Abstract
The unique hormonal, genetic and epigenetic environments of males and females during development and adulthood shape the neural circuitry of the brain. These differences in neural circuitry result in sex-typical displays of social behaviours such as mating and aggression. Like other neural circuits, those underlying sex-typical social behaviours weave through complex brain regions that control a variety of diverse behaviours. For this reason, the functional dissection of neural circuits underlying sex-typical social behaviours has proved to be difficult. However, molecularly discrete neuronal subpopulations can be identified in the heterogeneous brain regions that control sex-typical social behaviours. In addition, the actions of oestrogens and androgens produce sex differences in gene expression within these brain regions, thereby highlighting the neuronal subpopulations most likely to control sexually dimorphic social behaviours. These conditions permit the implementation of innovative genetic approaches that, in mammals, are most highly advanced in the laboratory mouse. Such approaches have greatly advanced our understanding of the functional significance of sexually dimorphic neural circuits in the brain. In this review, we discuss the neural circuitry of sex-typical social behaviours in mice while highlighting the genetic technical innovations that have advanced the field.
Keywords: sex differences, sexual dimorphism, mating, aggression, sex hormones
1. Introduction
Sexually reproducing animals exhibit sex-typical displays of social behaviours, such as mating and aggression. Such sexual dimorphisms in behaviour can be qualitative or quantitative in nature, and they arise from sexually differentiated neural circuits, which in turn are shaped by the varying hormonal, genetic and epigenetic environments of males and females during development and adulthood [1–6]. Hormones such as oestrogens and androgens exert their effects by binding to their respective membrane-bound and nuclear receptors [7–8]. Nuclear oestrogen and androgen receptors (ARs) are transcription factors that can bind to hormone-responsive DNA elements to regulate expression of target genes [6,9], and, in neurons, presumably lead to changes in neuronal connectivity and excitability and ultimately, behaviour.
Many sex differences in gene expression have been identified [10–18], and hormone-driven sex differences in gene expression highlight neuronal subpopulations within the brain areas implicated in sex-typical social behaviours that are most likely to control these behaviours. This information is useful because the neural circuits underlying these behaviours, like many neural circuits, are embedded in brain regions comprising a tangled mixture of neurons that control diverse behaviours. For example, many studies implicate the bed nucleus of the stria terminalis (BNST) in social behaviours [19–26]. However, the BNST is also implicated in other behaviours such as stress responses, reward responses and salt intake [27–35]. With traditional approaches, it is not possible to prospectively identify or manipulate the relevant subpopulations within the BNST that control sex-typical social behaviours. Hormone-dependent gene expression patterns define specific pools of neurons in the BNST as well as other heterogeneous brain regions that control sex-typical social behaviours [10,36–40]. The use of genetic strategies to selectively study and manipulate such molecularly defined neuronal subpopulations within the BNST and elsewhere will be essential in the attempt to untangle and understand the neural circuits underlying sexually dimorphic behaviour.
This review provides an overview of our current understanding of the neural circuits underlying sexually dimorphic social behaviours while highlighting genetic approaches that continue to advance the field. Important contributions to our understanding of the neural circuitry of sex-typical social behaviours have also been made by work on other organisms, including humans (e.g. [41–53]). However, in mammals, state-of-the-art genetic tools are currently most powerfully implemented using the laboratory mouse, and therefore, this review will focus on the neural pathways underlying social interactions in rodents. We discuss the role of sensory input in the control of social behaviours, the brain regions implicated in social behaviours, the behavioural significance of molecularly defined neuronal pools within these brain areas and future directions for the study of sexually dimorphic social behaviours.
2. Sensory input driving sexually dimorphic social behaviours
Sex-typical social behaviours occur in response to sensory cues from the environment [54–62]. The predominant sensory cues that trigger these behaviours, in mice and many other animals, are pheromones detected by chemosensory neurons in two epithelia in the nose: the main olfactory epithelium (MOE) and the vomeronasal organ (VNO) [63]. Neurons in the MOE and VNO express G-protein-coupled chemosensory receptors from unrelated gene families [64] and detect volatile and contact-based pheromones, respectively [65–66]. The importance of pheromone signalling via the VNO for the control of sex-typical social behaviours has long been established by traditional lesioning studies [67]. However, implementation of genetic approaches has revealed previously unappreciated complexity of the chemosensory control of these behaviours [54–62].
The generation of genetically modified mice bearing a loss of function for specific genes has been a fundamental tool in the dissection of the neural circuits underlying sex-typical social behaviours. These techniques will continue to be a valuable resource for research as recent advances in the application of CRISPR-Cas systems for gene editing have greatly increased the speed and efficiency of generating genetic knockouts [68–70]. The use of targeted gene knockouts has made it possible to study the contributions of contact-based VNO or volatile MOE sensory input for the display of sex-typical social behaviours by genetically disabling one signalling pathway while keeping the other functionally intact. These studies conducted in many laboratories, including ours, reveal that mating and aggression behaviours require the coordinated function of both epithelia [54–61].
Targeted deletion of Trpc2, a cation channel expressed in the VNO but not MOE, results in mice with a functionally disabled VNO ([58,60], but see [71,72]). Male Trpc2 knockout mice exhibit male-typical mounting and copulatory behaviours towards not only females but also other males, and male-typical aggressive attacks are abrogated in these mice [58,60]. These findings suggest that contact-based male pheromones detected by the VNO normally inhibit mating and trigger aggression towards other males. Interestingly, female Trpc2 knockout mice also display high levels of male-typical mounting behaviour towards mice of either sex [57], suggesting that the neural circuit for male-typical mating behaviour is present in both sexes but is normally inhibited by sensory input from the VNO. The presence of a shared male-type mating circuitry in both sexes is consistent with prior classical endocrinological studies showing that adult females treated with testosterone will display male-typical mating behaviour [73]. Therefore, circulating testosterone can override the contact pheromone-based VNO-mediated sensory inhibition of male-typical mating behaviours. The importance of the detection of volatile pheromones by the MOE has been uncovered using mice with a targeted deletion of Cnga2, a cation channel expressed in the MOE but not VNO, leading to disabled MOE signalling. Male Cnga2 knockout mice exhibit a profound reduction in male-typical mounting and copulatory behaviours as well as male-typical aggressive attacks [59,61,74]. Thus, both male-typical mating and aggression behaviours require sensory input from the MOE. Studies using these mutants also demonstrate that the VNO and MOE are required for female-typical displays of sexually dimorphic social behaviours, such as sexual receptivity and maternal aggression [54].
Taken together, these genetic studies suggest a model in which sex differences in neural circuits underlying sexually dimorphic social interactions regulate the display of these behaviours such that contact-based VNO sensory input decreases and testosterone increases the probability of displaying male-typical mating, whereas both contact-based VNO and volatile MOE sensory input increase the probability of displaying male-typical aggression. Therefore, it appears that male-typical mating behaviours are normally inhibited in adult females by a functional VNO and the absence of high, male-typical levels of testosterone. Female mice rarely attack males unless nursing, and there appears to be no qualitative sex difference in the expression of the chemoreceptors [65]. Therefore, the lack of male-typical aggression in females is most likely regulated by a sexual dimorphism downstream of chemosensory input in the underlying neural circuit.
3. Neural circuits underlying sexually dimorphic social behaviours
(a). Overview of brain regions implicated in sexually dimorphic social behaviours
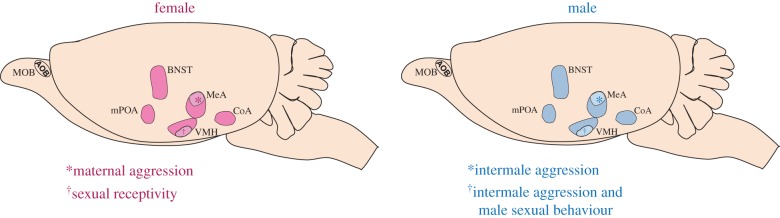
The sensory inputs of the VNO and MOE maintain their anatomic segregation with central projections to the accessory olfactory bulb (AOB) and the main olfactory bulb (MOB), respectively [63,66]. AOB projection neurons send axons to the medial amygdala (MeA) as well as the posteromedial cortical amygdala [75–77]. By contrast, MOB projection neurons send axons to several cortical regions, including the posterolateral cortical amygdala [76,78–80]. As discussed above, both the VNO and MOE regulate sex-typical social behaviours, indicating an interaction between or convergence of these two chemosensory circuits. Circuit mapping reveals a convergence of the connections of the projection targets of the AOB and MOB in the BNST and several hypothalamic nuclei [81–85]. In addition, some, but not all, studies report MOB projections directly to the MeA, thereby providing another site of convergence of the VNO and MOE pathways [78,80,83,86]. Neurons in the MeA, in turn, project to targets including the ventromedial hypothalamus (VMH), the BNST and the medial preoptic area (mPOA). These regions are interconnected, and decades of lesion or stimulation studies of these regions have revealed their importance in the control of mating and aggression behaviours in both sexes [26–27,87–98]. How molecularly defined neurons within these brain regions interact to produce sex-typical behaviours, and whether these interconnected pathways comprise a single neural circuit or a set of separate neural circuits that control sex-typical mating and aggression behaviours, is currently unclear and under investigation (figure 1).
Figure 1.
Brain regions implicated in sexually dimorphic social behaviours. A shared set of brain regions has been implicated in the control of sexually dimorphic social behaviours in females and males, raising the issue of how neurons in these areas control distinct behaviours in the two sexes. A related issue is that the molecular identity of neurons in these functionally and molecularly heterogeneous brain regions is largely unknown. Recent molecular genetic studies have now identified discrete subsets of neurons, representing 10–20% of neurons in these regions, that control one or a few sexually dimorphic social behaviours but not other behaviours regulated by that entire brain region. Thus, the control of complex social behaviours appears to be modularly organized at the level of molecularly defined neurons. Another general principle emerging from these studies is that these neurons are functionally bivalent in the sense that they control distinct behaviours in both sexes. Such sexually dimorphic function is likely to be regulated by sex differences in gene expression or connectivity. Indeed, both the MeA and VMH express genes in a sexually dimorphic manner (e.g. [10,36,37]), and a recent study showed that VMH neurons regulating social behaviours project in a sex-typical manner [37]. AOB, accessory olfactory bulb; CoA, cortical amygdala encompassing both the posterolateral and posteromedial components that receive inputs from the MOB and AOB, respectively; MOB, main olfactory bulb. *, aromatase-expressing neurons in the posterodorsal MeA (MeApd) control maternal aggression and intermale aggression; †, progesterone receptor (PR)-expressing neurons in the ventrolateral subdivision of VMH (VMHvl) control mating behaviour in both sexes and aggression in males. (Online version in colour.)
(b). Sex differences in gene expression
The MeA, VMH, BNST and mPOA exhibit sex differences such that the number of neurons or innervation within these regions are different in males and females [10,36–37,40,99–101], and with few exceptions, subsets of adult neurons within these regions express one or more sex hormone receptors [10,36–37,40,102–104]. As discussed above, these receptors can regulate gene expression, and many hormone-dependent sex differences in gene expression have been identified, including in the sex hormone receptors themselves and the enzyme aromatase that converts testosterone into 17β-oestradiol [10–18,36–37,40]. For example, we have used genetic strategies to visualize the expression pattern of aromatase in the brain and discovered that it is expressed in a few discrete areas, including the posterodorsal MeA (MeApd) and the posteromedial part of medial division of the BNST (BNSTmpm). Aromatase-expressing neurons in the MeApd and BNSTmpm exhibit sex differences in cell number, and this sexual dimorphism is masculinized by neonatal oestrogen exposure [36]. Notably, this masculinization of the number of aromatase-expressing neurons is accompanied by a masculinization of aggression behaviour in females even in the absence of adult supplementation of testosterone.
Numerous, novel sex differences in gene expression patterns within the MeA, mPOA, VMH and BNST have been identified [10]. Using genome-wide expression profiling in conjunction with in situ hybridization, we discovered that the regulation of sexually dimorphic gene expression patterns is complex as many individual genes are upregulated in different brain regions in the two sexes [10]. Consistent with this complexity, we showed that in males many, but not all, of the sex differences in gene expression are dependent upon adult testosterone. However, in females, the sex differences in gene expression are mostly independent of adult ovarian hormones, suggesting a developmental pre-patterning of the dimorphic transcriptional programme in females. Moreover, we demonstrated that these sexually dimorphic genes play important, modular roles in sexually dimorphic social behaviours. Mice singly homozygous null for these genes exhibit specific deficits in one or more components of male or female mating behaviour (Brs3, Sytl4 and Cckar), male-typical aggression (Brs3) or maternal care (Irs4), while other sex-typical behaviours remain unaffected. Each of these dimorphic genes is expressed in specific neurons within the MeA, VMH or other areas, implicating one or more neuronal pools in distinct components of sex-typical social behaviours. These findings indicate that sex-typical social behaviours are modular in that specific components of behaviour (such as male-typical aggressive attacks and male-typical marking behaviour) are controlled by distinct sets of genes. For example, Cckar, a G-protein-coupled receptor for the neuropeptide cholecystokinin, is expressed in a subset of neurons within the VMH, and Cckar is required for female sexual receptivity but not for maternal behaviours and the oestrous cycle [10]. Thus, unlike castrates or animals mutant for sex hormone receptors that exhibit global deficits in sex-typical behaviours, mice mutant for genes that are downstream of sex hormone signalling exhibit phenotypes restricted to specific components of sexually dimorphic behaviours. These findings suggest a model in which the components of individual dimorphic behaviours are controlled in a modular manner by genetically separable pathways comprising sexually dimorphic molecularly defined neuronal subpopulations.
(c). Genetic dissection of molecularly defined neuronal populations underlying sexually dimorphic behaviours
The utilization of conditional genetic approaches such as the Cre-lox system to study molecularly defined sexually dimorphic neuronal subpopulations has greatly advanced our understanding of the neural circuits underlying sex-typical social behaviours. The MeA, VMH, BNST and mPOA each contains a complex mixture of molecularly discrete populations of neurons intermingled with each other [10,36–37,40,105–106]. Therefore, the neural pathways underlying sex-typical social behaviours are embedded within an assortment of neural circuits involved in many unrelated behaviours. The use of conditional genetic approaches is necessary to study the neurons relevant for social behaviours in isolation from their functionally distinct neighbours [107–109]. The use of mice genetically modified to express Cre in molecularly defined neuronal subpopulations of neurons in conjunction with virally encoded Cre-dependent transgenes has permitted projection-mapping as well as functional characterization of these neurons. The viruses used in such studies are stereotactically delivered to the region of interest where they indiscriminately infect all neurons, but the virally encoded transgene is expressed only in neurons that express Cre.
We recently used this genetic approach to study the role of the MeA in sex-typical social behaviours [38]. The MeA is a large complex that controls many diverse behaviours, including dimorphic patterns of mating and aggression, social memory, stress, anxiety and defensive response to predators [38,110–113]. The functional diversity of the MeA is reflected in its molecular heterogeneity and the diversity of its projections [10,105,114–116]. It is not clear how the MeA integrates sensory and physiological cues to regulate such an array of diverse behaviours. We have discovered a sexually dimorphic subpopulation of neurons in the MeApd that expresses the enzyme aromatase such that there are more aromatase-expressing neurons in males compared with females [36]. Aromatase itself is required for the normal display of male-typical mating and aggression behaviours [117–120]. To study the functional significance of the aromatase-expressing neuronal subpopulation within the MeApd, we specifically ablated these neurons in adult mice [38], using a virally encoded Cre-dependent designer executioner caspase-3 [37,121]. These studies were done in genetically modified mice that we generated in order to express Cre in aromatase-expressing cells (arocre mice). Targeted delivery of the virally encoded caspase-3 to the MeA specifically induced apoptosis of aromatase-expressing MeApd neurons in arocre but not control wild-type mice. Importantly, this caspase-3-induced apoptosis is restricted to Cre-expressing neurons and spares neighbouring neurons that do not express aromatase (Cre) [37–38,121]. Ablation of these neurons in males significantly reduced specific components of male-typical aggression while male-typical mating behaviours were unaffected, whereas ablation of these neurons in females significantly reduced specific components of maternal aggression while maternal care and female-typical mating behaviours were unaffected. Furthermore, using this approach to exclusively express DREADD/Gi, an engineered inhibitory G-protein-coupled receptor activated by a heterologous ligand [122], in aromatase-expressing MeApd neurons, we found that pharmacogenetic inhibition of aromatase-expressing MeApd neurons recapitulated these behavioural deficits in both sexes. The sensory, hormonal and contextual requirements for male-typical and maternal aggression are very different [123–125]. Therefore, aromatase-expressing MeApd neurons appear to be functionally bivalent in that they control distinct forms of aggression in each sex. In addition, our findings demonstrate that it is possible to dissociate specific components of a complex social display such as aggression without altering other features of social interactions. Indeed, our manipulations show that it is possible to dissociate maternal care of pups from maternal defence of pups from intruders. The entire MeA is critical for aggression as well as mating behaviours in both sexes [105–106,126–129], and a recent study described the important contributions of GABAergic MeApd neurons to both male-typical mating and aggression behaviours [113]. Importantly, aromatase-expressing neurons are a subset of the population of GABAergic neurons within the MeApd [38]. Therefore, by studying a small molecularly defined subpopulation of neurons expressing a gene (aromatase) functionally important for social behaviours, we have identified a subset of neurons within the MeApd that specifically regulate intermale and maternal aggression.
For almost a century, experiments have implicated the VMH or adjacent hypothalamic regions in modulating aggression behaviour in diverse species [93,130–131]. Only recently has the ventrolateral VMH (VMHvl) been implicated as a key aggression-eliciting centre in the male mouse [94]. In vivo recordings in freely moving male mice reveal an increase in VMHvl activity during male-typical aggression, and optogenetic stimulation of VMHvl neurons elicits indiscriminate aggressive attacks towards both males and females. The VMH is molecularly heterogeneous and regulates both female sexual receptivity and male-typical aggression behaviours as well as defensive reactions to predators and energy balance in diverse animals, including humans [89,93–97,130,132–146]. Lesions and other manipulations that do not target molecularly defined neurons within the VMH can yield conflicting behavioural phenotypes [96–97,135,137], perhaps owing to non-targeted manipulation of these heterogeneous neurons or destruction of fibres of passage within this region. Indeed, response profiles of the neural activity of VMHvl neurons are complex and heterogeneous, with some subpopulations responding maximally during male-typical aggression behaviour and others responding maximally during other behaviours [147]. To identify the VMH neurons that specifically regulate sex-typical social behaviours, we used the genetic strategy discussed above to ablate progesterone receptor (PR)-expressing neurons in the VMHvl of adult mice that we genetically modified to express Cre in PR-expressing cells [37]. PR-expressing VMHvl neurons are a subset of neurons within the VMHvl, which itself represents a subset of neurons within the VMH. PR expression is sexually dimorphic such that there are more PR-expressing VMHvl neurons in females than males. We discovered that ablation of these PR-expressing neurons in the adult female VMHvl causes a profound reduction in sexual receptivity, and ablation of the corresponding neurons in males causes a significant reduction in both male-typical mating and aggression behaviour. Thus, these PR-expressing VMHvl neurons, like aromatase-expressing MeApd neurons, are functionally bivalent in that they regulate distinct dimorphic behaviours in the two sexes (i.e. sexual receptivity in females and male-typical mating and aggression in males).
These studies in the MeA and VMH therefore suggest a principle whereby a common pool of molecularly defined neurons is present in both sexes, but sex differences in gene expression or the neural circuit within which these neurons are embedded enable these pathways to transform sensory input into sexually dimorphic behaviours [6]. In both instances, characterization of neurons expressing a functionally important gene (aromatase, PR) reveals that these neurons regulate specific aspects of sexually dimorphic behaviour, whereas the genes themselves are expressed in multiple brain regions and regulate multiple aspects of these behaviours. Moreover, PR-expressing neurons in the VMHvl also express Cckar in a sexually dimorphic manner [10] such that there is little Cckar expression in males in this region, and its expression peaks in females during oestrus, a period of maximal sexual receptivity. As discussed earlier, females null for Cckar have a significant and specific reduction in sexual receptivity. Strikingly, ablation of PR- and Cckar-expressing VMHvl neurons leads to a correspondingly profound and specific diminution in sexual receptivity [37]. We therefore anticipate that other genes whose expression is regulated by sex hormone signalling (e.g. [10]) will similarly highlight neuronal subsets that also regulate sexually dimorphic behaviour in a modular manner.
How PR-expressing VMHvl neurons influence both male-typical mating and aggression behaviour is unclear. In one scenario, the same neurons mediate both male-typical mating and aggression behaviour via distinct patterns of neural activity. A recent study provides support for this scenario [148]. The vast majority of PR-expressing neurons in the VMHvl also express oestrogen receptor α (ERα or Esr1) [37]. Optogenetic stimulation of ERα-expressing VMHvl neurons elicits male-typical aggressive attacks in male mice [148]. Surprisingly, weaker optogenetic stimulation of these neurons elicits male-typical mating behaviour, rather than aggression, towards both females and males. These findings suggest that changes in neural activity in the VMHvl resulting in an increase in the number of active neurons or the average level of activity per neuron promotes a transition from male-typical mating to aggression behaviour. Alternatively, the subpopulation of ERα-expressing neurons in the VMHvl could consist of at least two molecularly distinct neuronal subsets, with one regulating male-typical mating and the other aggression. It is possible that different intensities of optogenetic stimulation preferentially recruit one or the other population to drive mating or aggression. Future studies manipulating the activity of the specific projections of molecularly defined neuronal subsets to identified target regions will greatly inform our understanding of the functional connectivity of the neural circuitry underlying sex-typical social behaviours.
4. Future directions of the study of sexually dimorphic social behaviours
A more mechanistic understanding of the neural circuits underlying sex-typical social behaviours will require a comprehensive inter-disciplinary effort using innovative genetic strategies to map the anterograde and retrograde connectivity of molecularly defined subpopulations of neurons, followed by the optogenetic manipulation of specific projection targets and recording of the activity patterns in vivo of those same molecularly defined subpopulations during sex-typical social behaviours. Such anatomic circuit mapping endeavours will rely on recent advances in optogenetics as well as virally encoded trans-synaptic tracers [149–153] to facilitate the characterization of the neural connectivity of genetically defined pools of neurons. In addition to anatomic and optogenetic circuit mapping, it will be critical to use recording or imaging approaches to define activity patterns in molecularly defined neuronal populations. Recent advances in genetically encoded calcium sensors [154–155] in conjunction with advances in in vivo imaging via fibre optic cables or microscopy [156–159] will enable the recording of neural activity of genetically defined subsets of neurons during the display of sex-typical social behaviours. Such studies will provide insights into the information processed by molecularly defined neuronal pools at different levels in the neural circuits underlying sex-typical social behaviours.
In addition to an anatomic and functional delineation of neural circuits underlying sex-typical social behaviours, it will be important to understand the role of sexually dimorphic gene expression patterns in these behaviours. The use of conditional gene knockouts will address issues relating to the role of the gene in the development and function of the neural circuit, and it will also serve to localize gene action to specific neuronal populations that regulate the behaviour. For example, we and others have studied the functional role of the AR in the brain in male-typical social behaviours [39,160]. Constitutive, systemic deletion of AR leads to feminized external physical characteristics and demasculinized behaviours (tfm mice). However, these tfm males undergo testicular atrophy shortly after the critical period, leaving open the question of whether testosterone signalling via AR is required perinatally to masculinize the brain in an organizational manner. Alternatively, early masculinization of the brain may be solely regulated by oestrogens that are synthesized locally via aromatization of circulating testosterone. Targeted deletion of AR in the nervous system has shed light on this important question. Male mice in which AR is deleted in neural precursors but unaltered in other cells before the organizational critical period are externally masculinized and have physiological levels of circulating testosterone. Moreover, these mutant males exhibit qualitatively male-typical displays of social behaviours with a significant, quantitative reduction in various aspects of reproductive and territorial behaviours [39]. Taken together with classic endocrinological and other genetic studies, the results from a nervous system-restricted deletion of AR show that a functional AR is not required to masculinize the brain during the organizational period, but it is required for the normal intensity of male-typical social behaviours in adult life. In future studies, it will be important to use intersectional genetic strategies, with mice expressing Cre in specific neurons or targeted delivery of virally encoded Cre, to further localize the requirement of AR in specific neuronal subsets for the normal intensity of male-typical behavioural displays. The spatial and temporal disruption of other sexually dimorphically expressed genes will similarly provide important insights into the molecular control of neural circuits underlying these social behaviours.
5. Conclusion
Outstanding progress has been made in our understanding of how the brain generates sexually dimorphic behaviours, and the application of the innovative genetic strategies discussed in this review will provide important new mechanistic insights into the molecular and neural control of sex-typical behaviours. The modular or specialized function of discrete molecularly defined neuronal populations uncovered by recent studies is a common theme emerging from the genetic studies of sex-typical social interactions in mice [6]. It will be important to test whether other complex behaviours are regulated in a similarly modular manner at the level of genes and neurons.
Healthy social interactions are an essential component of well-being and personal and professional success, and deficits in social interactions contribute to the emotional and financial burden of many neurological and psychiatric disorders such as Alzheimer's disease and autism spectrum disorders. Furthermore, many neuro-psychiatric disorders exhibit sex differences in incidence, prevalence or disease outcome [161–165]. The reasons underlying these sex differences are poorly understood. We anticipate that insights from studies in mice using state-of-the-art genetic tools will reveal how sex differences in the brain translate into sexual dimorphisms in social behaviour in health and also render the brain differentially susceptible to disease between men and women.
Acknowledgements
The authors acknowledge comments from members of the Shah laboratory.
Authors' contributions
Both authors contributed equally to the drafting of the manuscript, and both authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by grants from the Ellison Medical Foundation (N.M.S.) and NIH (T32HD007263 to D.W.B. and R01NS049488, R01NS083872, R01MH108319 to N.M.S.)
References
- 1.Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382. ( 10.1016/j.yhbeh.2009.03.015) [DOI] [PubMed] [Google Scholar]
- 2.McCarthy MM. 1994. Molecular aspects of sexual differentiation of the rodent brain. Psychoneuroendocrinology 19, 415–427. ( 10.1016/0306-4530(94)90029-9) [DOI] [PubMed] [Google Scholar]
- 3.Arnold AP. 2009. The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 55, 570–578. ( 10.1016/j.yhbeh.2009.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy MM. 2009. The epigenetics of sex differences in the brain. J. Neurosci. 29, 12 815–12 823. ( 10.1523/JNEUROSCI.3331-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy MM, Arnold AP. 2011. Reframing sexual differentiation of the brain. Nat. Neurosci. 14, 677–683. ( 10.1038/nn.2834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang CF, Shah NM. 2014. Representing sex in the brain, one module at a time. Neuron 82, 261–278. ( 10.1016/j.neuron.2014.03.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangelsdorf DJ, et al. 1995. The nuclear receptor superfamily: the second decade. Cell 83, 835–839. ( 10.1016/0092-8674(95)90199-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ervin KS, Lymer JM, Matta R, Clipperton-Allen AE, Kavaliers M, Choleris E. 2015. Estrogen involvement in social behavior in rodents: rapid and long-term actions. Horm. Behav. 74, 53–76. ( 10.1016/j.yhbeh.2015.05.023) [DOI] [PubMed] [Google Scholar]
- 9.Beato M, Sánchez-Pacheco A. 1996. Interaction of steroid hormone receptors with the transcription initiation complex. Endocr. Rev. 17, 587–609. ( 10.1210/edrv-17-6-587) [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, Shah NM. 2012. Modular genetic control of sexually dimorphic behaviors. Cell 148, 596–607. ( 10.1016/j.cell.2011.12.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewing P, Shi T, Horvath S, Vilain E. 2003. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res. Mol. Brain Res. 118, 82–90. ( 10.1016/S0169-328X(03)00339-5) [DOI] [PubMed] [Google Scholar]
- 12.Dewing P, et al. 2006. Direct regulation of adult brain function by the male-specific factor SRY. Curr. Biol. 16, 415–420. ( 10.1016/j.cub.2006.01.017) [DOI] [PubMed] [Google Scholar]
- 13.Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S. 2007. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev. Neurobiol. 67, 1371–1381. ( 10.1002/dneu.20507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnidze K, Pfaff DW, Mong JA. 2010. Gene expression in neuroendocrine cells during the critical period for sexual differentiation of the brain. Prog. Brain Res. 186, 97–111. ( 10.1016/B978-0-444-53630-3.00007-5) [DOI] [PubMed] [Google Scholar]
- 15.Rinn JL, Rozowsky JS, Laurenzi IJ, Petersen PH, Zou K, Zhong W, Gerstein M, Snyder M. 2004. Major molecular differences between mammalian sexes are involved in drug metabolism and renal function. Dev. Cell 6, 791–800. ( 10.1016/j.devcel.2004.05.005) [DOI] [PubMed] [Google Scholar]
- 16.Vries GJ. 1990. Sex differences in neurotransmitter systems. J. Neuroendocrinol. 2, 1–13. ( 10.1111/j.1365-2826.1990.tb00385.x) [DOI] [PubMed] [Google Scholar]
- 17.Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. 2005. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Brain Res. Dev. Brain Res. 157, 34–41. ( 10.1016/j.devbrainres.2005.03.001) [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. 2006. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 16, 995–1004. ( 10.1101/gr.5217506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veening JG, Coolen LM. 1998. Neural activation following sexual behavior in the male and female rat brain. Behav. Brain Res. 92, 181–193. ( 10.1016/S0166-4328(97)00190-3) [DOI] [PubMed] [Google Scholar]
- 20.Coolen LM, Peters HJ, Veening JG. 1997. Distribution of Fos immunoreactivity following mating versus anogenital investigation in the male rat brain. Neuroscience 77, 1151–1161. ( 10.1016/S0306-4522(96)00542-8) [DOI] [PubMed] [Google Scholar]
- 21.Gammie SC, Nelson RJ. 2001. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 898, 232–241. ( 10.1016/S0006-8993(01)02189-8) [DOI] [PubMed] [Google Scholar]
- 22.Tobiansky DJ, Hattori T, Scott JM, Nutsch VL, Roma PG, Dominguez JM. 2012. Mating-relevant olfactory stimuli activate the rat brain in an age-dependent manner. Neuroreport 23, 1077–1083. ( 10.1097/WNR.0b013e32835b6ec1) [DOI] [PubMed] [Google Scholar]
- 23.Numan M, Numan MJ. 1997. Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. J. Neuroendocrinol. 9, 369–384. ( 10.1046/j.1365-2826.1997.t01-1-00597.x) [DOI] [PubMed] [Google Scholar]
- 24.Valcourt RJ, Sachs BD. 1979. Penile reflexes and copulatory behavior in male rats following lesions in the bed nucleus of the stria terminalis. Brain Res. Bull. 4, 131–133. ( 10.1016/0361-9230(79)90068-6) [DOI] [PubMed] [Google Scholar]
- 25.Liu YC, Salamone JD, Sachs BD. 1997. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J. Neurosci. 17, 5245–5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emery DE, Sachs BD. 1976. Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiol. Behav. 17, 803–806. ( 10.1016/0031-9384(76)90044-5) [DOI] [PubMed] [Google Scholar]
- 27.Erb S, Stewart J. 1999. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J. Neurosci. 19, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zardetto-Smith AM, Beltz TG, Johnson AK. 1994. Role of the central nucleus of the amygdala and bed nucleus of the stria terminalis in experimentally-induced salt appetite. Brain Res. 645, 123–134. ( 10.1016/0006-8993(94)91645-4) [DOI] [PubMed] [Google Scholar]
- 29.Meloni EG, Jackson A, Gerety LP, Cohen BM, Carlezon WA Jr. 2006. Role of the bed nucleus of the stria terminalis (BST) in the expression of conditioned fear. Ann. NY Acad. Sci. 1071, 538–541. ( 10.1196/annals.1364.059) [DOI] [PubMed] [Google Scholar]
- 30.Henke PG. 1984. The bed nucleus of the stria terminalis and immobilization-stress: unit activity, escape behaviour, and gastric pathology in rats. Behav. Brain Res. 11, 35–45. ( 10.1016/0166-4328(84)90006-8) [DOI] [PubMed] [Google Scholar]
- 31.Schulz D, Canbeyli RS. 2000. Lesion of the bed nucleus of the stria terminalis enhances learned despair. Brain Res. Bull. 52, 83–87. ( 10.1016/S0361-9230(00)00235-5) [DOI] [PubMed] [Google Scholar]
- 32.Sánchez MM, Aguado F, Sánchez-Toscano F, Saphier D. 1995. Effects of prolonged social isolation on responses of neurons in the bed nucleus of the stria terminalis, preoptic area, and hypothalamic paraventricular nucleus to stimulation of the medial amygdala. Psychoneuroendocrinology 20, 525–541. ( 10.1016/0306-4530(94)00083-M) [DOI] [PubMed] [Google Scholar]
- 33.Hammack SE, Richey KJ, Watkins LR, Maier SF. 2004. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav. Neurosci. 118, 443–448. ( 10.1037/0735-7044.118.2.443) [DOI] [PubMed] [Google Scholar]
- 34.McHenry JA, Rubinow DR, Stuber GD. 2015. Maternally responsive neurons in the bed nucleus of the stria terminalis and medial preoptic area: putative circuits for regulating anxiety and reward. Front. Neuroendocrinol. 38, 65–72. ( 10.1016/j.yfrne.2015.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gafford GM, Ressler KJ. 2015. GABA and NMDA receptors in CRF neurons have opposing effects in fear acquisition and anxiety in central amygdala vs. bed nucleus of the stria terminalis. Horm. Behav. 76: 136–142. ( 10.1016/j.yhbeh.2015.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM. 2009. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell 139, 61–71. ( 10.1016/j.cell.2009.07.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM. 2013. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909. ( 10.1016/j.cell.2013.04.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unger EK, Burke KJ Jr, Yang CF, Bender KJ, Fuller PM, Shah NM. 2015. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 10, 453–462. ( 10.1016/j.celrep.2014.12.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda SI, Harada N, Shah NM. 2010. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66, 260–272. ( 10.1016/j.neuron.2010.03.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. 2004. Visualizing sexual dimorphism in the brain. Neuron 43, 313–319. ( 10.1016/j.neuron.2004.07.008) [DOI] [PubMed] [Google Scholar]
- 41.Baum MJ. 2003. Activational and organizational effects of estradiol on male behavioral neuroendocrine function. Scand. J. Psychol. 44, 213–220. ( 10.1111/1467-9450.00338) [DOI] [PubMed] [Google Scholar]
- 42.Crews D, Moore MC. 2005. Historical contributions of research on reptiles to behavioral neuroendocrinology. Horm. Behav. 48, 384–394. ( 10.1016/j.yhbeh.2005.04.003) [DOI] [PubMed] [Google Scholar]
- 43.Dickson BJ. 2008. Wired for sex: the neurobiology of Drosophila mating decisions. Science 322, 904–909. ( 10.1126/science.1159276) [DOI] [PubMed] [Google Scholar]
- 44.Haller J. 2013. The neurobiology of abnormal manifestations of aggression: a review of hypothalamic mechanisms in cats, rodents and humans. Brain Res. Bull. 93, 97–109. ( 10.1016/j.brainresbull.2012.10.003) [DOI] [PubMed] [Google Scholar]
- 45.Manoli DS, Meissner GW, Baker BS. 2006. Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci. 29, 444–451. ( 10.1016/j.tins.2006.06.006) [DOI] [PubMed] [Google Scholar]
- 46.Moore FL, Boyd SK, Kelley DB. 2005. Historical perspective: hormonal regulation of behaviors in amphibians. Horm. Behav. 48, 373–383. ( 10.1016/j.yhbeh.2005.05.011) [DOI] [PubMed] [Google Scholar]
- 47.Newman SW, Parfitt DB, Kollack-Walker S. 1997. Mating-induced c-fos expression patterns complement and supplement observations after lesions in the male Syrian hamster brain. Ann. NY Acad. Sci. 807, 239–259. ( 10.1111/j.1749-6632.1997.tb51924.x) [DOI] [PubMed] [Google Scholar]
- 48.Perkins A, Roselli CE. 2007. The ram as a model for behavioral neuroendocrinology. Horm. Behav. 52, 70–77. ( 10.1016/j.yhbeh.2007.03.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Portman DS. 2007. Genetic control of sex differences in C. elegans neurobiology and behavior. Adv. Genet. 59, 1–37. ( 10.1016/S0065-2660(07)59001-2) [DOI] [PubMed] [Google Scholar]
- 50.Siwicki KK, Kravitz EA. 2009. Fruitless, doublesex and the genetics of social behavior in Drosophila melanogaster. Curr. Opin. Neurobiol. 19, 200–206. ( 10.1016/j.conb.2009.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres CV, Sola RG, Pastor J, Pedrosa M, Navas M, Garcia-Navarrete E, Ezquiaga E, García-Camba E. 2013. Long-term results of posteromedial hypothalamic deep brain stimulation for patients with resistant aggressiveness. J. Neurosurg. 119, 277–287. ( 10.3171/2013.4.jns121639) [DOI] [PubMed] [Google Scholar]
- 52.Wade J, Arnold AP. 2004. Sexual differentiation of the zebra finch song system. Ann. NY Acad. Sci. 1016, 540–559. ( 10.1196/annals.1298.015) [DOI] [PubMed] [Google Scholar]
- 53.Wallen K. 2005. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front. Neuroendocrinol. 26, 7–26. ( 10.1016/j.yfrne.2005.02.001) [DOI] [PubMed] [Google Scholar]
- 54.Fraser EJ, Shah NM. 2014. Complex chemosensory control of female reproductive behaviors. PLoS ONE 9, e90368 ( 10.1371/journal.pone.0090368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasen NS, Gammie SC. 2009. Trpc2 gene impacts on maternal aggression, accessory olfactory bulb anatomy and brain activity. Genes Brain Behav. 8, 639–649. ( 10.1111/j.1601-183X.2009.00511.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hasen NS, Gammie SC. 2011. Trpc2-deficient lactating mice exhibit altered brain and behavioral responses to bedding stimuli. Behav. Brain Res. 217, 347–353. ( 10.1016/j.bbr.2010.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kimchi T, Xu J, Dulac C. 2007. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448, 1009–1014. ( 10.1038/nature06089) [DOI] [PubMed] [Google Scholar]
- 58.Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. 2002. Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl Acad. Sci. USA 99, 6376–6381. ( 10.1073/pnas.082127599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandiyan VS, Coats JK, Shah NM. 2005. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat. Neurosci. 8, 1660–1662. ( 10.1038/nn1589) [DOI] [PubMed] [Google Scholar]
- 60.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. 2002. Loss of sex discrimination and male–male aggression in mice deficient for TRP2. Science 295, 1493–1500. ( 10.1126/science.1069259) [DOI] [PubMed] [Google Scholar]
- 61.Wang Z, Balet Sindreu C, Li V, Nudelman A, Chan GC-K, Storm DR. 2006. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J. Neurosci. 26, 7375–7379. ( 10.1523/JNEUROSCI.1967-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. 2014. Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330. ( 10.1038/nature13307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zufall F, Leinders-Zufall T. 2007. Mammalian pheromone sensing. Curr. Opin. Neurobiol. 17, 483–489. ( 10.1016/j.conb.2007.07.012) [DOI] [PubMed] [Google Scholar]
- 64.Liberles SD. 2014. Mammalian pheromones. Annu. Rev. Physiol. 76, 151–175. ( 10.1146/annurev-physiol-021113-170334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Touhara K, Vosshall LB. 2009. Sensing odorants and pheromones with chemosensory receptors. Annu. Rev. Physiol. 71, 307–332. ( 10.1146/annurev.physiol.010908.163209) [DOI] [PubMed] [Google Scholar]
- 66.Dulac C, Wagner S. 2006. Genetic analysis of brain circuits underlying pheromone signaling. Annu. Rev. Genet. 40, 449–467. ( 10.1146/annurev.genet.39.073003.093937) [DOI] [PubMed] [Google Scholar]
- 67.Wysocki CJ, Lepri JJ. 1991. Consequences of removing the vomeronasal organ. J. Steroid Biochem. Mol. Biol. 39, 661–669. ( 10.1016/0960-0760(91)90265-7) [DOI] [PubMed] [Google Scholar]
- 68.Doudna JA, Charpentier E. 2014. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 ( 10.1126/science.1258096) [DOI] [PubMed] [Google Scholar]
- 69.Hsu PD, Lander ES, Zhang F. 2014. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278. ( 10.1016/j.cell.2014.05.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sander JD, Joung JK. 2014. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355. ( 10.1038/nbt.2842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim S, Ma L, Jensen KL, Kim MM, Bond CT, Adelman JP, Yu CR. 2012. Paradoxical contribution of SK3 and GIRK channels to the activation of mouse vomeronasal organ. Nat. Neurosci. 15, 1236–1244. ( 10.1038/nn.3173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Omura M, Mombaerts P. 2014. Trpc2-expressing sensory neurons in the main olfactory epithelium of the mouse. Cell Rep. 8, 583–595. ( 10.1016/j.celrep.2014.06.010) [DOI] [PubMed] [Google Scholar]
- 73.Edwards DA, Burge KG. 1971. Early androgen treatment and male and female sexual behavior in mice. Horm. Behav. 2, 49–58. ( 10.1016/0018-506X(71)90037-7) [DOI] [Google Scholar]
- 74.Yoon H, Enquist LW, Dulac C. 2005. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123, 669–682. ( 10.1016/j.cell.2005.08.039) [DOI] [PubMed] [Google Scholar]
- 75.von Campenhausen H, Mori K. 2000. Convergence of segregated pheromonal pathways from the accessory olfactory bulb to the cortex in the mouse. Eur. J. Neurosci. 12, 33–46. ( 10.1046/j.1460-9568.2000.00879.x) [DOI] [PubMed] [Google Scholar]
- 76.Scalia F, Winans SS. 1975. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J. Comp. Neurol. 161, 31–55. ( 10.1002/cne.901610105) [DOI] [PubMed] [Google Scholar]
- 77.Winans SS, Scalia F. 1970. Amygdaloid nucleus: new afferent input from the vomeronasal organ. Science 170, 330–332. ( 10.1126/science.170.3955.330) [DOI] [PubMed] [Google Scholar]
- 78.Kang N, Baum MJ, Cherry JA. 2011. Different profiles of main and accessory olfactory bulb mitral/tufted cell projections revealed in mice using an anterograde tracer and a whole-mount, flattened cortex preparation. Chem. Senses 36, 251–260. ( 10.1093/chemse/bjq120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shipley MT, Adamek GD. 1984. The connections of the mouse olfactory bulb: a study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Res. Bull. 12, 669–688. ( 10.1016/036J.9230(84)90148-5) [DOI] [PubMed] [Google Scholar]
- 80.Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. 2011. Distinct representations of olfactory information in different cortical centres. Nature 472, 213–216. ( 10.1038/nature09868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kevetter GA, Winans SS. 1981. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the ‘vomeronasal amygdala’. J. Comp. Neurol. 197, 81–98. ( 10.1002/cne.901970107) [DOI] [PubMed] [Google Scholar]
- 82.Kevetter GA, Winans SS. 1981. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the ‘olfactory amygdala’. J. Comp. Neurol. 197, 99–111. ( 10.1002/cne.901970108) [DOI] [PubMed] [Google Scholar]
- 83.Licht G, Meredith M. 1987. Convergence of main and accessory olfactory pathways onto single neurons in the hamster amygdala. Exp. Brain Res. 69, 7–18. ( 10.1007/BF00247024) [DOI] [PubMed] [Google Scholar]
- 84.Meredith M. 1998. Vomeronasal, olfactory, hormonal convergence in the brain. Cooperation or coincidence? Ann. NY Acad. Sci. 855, 349–361. ( 10.1111/j.1749-6632.1998.tb10593.x) [DOI] [PubMed] [Google Scholar]
- 85.Shipley MT, Murphy AZ, Rizvi TA, Ennis M, Behbehani MM. 1996. Olfaction and brainstem circuits of reproductive behavior in the rat. Prog. Brain Res. 107, 355–377. ( 10.1016/S0079-6123(08)61876-2) [DOI] [PubMed] [Google Scholar]
- 86.Kang N, Baum MJ, Cherry JA. 2009. A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur. J. Neurosci. 29, 624–634. ( 10.1111/j.1460-9568.2009.06638.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colpaert FC, Wiepkema PR. 1976. Effects of ventromedial hypothalamic lesions on spontaneous intraspecies aggression in male rats. Behav. Biol. 16, 117–125. ( 10.1016/S0091-6773(76)91225-6) [DOI] [PubMed] [Google Scholar]
- 88.Commins D, Yahr P. 1984. Lesions of the sexually dimorphic area disrupt mating and marking in male gerbils. Brain Res. Bull. 13, 185–193. ( 10.1016/0361-9230(84)90020-0) [DOI] [PubMed] [Google Scholar]
- 89.Goy RW, Phoenix CH. 1963. Hypothalamic regulation of female sexual behaviour; establishment of behavioural oestrus in spayed guinea-pigs following hypothalamic lesions. J. Reprod. Fertil. 5, 23–40. ( 10.1530/jrf.0.0050023) [DOI] [PubMed] [Google Scholar]
- 90.Hennessey AC, Wallen K, Edwards DA. 1986. Preoptic lesions increase the display of lordosis by male rats. Brain Res. 370, 21–28. ( 10.1016/0006-8993(86)91100-5) [DOI] [PubMed] [Google Scholar]
- 91.Kondo Y, Shinoda A, Yamanouchi K, Arai Y. 1990. Role of septum and preoptic area in regulating masculine and feminine sexual behavior in male rats. Horm. Behav. 24, 421–434. ( 10.1016/0018-506X(90)90019-T) [DOI] [PubMed] [Google Scholar]
- 92.Kondo Y, Sachs BD, Sakuma Y. 1998. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav. Brain Res. 91, 215–222. [PubMed] [Google Scholar]
- 93.Kruk MR, van der Poel AM, de Vos-Frerichs TP. 1979. The induction of aggressive behaviour by electrical stimulation in the hypothalamus of male rats. Behaviour 70, 292–322. ( 10.1163/156853979X00106) [DOI] [PubMed] [Google Scholar]
- 94.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. 2011. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226. ( 10.1038/nature09736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Olivier B, Wiepkema PR. 1974. Behaviour changes in mice following electrolytic lesions in the median hypothalamus. Brain Res. 65, 521–524. ( 10.1016/0006-8993(74)90241-8) [DOI] [PubMed] [Google Scholar]
- 96.Pfaff DW, Sakuma Y. 1979. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J. Physiol. 288, 203–210. ( 10.1113/jphysiol.1979.sp012691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pfaff DW, Sakuma Y. 1979. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J. Physiol. 288, 189–202. ( 10.1113/jphysiol.1979.sp012690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamanouchi K, Arai Y. 1985. Presence of a neural mechanism for the expression of female sexual behaviors in the male rat brain. Neuroendocrinology 40, 393–397. ( 10.1159/000124104) [DOI] [PubMed] [Google Scholar]
- 99.Dugger BN, Morris JA, Jordan CL, Breedlove SM. 2007. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm. Behav. 51, 195–201. ( 10.1016/j.yhbeh.2006.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guillamon A, Segovia S. 1997. Sex differences in the vomeronasal system. Brain Res. Bull. 44, 377–382. ( 10.1016/S0361-9230(97)00217-7) [DOI] [PubMed] [Google Scholar]
- 101.Raisman G, Field PM. 1971. Sexual dimorphism in the preoptic area of the rat. Science 173, 731–733. ( 10.1126/science.173.3998.731) [DOI] [PubMed] [Google Scholar]
- 102.Grgurevic N, Büdefeld T, Spanic T, Tobet SA, Majdic G. 2012. Evidence that sex chromosome genes affect sexual differentiation of female sexual behavior. Horm. Behav. 61, 719–724. ( 10.1016/j.yhbeh.2012.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. 1997. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor α gene. Horm. Behav. 32, 176–183. ( 10.1006/hbeh.1997.1419) [DOI] [PubMed] [Google Scholar]
- 104.Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL, Handa RJ. 2014. Estrogen receptor β expression in the mouse forebrain: age and sex differences. J. Comp. Neurol. 522, 358–371. ( 10.1002/cne.23400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi GB, Dong H-W, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. 2005. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron 46, 647–660. ( 10.1016/j.neuron.2005.04.011) [DOI] [PubMed] [Google Scholar]
- 106.Swanson LW. 2000. Cerebral hemisphere regulation of motivated behavior. Brain Res. 886, 113–164. ( 10.1016/S0006-8993(00)02905-X) [DOI] [PubMed] [Google Scholar]
- 107.Juntti SA, Coats JK, Shah NM. 2008. A genetic approach to dissect sexually dimorphic behaviors. Horm. Behav. 53, 627–637. ( 10.1016/j.yhbeh.2007.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sauer B. 1998. Inducible gene targeting in mice using the cre/lox system. Methods 14, 381–392. ( 10.1006/meth.1998.0593) [DOI] [PubMed] [Google Scholar]
- 109.Branda CS, Dymecki SM. 2004. Talking about a revolution: the impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 6, 7–28. ( 10.1016/S1534-5807(03)00399-X) [DOI] [PubMed] [Google Scholar]
- 110.Chen SW, Shemyakin A, Wiedenmayer CP. 2006. The role of the amygdala and olfaction in unconditioned fear in developing rats. J. Neurosci. 26, 233–240. ( 10.1523/JNEUROSCI.2890-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cooke BM, Hegstrom CD, Keen A, Breedlove SM. 2001. Photoperiod and social cues influence the medial amygdala but not the bed nucleus of the stria terminalis in the Siberian hamster. Neurosci. Lett. 312, 9–12. ( 10.1016/S0304-3940(01)02173-5) [DOI] [PubMed] [Google Scholar]
- 112.Davis M. 1992. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 15, 353–375. ( 10.1146/annurev.ne.15.030192.002033) [DOI] [PubMed] [Google Scholar]
- 113.Hong W, Kim DW, Anderson DJ. 2014. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 158, 1348–1361. ( 10.1016/j.cell.2014.07.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Knapska E, Radwanska K, Werka T, Kaczmarek L. 2007. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol. Rev. 87, 1113–1173. ( 10.1152/physrev.00037.2006) [DOI] [PubMed] [Google Scholar]
- 115.Sah P, Faber ES, Lopez De Armentia M, Power J. 2003. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 83, 803–834. ( 10.1152/physrev.00002.2003) [DOI] [PubMed] [Google Scholar]
- 116.Swanson LW. 2003. The amygdala and its place in the cerebral hemisphere. Ann. NY Acad. Sci. 985, 174–184. ( 10.1111/j.1749-6632.2003.tb07081.x) [DOI] [PubMed] [Google Scholar]
- 117.Bakker J, Honda S, Harada N, Balthazart J. 2003. The aromatase knockout (ArKO) mouse provides new evidence that estrogens are required for the development of the female brain. Ann NY Acad Sci. 1007, 251–262. ( 10.1196/annals.1286.024) [DOI] [PubMed] [Google Scholar]
- 118.Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. 2001. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19). J. Endocrinol. 168, 217–220. ( 10.1677/joe.0.1680217) [DOI] [PubMed] [Google Scholar]
- 119.Harada N, Wakatsuki T, Aste N, Yoshimura N, Honda SI. 2009. Functional analysis of neurosteroidal oestrogen using gene-disrupted and transgenic mice. J. Neuroendocrinol. 21, 365–369. ( 10.1111/j.1365-2826.2009.01857.x) [DOI] [PubMed] [Google Scholar]
- 120.Honda S, Harada N, Ito S, Takagi Y, Maeda S. 1998. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem. Biophys. Res. Commun. 252, 445–449. ( 10.1006/bbrc.1998.9672) [DOI] [PubMed] [Google Scholar]
- 121.Morgan CW, Julien O, Unger EK, Shah NM, Wells JA. 2014. Turning on caspases with genetics and small molecules. Methods Enzymol. 544, 179–213. ( 10.1016/B978-0-12-417158-9.00008-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sternson SM, Roth BL. 2014. Chemogenetic tools to interrogate brain functions. Annu. Rev. Neurosci. 37, 387–407. ( 10.1146/annurev-neuro-071013-014048) [DOI] [PubMed] [Google Scholar]
- 123.Demas GE, Kriegsfeld LJ, Blackshaw S, Huang P, Gammie SC, Nelson RJ, Snyder SH. 1999. Elimination of aggressive behavior in male mice lacking endothelial nitric oxide synthase. J. Neurosci. 19, RC30, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gammie SC, Nelson RJ. 1999. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J. Neurosci. 19, 8027–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Svare B, Gandelman R. 1976. Suckling stimulation induces aggression in virgin female mice. Nature 260, 606–608. ( 10.1038/260606a0) [DOI] [PubMed] [Google Scholar]
- 126.Baum MJ, Bakker J. 2013. Roles of sex and gonadal steroids in mammalian pheromonal communication. Front. Neuroendocrinol. 34, 268–284. ( 10.1016/j.yfrne.2013.07.004) [DOI] [PubMed] [Google Scholar]
- 127.Bergan JF, Ben-Shaul Y, Dulac C. 2014. Sex-specific processing of social cues in the medial amygdala. eLife 3, e02743 ( 10.7554/eLife.02743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.DiBenedictis BT, Ingraham KL, Baum MJ, Cherry JA. 2012. Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol. Behav. 105, 554–559. ( 10.1016/j.physbeh.2011.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sokolowski K, Corbin JG. 2012. Wired for behaviors: from development to function of innate limbic system circuitry. Front. Mol. Neurosci. 5, 55 ( 10.3389/fnmol.2012.00055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hess WR, Akert K. 1955. Experimental data on role of hypothalamus in mechanism of emotional behavior. AMA Arch. Neurol. Psychiatry 73, 127–129. ( 10.1001/archneurpsyc.1955.02330080005003) [DOI] [PubMed] [Google Scholar]
- 131.Bard P. 1928. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. Am. J. Physiol. 84, 490–515. [Google Scholar]
- 132.Cheung CC, Krause WC, Edwards RH, Yang CF, Shah NM, Hnasko TS, Ingraham HA. 2015. Sex-dependent changes in metabolism and behavior, as well as reduced anxiety after eliminating ventromedial hypothalamus excitatory output. Mol. Metab. 4, 857–866. ( 10.1016/j.molmet.2015.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hetherington AW, Ranson SW. 1940. Hypothalamic lesions and adiposity in the rat. Anat. Rec. 78, 149–172. ( 10.1002/ar.1090780203) [DOI] [Google Scholar]
- 134.King BM. 2006. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol. Behav. 87, 221–244. ( 10.1016/j.physbeh.2005.10.007) [DOI] [PubMed] [Google Scholar]
- 135.Kow LM, Harlan RE, Shivers BD, Pfaff DW. 1985. Inhibition of the lordosis reflex in rats by intrahypothalamic infusion of neural excitatory agents: evidence that the hypothalamus contains separate inhibitory and facilitatory elements. Brain Res. 341, 26–34. ( 10.1016/0006-8993(85)91468-4) [DOI] [PubMed] [Google Scholar]
- 136.Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA. 2007. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J. Neurosci. 27, 13624–13634. ( 10.1523/JNEUROSCI.2858-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.La Vaque TJ, Rodgers CH. 1975. Recovery of mating behavior in the female rat following VMH lesions. Physiol. Behav. 14, 59–63. ( 10.1016/0031-9384(75)90142-0) [DOI] [PubMed] [Google Scholar]
- 138.Mathews D, Edwards DA. 1977. The ventromedial nucleus of the hypothalamus and the hormonal arousal of sexual behaviors in the female rat. Horm. Behav. 8, 40–51. ( 10.1016/0018-506X(77)90019-8) [DOI] [PubMed] [Google Scholar]
- 139.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. 2006. RNAi-mediated silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc. Natl Acad. Sci. USA 103, 10 456–10 460. ( 10.1073/pnas.0603045103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S. 2007. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl Acad. Sci. USA 104, 2501–2506. ( 10.1073/pnas.0610787104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reeves AG, Plum F. 1969. Hyperphagia, rage, and dementia accompanying a ventromedial hypothalamic neoplasm. Arch. Neurol. 20, 616–624. ( 10.1001/archneur.1969.00480120062005) [DOI] [PubMed] [Google Scholar]
- 142.Robarts DW, Baum MJ. 2007. Ventromedial hypothalamic nucleus lesions disrupt olfactory mate recognition and receptivity in female ferrets. Horm. Behav. 51, 104–113. ( 10.1016/j.yhbeh.2006.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Silva BA, Mattucci C, Krzywkowski P, Murana E, Illarionova A, Grinevich V, Canteras NS, Ragozzino D, Gross CT. 2013. Independent hypothalamic circuits for social and predator fear. Nat. Neurosci. 16, 1731–1733. ( 10.1038/nn.3573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Swaab D. 2003. Chapter 9 The ventromedial nucleus (VMN; nucleus of Cajal). Handb. Clin. Neurol. 79, 239–242. ( 10.1016/S0072-9752(03)80016-7) [DOI] [PubMed] [Google Scholar]
- 145.Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Agmo A. 2010. Estrogen-induced sexual incentive motivation, proceptivity and receptivity depend on a functional estrogen receptor α in the ventromedial nucleus of the hypothalamus but not in the amygdala. Neuroendocrinology 91, 142–154. ( 10.1159/000255766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Agmo A. 2010. The role of the estrogen receptor α in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav. Brain Res. 210, 211–220. ( 10.1016/j.bbr.2010.02.033) [DOI] [PubMed] [Google Scholar]
- 147.Falkner AL, Dollar P, Perona P, Anderson DJ, Lin D. 2014. Decoding ventromedial hypothalamic neural activity during male mouse aggression. J. Neurosci. 34, 5971–5984. ( 10.1523/JNEUROSCI.5109-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee H, Kim DW, Remedios R, Anthony TE, Chang A, Madisen L, Zeng H, Anderson DJ. 2014. Scalable control of mounting and attack by ESR1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632. ( 10.1038/nature13169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lo L, Anderson DJ. 2011. A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 72, 938–950. ( 10.1016/j.neuron.2011.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Luo L, Callaway EM, Svoboda K. 2008. Genetic dissection of neural circuits. Neuron 57, 634–660. ( 10.1016/j.neuron.2008.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Petreanu L, Huber D, Sobczyk A, Svoboda K. 2007. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668. ( 10.1038/nn1891) [DOI] [PubMed] [Google Scholar]
- 152.Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. 2010. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl Acad. Sci. USA 107, 21 848–21 853. ( 10.1073/pnas.1011756107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. 2007. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647. ( 10.1016/j.neuron.2007.01.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Akerboom J, et al. 2012. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13 819–13 840. ( 10.1523/JNEUROSCI.2601-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen TW, et al. 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. ( 10.1038/nature12354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. 2013. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242. ( 10.1038/nature11846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen Y, Lin YC, Kuo TW, Knight ZA. 2015. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841. ( 10.1016/j.cell.2015.01.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gunaydin LA, et al. 2014. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551. ( 10.1016/j.cell.2014.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hamel EJ, Grewe BF, Parker JG, Schnitzer MJ. 2015. Cellular level brain imaging in behaving mammals: an engineering approach. Neuron 86, 140–159. ( 10.1016/j.neuron.2015.03.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. 2009. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J. Neurosci. 29, 4461–4470. ( 10.1523/JNEUROSCI.0296-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bangasser DA, Valentino RJ. 2014. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 35, 303–319. ( 10.1016/j.yfrne.2014.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Davies W. 2014. Sex differences in Attention Deficit Hyperactivity Disorder: candidate genetic and endocrine mechanisms. Front. Neuroendocrinol. 35, 331–346. ( 10.1016/j.yfrne.2014.03.003) [DOI] [PubMed] [Google Scholar]
- 163.Li R, Singh M. 2014. Sex differences in cognitive impairment and Alzheimer's disease. Front. Neuroendocrinol. 35, 385–403. ( 10.1016/j.yfrne.2014.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Smith KM, Dahodwala N. 2014. Sex differences in Parkinson's disease and other movement disorders. Exp. Neurol. 259, 44–56. ( 10.1016/j.expneurol.2014.03.010) [DOI] [PubMed] [Google Scholar]
- 165.Werling DM, Geschwind DH. 2013. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 26, 146–153. ( 10.1097/WCO.0b013e32835ee548) [DOI] [PMC free article] [PubMed] [Google Scholar]