6) | 23.2 (2.6) | 24.6 (2.8) | 24.6 (2.8) | 24.6 (2.8) |
| 3 minute rule | 2.2 (1.5) | 4.5 (1.5) | -8.7 (2.7) | 5.9 (2.7) | -13.7 (2.9)* |
| 5 minute rule | 3.4 (1.1) | 5.5 (1.7) | -7.6 (2.8) | -4.8 (2.7) | -11.4 (2.8)* |
| 10 minute rule | 4.4 (1.4) | 6.6 (1.7)* | -1.8 (2.7) | 0.5 (2.5) | -5.0 (2.8) |
| 15 minute rule | 4.9 (1.6) | 8.7 (2.4)* | 2.0 (2.7) | 4.6 (3.1) | -2.0 (2.7) |
| 20 minute rule | 7.9 (2.5) | 9.8 (2.5)* | 4.8 (3.4) | 8.9 (3.2) | -1.3 (2.6) |
|
| |||||
|
Adolescent AMI n=21, PRMM n=18 |
AMI: Sadeh | AMI: Cole-Kripke | PRMM: Mediumb | PRMM: Low | PRMM: High |
|
| |||||
| PSG | 23.9 (4.2) | 23.9 (4.2) | 22.1 (4.4) | 22.1 (4.4) | 22.1 (4.4) |
| 3 minute rule | -4.7 (3.7) | -8.3 (4.2) | -14.3 (4.5) | -12.4 (4.5) | -16.3 (4.4) |
| 5 minute rule | -4.1 (3.8) | -8.0 (4.2) | -14.1 (4.4) | -12.7 (4.5) | -15.6 (4.6) |
| 10 minute rule | -4.1 (3.8) | -7.4 (4.4) | -11.6 (3.6) | -7.2 (3.3) | -14.5 (4.4) |
| 15 minute rule | -4.1 (3.8) | -7.4 (4.4) | -5.7 (3.5) | -2.5 (2.8) | -11.6 (4.1) |
| 20 minute rule | -2.5 (2.7) | -2.2 (2.6) | -4.2 (3.1) | -0.6 (3.9) | -7.1 (3.1) |
Note. Negative values indicate an underestimate of SOL compared to PSG, positive values indicate an overestimate of SOL compared to PSG.
AMI = Ambulatory Monitoring Inc., Motionlogger Sleep Watch; PRMM = Phillips Respironics Mini-Mitter Actiwatch-2; PSG = Polysomnography
Multivariate analysis (Wilks' Lambda) significant, p≤.001
Multivariate analysis (Wilks' Lambda) significant, p≤.01
Multivariate analysis (Wilks' Lambda) significant, p≤.05
Post-hoc comparison vs. PSG, p≤.001
For preschoolers the AMI device significantly overestimated sleep onset latency for both the Sadeh, F(5, 24)=8.8, p<.001, and Cole-Kripke, F(5, 24)=20.4, p<.001, algorithms. Pairwise comparisons found no statistical differences between the Sadeh algorithm and PSG, with the longer scoring rules (10 and 15 min) significantly different than PSG for the Cole-Kripke algorithm.
For school-aged children, the Sadeh F(5, 35)=2.7, p=.04 and Cole-Kripke F(5,35)=3.8, p=.007 algorithms also overestimated SOL, with pairwise comparisons finding a significant difference between PSG and the longer scoring rules (10, 15, and 20 min) for only the Cole-Kripke algorithm.
Although a significant difference was not found for adolescents, the AMI device underestimated sleep onset latency in this group. Notably, the 20 minute rule (both Sadeh and Cole-Kripke algorithms) provided the closest estimate of PSG sleep onset latency for adolescents.
PRMM (Figure 2 and Table 1)
Figure 2.
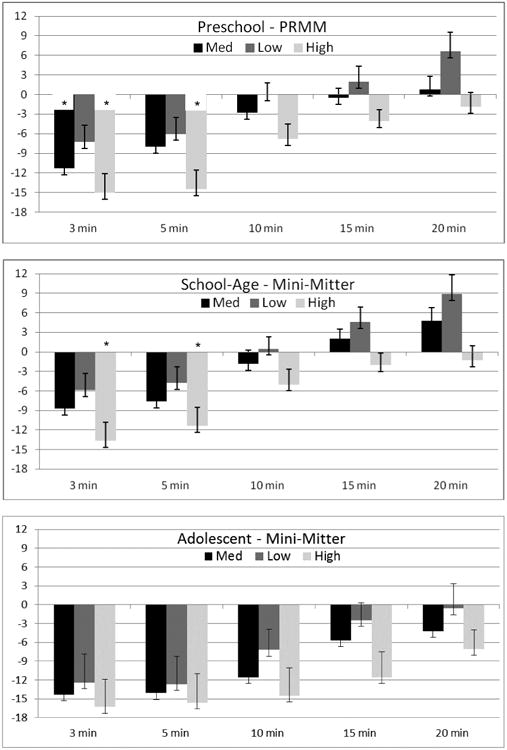
Difference in sleep onset latency (SOL) between PRMM actigraphy and PSG by age group. PSG is indicated by the zero (0) axis. Positive values indicate overestimation of SOL by actigraphy, and negative values indicate underestimation of SOL by actigraphy. * indicates signficiant post-hoc comparison between actigraphy and PSG (p ≤ .001).
For preschoolers, a significant difference between PSG sleep onset latency and the other scoring rules was found for all three sensitivity settings: Medium, F(5, 24)=3.0, p=.03, Low, F(5, 24)=3.6, p=.02, and High, F(5, 24)=4.7, p=.004. Pairwise comparisons found significant differences between PSG and the 3 minute rule for both the Medium and High setting, and the 5 minute rule for the High setting.
For school-aged children, significant differences between PSG sleep onset latency and the other scoring rules were also found for all three sensitivity settings: Medium, F(5, 35)=4.3, p=.004, Low, F(5, 35)=3.9, p=.006, and High, F(5, 35)=4.9, p=.002. Pairwise comparisons found significant differences between PSG and both the 3 minute and the 5 minute rule for the High setting.
For adolescents, a significant difference was found only at the Medium setting, F(5, 13)=4.1, p=.02. The other settings, while not significant, may have been underpowered to detect differences: Low, F(5, 13)=2.6, p=.08, and High, F(5, 13)=2.4, p=.10. Longer scoring rules (in particular the 20 minute rule) provided the closest estimate of sleep onset latency for adolescents.
Sleep Onset Latency and Sleep Disordered Breathing
AMI (Figure 3 and Table 2)
Figure 3.
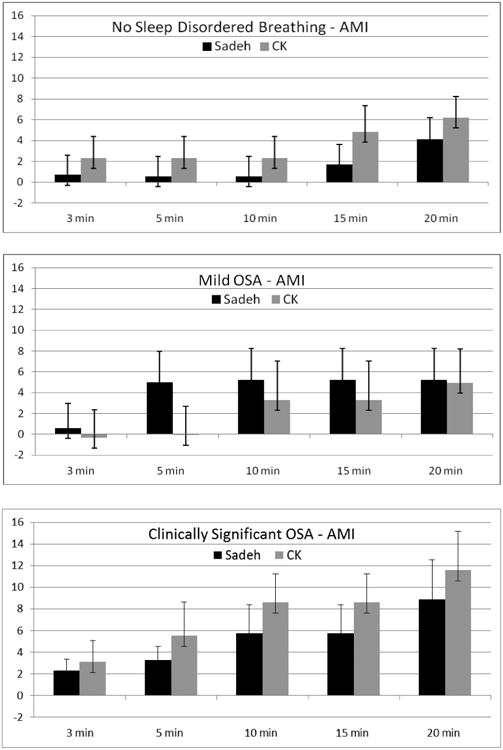
Difference in sleep onset latency (SOL) between AMI actigraphy and PSG by SDB. PSG is indicated by the zero (0) axis. Positive values indicate overestimation of SOL by actigraphy, and negative values indicate underestimation of SOL by actigraphy. * indicates signficiant post-hoc comparison between actigraphy and PSG (p ≤ .001).
Table 2. Mean (SE) Values for Polysomnography and Over/Underestimation (SE) of Sleep Onset Latency for Actigraphy Settings by Sleep Disordered Breathing.
|
No SDB AMI n=45, PRMM n=44 |
AMI: Sadeh | AMI: Cole-Kripkec | PRMM: Mediuma | PRMM: Lowb | PRMM: Higha |
|---|---|---|---|---|---|
| PSG | 27.3 (2.7) | 27.3 (2.7) | 27.7 (2.8) | 27.7 (2.8) | 27.7 (2.8) |
| 3 minute rule | 0.7 (1.9) | 2.3 (2.1) | -11.7 (2.3)* | -7.1 (2.2) | -15.9 (2.4)* |
| 5 minute rule | 0.6 (1.9) | 2.3 (2.1) | -9.0 (2.2)* | -6.2 (2.2) | -14.0 (2.4) |
| 10 minute rule | 0.6 (1.9) | 2.3 (2.1) | -3.4 (1.5) | 0.8 (1.6) | -5.8 (1.8) |
| 15 minute rule | 1.7 (1.9) | 4.8 (2.5) | -2.5 (1.5) | 1.1 (1.9) | -4.8 (1.8) |
| 20 minute rule | 4.1 (2.1) | 6.2 (2.0) | 0.6 (2.4) | 4.4 (2.7) | -3.3 (1.2) |
|
| |||||
|
Mild OSA AMI n=29, PRMM n=27 |
AMI: Sadeh | AMI: Cole-Kripke | PRMM: Mediumb | PRMM: Lowc | PRMM: Highb |
|
| |||||
| PSG | 23.6 (3.1) | 23.6 (3.1) | 24.5 (3.5) | 24.5 (3.4) | 24.5 (3.4) |
| 3 minute rule | 0.6 (2.3) | 0.3 (2.7) | -11.3 (3.5) | -9.8 (3.3) | -15.8 (3.4)* |
| 5 minute rule | 5.0 (3.0) | 0.0 (2.7) | -10.4 (3.4) | -8.2 (3.2) | -14.6 (3.5)* |
| 10 minute rule | 5.2 (3.0) | 3.3 (3.7) | -7.5 (2.8) | -1.9 (2.2) | -11.2 (3.4) |
| 15 minute rule | 5.2 (3.0) | 3.3 (3.7) | 0.5 (2.3) | 2.1 (2.6) | -4.8 (2.4) |
| 20 minute rule | 5.2 (3.0) | 5.0 (3.2) | 1.8 (2.7) | 3.2 (2.9) | -4.7 (2.4) |
|
| |||||
|
Mod/Severe OSA AMI n=15, PRMM n=16 |
AMI: Sadeh | AMI: Cole-Kripkeb | PRMM: Medium | PRMM: Lowb | PRMM: High |
|
| |||||
| PSG | 15.6 (2.8) | 15.6 (2.8) | 16.2 (2.6) | 16.2 (2.6) | 16.2 (2.6) |
| 3 minute rule | 2.5 (1.1) | 3.1 (1.9) | -3.8 (3.8) | -2.1 (3.9) | -6.1 (3.4) |
| 5 minute rule | 3.3 (1.3) | 5.5 (3.1) | -3.3 (3.8) | -2.6 (3.8) | -5.9 (4.0) |
| 10 minute rule | 5.7 (2.6) | 8.6 (2.6) | 3.3 (3.8) | 2.6 (4.4) | -2.8 (4.7) |
| 15 minute rule | 5.7 (2.6) | 8.6 (2.6) | 7.8 (4.2) | 10.1 (5.0) | -0.1 (5.4) |
| 20 minute rule | 8.9 (3.6) | 8.6 (3.6) | 8.4 (4.5) | 17.3 (5.1) | 6.4 (5.1) |
Note. Negative values indicate an underestimate of SOL compared to PSG, positive values indicate an overestimate of SOL compared to PSG.
AMI = Ambulatory Monitoring Inc., Motionlogger Sleep Watch; PRMM = Phillips Respironics Mini-Mitter Actiwatch-2; PSG = Polysomnography
Multivariate analysis (Wilks' Lambda) significant, p≤.001
Multivariate analysis (Wilks' Lambda) significant, p≤.01
Multivariate analysis (Wilks' Lambda) significant, p≤.05
Post-hoc comparison vs. PSG, p≤.001
Significant differences were found between PSG and the different immobility scoring rules for only the Cole-Kripke algorithm for children with no SDB, F(3, 42)=3.8, p=.02, or clinically significant OSA, F(4, 11)=5.7, p=.01. For all three SDB groups and across both algorithms, the AMI device overestimated sleep onset latency. However, none of these differences were statistically significant.
PRMM (Figure 4 and Table 2)
Figure 4.
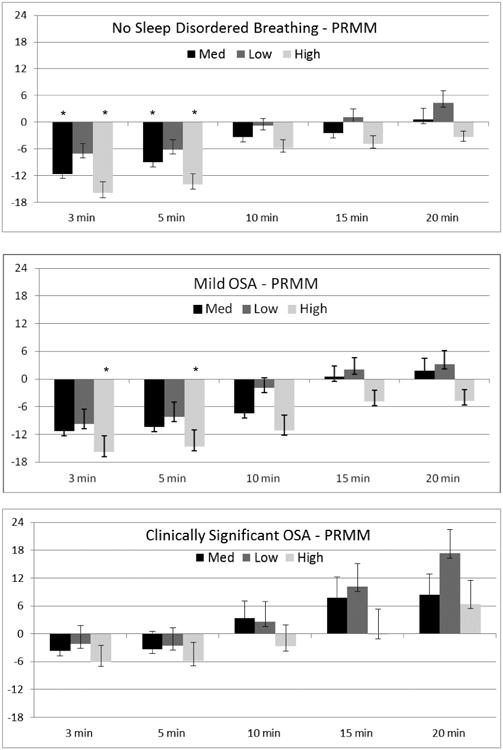
Difference in sleep onset latency (SOL) between PRMM actigraphy and PSG by SDB. PSG is indicated by the zero (0) axis. Positive values indicate overestimation of SOL by actigraphy, and negative values indicate underestimation of SOL by actigraphy. * indicates signficiant post-hoc comparison between actigraphy and PSG (p ≤ .001).
Unlike the AMI device, the PRMM device significantly underestimated sleep onset latency compared to PSG for both children with no SDB (Medium: F(5,39)=5.5, p=.001; Low: F(5,39)=3.7, p=.007; and High: F(5,39)=9.6, p<.001) and those with mild OSA (Medium: F(5,22)=3.2, p=.02; Low: F(5,22)=2.9, p=.04; and High: F(5,22)=5.3, p=.002). For children with clinically significant OSA, a significant difference between scoring rules was found only for the Low setting, F(5,11)=5.2, p=.01. For children with no SDB, pairwise comparisons show significant differences between PSG and the 3 and 5 minute scoring rules for the Medium and High sensitivity settings. For children with mild OSA, significant differences between PSG and both the 3 and 5 minute rules were also found, but only for the High setting. For children with clinically significant OSA, no statistically significant differences between PSG and actigraphy were found.
Discussion
This is the first paper to our knowledge that has examined differences in immobility as a measure of sleep onset latency in children and adolescents, comparing two commonly used brands of actigraphs [2] to overnight polysomnography. Our results highlight that sleep onset latency estimates differ across devices and scoring algorithms/sensitivities, and there is not one single rule or algorithm that could be simply used across ages and groups of children with and without SDB. However, this paper provides some initial guidance for researchers and clinicians on choosing a scoring rule that depends on the device, the scoring algorithm, and the individual research study or clinical population of interest.
With the increasing use of actigraphy in both research and clinical settings, it is critical to ensure that the sleep-wake estimations are as accurate as possible. This study adds to the previously recommended practice parameters for the use of actigraphy published by the American Academy of Sleep Medicine [9] by showing that the choice of a scoring rule may impact sleep onset latency estimates. Further, this study shows the need to not simply rely on the default settings provided by the proprietary software used for each type of device. Researchers are encouraged to override these default settings, instead choosing a scoring rule that best fits the characteristics of their study population (e.g., preschoolers).
Although no single rule or algorithm performed well across different ages and SDB groups, some patterns emerged that may be useful for researchers and clinicians. For the AMI Sadeh algorithm, none of the scoring rules produced greater than an 8 minute difference between PSG and actigraphic SOL (which may not be clinically meaningful). That said, as expected the smallest differences in SOL estimates were found with the 3 minute rule for both preschool and school-aged children and with the 20 minute rule for adolescents. A similar pattern (shorter rules performed better for children and longer rules for adolescents) can be seen with the Cole-Kripke algorithm, with the greatest difference between actigraphy and PSG just under 10 minutes.
For the PRMM device, a somewhat different picture emerged. The Medium sensitivity level (the most commonly used one in pediatric research) provided the most accurate estimate of SOL with longer rules (15 or 20 minutes for preschoolers, 10 or 15 minutes for school-aged children, and 20 minutes for adolescents). While the need for a longer scoring rule in adolescents would be expected based on motionless wakefulness (e.g., adolescent can lie quietly for prolonged periods of time even if not sleeping), we did not expect the same to be true for younger children. These results likely reflect the differences between actigraphy brands in terms of the technology used and algorithms applied, suggesting caution when comparing results from two different brands of actigraphy [2,5]. Further, it was notable that in some cases the PRMM device underestimated SOL by up to 16 minutes, which could be clinically meaningful and/or impact research outcomes. Finally, our results also differ from those found by Chae and colleagues, where the 5 minute rule was the best estimate of SOL in adults.[4]
In terms of sleep disordered breathing, clear differences between the devices were again notable. For the AMI device, the shorter scoring rules were most accurate for both scoring algorithms and across groups, with the greatest difference in estimates between PSG and actigraphic SOL less than 9 minutes. The opposite was true for the PRMM device with longer scoring rules more accurate for those with no or mild SDB, while in general shorter immobility scoring rules provided more accurate sleep onset latency for youth with clinically significant SDB. It is important to again note that some of the PRMM estimates across groups were as high as 17 minutes.
There are several limitations to this study. First, measurement was only a single night in the sleep lab, where more movement may have occurred during sleep onset due to the discomfort of the wires and being unfamiliar with the sleep lab. Second, although our overall sample size was larger than many validation studies, group comparisons across developmental age group and SDB status resulted in smaller sample sizes. Third, our findings may have been influenced by collapsing the PSG data into 1-minute epochs. Finally, in this study we were able to clearly identify the start of sleep onset latency for actigraphy by using the PSG “lights off” time. Outside of the sleep lab, the measurement of sleep onset latency will always be limited by the requirement of a parent or youth to record their attempted sleep onset time by sleep diary or an event marker. Because of these limitations, additional research is clearly needed in this area that includes larger samples of children and adolescents.
Despite these limitations, this study provides novel data on different scoring rules for sleep immobility as a measure of sleep onset latency for actigraphy in a pediatric population. Other strengths of the study include the direct comparison with polysomnography, the broad age range, and the inclusion of youth with and without sleep disordered breathing. In conclusion, it is important to note that this study was not done to demonstrate whether one brand of actigraphic device is better than another in its measurement of immobility and sleep onset latency, but rather to provide researchers and clinicians some baseline information to guide decisions about scoring rules. Studies such as this one help us to better understand the strengths and limitations of actigraphy in research and clinical practice. However, larger studies are clearly needed to further investigate the differences in devices, scoring algorithms/sensitivies, as well as sleep onset latency scoring rules to ensure accurate and valid estimates of sleep in children.
Acknowledgments
The authors thank the children and families who participated in this study; the Children's Hospital of Philadelphia Sleep Lab for their assistance with this project; and Hawley Montgomery-Downs for her helpful review and comments on this paper. This study was funded in part by NIH MH077662.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Contributor Information
Lisa J. Meltzer, Department of Pediatrics, National Jewish Health.
Colleen M. Walsh, Center for Sleep and Respiratory Neurobiology, University of Pennsylvania School of Medicine
Ashley A. Peightal, Department of Pediatrics, National Jewish Health
References
- 1.Sadeh A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med Rev. 2011;15:259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16:463–475. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 4.Chae KY, Kripke DF, Poceta JS, Shadan F, Jamil SM, Cronin JW, Kline LE. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10:621–625. doi: 10.1016/j.sleep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer LJ, Walsh CM, Traylor J, Westin AM. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2012;35:159–166. doi: 10.5665/sleep.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- 7.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep. 1994;17:201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 8.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 9.Morgenthaler TI, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A, Jr, Coleman J, Lee-Chiong T, Pancer J, Swick TJ. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 10.Jenni OG, Carskadon MA. SRS Basics of Sleep Guide. Sleep Researcher Society; Westchester, IL: 2005. Normal human sleep at different ages: Infants to adolescents; pp. 11–19. [Google Scholar]
- 11.Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz ES, Marcus CL. Diagnosis of obstructive sleep apnea syndrome in infants and children. In: Sheldon SH, Ferber R, Kryger MH, editors. Principles and Practice of Pediatric Sleep Medicine. Elsevier Saunders; Philadelphia: 2005. pp. 197–210. [Google Scholar]
- 13.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]
- 14.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 15.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–878. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 16.Marcus CL, Brooks LJ, Ward SD, Draper KA, Gozal D, Halbower AC, Jones J, Lehmann C, Schechter MS, Sheldon S, Shiffman RN, Spruyt K. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]


