Abstract
Acquired protection from Plasmodium falciparum placental malaria, a major cause of maternal, fetal, and infant morbidity, is mediated by IgG specific for the P. falciparum erythrocyte membrane protein 1 variant VAR2CSA. This protein enables adhesion of P. falciparum-infected erythrocytes to chondroitin sulfate A in the intervillous space. Although interclonal variation of the var2csa gene is lower than that among var genes in general, VAR2CSA-specific Abs appear to target mainly polymorphic epitopes. This has raised doubts about the feasibility of VAR2CSA-based vaccines. We used eight human monoclonal IgG Abs from affinity-matured memory B cells of P. falciparum-exposed women to study interclonal variation and functional importance of Ab epitopes among placental and peripheral parasites from East and West Africa. Most placental P. falciparum isolates were labeled by several mAbs, whereas peripheral isolates from children were essentially nonreactive. The mAb reactivity of peripheral isolates from pregnant women indicated that some were placental, whereas others had alternative sequestration foci. Most of the mAbs were comparable in their reactivity with bound infected erythrocytes (IEs) and recombinant VAR2CSA and interfered with IE and/or VAR2CSA binding to chondroitin sulfate A. Pair-wise mAb combinations were more inhibitory than single mAbs, and all of the mAbs together was the most efficient combination. Each mAb could opsonize IEs for phagocytosis, and a combination of the eight mAbs caused phagocytosis similar to that of plasma IgG-opsonized IEs. We conclude that functionally important Ab epitopes are shared by the majority of polymorphic VAR2CSA variants, which supports the feasibility of VAR2CSA-based vaccines against placental malaria.
Placental malaria is a major cause of maternal anemia, fetal growth retardation and prematurity, and perinatal and infant morbidity and mortality in areas of stable transmission of Plasmodium falciparum parasites (1). The syndrome is caused by a selective accumulation of infected erythrocytes (IEs) in the placenta, which leads to maternal anemia and placental inflammation that compromise the function of the feto-placental unit. Placental IE sequestration is mediated by the VAR2CSA member of the Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) family of high-molecular mass adhesins that the parasite can express on the surface of IEs (2–5). VAR2CSA has selective affinity for the chondroitin sulfate A (CSA) chains of intervillous chondroitin sulfate proteoglycan, and parasites expressing this PfEMP1 variant do not appear to cause significant infections except in pregnant women (6).
Clinically relevant IE adhesion to CSA appears largely restricted to VAR2CSA-expressing parasites, because parasites where the encoding var2csa gene has been knocked out lose or show greatly reduced capacity to acquire this adhesion phenotype (7, 8). In agreement with this finding, protective immunity to placental malaria is associated with VAR2CSA-specific IgG, which probably acts by blocking adhesion of IEs to CSA and by opsonizing IEs for phagocytosis (3, 9, 10). Primigravidae in areas of stable P. falciparum transmission are susceptible to placental malaria because they do not possess significant levels of VAR2CSA-specific IgG (11–13). However, protective VAR2CSA-specific immunity is acquired rapidly after exposure to placenta-sequestering parasites, and multigravid women generally have high levels of VAR2CSA-specific IgG and are largely protected from harmful placental parasitemia (11, 14).
These findings have raised hopes that VAR2CSA-specific vaccines can be developed that would protect pregnant women from the adverse consequences of placental malaria if given prior to their first pregnancy (15, 16). However, this optimism is tempered by the fact that VAR2CSA is a high-molecular mass protein (350 kDa) composed of six so-called Duffy binding-like (DBL) domains and a cysteine-rich interdomain region domain, which each display considerable polymorphism (2, 17). Each P. falciparum genome contains one or more var2csa genes (18, 19), and although var2csa is one of the most interclonally conserved var genes (20), most Ab epitopes on surface-expressed native VAR2CSA nevertheless appear to be polymorphic (21, 22). The feasibility of VAR2CSA-based vaccines hinges on the presence of interclonally conserved and functionally important Ab epitopes. Experimental vaccination of animals with single-domain VAR2CSA constructs can induce Abs that inhibit CSA-specific in vitro adhesion of erythrocytes infected by different clones of P. falciparum to the same degree as plasma IgG from exposed women (23, 24). However, the inhibitory capacity of such antisera is much lower than that of antisera to recombinant full-length VAR2CSA (25). This might suggest that exposure to native full-length VAR2CSA on the surface of IEs focuses the naturally acquired Ab response on variant-specific rather than cross-reactive epitopes and that this frustrates the development of protective immunity. In the current study, we therefore set out to investigate the degree of interclonal conservation and functional significance of epitopes recognized by a panel of human monoclonal IgG1 Abs produced by EBV-immortalized affinity-maturated memory B cells from P. falciparum-exposed multigravidae.
Materials and Methods
Human mAbs and immune plasma
The isolation and characterization of the eight human monoclonal IgG1 Abs (PAM1.4, PAM2.8, PAM3.1, PAM4.7, PAM5.2, PAM6.1, PAM7.5, and PAM8.1) used in this study have been described in detail elsewhere (26). All but PAM1.4 specifically recognize epitopes in either the DBL3-X or the DBL5-ε domain of the placental malaria-specific PfEMP1 variant VAR2-CSA (Table I). Except where specifically indicated, purified monoclonal IgG preparations were used throughout the current study. These were obtained from monoclonal B cell supernatants by dia-filtration on an ÄKTAcrossflow system (GE Life Sciences, Brøndby, Denmark), followed by affinity purification on protein A columns (ProPur Spin Columns; Nunc, Roskilde, Denmark) according to the manufacturers’ instructions. Satisfactory IgG purity was verified by SDS-PAGE. In addition to the mAbs, we used panels of plasma obtained either from P. falciparum-exposed multigravidae and men or from nonexposed control donors to test the type of parasite-encoded variant surface Ags (VSAs) expressed on the surface of IEs (see below).
Table I.
Domain specificity and binding characteristics of the human monoclonal VAR2CSA-specific IgG Abs included in the study
| mAb | Domain Specificity | Reactivitya (% and [95% CI]) |
kon (M−1 s−1) | KD (M) |
|---|---|---|---|---|
| PAM1.4 | Unknownb | 69% [44–86%] | 2.3 × 104 | 8.4 × 10−9 |
| PAM2.8 | DBL3-X | 56% [33–77%] | 1.3 × 104 | 4.8 × 10−10 |
| PAM3.10 | DBL5-ε | 56% [33–77%] | 5.7 × 104 | 0.1 × 10−9 |
| PAM4.7 | DBL5-ε | 31% [14–56%] | Not tested | Not tested |
| PAM5.2 | DBL5-ε | 50% [28–72%] | 6.9 × 104 | 0.3 × 10−9 |
| PAM6.1 | DBL3-X | 73% [48–89%] | ND (low) | ND (low) |
| PAM7.5 | DBL5-ε | 64% [39–84%] | 4.5 × 104 | 0.2 × 10−9 |
| PAM8.1 | DBL3-X | 63% [39–82%] | 3.2 × 104 | 5.5 × 10−9 |
Percentage of the placental isolates tested that was labeled by the indicated Ab (PAM6.1 was only tested against 15 isolates and PAM7.5 only against 14 isolates).
Indirect evidence indicates that PAM1.4 recognizes a conformational and probably discontinuous epitope in VAR2CSA (26).
Plasmodium falciparum laboratory lines and field isolates
We used the long-term in vitro-propagated P. falciparum parasites 3D7, FCR3, and HB3 grown in O+ erythrocytes without human serum as described (27). Ab reactivity with IEs was tested on unselected cultures and cultures selected for IE adhesion to CSA by repeated panning on the human choriocarcinoma cell line BeWo as described (28). CSA-adhering IEs expressed VSAs with the sex-specific and parity-dependent plasma Ab recognition pattern characteristic of P. falciparum parasites involved in the pathogenesis of placental malaria (VSAPM) and described in detail elsewhere (13, 14). Unselected IEs did not express VSAPM.
To determine mAb reactivity with primary field isolates, we used 68 P. falciparum isolates collected in Korogwe, northeastern Tanzania (29), and 22 isolates collected at Comé, southwestern Benin (30). The isolates were obtained either from the peripheral blood of children below the age of five years (n = 15) and pregnant women (n = 59) or from placental blood at delivery (n = 16) as part of studies approved by the relevant ethical review boards. The isolates were maintained in vitro for no more than a few days before testing. In addition to the above-mentioned laboratory lines, we used primary field isolates obtained from the placentas of four women delivering at Morogoro Regional Hospital, central Tanzania, in assays of the ability of the mAbs to inhibit adhesion to CSA ex vivo.
Ab reactivity with the surface of P. falciparum-infected erythrocytes
The ability of the mAbs and immune plasma to label the surface of intact and unfixed late-stage IEs was tested essentially as described previously (26, 31, 32). In brief, erythrocytes infected by late-stage parasites were enriched by exposure of parasite cultures to a strong magnetic field (31, 33) and labeled by ethidium bromide (EtBr; 10 mg/ml) and either monoclonal or plasma Abs (30 min, 4°C) followed by secondary, FITC-conjugated goat anti-human IgG Ab (Beckman Coulter, Fullerton, CA) (30 min, 4°C). Ab surface labeling of EtBr-positive IEs then was quantified by flow cytometry, followed by analysis of collected data files using WinList software (Verity Software House, Topsham, ME).
Ab reactivity with recombinant P. falciparum VAR2CSA
The full-length ectodomain of VAR2CSA from the FCR3 parasite line (FCR3-FV2) was expressed in baculovirus-infected insect cells as described (25). The corresponding full-length VAR2CSA ectodomain from clone 3D7 (3D7-FV2) was cloned from H57 to N2641 and expressed and purified as described previously (25). ELISA plates (Nunc) were coated with either 3D7-FV2 or FCR3-FV2 (1 µg/ml, 1 h, 37°C). Unbound FV2 was removed by one wash using ELISA washing buffer [PBS with 1% Triton X-100 and 0.5 M NaCl (pH 7.4)] prior to blocking for 1 h with the same buffer. Blocking of the plates and all incubations with mAbs were done at 37°C for 1 h with gentle shaking. After three washes in ELISA washing buffer, a 1:3000 dilution of HRP-conjugated rabbit anti-human IgG (P0214; Dako, Glostrup, Denmark) was added to each well. Plates were finally washed three times prior to visualization of the Ab reactivity using ortho-phenylenediamine substrate and H2O2. The color reaction was stopped by adding H2SO4, and the OD was measured at 492 nm.
Ab affinity for recombinant P. falciparum VAR2CSA
Affinities of the mAbs for FCR3-FV2 were determined by surface plasmon resonance assay using a Biacore T100 instrument (Biacore, Little Chalfont, U.K.). In brief, FCR3-FV2 was coupled to surface 2 of a CM5 chip (Biacore) using the amine coupling protocol. Channels 1 and 2 were activated with a mixture of 0.2 M 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and 50 mM N-hydroxysuccinimide. FCR3-FV2 was diluted to a concentration of 100 µg/ml in 10 mM sodium acetate (pH 5) and passed through channel 2 until a response of 400 relative units was achieved. The remaining reactive succinimide ester groups on both surfaces then were blocked using 1 M ethanolamine (pH 8.5). The chip was equilibrated using HEPES-buffered saline [10 mM HEPES (pH 7.4), 150 mM NaCl, 50 µM EDTA, and 0.05% Tween 20)]. Human mAbs were exchanged into HEPES-buffered saline and injected over both channel 1 and 2 at a flow rate of 50 µl/min. The specific binding signal was obtained from a subtraction of the measurement response of channel 2 from that of channel 1. The surfaces of both channels were regenerated with injections of 15 µl of regenerating buffer (5 mM NaOH and 1 M NaCl). The BIAevaluation software (version 1.1.1) was used to analyze the kinetics of association and dissociation.
Ab-mediated inhibition of recombinant P. falciparum VAR2CSA binding to human chondroitin sulfate proteoglycan
mAb interference with the binding of FCR3-FV2 to human chondroitin sulfate proteoglycan (CSPG-h) was evaluated similarly by surface plasmon resonance assay, essentially as described elsewhere (25). In these experiments, FCR3-FV2 (5 nM) was preincubated with different concentrations of mAb (0–40 µg/ml), followed by assessment of binding of the mAb-labeled FCR3-FV2 to a streptavidin chip coated with biotinylated CSPG-h from human placenta (MRA-769; Malaria Research and Reference Reagent Resource Center, ATCC, Manassas, VA; Ref. 34). Data were analyzed using BIAevaluation software.
Ab-mediated inhibition of IE adhesion to CSA
Static and flow assays were employed to evaluate the capacity of the human mAbs to interfere with CSA-specific adhesion of IEs. The static assay, which was used in Tanzania for the study of placental IEs ex vivo, has been described in detail elsewhere (35). Briefly, late-stage–infected IEs were preincubated with the eight mAbs (B cell supernatants at 1:5 dilution) and allowed to adhere to CSA-coated Petri dishes (20 min, room temperature). Nonadhering cells were removed by an automated washing system, and the number of adhering IEs was quantified by microscopy and compared with the level of binding in the presence of an unrelated mAb. For the flow-based assay Vena8 channels (Cellix, Dublin, Ireland) were coated overnight at 4°C in a humid chamber with 20 µg/ml decorin (Sigma-Aldrich, St. Louis, MO) in PBS. The channels were blocked with 2% BSA for 1 h at room temperature. IEs were prepared and adjusted to 2% parasitemia and 1% hematocrit in culture media and incubated (30 min, 37°C) in PBS with mAbs (120 µg/ml) or soluble CSA (50 µg/ml). The IE suspension (40 µl) was loaded into the channel using the VenaFlux Platform (Cellix) and a shear stress of 0.075 Pa. Ten digital 20-frame movie sequences were recorded through a Leica microscope (magnification ×20) at ten random positions along the channel 5–8 min after inoculation of the sample. The number of bound IEs at each position was determined using Image-Pro Analyzer 7.0 software (MediaCybernetics, Bethesda, MD).
Ab opsonization for phagocytosis
We measured the opsonizing capacity of the human mAbs by a recently described flowcytometry assay (36). In brief, magnet-enriched late-stage IEs (2 × 108 per milliliter) were labeled by EtBr (10 mg/ml, 10 min, room temperature), washed twice in RPMI 1640 medium supplemented with 2% FCS, and exposed to monoclonal B cell supernatants (1:5 dilution, 30 min, room temperature). After two additional washes, the opsonized IEs were incubated at a 20:1 ratio with the human monocytic leukemia line THP-1 (TIB-202; LGC Standards, Borås, Sweden) on a rocking table (30 min, 37°C). IEs that had not been phagocytosed by the THP-1 cells were lysed (15 mM NH4Cl, 10 mM NaHCO3, and 1 mM EDTA; 3 min with shaking for 30 s). The THP-1 cells then were washed twice in ice-cold PBS, supplemented with 2% FCS, and the percentage of EtBr-positive cells was measured on a FACScan flow cytometer (BD Biosciences, San Jose, CA), with subsequent data analysis in WinList software (Verity Software House).
Statistical analysis
Confidence intervals for proportions and the statistical significance of differences in proportions were calculated as described in Ref. 37, using the accompanying software (http://www.som.soton.ac.uk/cia/).
Results
VSAPM-specific human mAbs selectively recognize parasite isolates obtained from pregnant women from East and West Africa
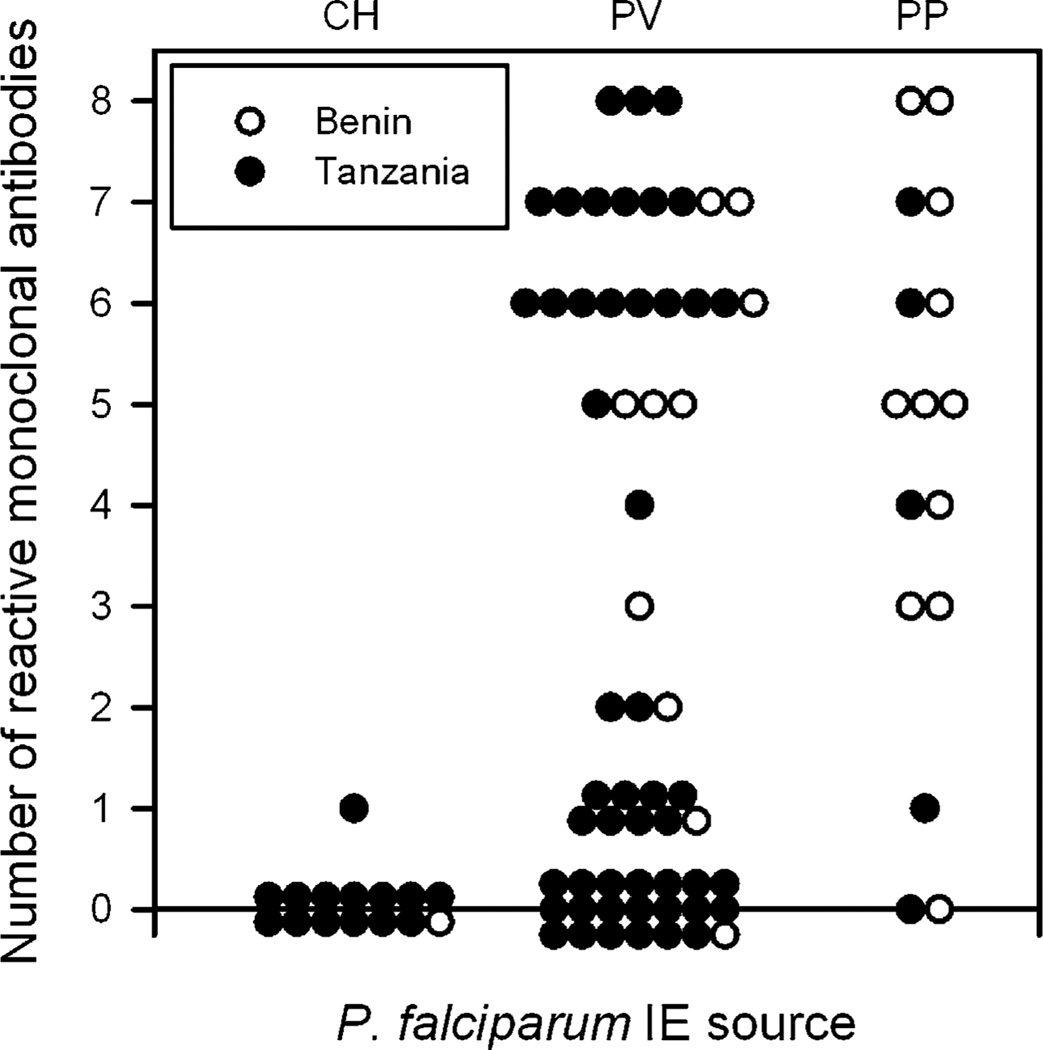
We have described previously a panel of eight human monoclonal IgG Abs (Table I), produced by EBV-immortalized B cell clones derived from P. falciparum-exposed multigravidae, which reacts specifically with polymorphic epitopes in Ags expressed on the surface of P. falciparum IEs selected in vitro for adhesion to CSA (26). After selection, erythrocytes infected by the laboratory line FCR3 (FCR3-BeWo) were recognized by all eight mAbs, whereas BeWo-selected 3D7 was recognized by all but PAM4.7 and PAM8.1, in accordance with the earlier study (26). BeWo-selected HB3 IEs were recognized by all mAbs except PAM4.7 (data not shown). Unselected 3D7, FCR3, and HB3 were not recognized by any of the mAbs (data not shown). The reactivity of the panel of mAbs with field isolates has not been documented previously. To verify the in vivo relevance of the epitopes recognized by the mAbs, we therefore tested their ex vivo reactivity with IEs obtained from two independent series of P. falciparum-infected children and pregnant women (Fig. 1). In the first series of 22 primary parasite isolates from Benin, we found that 10/11 (91%; 95% confidence interval [62–98%]) placental isolates showed reactivity with at least one of the mAbs, whereas 7/11 (64% [35–86%]) reacted with at least five mAbs. Reactivity with peripheral blood isolates from pregnant women was also high, because 9/10 (90% [60–98%]) reacted with one or more mAbs and 6/10 (60% [31–83%]) reacted with at least five. IEs from the peripheral blood of the single malarious child from Benin that we tested did not react with any of the eight mAbs.
FIGURE 1.
mAb recognition of P. falciparum-infected erythrocytes obtained from naturally infected individuals from Benin (○) and Tanzania (●). IEs obtained from the placenta at delivery, peripheral venous blood of pregnant women, or peripheral blood of children with malaria were labeled with each of the eight mAbs and analyzed by flow cytometry. The number of mAbs showing significant labeling was recorded for each isolate. CH, children with malaria; PP, placenta; PV, peripheral venous blood.
The mAbs in our panel were derived from B cell donors in Ghana (26), not far from the study site in Benin. We therefore proceeded to test whether IEs from a geographically distant location would be similarly well recognized by the panel mAbs. For this, we used a series of 68 primary parasite isolates from Korogwe, northeastern Tanzania, ~4500 km to the southeast of Ghana. We found that 4/5 (80% [38–96%]) of placental IEs reacted with at least one of the mAbs, whereas 2/5 (40% [12–77%]) reacted with five or more. Although these proportions were lower than those observed in the series from Benin, the differences were not statistically significant (p > 0.6 in both cases; Fisher’s exact test). Similarly, 29/49 (59% [45–72%]) of peripheral blood IEs from pregnant women reacted with at least one of the mAbs, whereas 18/49 (37% [25–51%]) reacted with five or more. Again, the proportions from Benin and Tanzania were not statistically different (p = 0.08 and p = 0.29, respectively). Only one of the 14 tested peripheral blood IE samples from Tanzanian children showed weak reactivity with PAM2.8, whereas all of the others were completely negative. Due to technical difficulties, only 65/90 IE samples were tested against all eight mAbs. Of the remainder, one isolate was tested against five, six against six, and 18 against seven mAbs. The above reactivity figures are thus conservative, but reanalysis using the fraction of reactive mAbs among tested mAbs yielded results that were comparable to those presented above (data not shown).
Assuming that all of the placental IE isolates were actually CSA-adhering and placenta-sequestering and that none of the pediatric IE isolates would be capable of placental sequestration, these results indicate that reactivity with at least one of the eight mAbs identifies IEs from a placental infection with 93% (nonreactivity with IE samples from 14/15 children) specificity and 88% (reactivity with IE samples from 14/16 placentas) sensitivity. The corresponding figures if requiring reactivity with at least two of the mAbs in the panel would be 100 and 81%, respectively. Accordingly, between 58 and 73% (34 or 43 of the 59 PV samples) of the IE samples obtained from peripheral venous blood appear to have originated from a placental sequestration focus. Individual human mAbs identified between one third and three quarters of the placental isolates tested (Table I), whereas the minimal mAb combinations that identified all of the placental isolates except the two completely nonreactive ones (Fig. 1) were PAM1.4 in combination with PAM6.1, PAM7.5, or PAM8.1 (data not shown).
We next examined whether there was any relationship between either overall mAb reactivity or reactivity with particular mAbs on the one hand and gravidity on the other. We did not identify any significant relationships of this kind (data not shown).
Overall, these results confirm and extend our previous results that the mAbs in our panel recognize polymorphic epitopes in IE VSAs selectively expressed by CSA-adhering IEs (26) and document that VSAs expressed following in vitro selection of parasites for mAb reactivity (38) or adhesion to CSA (26) are indistinguishable from those expressed by placental parasites. More strikingly, the results document that despite this interclonal diversity, the majority of placental parasites and parasites from the peripheral blood of pregnant women (probably from a placental sequestration focus) were recognized by several of the mAbs. This strongly indicates that the variant Ags expressed on the surface of erythrocytes infected by such parasites possess several Ab epitopes shared among many variants.
Reactivity with native and recombinant VAR2CSA
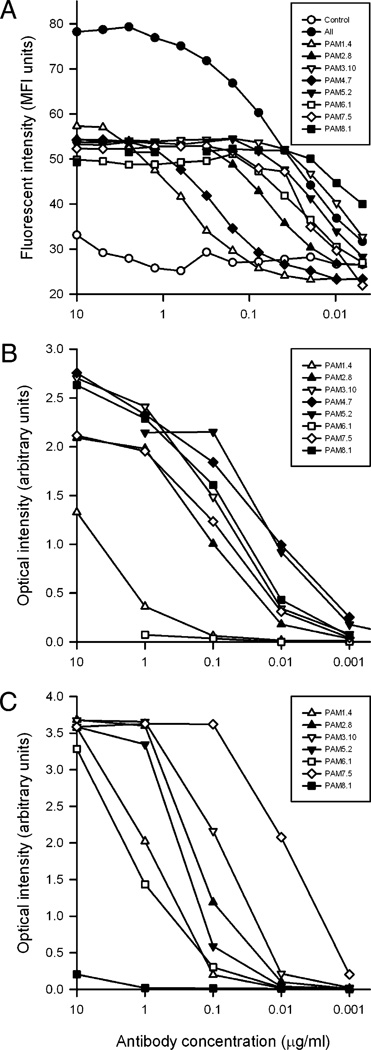
To study the binding properties of the human mAbs in more detail, we first analyzed the relationship between Ab concentration and labeling of FCR3-BeWo–infected erythrocytes by flow cytometry (Fig. 2A). Significant labeling could be detected at Ab concentrations >0.01 µg/ml for all mAbs except PAM1.4 and PAM4.7, which required >0.1 µg/ml. No increase in labeling was seen >0.2 µg/ml, except for PAM1.4 and PAM4.7, where labeling did not reach a plateau until ~2 µg/ml. Labeling with a combination of all eight mAbs (in equal amounts) in the panel resulted in a substantially higher plateau than that observed when using the mAbs individually. This fits our earlier evidence that the mAbs are specific for nonoverlapping, domain-specific epitopes in the PfEMP1 variant VAR2CSA that is expressed on the surface of placentasequestering, CSA-adhering IEs (Table I and Ref. 26).
FIGURE 2.
mAb reactivity with native and recombinant VAR2CSA. Erythrocytes infected by the VAR2CSA-expressing P. falciparum parasite line FCR3-BeWo (A) or recombinant full-length constructs of P. falciparum FCR3-VAR2CSA (B) or 3D7-VAR2CSA (C) were incubated with varying concentrations of mAbs. Reactivity was tested by flow cytometry (A) or ELISA (B, C). A pool of all eight mAbs (All) and a mAb of unrelated specificity (Control) were included in the flow cytometry experiments as positive and negative controls, respectively. Each experiment was performed at least twice. Total Ab concentrations are given, and the results of a typical experiment are shown. Ab labels are as in Fig. 1.
To substantiate this specificity for VAR2CSA, we used ELISA to test the reactivity of the mAbs with FCR3-FV2 (25) and 3D7-FV2. Most of the mAbs specifically recognized both FV2 constructs at Ab concentrations ≥0.01 µg/ml (Fig. 2B, 2C). The exceptions were PAM1.4 (which reacted poorly with FCR3-FV2), PAM6.1 (which was nonreactive with FCR3-FV2), and PAM8.1 (which was nonreactive with 3D7-FV2). The reactivity of PAM1.4 with both FV2 constructs confirms that VAR2CSA is indeed the target of this mAb, despite the lack of PAM1.4 reactivity with single-domain constructs of the Ag (26). The differential reactivity of PAM8.1 with 3D7-FV2 and FCR3-FV2 was expected, because native 3D7-VAR2CSA (and recombinant 3D7-VAR2CSA-DBL3-X) lack the PAM8.1 epitope (26). However, the absence of PAM6.1 reactivity with FRC3-FV2 was unexpected, because this mAb reacts with the native FCR3 variant of VAR2CSA on the surface of IEs (26). This discrepancy indicates that FCR3-FV2 correctly reproduces many but not all Ab epitopes present in the corresponding native Ag.
In a final set of mAb specificity experiments, we determined the VAR2CSA affinity of seven of the mAbs in a surface plasmon resonance assay by flowing them over a Biacore chip surface coupled with FCR3-FV2. Association rates (kon) were high and similar for all tested mAbs, except PAM6.1, which bound very poorly to FCR3-FV2 (Table I). Dissociation rates (koff) were extremely slow, making fitting inaccurate and thereby affecting the calculation of dissociation constants (KD = koff/kon). With this caveat in mind, the data indicate that apart from PAM6.1 all of the tested mAbs had a high affinity for FCR3-FV2 and displayed similar binding characteristics. The poor affinity of PAM6.1 for FCR3-FV2 in the Biacore experiments agrees with the ELISA data.
Overall, these results confirm and extend our previous data that the molecular target of the mAbs on the surface of IEs is indeed VAR2CSA and that the mAbs have high affinities for this Ag (26, 39). Furthermore, the data reinforce the evidence that VAR2CSA has several Ab epitopes shared among many of its polymorphic variants.
Human mAbs specifically interfere with the binding of recombinant VAR2CSA to CSPG-h
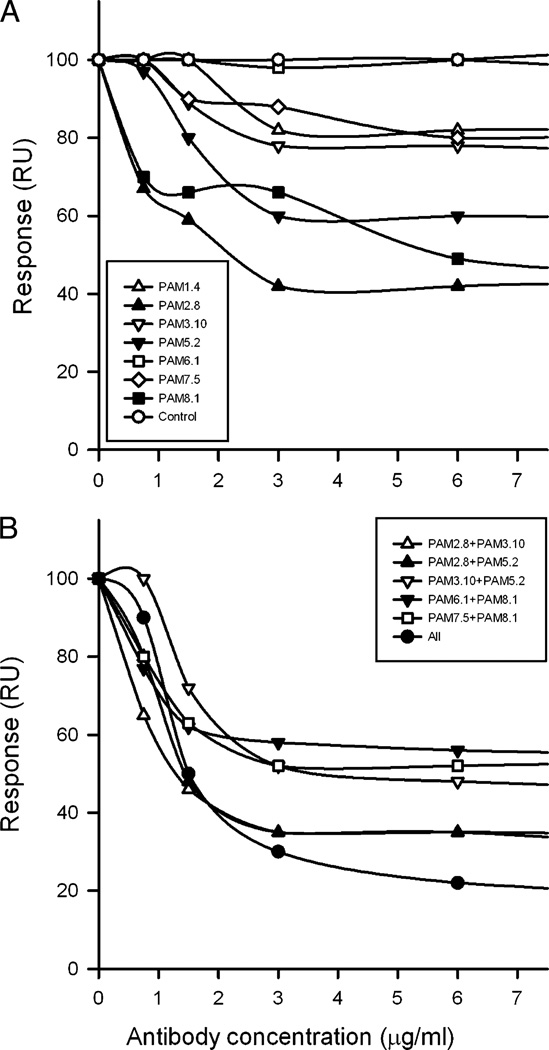
It is generally assumed that an important effector function of protective IgG against placental malaria is interference with CSA-specific adhesion of IEs in the intervillous space (11), and it was shown recently that IgG purified from rat FCR3-FV2–specific antisera very effectively inhibits the interaction between FCR3-FV2 and the CSA moieties of CSPG-h (25). We used a similar Biacore setup to test how preincubation of FCR3-FV2 with seven of the human mAbs individually and in various combinations affected the ability of this molecule to subsequently bind to a CSPG-h–coated chip surface (Fig. 3). Preincubation with single mAbs showed that some (PAM2.8, PAM5.2, and PAM8.1) inhibited binding >40% at concentrations ≥3 µg/ml, whereas others (PAM1.4, PAM3.10, and PAM7.5) were less effective (~20% inhibition) or completely ineffective (PAM6.1) (Fig. 3A). Both the DBL3-X and the DBL5-ε domains have been implicated in the interaction of VAR2CSA with CSA (40), although the results of single-domain constructs should be interpreted with caution (41). We found both DBL3-X–specific and DBL5-ε–specific mAbs among those inhibiting the binding of FCR3-FV2 to CSPG-h, and there was therefore no clear correlation between the domain specificity of the mAbs and their ability to interfere with FCR3-FV2 binding to CSPG-h. The inability of PAM6.1 to interfere with binding of FCR3-FV2 was expected from the apparent lack of the PAM6.1 epitope in the recombinant construct.
FIGURE 3.
mAb interference with the binding of recombinant full-length FCR3-VAR2CSA (FCR3-FV2) to CSPG-h. FCR3-FV2 was preincubated with the indicated concentrations of single mAbs (A) or the indicated combinations of Abs (B) before flowing FCR3-FV2 past CSPG-h coupled to the surface of a Biacore chip. Ab labels are as in Fig. 1.
Pair-wise combinations of the mAbs performed better than the Abs in isolation. As an example, PAM2.8 and PAM3.10 at 1.5 µg/ml inhibited the binding of FCR3-FV2 to CSPG-h ~40 and 10%, respectively, when used alone (Fig. 3A), whereas these mAbs together (at a final concentration of 3 µg/ml) inhibited the interaction ~;65% (Fig. 3B). Thus, combining mAbs augmented their inhibitory effect. In line with this, a combination of all seven mAbs was most effective in inhibiting the interaction between FCR3-FV2 and CSPG-h, showing ~70% inhibition at concentrations of ≥3 µg/ml (Fig. 3B). Increasing the mAb concentrations to 6–40 µg/ml did not cause any further increase in the inhibitory effect of single mAbs or combinations of them (data not shown). Three conclusions can be drawn from these experiments. First, the interaction between soluble full-length recombinant VAR2CSA and its receptor CSPG-h is markedly affected by epitopes in DBL3-X and DBL5-ε of VAR2-CSA. Second, the results are consistent with the presence of several functionally important and interclonally conserved Ab epitopes in VAR2CSA, because the mAbs tested in this study were not raised against FCR3-FV2 but rather against unknown naturally occurring variants of VAR2CSA. Third, combinations of mAbs worked better than single mAbs, although the effect appears to be less than simply additive. Targeting relatively few critical Ab epitopes thus might be sufficient to significantly reduce VAR2CSA-dependent adhesion of IEs to CSA. We therefore next studied the ability of the human mAbs to inhibit the CSA-specific adhesion of IEs.
Human mAbs can inhibit CSA-specific adhesion of IEs
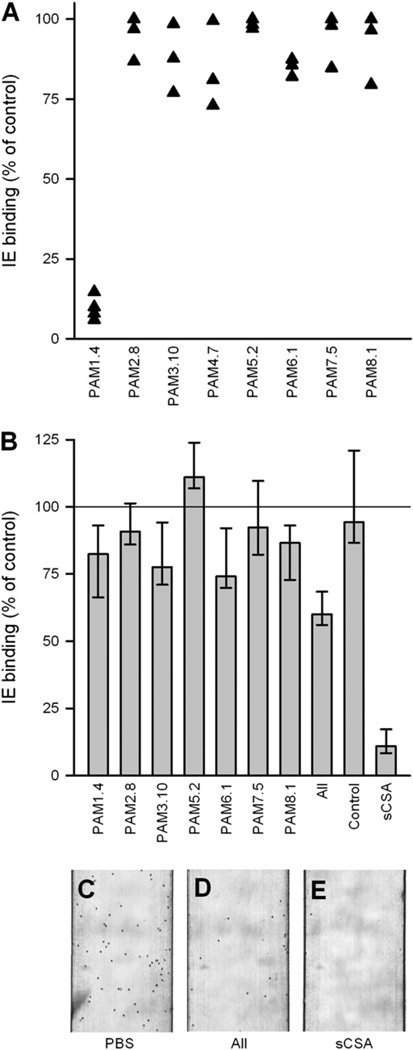
Preliminary static adhesion inhibition assays with manual washing away of nonadhering erythrocytes suggested that some of the human mAbs were indeed able to significantly reduce adhesion of IEs to CSA, but the assay was prone to substantial interassay variation (data not shown). Instead, we conducted a series of experiments in Tanzania with a more standardized assay adapted to field conditions (35). The results showed that PAM1.4 essentially abrogated CSA-specific adhesion of 4/4 placental isolates, whereas the adhesion-inhibitory capacity of the remaining mAbs was substantially lower (only three isolates were tested with mAbs other than PAM1.4) (Fig. 4A).
FIGURE 4.
mAb interference with the binding of P. falciparum IEs to CSA. The ability of the Abs to interfere with static CSA-specific adhesion of placental primary isolates (B cell supernatants at 1:5 dilution) (A) or flow adhesion of CSA-selected FCR3 IEs (purified mAbs at a final concentration of 120 µg/ml) (B) was measured. Soluble CSA was used at a concentration of 50 µg/ml. Ab labels are as in Fig. 1. Typical flow assay micrograph frames of CSA-adhering IEs after incubation in PBS (C), a pool of all Abs (D), and sCSA (E) also are shown. sCSA, soluble CSA.
We proceeded to study the adhesion-inhibitory capacity of the mAbs under arguably more physiologically relevant flow conditions. In these experiments using CSA-adhering FCR3 IEs we found that PAM1.4, PAM3.10, PAM6.1, and PAM8.1 were moderately inhibitory, whereas PAM2.8, PAM5.2, and PAM7.5 had little or no effect (Fig. 4B). A combination of all seven tested mAbs was more inhibitory than any of the mAbs separately and approximately half as effective as soluble CSA. These results correspond well with the Tanzanian data (Fig. 4A). The limited capacity of PAM1.4 to inhibit adhesion of FCR3 IEs compared with the Tanzanian parasites is likely to be a consequence of the relatively low reactivity of PAM1.4 with FCR3 IEs (Fig. 2A). The IE adhesion data also corresponded well with the Biacore data, except for PAM2.8 and PAM5.2, which were highly inhibitory in the Biacore assay (Fig. 3A) but showed very limited (PAM2.8) or no (PAM5.2) effect ex vivo (Fig. 4A) or in vitro (Fig. 4B). Although a biophysical assay involving individual VAR2CSA molecules in solution is undoubtedly more sensitive to interference than an in vitro assay involving multiple membrane-bound molecules, it is probably less informative of biological relevance. We therefore put more weight on the results of the latter assay.
Overall, these results underpin the hypothesis that few Ab specificities are sufficient to substantially interfere with receptor-specific adhesion of IEs to CSA.
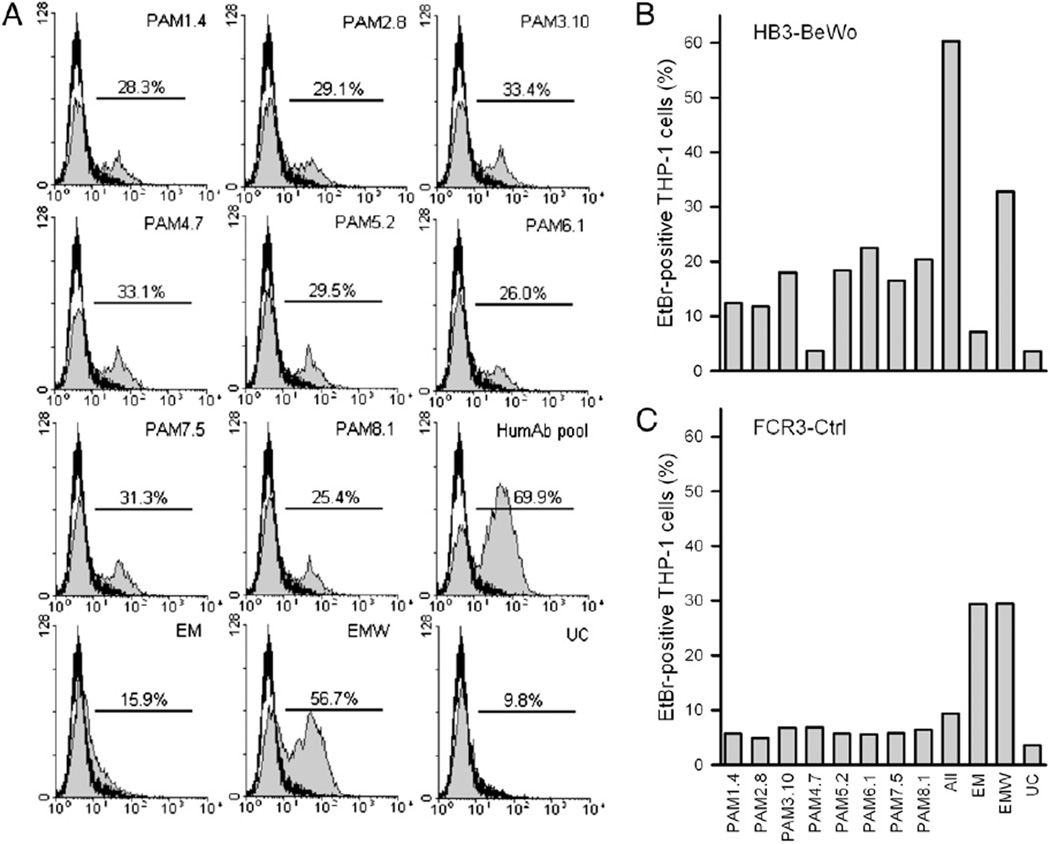
Human mAbs can efficiently and additively opsonize IEs for phagocytosis
Previous data indicate that protection against placental malaria involves other effector functions than inhibition of CSA-specific IE adhesion (14). Furthermore, the compromised Ab-mediated immunity to placental malaria in HIV-infected women involves a reduced capacity to opsonize IEs for phagocytosis (42, 43). We therefore completed this study by investigating the ability of the human mAbs to opsonize IEs for phagocytosis. With a single exception, each of the eight mAbs efficiently opsonized CSA-adhering FCR3 and HB3 IEs for phagocytosis (Fig. 5A, 5B). The exception was PAM4.7, which could not opsonize HB3-BeWo IEs (Fig. 5B), in line with the nonreactivity of this mAb with CSA-adhering HB3 IEs (see above). The opsonizing capacity of the individual human mAbs was additive, corresponding to their reactivity with different epitopes in VAR2CSA (26). The opsonizing capacity of a pool of all eight mAbs equaled or exceeded that of presumably highly polyclonal Abs in pooled plasma from P. falciparum-exposed multigravid women (Fig. 5A, 5B). Abs in a pool of plasma from sympatric P. falciparum-exposed men did not opsonize the CSA-adhering IEs (FCR3-BeWo and HB3-BeWo) (Fig. 5A, 5B) but efficiently opsonized IEs expressing VSAs not related to pregnancy and not mediating IE adhesion to CSA (FCR3-Ctrl; Fig. 5C). This finding confirms the pregnancy specificity of the IE surface Ags expressed by BeWo-selected parasite lines (28). A plasma pool from P. falciparum-unexposed control donors did not contain Abs capable of opsonizing any of the parasite lines for phagocytosis by THP-1 cells (Fig. 5). This finding documents that an Ag-specific interaction between IEs and Abs is required for opsonization to occur. Together, these results confirm the expected simple relationship between the ability of VSA-specific IgG to label intact IEs and the ability to opsonize them for phagocytosis.
FIGURE 5.
Phagocytosis of opsonized P. falciparum IEs. Erythrocytes infected by EtBr-labeled P. falciparum FCR3-BeWo (A), HB3-BeWo (B), and FCR3-Ctrl (C) were opsonized with human mAbs (B cell supernatants at 1:5 dilution) either alone (PAM1.4, PAM2.8, PAM3.10, PAM4.7, PAM5.2, PAM6.1, PAM7.5, or PAM8.1), in combination (All), or with plasma from P. falciparum-exposed men, P. falciparum-exposed multigravid women, or unexposed controls and coincubated with THP-1 cells. The difference in phagocytosis in the absence (open curve) and presence of Abs (shaded curves) is shown in A. Each experiment was performed at least twice. The results of a typical experiment are shown in as the percentage of THP-1 cells that became EtBr-positive after coincubation with EtBr-labeled IEs. EM, exposed men; EMW, exposed multigravid women; UC, unexposed controls.
Discussion
Placental sequestration of IEs to the CSA chains of CSPG-h in the intervillous space is mediated by the PfEMP1 variant VAR2CSA (3, 7, 8). The var2csa gene encoding this protein is polymorphic and composed of variable regions with interspersed conserved stretches (20). Although naturally acquired Abs appear to target mainly polymorphic epitopes of VAR2CSA (21, 26), protective immunity to placental malaria nevertheless is acquired over just a few pregnancies in areas of intense P. falciparum transmission. It therefore seems reasonable to speculate that at least some functionally important Ab epitopes are present in many polymorphic variants of VAR2CSA, and there is evidence that such conserved regions of VAR2CSA do exist (5, 22, 44). These findings have raised hopes that development of VAR2CSA-based vaccines against the syndrome is feasible (15, 16). The main protective mechanism is likely to be inhibition of CSA-dependent adhesion of IEs in the placenta (11), although phagocytic clearance of opsonized IEs also might be involved (43, 45). In the current study, we set out to study the interclonal diversity and functional significance of epitopes defined by their reactivity with mAbs acquired in response to placental malaria infection.
We found that placental IE isolates from East and West Africa could be identified by their reactivity with these mAbs with high specificity and sensitivity (Fig. 1). In fact, the majority of the isolates were recognized by several or even all of the mAbs, supporting the notion of prevalent epitopes on IE surface adhesins expressed by many different placenta-sequestering P. falciparum isolates. Although earlier studies have reported similar Ab recognition of VSAs expressed by geographically distant P. falciparum parasites (11, 46, 47), the preservation of specific VSA epitopes with the precision afforded by monoclonal reagents has not been demonstrated previously. We also could show evidence that IEs obtained from the peripheral blood of a large proportion of pregnant women originated from infections with a placental sequestration focus, in line with previous evidence (48, 49). Finally, we observed low levels of IE reactivity with one of the VAR2CSA-specific mAbs in one of 15 children (Fig. 1). This was an unexpected finding, because VAR2CSA expression is assumed to be restricted to placenta-sequestering parasites (6). However, var2csa can be transcribed at low levels by parasites isolated from some non-pregnant patients, including this particular isolate (P. Magistrado, unpublished observations). Furthermore, Abs in the serum of P. falciparum-exposed men and children occasionally show low-level reactivity with VAR2CSA-expressing IEs, indicating that expression of the protein can occur outside pregnancy (50). We currently are investigating this issue in more detail.
Identification of functionally important epitopes has been frustrated by the inability to generate full-length recombinant PfEMP1 constructs. Studies of VAR2CSA single-domain constructs have shown that several bind to CSA (40, 51, 52), but so do similar constructs from many other, placental malaria-unrelated PfEMP1 variants (41). This indicates that the properties of full-length PfEMP1 molecules cannot be reliably predicted from studies of single PfEMP1 domains (53). The recent reports on full-length VAR2CSA proteins underpin this claim (25, 54), because their affinity for CSA is many-fold higher than that of single-domain constructs. One of the mAbs (PAM6.1) did not label one of our full-length VAR2CSA constructs (FCR3-FV2; Fig. 2B) as expected but did react with the other (3D7-FV2; Fig. 2C), indicating that full-length constructs may not always accurately reproduce all of the Ab epitopes present in the native molecule. The remaining mAbs did bind to FV2 with roughly similar characteristics (Table I), and even if we did not determine the binding properties of the PAM4.7 mAb to FV2 for technical reasons, our previous data show that this mAb also binds to recombinant VAR2CSA with high affinity (39). In contrast, we observed marked differences in mAb ability to interfere with the interaction between CSA on the one hand and either recombinant (Fig. 3) or native (Fig. 4) VAR2CSA on the other. Three mAbs (PAM1.4, PAM3.10, and PAM8.1) consistently inhibited binding in both types of assays (albeit to varying degrees), whereas two mAbs (PAM2.8 and PAM5.2) did not inhibit IE binding but were able to interfere with the binding of soluble VAR2CSA to CSA. In contrast, PAM6.1 reduced IE binding to CSA but did not affect the binding of soluble VAR2CSA to CSA, whereas PAM7.5 did not perform convincingly in either assay. Inhibitory mAbs targeted the DBL3-X domain, which appears to form part of the minimal CSA-binding domain of FV2 (M. Dahlbäck L.M. Jørgensen, M.A. Nielsen, S.B. Ditlev, M. Resende, V.V. Pinto, D.E. Arnot, T.G. Theander, and A. Salanti, submitted for publication), as well as the DBL5-ε domain, which appears to be outside it. The inhibitory capacity of the DBL5-ε–specific mAbs may be due to steric hindrance. In this context, it is worth noticing that antisera against DBL4-ε (not implicated in CSA adhesion) also strongly inhibit adhesion of IEs to CSA (24). Combinations of Ab specificities were consistently more inhibitory than single Ab specificities (Figs. 3B, 4B), which is in agreement with the idea that Ab binding to several epitopes can affect VAR2CSA binding to CSA.
The above results indicate that Ab obstruction of just a few key epitopes is sufficient to markedly impede binding of VAR2CSA-expressing IEs to CSA, in line with a previous experimental immunization study (24), and that placental infection induces substantial cross-reactive immunity. However, they also emphasize that not all adhesion-inhibitory epitopes are present in all parasite variants and that Ab-mediated inhibition therefore is likely to be at least partially variant-specific, a conclusion further supported by data from experimental immunizations with FV2 (A. Salanti, unpublished observations).
The ability to inhibit CSA-specific IE adhesion is highly likely to be a key characteristic of protective IgG but may not be the only one. Naturally acquired Ab response to CSA-adhering IEs is dominated completely by the cytophilic Ab subclasses IgG1 and IgG3 (55). This and the impaired opsonizing capacity of plasma IgG from HIV-infected multigravidae (43), who are much more susceptible to placental malaria than their HIV-negative peers (56), point to phagocytic clearance of opsonized IEs as an alternative or additional mechanism of Ab-mediated protection. The data presented in this study show that all tested monoclonal IgG1 Abs efficiently opsonized IEs for phagocytosis, that this is due to a VAR2CSA-specific interaction with the IEs (because PAM4.7 could not opsonize HB3-BeWo-IEs), and that the effect is additive with as few as eight different Ab specificities being sufficient to exceed the opsonizing capacity of immune plasma from P. falciparum-exposed multigravidae. These results corroborate an important role for Ab-dependent phagocytosis as an effector mechanism in acquired protective immunity to placental malaria.
In conclusion, we have presented several lines of evidence that CSA-adhering P. falciparum-infected IEs express multiple Ab epitopes that are not only shared by many variants but are also functionally important by being involved in IE adhesion. Our data do not support the concern that exposure to native VAR2CSA on the IE surface selectively focuses the naturally acquired Ab response on variant-specific rather than cross-reactive epitopes. This is encouraging with respect to current efforts to develop VAR2CSA-based vaccines to protect women against placental malaria, because it indicates that relatively few epitopes are sufficient to induce an Ab response that can interfere substantially with IE adhesion to CSA in the placenta and efficiently opsonize IEs for phagocytosis.
Acknowledgments
We thank Kirsten Pihl, Katrine Vegener, and Maiken Visti for excellent technical assistance and Achille Massougbodji and Nadine Fievet for managing the study site in Benin. This paper is published with the permission of the National Institute for Medical Research, Moshi, Tanzania.
This work was supported by the Bill and Melinda Gates Foundation (Grant 47029), the Danish Medical Research Council (Grant 271-07-0301), the Danish Consultative Research Committee for Development Research (Grant 87-08-KU), the European Community’s Sixth Framework Program (Grant LSHP-CT-2006-036838), the European Community’s Seventh Framework Programs (Grants 200889 and 242095), Rigshospitalet (Grant 9615.05330), and the University of Copenhagen (Program of Excellence in Membrane Topology and Quaternary Structure of Key Membrane Proteins Involved in Plasmodium falciparum Malaria Pathogenesis and Immunity). M.K.H. is a Royal Society University Research Fellow and was supported by grants from the Royal Society and the Wellcome Trust. P.K. was supported by a scholarship from the Royal Thai Government.
Abbreviations used in this paper
- CH
children with malaria
- CSA
chondroitin sulfate A
- CSPG-h
human chondroitin sulfate proteoglycan
- DBL
Duffy binding-like
- EM
exposed men
- EMW
exposed multigravid women
- EtBr
ethidium bromide
- IE
infected erythrocyte
- PfEMP1
Plasmodium falciparum erythrocyte membrane protein 1
- PP
placenta
- PV
peripheral venous blood
- sCSA
soluble CSA
- UC
unexposed control
- VSA
variant surface Ag
- VSAPM
placental malaria-specific VSA
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Rogerson SJ, Hviid L, Duffy PE, Leke RFG, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 2.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 3.Salanti A, Dahlbäck M, Turner L, Nielsen MA, Barfod L, Magistrado P, Jensen AT, Lavstsen T, Ofori MF, Marsh K, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuikue Ndam NG, Salanti A, Bertin G, Dahlbäck M, Fievet N, Turner L, Gaye A, Theander TG, Deloron P. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J. Infect. Dis. 2005;192:331–335. doi: 10.1086/430933. [DOI] [PubMed] [Google Scholar]
- 5.Magistrado P, Salanti A, Tuikue Ndam NG, Mwakalinga SB, Resende M, Dahlbäck M, Hviid L, Lusingu J, Theander TG, Nielsen MA. VAR2CSA expression on the surface of placenta-derived Plasmodium falciparum-infected erythrocytes. J. Infect. Dis. 2008;198:1071–1074. doi: 10.1086/591502. [DOI] [PubMed] [Google Scholar]
- 6.Hviid L, Salanti A. VAR2CSA and protective immunity against pregnancy-associated Plasmodium falciparum malaria. Parasitology. 2007;134:1871–1876. doi: 10.1017/S0031182007000121. [DOI] [PubMed] [Google Scholar]
- 7.Viebig NK, Gamain B, Scheidig C, Lépolard C, Przyborski J, Lanzer M, Gysin J, Scherf A. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Rep. 2005;6:775–781. doi: 10.1038/sj.embor.7400466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffy MF, Maier AG, Byrne TJ, Marty AJ, Elliott SR, O’Neill MT, Payne PD, Rogerson SJ, Cowman AF, Crabb BS, Brown GV. VAR2CSA is the principal ligand for chondroitin sulfate A in two allogeneic isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 2006;148:117–124. doi: 10.1016/j.molbiopara.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 2003;71:6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet. 2004;363:283–289. doi: 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 11.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 12.Beeson JG, Brown GV, Molyneux ME, Mhango C, Dzinjalamala F, Rogerson SJ. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 1999;180:464–472. doi: 10.1086/314899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricke CH, Staalsoe T, Koram K, Akanmori BD, Riley EM, Theander TG, Hviid L. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 2000;165:3309–3316. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 14.Staalsoe T, Megnekou R, Fievét N, Ricke CH, Zornig HD, Leke R, Taylor DW, Deloron P, Hviid L. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that are associated with protection against placental parasitemia. J. Infect. Dis. 2001;184:618–626. doi: 10.1086/322809. [DOI] [PubMed] [Google Scholar]
- 15.Smith JD, Deitsch KW. Pregnancy-associated malaria and the prospects for syndrome-specific antimalaria vaccines. J. Exp. Med. 2004;200:1093–1097. doi: 10.1084/jem.20041974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hviid L. The role of Plasmodium falciparum variant surface antigens in protective immunity and vaccine development. Hum. Vaccin. 2010;6:84–89. doi: 10.4161/hv.6.1.9602. [DOI] [PubMed] [Google Scholar]
- 17.Rask TS, Hansen DA, Theander TG, Gorm Pedersen A, Lavstsen T. Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes—divide and conquer. PLoS Comput. Biol. 2010;6:e1000983. doi: 10.1371/journal.pcbi.1000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brolin KJ, Ribacke U, Nilsson S, Ankarklev J, Moll K, Wahlgren M, Chen Q. Simultaneous transcription of duplicated var2csa gene copies in individual Plasmodium falciparum parasites. Genome Biol. 2009;10:R117. doi: 10.1186/gb-2009-10-10-r117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sander AF, Salanti A, Lavstsen T, Nielsen MA, Magistrado P, Lusingu J, Ndam NT, Arnot DE. Multiple var2csa-type PfEMP1 genes located at different chromosomal loci occur in many Plasmodium falciparum isolates. PLoS One. 2009;4:e6667. doi: 10.1371/journal.pone.0006667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bockhorst J, Lu F, Janes JH, Keebler J, Gamain B, Awadalla P, Su XZ, Samudrala R, Jojic N, Smith JD. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol. Biochem. Parasitol. 2007;155:103–112. doi: 10.1016/j.molbiopara.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Dahlbäck M, Rask TS, Andersen PH, Nielsen MA, Ndam NT, Resende M, Turner L, Deloron P, Hviid L, Lund O, et al. Epitope mapping and topographic analysis of VAR2CSA DBL3X involved in P. falciparum placental sequestration. PLoS Pathog. 2006;2:e124. doi: 10.1371/journal.ppat.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen P, Nielsen MA, Resende M, Rask TS, Dahlbäck M, Theander T, Lund O, Salanti A. Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog. 2008;4:e42. doi: 10.1371/journal.ppat.0040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez P, Viebig NK, Dechavanne S, Lépolard C, Gysin J, Scherf A, Gamain B. Var2CSA DBL6-epsilon domain expressed in HEK293 induces limited cross-reactive and blocking antibodies to CSA binding parasites. Malar. J. 2008;7:170. doi: 10.1186/1475-2875-7-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen MA, Pinto VV, Resende M, Dahlbäck M, Ditlev SB, Theander TG, Salanti A, Salanti A. Induction of adhesion-inhibitory antibodies against placental Plasmodium falciparum parasites by using single domains of VAR2CSA. Infect. Immun. 2009;77:2482–2487. doi: 10.1128/IAI.00159-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khunrae P, Dahlbäck M, Nielsen MA, Andersen G, Ditlev SB, Resende M, Pinto VV, Theander TG, Higgins MK, Salanti A. Full-length recombinant Plasmodium falciparum VAR2CSA binds specifically to CSPG and induces potent parasite adhesion-blocking antibodies. J. Mol. Biol. 2010;397:826–834. doi: 10.1016/j.jmb.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barfod L, Bernasconi NL, Dahlbäck M, Jarrossay D, Andersen PH, Salanti A, Ofori MF, Turner L, Resende M, Nielsen MA, et al. Human pregnancy-associated malaria-specific B cells target polymorphic, conformational epitopes in VAR2CSA. Mol. Microbiol. 2007;63:335–347. doi: 10.1111/j.1365-2958.2006.05503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cranmer SL, Magowan C, Liang J, Coppel RL, Cooke BM. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 1997;91:363–365. doi: 10.1016/s0035-9203(97)90110-3. [DOI] [PubMed] [Google Scholar]
- 28.Haase RN, Megnekou R, Lundquist M, Ofori MF, Hviid L, Staalsoe T. Plasmodium falciparum parasites expressing pregnancy-specific variant surface antigens adhere strongly to the choriocarcinoma cell line BeWo. Infect. Immun. 2006;74:3035–3038. doi: 10.1128/IAI.74.5.3035-3038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drakeley CJ, Carneiro I, Reyburn H, Malima R, Lusingu JP, Cox J, Theander TG, Nkya WM, Lemnge MM, Riley EM. Altitude-dependent and -independent variations in Plasmodium falciparum prevalence in northeastern Tanzania. J. Infect. Dis. 2005;191:1589–1598. doi: 10.1086/429669. [DOI] [PubMed] [Google Scholar]
- 30.Yadouleton AW, Padonou G, Asidi A, Moiroux N, Bio-Banganna S, Corbel V, N’guessan R, Gbenou D, Yacoubou I, Gazard K, Akogbeto MC. Insecticide resistance status in Anopheles gambiae in southern Benin. Malar. J. 2010;9:83. doi: 10.1186/1475-2875-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staalsoe T, Giha HA, Dodoo D, Theander TG, Hviid L. Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry. 1999;35:329–336. doi: 10.1002/(sici)1097-0320(19990401)35:4<329::aid-cyto5>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 32.Tuikue Ndam NG, Fievet N, Bertin G, Cottrell G, Gaye A, Deloron P. Variable adhesion abilities and overlapping antigenic properties in placental Plasmodium falciparum isolates. J. Infect. Dis. 2004;190:2001–2009. doi: 10.1086/425521. [DOI] [PubMed] [Google Scholar]
- 33.Paul F, Roath S, Melville D, Warhurst DC, Osisanya JO. Separation of malaria-infected erythrocytes from whole blood: use of a selective high-gradient magnetic separation technique. Lancet. 1981;318:70–71. doi: 10.1016/s0140-6736(81)90414-1. [DOI] [PubMed] [Google Scholar]
- 34.Alkhalil A, Achur RN, Valiyaveettil M, Ockenhouse CF, Gowda DC. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J. Biol. Chem. 2000;275:40357–40364. doi: 10.1074/jbc.M006399200. [DOI] [PubMed] [Google Scholar]
- 35.Fried M, Duffy PE. Analysis of CSA-binding parasites and anti-adhesion antibodies. Methods Mol. Med. 2002;72:555–560. doi: 10.1385/1-59259-271-6:555. [DOI] [PubMed] [Google Scholar]
- 36.Tippett E, Fernandes LA, Rogerson SJ, Jaworowski A. A novel flow cytometric phagocytosis assay of malaria-infected erythrocytes. J. Immunol. Methods. 2007;325:42–50. doi: 10.1016/j.jim.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with Confidence. 2nd. London, U.K.: British Medical Journal; 2000. [Google Scholar]
- 38.Soerli J, Barfod L, Lavstsen T, Bernasconi NL, Lanzavecchia A, Hviid L. Human monoclonal IgG selection of Plasmodium falciparum for the expression of placental malaria-specific variant surface antigens. Parasite Immunol. 2009;31:341–346. doi: 10.1111/j.1365-3024.2009.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joergensen LM, Salanti A, Dobrilovic T, Barfod L, Hassenkam T, Theander TG, Hviid L, Arnot DE. The kinetics of antibody binding to Plasmodium falciparum VAR2CSA PfEMP1 antigen and modelling of PfEMP1 antigen packing on the membrane knobs. Malar. J. 2010;9:100. doi: 10.1186/1475-2875-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resende M, Nielsen MA, Dahlbäck M, Ditlev SB, Andersen P, Sander AF, Ndam NT, Theander TG, Salanti A. Identification of glycosaminoglycan binding regions in the Plasmodium falciparum encoded placental sequestration ligand, VAR2CSA. Malar. J. 2008;7:104. doi: 10.1186/1475-2875-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resende M, Ditlev SB, Nielsen MA, Bodevin S, Bruun S, Pinto VV, Clausen H, Turner L, Theander TG, Salanti A, Dahlbäck M. Chondroitin sulphate A (CSA)-binding of single recombinant Duffy-binding-like domains is not restricted to Plasmodium falciparum Erythrocyte Membrane Protein 1 expressed by CSA-binding parasites. Int. J. Parasitol. 2009;39:1195–1204. doi: 10.1016/j.ijpara.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Mount AM, Mwapasa V, Elliott SR, Beeson JG, Tadesse E, Lema VM, Molyneux ME, Meshnick SR, Rogerson SJ. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet. 2004;363:1860–1867. doi: 10.1016/S0140-6736(04)16354-X. [DOI] [PubMed] [Google Scholar]
- 43.Keen J, Serghides L, Ayi K, Patel SN, Ayisi J, van Eijk A, Steketee R, Udhayakumar V, Kain KC. HIV impairs opsonic phagocytic clearance of pregnancy-associated malaria parasites. PLoS Med. 2007;4:e181. doi: 10.1371/journal.pmed.0040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avril M, Kulasekara BR, Gose SO, Rowe C, Dahlbäck M, Duffy PE, Fried M, Salanti A, Misher L, Narum DL, Smith JD. Evidence for globally shared, cross-reacting polymorphic epitopes in the pregnancy-associated malaria vaccine candidate VAR2CSA. Infect. Immun. 2008;76:1791–1800. doi: 10.1128/IAI.01470-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groux H, Gysin J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res. Immunol. 1990;141:529–542. doi: 10.1016/0923-2494(90)90021-p. [DOI] [PubMed] [Google Scholar]
- 46.Aguiar JC, Albrecht GR, Cegielski P, Greenwood BM, Jensen JB, Lallinger G, Martinez A, McGregor IA, Minjas JN, Neequaye J, et al. Agglutination of Plasmodium falciparum-infected erythrocytes from east and west African isolates by human sera from distant geographic regions. Am. J. Trop. Med. Hyg. 1992;47:621–632. doi: 10.4269/ajtmh.1992.47.621. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen MA, Vestergaard LS, Lusingu J, Kurtzhals JA, Giha HA, Grevstad B, Goka BQ, Lemnge MM, Jensen JB, Akanmori BD, et al. Geographical and temporal conservation of antibody recognition of Plasmodium falciparum variant surface antigens. Infect. Immun. 2004;72:3531–3535. doi: 10.1128/IAI.72.6.3531-3535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen-Dinh P, Steketee RW, Greenberg AE, Wirima JJ, Mulenda O, Williams SB. Rapid spontaneous postpartum clearance of Plasmodium falciparum parasitaemia in African women. Lancet. 1988;332:751–752. doi: 10.1016/s0140-6736(88)90229-2. [DOI] [PubMed] [Google Scholar]
- 49.Ofori MF, Staalsoe T, Bam V, Lundquist M, David KP, Browne ENL, Akanmori BD, Hviid L. Expression of variant surface antigens by Plasmodium falciparum parasites in the peripheral blood of clinically immune pregnant women indicates ongoing placental infection. Infect. Immun. 2003;71:1584–1586. doi: 10.1128/IAI.71.3.1584-1586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beeson JG, Ndungu F, Persson KE, Chesson JM, Kelly GL, Uyoga S, Hallamore SL, Williams TN, Reeder JC, Brown GV, Marsh K. Antibodies among men and children to placental-binding Plasmodium falciparum-infected erythrocytes that express var2csa. Am. J. Trop. Med. Hyg. 2007;77:22–28. [PubMed] [Google Scholar]
- 51.Gamain B, Trimnell AR, Scheidig C, Scherf A, Miller LH, Smith JD. Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2CSA gene transcribed in CSA-binding parasites. J. Infect. Dis. 2005;191:1010–1013. doi: 10.1086/428137. [DOI] [PubMed] [Google Scholar]
- 52.Avril M, Gamain B, Lépolard C, Viaud N, Scherf A, Gysin J. Characterization of anti-var2CSA-PfEMP1 cytoadhesion inhibitory mouse monoclonal antibodies. Microbes Infect. 2006;8:2863–2871. doi: 10.1016/j.micinf.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Dahlbäck M, Nielsen MA, Salanti A. Can any lessons be learned from the ambiguous glycan binding of PfEMP1 domains? Trends Parasitol. 2010;26:230–235. doi: 10.1016/j.pt.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava A, Gangnard S, Round A, Dechavanne S, Juillerat A, Raynal B, Faure G, Baron B, Ramboarina S, Singh SK, et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc. Natl. Acad. Sci. USA. 2010;107:4884–4889. doi: 10.1073/pnas.1000951107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Megnekou R, Staalsoe T, Taylor DW, Leke R, Hviid L. Effects of pregnancy and intensity of Plasmodium falciparum transmission on immunoglobulin G subclass responses to variant surface antigens. Infect. Immun. 2005;73:4112–4118. doi: 10.1128/IAI.73.7.4112-4118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ter Kuile FO, Parise ME, Verhoeff FH, Udhayakumar V, Newman RD, van Eijk AM, Rogerson SJ, Steketee RW. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am. J. Trop. Med. Hyg. 2004;71(2 Suppl):41–54. [PubMed] [Google Scholar]