Abstract
Men and women sleep differently. While much is known about the mechanisms that drive sleep, the reason for these sex differences in sleep behaviour is unknown and understudied. Historically, women and female animals are underrepresented in studies of sleep and its disorders. Nevertheless, there is a growing recognition of sex disparities in sleep and rhythm disorders. Women typically report poorer quality and more disrupted sleep across various stages of life. Findings from clinical and basic research studies strongly implicate a role for sex steroids in sleep modulation. Understanding how neuroendocrine mediators and sex differences influence sleep is central to advancing our understanding of sleep-related disorders. The investigation into sex differences and sex steroid modulation of sleep is in its infancy. Identifying the mechanisms underlying sex and gender differences in sleep will provide valuable insights leading to tailored therapeutics that benefit each sex. The goal of this review is to discuss our current understanding of how biological sex and sex steroids influence sleep behaviour from both the clinical and pre-clinical perspective.
Keywords: sleep, oestrogens, testosterone, progesterone, ventrolateral preoptic area, sleep circuits
1. Introduction
Emerging clinical evidence suggests that sleep dysregulation may have more severe health consequences for women than men. Compared to men and boys, women and girls are twice as likely to experience sleep disruptions and insomnia throughout their lifespan [1]. While much is known about the mechanics of sleep (primarily from studies in males), the exact influences of sex steroids over sleep and basic sex differences in sleep mechanisms remain significant gaps in our knowledge. This lack of knowledge has significant implications when one considers that the majority of sleep studies are done in men or male animals, suggesting that treatment generalized to the male physiology may not effectively alleviate sleep disruptions in women. There is heuristic value in comparing and contrasting sleep between the sexes. Understanding the mechanisms that influence sleep in females will provide valuable insights that may lead to tailored therapeutics that benefit both men and women.
The objective of this review is to discuss our current understanding of how biological sex and sex steroids influence sleep behaviour from both the clinical and pre-clinical perspective. As investigations into how biological sex and sex steroids influence sleep are in their infancy, this review will also highlight significant knowledge gaps. The circadian timing system, which has reported sex differences and is influenced by sex steroids (for review see [2]), is a regulator of sleep; however, this review will mainly focus on sex differences in sleep behaviour and the underlying sleep circuits.
2. Overview of sleep
Sleep, in most organisms, is a behavioural state best characterized by diminished responsiveness to external stimuli coincident with changes in cortical brain activity and muscle tone [3,4]. Although the amount and timing of sleep vary greatly among species, the occurrence and biological need for sleep is evolutionarily conserved [5]. In mammals, distinct patterns of neuronal activity mark changes in vigilance states. Monitoring this neuronal activity via electroencephalography (EEG) provides an accurate and quantifiable assessment of changes in sleep states and patterns. Across most mammalian species, EEG analysis reveals three basic vigilance states including (i) wake, which is characterized by high-frequency, low-amplitude EEGs, (ii) non-rapid eye movement sleep (NREMS), which consists of slower frequency, higher amplitude EEGs; and (iii) rapid eye movement sleep (REMS), which is best characterized by a return to high-frequency, low-amplitude EEGs (for review see [6]) from NREMS states. Healthy sleep patterns occur in cycles, starting with the stages of NREMS (N1–N3; table 1) and progressing to REMS. On average, sleep cycles in adult humans occur about every 90 min, resulting in approximately three to five cycles per sleep period [7]. Sleep that is disturbed by frequent awakenings (i.e. fragmented sleep), extended periods of arousals and/or diminished slow wave sleep (SWS) (N3) results in daytime sleepiness and impaired daytime function [8].
Table 1.
Description of human sleep stages and defining characteristics of EEG wave forms.
| human sleep stages | |
|---|---|
| stage | behaviour |
| NREMS | |
| N1 Sleep | demarcates the transition period from wakefulness into sleep and is characterized by drowsiness and a low arousal threshold |
| N2 Sleep | a deeper sleep than N1 where brain activity, breathing and heart rate begin to slow and the arousal threshold increases. Unique to N2 sleep are sleep spindles and K-complexes (see below) |
| N3 Sleep | typically referred to as slow wave sleep (SWS) or delta sleep for the predominance of the low-frequency, high-amplitude delta waves in the EEG, this is the deepest stage of sleep. The N3 sleep stage is generally accepted as a regenerative period |
| REMS | |
| REM sleep | sleep phase characterized by rapid side-to-side movement of the eyes, muscle atonia and a mixture of high-frequency, low-amplitude brain waves similar to those present in the waking state |
| characteristics of EEG wave forms | |
|---|---|
| term | description |
| sleep spindles | short bursts of high-frequency activity (11–16 Hz) and are thought to be involved in synaptic plasticity and learning and memory |
| K-complexes | common during the transition into stage N3, present as high-amplitude bi- or tri-phasic EGG waveforms that are either spontaneous or evoked by sensory stimuli. K-complexes, as well as sleep spindles, have been postulated to shield the sleep state from sensory stimuli |
| slow-wave activity | the quantification of the amount of delta frequencies in SWS is an accepted neurophysiological marker of sleep depth and intensity |
Despite our understanding of sleep behaviour, the functional significance of why we need sleep remains enigmatic. One prevailing theory with strong supportive evidence is that sleep serves a restorative function for the brain and body. Chronic insufficient sleep is a risk factor for a variety of psychological [9–13], neurological [14–19] and neurodegenerative pathologies [16], as well as cardiovascular and metabolic dysfunctions [20–24]. More recent findings from clinical studies reveal that women suffering from sleep disturbances and insufficient sleep are at greater risk compared with men for mood disorders such as depression [25], as well as metabolic [26] and cardiovascular dysfunction [23,27–29]. Given the increased risks to psychological and physiological well-being, sleep disorders among women constitute a significant public health concern. Yet, surprisingly little is known about the mechanisms through which biological sex and/or gender (i.e. one's sense of self as male or female) influences sleep and the development of sleep disorders.
3. Clinical perspective
(a). Sex differences in sleep
As sleep is a highly evolutionarily conserved behaviour, the possibility that men and women sleep differently might not be immediately evident. In the limited number of polysomnography sleep studies (PSG; an objective method for analysing sleep and sleep architecture that includes EEGs) of healthy subjects where biological sex is considered as a variable, findings of sex differences in sleep are mixed. Differences in study design, variability in the populations and low numbers of subjects may contribute to these mixed results. Consistent across the studies that do report sex differences are the findings that women have better PSG-defined sleep quality than men [30–36]. More striking is that this general finding is consistent across multiple approaches. In a sleep laboratory study including 31 healthy volunteers with an average age of approx. 20 years, PSG measures indicate that women have significantly longer total sleep time and less total wake time, a shorter sleep onset latency including time to N1 and N2, and better sleep efficiency than men [36] (but see [37,38]). Similarly, a cross-sectional analysis of portable (i.e. in-home) PSG measures from 2685 participants with an average age of approximately 62 years reports that men had evidence of lighter sleep when compared with women of matched ages [35]. Specifically, men accumulate a greater percentage of N1 and N2 stages with a reduction in the per cent of deep SWS (N3 stage) and REMS. Consistent with the suggestion of poor sleep, men in this study also exhibit a higher arousal index and lower sleep efficiency. Another significant study of note is a meta-analysis of quantitative sleep parameters where a subset of studies used PSG or actigraphy in healthy adult men and women [34]. When analysed by sex, the findings indicate that women have greater total sleep times (with less N2 stage sleep) and a greater percentage of SWS than age-matched men. Consistent across a number of studies is the finding that slow wave activity (SWA), a measure of sleep intensity during SWS, is greater in women across ages [33,39–42] and is less affected by aging in women [41]. Following sleep deprivation (SD), women also have a greater SWA in recovery sleep, suggesting that sleep debt accumulates more quickly in women giving rise to sex differences in the ability to recover from sleep loss [43].
Despite the findings that healthy women objectively have better quality sleep than men, a paradox exists. Women across a wide age range report more sleep problems. In subjective studies and self-assessments, women report disrupted and insufficient sleep more frequently than men [44–48]. They report poorer sleep quality, difficulties falling asleep, frequent night awakenings and longer periods of time awake throughout the night [47,48]. It is unclear what accounts for this discordance between the subjective and objective sleep findings.
Given that sleep is tightly linked to circadian timing, a desynchrony between circadian timing and sleep behaviour in women may be a contributing factor. Sex differences exist in the circadian timing of sleep; women tend to go to bed earlier and wake up earlier than men [32,49,50]. Retrospective analyses of the circadian timing system in men and women with similar sleep times and durations find that women have an early timing of circadian rhythms, particularly for endogenous temperature and melatonin [51], partly as a consequence of a significantly shorter circadian period [52]. Together, these studies suggest that circadian timing in women is even earlier than the sex difference in sleep timing would predict. Thus, women may be sleeping at later circadian times, which could contribute to the higher prevalence of insomnia (see below) and/or perception of less restorative sleep.
Sleep-independent factors such as mood may also contribute to the perception of poorer sleep quality in the absence of PSG sleep disturbances in women. In a PSG study of women with premenstrual syndrome, poorer subjective sleep quality correlates with higher anxiety and more perceived night-time awakenings in the absence of objectively defined measures of poor sleep, suggesting that sleep quality assessments are strongly impacted by anxiety, depressive symptoms and affective disorders [53]. Nevertheless, traditional measures of sleep quality such as total sleep time, sleep onset, arousal frequency and SWS/SWA, which have been historically based on male physiology, may not be sufficient to detect poor sleep in women. Thus there is a clear need for better in-depth studies of sleep intensity as measured via quantitative EEG or spectral analysis in women.
(b). Biological sex is a major risk factor for insomnia
While sex differences exist in the prevalence and type of sleep disorders (reviewed in [54,55]), insomnia presents one of the more striking sex biases. Insomnia is marked by the persistent difficulty initiating and maintaining sleep, waking too early and an association with non-restorative or poor sleep quality [56]. While insomnia is the most common sleep complaint reported in primary care settings, estimates of its prevalence in the general population are highly variable owing to the inconsistencies in diagnostic criteria. Numerous population-based studies across multiple counties strongly indicate that approximately 30% of the sample population report one or more insomnia symptoms [57].
The 2005 State of the Science Conference identified sex and age as the top risk factors for insomnia [58]. Women are at a 40% greater risk for insomnia throughout their lifetime compared with men [34,48,59] (figure 1). Studies from multiple countries indicate that the increased prevalence of insomnia in women compared to age-matched men is a global phenomenon (for review see [61]), suggesting that a woman's physiology is a significant consideration in insomnia. Indeed, changes in ovarian steroid production, such as those occurring during puberty and the menopausal transition, are markedly associated with an increased prevalence of insomnia and poor sleep compared with age-matched males [60,62–64] (see below).
Figure 1.
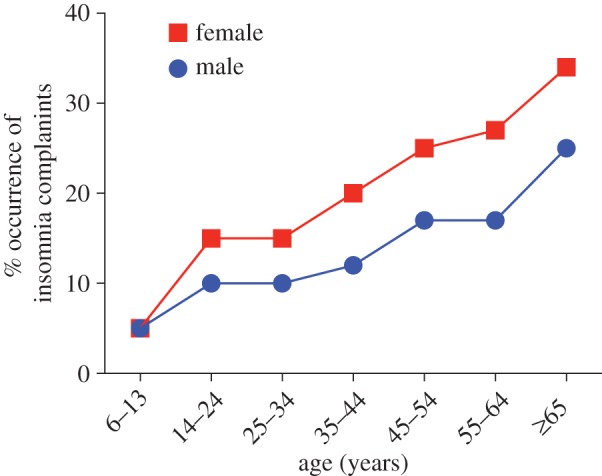
Prevalence of insomnia complaints by sex and age in a general population. Sleep disorders among women are a significant public health issue: sleep complaints such as insufficient sleep and insomnia are more prevalent in women. This sex difference in insomnia emerges after puberty, suggesting that hormonal events underlying puberty may be involved. Adapted from [1] and based on [47,58,60]. (Online version in colour.)
Insomnia may be a contributing factor to the development and perpetuation of depressive illnesses [64]. The occurrence of insomnia in women and girls is strongly associated with a twofold greater risk of depression [25]. Like insomnia, increased risk for depression and affective disorders in women emerges at the time of puberty and is linked to fluctuations in the ovarian steroidal milieu [62,63]. While ovarian steroids and biological sex are implicated as risk factors for sleep disruptions and depression, the relationship among these factors is poorly understood. Thus, a better understanding of the mechanisms underlying ovarian steroid modulation of sleep in women may serve to uncover novel therapies for the treatment of depression in women.
(c). Sex steroids influence sleep
The major gonadal (or sex) steroids, namely testosterone in men and oestrogens and progestins in women, are implicated in the modulation of sleep behaviours and have been extensively reviewed [65]. While there is currently a paucity of clinical studies directly comparing sex differences in the effects of sex steroids on sleep, a general consensus from the current literature is that sleep in women is more sensitive to changes in the ovarian steroidal milieu.
It is unclear if sex steroids, mainly testosterone, affect sleep in men. Testosterone secretion is tightly linked to sleep cycles, with peak levels occurring just before or after REMS onset [66]. Insufficient and/or fragmented sleep blocks the nocturnal increase in testosterone [67–69]. Yet, findings that fluctuations in testosterone levels affect sleep in men remain inconsistent. A cohort study in men aged 65 years and older reported an association of lower testosterone levels with decreased sleep efficiency, increased nocturnal awakenings and less time in SWS [70]. Conversely, high-dose testosterone replacement in older men and the use of anabolic androgenic steroids in healthy young men are associated with a reduction in sleep efficiency and total sleep time [71,72]. A number of studies suggest that testosterone is linked to a worsening of obstructive sleep apnoea in men [71,73–75]; however, blocking androgen action, via flutamide administration, does not affect sleep architecture or breathing parameters in men with sleep apnoea [76]. Interestingly, androgen deprivation therapy (ADT) for prostate cancer is highly associated with insomnia, potentially as a consequence of an increased occurrence of hot flushes and night sweats [77,78]. Although rare, oestrogen therapy is an effective therapeutic for ADT-induced hot flushes [79–81]. However, it is unknown whether oestrogen therapy is effective in improving sleep quality (but see [82]) in androgen-deprived men.
While sex steroids and sex have been implicated as risk factors for sleep disruptions and insomnia, the relationship between ovarian steroids, primarily oestrogens/progesterone and normal sleep is poorly understood. In women, sleep complaints typically coincide with periods of ovarian steroid fluctuation such as puberty, the menstrual cycle, pregnancy and the menopausal transition and these findings have been extensively reviewed [65,83,84]. Compared to sleep across the menstrual cycle and the menopausal transition, much less is known about the influence of ovarian steroids over sleep during puberty and pregnancy. As previously discussed, changes in the levels of ovarian steroids at the onset of puberty are associated with an increased prevalence of sleep disruptions. During pregnancy, women experience significant changes in sleep; however, it is difficult to parse out the direct effects of hormonal changes from those caused by physiological changes owing to the growth and development of the fetus. As early as the first trimester, women experience increased fatigue and report poor sleep quality and restless sleep. Studies indicate that initially total sleep is increased but then declines throughout the course of the pregnancy (reviewed in [85]). The menopausal transition is a well-described indicator of poor sleep, with the increased prevalence of sleep difficulties. The loss of ovarian oestradiol production is most likely involved in the sleep disturbances since oestrogen replacement therapy is effective at alleviating the sleep disruptions during this period [1,13,65,84]. Juxtaposed to sleep disturbances in the absence of ovarian steroids is the increased risk for sleep disturbances in women that emerges at the time of puberty [63]. Ovarian steroid fluctuations over the menstrual cycle are associated with an increased prevalence of sleep disruptions, but it remains unclear whether ovarian steroids benefit or hinder sleep in young women. The paucity of studies investigating sleep in women of reproductive age, the lack of consistency in experimental paradigms (i.e. hormonal profiles) and the small sample sizes of existing studies, largely contribute to our lack of understanding of the relationships between ovarian steroids and sleep in young women.
From the few studies using objective and/or subjective measures that find significant changes in sleep across the menstrual cycle in healthy women, a general conclusion is that sleep is most disturbed during the mid-luteal phase when ovarian steroid levels are still elevated but starting to decline (for review see [65,83,84]). A recent laboratory study of objective measures of sleep in mid-life women (approx. 48.8 years old) with and without insomnia reports that both groups of women experience increased awakenings and arousal during sleep and decreased SWS during the luteal versus the follicular phase. Moreover, in both groups, the sleep spindles in the luteal phase compared with the the follicular phase exhibit marked increases in number, duration and higher EEG spectral frequency (14–17 Hz) [86], which is in agreement with earlier reports in younger women [87,88]. Nevertheless, earlier PSG studies report stability of sleep across the menstrual cycle [89,90]. An important consideration in these findings is that the small sample size may not adequately overcome the variability in individual sex steroid levels, metabolism or social/psychological factors that can impact sleep. The finding that exogenous hormones, like oral contraception, influence sleep in young women is clearer. Women taking oral contraceptives have increased N2 sleep [91] and REM sleep [92], and SWS sleep is reduced [91–93]. However, it is not clear from these studies whether oestrogens and/or progestins are contributing to the changes.
The menopausal transition is marked by erratic fluctuations and eventual decline in oestrogens (for review see [94]). Complaints about sleep quality are one of the most common symptoms of the menopause transition, being reported by 33–51% of women [95]. A general consensus drawn from numerous large studies is that the perception of poor and disrupted sleep increases during perimenopause [96–100]. Yet, objective measures of sleep do not reflect this worsening of sleep quality [101–103]. An understanding of the discordance between subjective sleep complaints and objectively measured sleep in peri- and postmenopausal women remains a significant gap in our knowledge. The extent to which sleep is disturbed during menopause may depend on the severity of menopausal symptoms. Hot flushes affect 75–85% of women across the menopausal transition and are associated with sleep disruption [96,100,103,104]. Nevertheless, the exact relationship between hot flushes and sleep disruption remains controversial. Hormone therapy (HT) is reported to improve sleep quality, further implicating a role for ovarian steroids, and oestrogens particularly, in sleep (for review see [105]). However, improved sleep with HT may be partially owing to the associated decline in vasomotor symptoms and not via actions on the sleep mechanisms.
Overall, sleep studies investigating the effects of sex steroids in both men and women have been rather inconsistent in their findings. Moreover, apart from the clinical finding that sex steroids may affect sleep behaviour and architecture, the mechanisms underlying how sex steroids influence the sleep circuitry remain a significant gap in our knowledge. The use of animal models is critical for advancing our understanding of the potential endocrine–sleep nexus.
4. Lessons from animal models
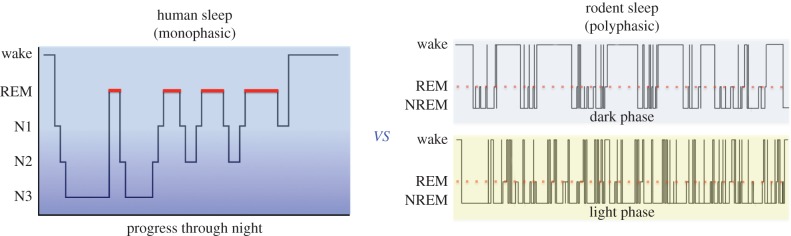
Given that human sleep is a complex behaviour easily influenced by perception as well as internal and environmental factors, studies in animal models, for which such confounds are minimized, can provide considerable insight into the biological basis of sex differences in sleep. Rodents are ideal models for sleep studies as (i) the basic vigilance states are easily measured via EEGs, (ii) the neurocircuitry and neurochemistry of sleep share similarities with humans and (iii) their sleep circuitry is amenable to pharmacological and genetic manipulations. However, unlike humans, rats and mice are polyphasic sleepers and cycle through many bouts of sleep (NREMS and REMS) and wake during both the dark (active) and light (quiescent) phases. This results in less consolidated vigilance states. Additionally, the majority of rodent species are nocturnal and acquire a higher percentage of sleep in the light phase, whereas more consolidated bouts of waking occur in the dark phase (for review see [106]). In contrast, human sleep is monophasic and occurs during a consolidated period typically at night (figure 2).
Figure 2.
Comparison of hypnograms representing typical sleep patterns in humans and rodents. Human sleep is monophasic and normally consists of three to five cycles of the sleep stages throughout the night. Longer bouts of NREMS stage N3 or SWS occur earlier in the night while REMS increases in duration and frequency as the night progresses. Rodent sleep is polyphasic, with wake, NREMS and REMS occurring throughout the light and dark phases. More sleep is acquired in the light phase, while the dark phases consist of consolidated periods of wake. (Online version in colour.)
(a). Sex differences in sleep behaviour
The paucity of basic studies investigating sex differences in sleep has resulted in mixed findings regarding the exact nature of these differences [107–112]. In general, gonadally intact female rodents spend less time in sleep states compared with males. In mice, females accumulate less total sleep and NREMS compared with males [107,110,112], whereas in intact rats only REMS is reported to be significantly less in female rats compared with males [111,113]. Comparison of sleep architecture in mice suggests that females, despite accumulating less total sleep, have more consolidated sleep bouts, consisting of longer bout durations with less state transitions and fewer arousals [110]. Moreover, NREMS delta power, a quantitative measure of sleep intensity that is analogous to SWA in humans, is higher in females during baseline sleep as well as in recovery sleep following SD [110]. These findings are in agreement with observations in humans.
Perhaps more striking is that in the absence of circulating sex steroids, these sex differences in sleep behaviour and architecture are eliminated [109,110], suggesting that sex differences in sleep are in part dependent on sex steroids. Recent findings in adult rats support this assertion and further suggest that sleep patterns in females remain sensitive to fluctuations in oestradiol while males on the other hand seem insensitive to changes in both oestradiol and testosterone [108]. Gonadectomized male and female rats exhibit no significant differences in any vigilance state. However, exogenous replacement of oestradiol in females significantly decreases dark phase NREMS and REMS to approximately 45% of baseline sleep. Indeed, the effect appears to be owing to sex and not the steroid, as testosterone induces a similar magnitude of change in females but oestradiol has no effect in males. Of note, the testosterone-mediated effects in females are most likely owing to the aromatization into oestradiol, as a non-aromatizable androgen, dihydrotestosterone, did not affect sleep behaviour in either males or females. In contrast, one study in male rats reports that chronic exposure to oestradiol induces arousal at the expense of sleep [114]. While long-term versus short-term exposure to oestradiol may account for the differences in the findings in male rats, the magnitude of change in males induced by chronic oestradiol exposure compared with females is uncertain because only males were examined in that particular study.
Taken together, the findings from the few animal studies that exist suggest that the sex differences are predominantly owing to the effects of ovarian sex steroids in females, a finding that is not unlike observations in humans. Normal sleep patterns in the female rat are exquisitely sensitive to the natural fluctuations of ovarian steroids (for review see [1]). Findings from a number of studies in rats generally agree that on the night of pro-estrus, when oestradiol and progesterone are elevated, both NREMS and REMS are significantly reduced compared with other phases of the oestrous cycle [115–118]. Ovariectomy eliminates the fluctuations in nocturnal sleep observed over the oestrous cycle, and exogenous oestradiol plus progesterone or oestradiol alone are sufficient to recapitulate the suppression of sleep observed during the night of pro-estrus [108,119–122]. To a lesser extent, this is also observed in mice [110,123,124]. In these studies, oestradiol predominately suppresses dark phase sleep and has little or no effect in the light phase.
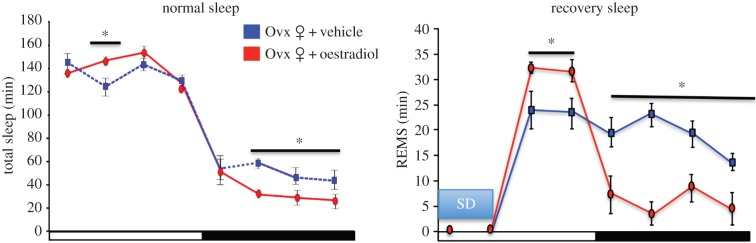
Oestradiol suppression of sleep in rodents may seem paradoxical to its effects in women. However, it is important to consider that the temporal organization of sleep may contribute to the oestrogenic effects. Typically, rodent species most often used in the laboratory are nocturnal, with the majority of their sleep episodes occurring during the day. An opposite pattern is observed in normal human sleep patterns. Thus, the effect of sex steroids on sleep might be chronotype dependent, such that oestradiol in rodents may work to consolidate sleep and wake behaviours to the appropriate phases. Indeed, oestradiol administered to ovariectomized rats increases the duration of total sleep in the light phase by a small but significant degree [108] (figure 3). Moreover, findings from SD studies further support that ovarian steroids and oestradiol in particular may facilitate recovery from sleep loss [121,122,125–127]. Following 6 h of SD, ovariectomized female rats treated with oestradiol and/or progesterone exhibit increases in REMS during the recovery sleep, while decreasing NREMS delta power [121]. A more recent study suggests that oestradiol facilitation of recovery sleep is phase dependent as oestradiol administered to ovariectomized rats enhances REMS recovery in the light phase, while suppressing sleep in the dark phase [125]. In this same study, oestradiol enhances NREMS delta power in the light phase without affecting NREMS recovery behaviour. As circadian timing tightly regulates sleep, these findings support the suggestion that oestradiol acts to consolidate and enhance sleep–wake activity to the appropriate time of day (figure 3).
Figure 3.
Oestradiol may act to consolidate and enhance sleep–wake activity to the appropriate time of day. Under normal sleep conditions, oestradiol administered to ovariectomized adult rats increases spontaneous total sleep (NREM and REM) in the light phase while suppressing total sleep in the dark phase, resulting in more consolidated wake bouts (not shown). Data redrawn from [108]. Following a 6-h bout of sleep deprivation (SD), oestradiol facilitated REM recovery sleep in the light phase allowing a return to baseline levels in the dark phase. In contrast, control SD females exhibited increased REM sleep throughout the dark phase [125]. Asterisks represent statistically significant differences between the treatment groups (significance level set at 0.05). Ovx, ovariectomized. (Online version in colour.)
While evidence that ovarian steroids modulate sleep in female rodents is clear, less is known about males. Nevertheless, sleep in male rodents seems insensitive to changes in sex steroids levels. Castration does not significantly change sleep or wakefulness in male rodents, suggesting a resilience of the male sleep circuitry to changes in testosterone levels [108,110,114]. Testosterone replacement, however, slightly increases NREMS during the dark phase in mice [124]. The observations in rodent models that sleep–wake patterns in males are less sensitive than those in females to fluctuating levels of sex steroids raises the intriguing possibility that the neural substrates mediating sleep may be sexually differentiated resulting in a greater plasticity and responsiveness to sex steroids in females than males.
(b). Sexual differentiation of sleep circuitry
The neural circuitry and mechanisms underlying sleep and wakefulness have been extensively studied (for review see [6]). However, studies investigating (i) whether sex differences exist and (ii) the precise targets of oestradiol action within these circuits are in their infancy. In rodents, sexual differentiation of the brain occurs during a sensitive developmental window when exposure to sex steroids results in the masculinization and defeminization of the neural substrates, whereas the absence of sex steroids leads to a feminization process (for review see [128]). In adulthood, the production and release of sex steroids activate these differentiated neural circuitries resulting in appropriate behaviours specific to the sex of the animals. This two-step process of developmental and adult exposure to sex steroids is classically referred to as the Organizational/Activational Hypothesis (for review see [129]). Early studies in rats have suggested that oestradiol effects on sleep behaviour might be owing to the organizing effects of sex steroid exposure during development [113,130]. A more recent study provides clear evidence supporting the hypothesis that sex differences in sleep are established by early programming effects of sex steroids [108]. Female rats exposed to a masculinizing dose of testosterone during the sensitive window for brain sexual differentiation exhibit male-like responsivity to oestradiol and testosterone in adulthood. Additionally, activity of sleep-active neurons in the ventrolateral preoptic area (VLPO), an established sleep-promoting nucleus (for review see [6]), exhibited male-like patterns in masculinized females illustrating for the first time that a component of the sleep circuitry is sensitive to the organizing effects of sex steroids.
Previous work in rodents implicates the VLPO in mediating oestradiol actions over sleep. In adult ovariectomized females, oestradiol decreases (i) the activation of sleep-active VLPO neurons [116] and (ii) downregulates the mRNA expression [131] and protein levels [116] of lipocalin-type prostaglandin D synthase (L-PGDS), the enzyme responsible for the production of prostaglandin D2 that potently promotes sleep [132], via actions in the VLPO. Unlike females, fluctuations in testosterone in adult males do not influence the activation states of sleep-active neurons or L-PGDS protein levels in the VLPO [116], further supporting that the male sleep–wake circuitry is less responsive to sex steroids.
The exact mechanisms mediating sex differences in and steroid modulation of sleep are far from being understood and most likely involve other nuclei in the sleep–wake circuitry (figure 4). Emerging evidence from female rats suggests that blocking oestradiol action directly in the median preoptic nucleus, a brain nucleus involved in the onset and regulation NREMS and REMS, attenuates oestradiol suppression of sleep [133]. Similarly, the wake-promoting hypocretin/orexin system in the lateral hypothalamus is highly sensitive to fluctuations in endogenous and exogenous ovarian steroids [134–138], suggesting that oestradiol may influence sleep–wake states via coordinated actions in arousal- and sleep-active cells. Given the interconnected circuitry and interactions between sleep homeostasis and circadian processes (for review see [139]), it is likely that sex differences in the circadian system exerts a level of influence over sleep behaviour. The suprachiasmatic nucleus (SCN), the master pacemaker for circadian rhythms, contains sex steroid receptors; however, androgen receptors predominate over oestrogen receptors, although oestrogen receptors are localized to regions projecting to the SCN (for review see [140]).
Figure 4.
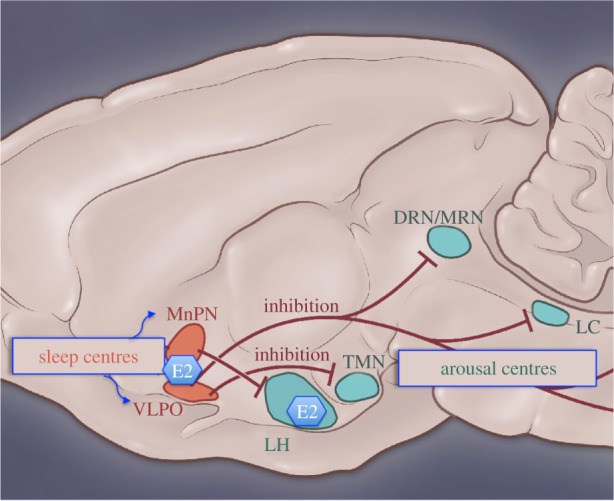
Simplified diagram of projections from sleep-regulating nuclei and potential sites of oestradiol action. Sleep-active neurons are present in the ventrolateral preoptic area (VLPO) and median preoptic nucleus (MnPN). Inhibitory projections from the VLPO and MnPN innervate the major arousal nuclei including the tubermammillary nucleus (TMN), the dorsal raphe nucleus (DRN), the locus coeruleus (LC) and the lateral hypothalamus (LH). MRN, median raphe nucleus. (Online version in colour.)
While the preponderance of evidence points to sex differences in sleep behaviours and the mechanisms governing these differences being largely dependent on sex hormones, exciting findings from a genetic mouse model suggest that sex chromosome complement contributes to the establishment of sex differences in sleep [112]. In the four core genotype mouse models, the sex chromosome complement (XY, XX) is opposite to phenotypic gonadal sex (for review see [141]). Animals represented in the four core genotypes are phenotypic males and females (as determined by the presence of testis or ovaries, respectively) but carry either XX or XY chromosome compliment resulting in sex chromosome complement being independent of gonadal sex. Following a period of SD, females with the XY compliment acquire more sleep during their mid-active phase and have higher NREM delta power than XX females, suggesting that the processes mediating recovery from sleep loss are partially dependent on sex chromosomes.
5. Conclusion
Sex steroids and biological sex are risk factors for sleep disruptions and insomnia. Yet, the exact influence of sex steroids over sleep remains a significant gap in our knowledge. Research directed to the understanding of the basic mechanisms of (i) sex differences in sleep and (ii) oestrogenic modulation of sleep is essential if we are to understand how interactions between the neuroendocrine and sleep circuitry systems influence the risk for sleep disorders in women and develop appropriate therapies that are informed by the female physiology. Indeed, this point is underscored by the recent Food and Drug Administration decision that women should receive half the recommended dose of Ambien (zolpidem), the commonly prescribed sleep-aid [55]. While this historic move to a sex-specific prescribing guideline is based on the discovery that women metabolize the drug more slowly than men, questions as to whether sex differences and/or ovarian steroid modulation of the sleep circuitry contribute to differences in sensitivity to zolpidem remain unanswered. Indeed, clinical evidence for sex differences in mechanisms regulating sleep is suggested by a study where a single oral dose of olanzapine (a second generation anti-psychotic) in healthy volunteers resulted in sex differences in the drug's effect on sleep. Women showed an increase in SWS, whereas men showed a decrease [142]. This finding supports the existence of sex differences in the mechanisms mediating sleep and underscores the importance of a better understanding of sleep processes in women.
Acknowledgements
This work was supported by National Heart, Lung, and Blood Institute (HL129138 awarded to J.A.M.).
Competing interests
The authors declare no competing financial interests.
Funding
We received no funding for this study.
References
- 1.Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R. 2011. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J. Neurosci. 31, 16 107–16 116. ( 10.1523/JNEUROSCI.4175-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey M, Silver R. 2014. Sex differences in circadian timing systems: implications for disease. Front. Neuroendocrinol. 35, 111–139. ( 10.1016/j.yfrne.2013.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendricks JC, Sehgal A, Pack AI. 2000. The need for a simple animal model to understand sleep. Prog. Neurobiol. 61, 339–351. ( 10.1016/S0301-0082(99)00048-9) [DOI] [PubMed] [Google Scholar]
- 4.Campbell SS, Tobler I. 1984. Animal sleep: a review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 8, 269–300. ( 10.1016/0149-7634(84)90054-X) [DOI] [PubMed] [Google Scholar]
- 5.Siegel JM. 2005. Clues to the functions of mammalian sleep. Nature 437, 1264–1271. ( 10.1038/nature04285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. 2010. Sleep state switching. Neuron 68, 1023–1042. ( 10.1016/j.neuron.2010.11.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dement W, Kleitman N. 1957. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr. Clin. Neurophysiol. 9, 673–690. ( 10.1016/0013-4694(57)90088-3) [DOI] [PubMed] [Google Scholar]
- 8.Guilleminault C, Partinen M, Quera-Salva MA, Hayes B, Dement WC, Nino-Murcia G. 1988. Determinants of daytime sleepiness in obstructive sleep apnea. Chest 94, 32–37. ( 10.1378/chest.94.1.32) [DOI] [PubMed] [Google Scholar]
- 9.Shechter A, Varin F, Boivin DB. 2010. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep 33, 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parry BL, Mendelson WB, Duncan WC, Sack DA, Wehr TA. 1989. Longitudinal sleep EEG, temperature, and activity measurements across the menstrual cycle in patients with premenstrual depression and in age-matched controls. Psychiatry Res. 30, 285–303. ( 10.1016/0165-1781(89)90020-6) [DOI] [PubMed] [Google Scholar]
- 11.Shechter A, Boivin DB. 2010. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int. J. Endocrinol. 2010, 259345 ( 10.1155/2010/259345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joffe H, Massler A, Sharkey KM. 2010. Evaluation and management of sleep disturbance during the menopause transition. Semin. Reprod. Med. 28, 404–421. ( 10.1055/s-0030-1262900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dzaja A, et al. 2005. Women's sleep in health and disease. J. Psychiatr. Res. 39, 55–76. ( 10.1016/j.jpsychires.2004.05.008) [DOI] [PubMed] [Google Scholar]
- 14.Mendez M, Radtke RA. 2001. Interactions between sleep and epilepsy. J. Clin. Neurophysiol. 18, 106–127. ( 10.1097/00004691-200103000-00003) [DOI] [PubMed] [Google Scholar]
- 15.Pasic Z, Smajlovic D, Dostovic Z, Kojic B, Selmanovic S. 2011. Incidence and types of sleep disorders in patients with stroke. Med. Arh. 65, 225–227. ( 10.5455/medarh.2011.65.225-227) [DOI] [PubMed] [Google Scholar]
- 16.Petit D, Gagnon JF, Fantini ML, Ferini-Strambi L, Montplaisir J. 2004. Sleep and quantitative EEG in neurodegenerative disorders. J. Psychosom. Res. 56, 487–496. ( 10.1016/j.jpsychores.2004.02.001) [DOI] [PubMed] [Google Scholar]
- 17.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. 1994. Gender differences in sleep disordered breathing in a community-based sample. Am. J. Respir. Crit. Care Med. 149, 722–726. ( 10.1164/ajrccm.149.3.8118642) [DOI] [PubMed] [Google Scholar]
- 18.Somers VK, et al. 2008. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 118, 1080–1111. ( 10.1161/CIRCULATIONAHA.107.189375) [DOI] [PubMed] [Google Scholar]
- 19.Vock J, Achermann P, Bischof M, Milanova M, Muller C, Nirkko A, Roth C, Bassetti CL. 2002. Evolution of sleep and sleep EEG after hemispheric stroke. J. Sleep Res. 11, 331–338. ( 10.1046/j.1365-2869.2002.00316.x) [DOI] [PubMed] [Google Scholar]
- 20.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. 2003. A prospective study of sleep duration and coronary heart disease in women. Arch. Intern. Med. 163, 205–209. ( 10.1001/archinte.163.2.205) [DOI] [PubMed] [Google Scholar]
- 21.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. 2006. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension 47, 833–839. ( 10.1161/01.HYP.0000217362.34748.e0) [DOI] [PubMed] [Google Scholar]
- 22.Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB. 2004. A prospective study of sleep duration and mortality risk in women. Sleep 27, 440–444. [DOI] [PubMed] [Google Scholar]
- 23.Cappuccio FP, et al. 2007. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension 50, 693–700. ( 10.1161/HYPERTENSIONAHA.107.095471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. 2004. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J. Am. Coll. Cardiol. 43, 678–683. ( 10.1016/j.jacc.2003.07.050) [DOI] [PubMed] [Google Scholar]
- 25.Krystal AD. 2004. Depression and insomnia in women. Clin. Cornerstone 6(Suppl. 1B), S19–S28. ( 10.1016/S1098-3597(04)80022-X) [DOI] [PubMed] [Google Scholar]
- 26.Lyytikainen P, Rahkonen O, Lahelma E, Lallukka T. 2011. Association of sleep duration with weight and weight gain: a prospective follow-up study. J. Sleep Res. 20, 298–302. ( 10.1111/j.1365-2869.2010.00903.x) [DOI] [PubMed] [Google Scholar]
- 27.Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, Marmot MG. 2007. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep 30, 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller MA, Kandala NB, Kivimaki M, Kumari M, Burnner EJ, Lowe GDO, Marmot MG, Cappuccio FP. 2009. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep 32, 857–864. [PMC free article] [PubMed] [Google Scholar]
- 29.Kronholm E, Laatikainen T, Peltonen M, Sippola R, Partonen T. 2011. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 12, 215–221. ( 10.1016/j.sleep.2010.07.021) [DOI] [PubMed] [Google Scholar]
- 30.Bixler EO, Papaliaga MN, Vgontzas AN, Lin HM, Pejovic S, Karataraki Maria, Vela-Bueno A, Chrousos GP. 2009. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J. Sleep Res. 18, 221–228. ( 10.1111/j.1365-2869.2008.00713.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitiello MV, Larsen LH, Moe KE. 2004. Age-related sleep change: gender and estrogen effects on the subjective–objective sleep quality relationships of healthy, noncomplaining older men and women. J. Psychosom. Res. 56, 503–510. ( 10.1016/S0022-3999(04)00023-6) [DOI] [PubMed] [Google Scholar]
- 32.Tonetti L, Fabbri M, Natale V. 2008. Sex difference in sleep-time preference and sleep need: a cross-sectional survey among Italian pre-adolescents, adolescents, and adults. Chronobiol. Int. 25, 745–759. ( 10.1080/07420520802394191) [DOI] [PubMed] [Google Scholar]
- 33.Dijk DJ, Beersma DG, Bloem GM. 1989. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep 12, 500–507. [DOI] [PubMed] [Google Scholar]
- 34.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. 2004. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27, 1255–1273. [DOI] [PubMed] [Google Scholar]
- 35.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. 2004. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch. Intern. Med. 164, 406–418. ( 10.1001/archinte.164.4.406) [DOI] [PubMed] [Google Scholar]
- 36.Goel N, Kim H, Lao RP. 2005. Gender differences in polysomnographic sleep in young healthy sleepers. Chronobiol. Int. 22, 905–915. ( 10.1080/07420520500263235) [DOI] [PubMed] [Google Scholar]
- 37.Armitage R, Hoffmann R, Trivedi M, Rush AJ. 2000. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 95, 201–213. ( 10.1016/S0165-1781(00)00178-5) [DOI] [PubMed] [Google Scholar]
- 38.Voderholzer U, Al-Shajlawi A, Weske G, Feige B, Riemann D. 2003. Are there gender differences in objective and subjective sleep measures? A study of insomniacs and healthy controls. Depress. Anxiety 17, 162–172. ( 10.1002/da.10101) [DOI] [PubMed] [Google Scholar]
- 39.Latta F, Leproult R, Tasali E, Hofmann E, Van Cauter E. 2005. Sex differences in delta and alpha EEG activities in healthy older adults. Sleep 28, 1525–1534. [DOI] [PubMed] [Google Scholar]
- 40.Mourtazaev MS, Kemp B, Zwinderman AH, Kamphuisen HA. 1995. Age and gender affect different characteristics of slow waves in the sleep EEG. Sleep 18, 557–564. [DOI] [PubMed] [Google Scholar]
- 41.Ehlers CL, Kupfer DJ. 1997. Slow-wave sleep: do young adult men and women age differently? J. Sleep Res. 6, 211–215. ( 10.1046/j.1365-2869.1997.00041.x) [DOI] [PubMed] [Google Scholar]
- 42.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. 2001. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). Psychophysiology 38, 232–242. ( 10.1111/1469-8986.3820232) [DOI] [PubMed] [Google Scholar]
- 43.Armitage R, Hoffmann RF. 2001. Sleep EEG, depression and gender. Sleep Med. Rev. 5, 237–246. ( 10.1053/smrv.2000.0144) [DOI] [PubMed] [Google Scholar]
- 44.Reyner LA, Horne JA, Reyner A. 1995. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. Sleep 18, 127–134. [PubMed] [Google Scholar]
- 45.Groeger JA, Zijlstra FR, Dijk DJ. 2004. Sleep quantity, sleep difficulties and their perceived consequences in a representative sample of some 2000 British adults. J. Sleep Res. 13, 359–371. ( 10.1111/j.1365-2869.2004.00418.x) [DOI] [PubMed] [Google Scholar]
- 46.Middelkoop HA, Smilde-van den Doel DA, Neven AK, Kamphuisen HA, Springer CP. 1996. Subjective sleep characteristics of 1,485 males and females aged 50–93: effects of sex and age, and factors related to self-evaluated quality of sleep. J. Gerontol. A Biol. Sci. Med. Sci. 51, M108–M115. ( 10.1093/gerona/51A.3.M108) [DOI] [PubMed] [Google Scholar]
- 47.Lindberg E, Janson C, Gislason T, Bjornsson E, Hetta J, Boman G. 1997. Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep 20, 381–387. [DOI] [PubMed] [Google Scholar]
- 48.Zhang B, Wing YK. 2006. Sex differences in insomnia: a meta-analysis. Sleep 29, 85–93. [DOI] [PubMed] [Google Scholar]
- 49.Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. 2007. Epidemiology of the human circadian clock. Sleep Med. Rev. 11, 429–438. ( 10.1016/j.smrv.2007.07.005) [DOI] [PubMed] [Google Scholar]
- 50.Adan A, Natale V. 2002. Gender differences in morningness–eveningness preference. Chronobiol. Int. 19, 709–720. ( 10.1081/CBI-120005390) [DOI] [PubMed] [Google Scholar]
- 51.Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SBS, Santhi N, Schoen MW, Czeisler CA, Duffy JF. 2010. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J. Biol. Rhythms 25, 288–296. ( 10.1177/0748730410374943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duffy JF, et al. 2011. Quantification of behavior Sackler Colloquium: sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl Acad. Sci. USA 108, 15 602–15 608. ( 10.1073/pnas.1010666108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baker FC, Sassoon SA, Kahan T, Palaniappan L, Nicholas CL, Trinder J, Colrain IM. 2012. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J. Sleep Res. 21, 535–545. ( 10.1111/j.1365-2869.2012.01007.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krishnan V, Collop NA. 2006. Gender differences in sleep disorders. Curr. Opin. Pulm. Med. 12, 383–389. ( 10.1097/01.mcp.0000245705.69440.6a) [DOI] [PubMed] [Google Scholar]
- 55.Mallampalli MP, Carter CL. 2014. Exploring sex and gender differences in sleep health: a Society for Women's Health Research Report. J. Womens Health 23, 553–562. ( 10.1089/jwh.2014.4816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ancoli-Israel S, Roth T. 1999. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep 22(Suppl 2), S347–S353. [PubMed] [Google Scholar]
- 57.Roth T. 2007. Insomnia: definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. 3, S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 58.National Institutes of Health. 2005. National Institutes of Health State of the Science Conference statement on manifestations and management of chronic insomnia in adults, June 13–15, 2005. Sleep 28, 1049–1057. [DOI] [PubMed] [Google Scholar]
- 59.Ohayon M. 1996. Epidemiological study on insomnia in the general population. Sleep 19, S7–S15. [DOI] [PubMed] [Google Scholar]
- 60.Mitchell ES, Woods NF. 1996. Symptom experiences of midlife women: observations from the Seattle Midlife Women's Health Study. Maturitas 25, 1–10. ( 10.1016/0378-5122(96)01047-X) [DOI] [PubMed] [Google Scholar]
- 61.Grewal R, Doghramji K. 2010. Epidemiology of insomnia. In Clinical handbook of insomnia (eds HP Attarian, C Schuman), pp 13–22. Totowa, NJ: Humana Press ( 10.1007/978-1-60327-042-7_2) [DOI] [Google Scholar]
- 62.Camhi SL, Morgan WJ, Pernisco N, Quan SF. 2000. Factors affecting sleep disturbances in children and adolescents. Sleep Med. 1, 117–123. ( 10.1016/S1389-9457(99)00005-2) [DOI] [PubMed] [Google Scholar]
- 63.Johnson EO, Roth T, Schultz L, Breslau N. 2006. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics 117, e247–e256. ( 10.1542/peds.2004-2629) [DOI] [PubMed] [Google Scholar]
- 64.Owens JF, Matthews KA. 1998. Sleep disturbance in healthy middle-aged women. Maturitas 30, 41–50. ( 10.1016/S0378-5122(98)00039-5) [DOI] [PubMed] [Google Scholar]
- 65.Lord C, Sekerovic Z, Carrier J. 2014. Sleep regulation and sex hormones exposure in men and women across adulthood. Pathol. Biol. 62, 302–310. ( 10.1016/j.patbio.2014.07.005) [DOI] [PubMed] [Google Scholar]
- 66.Wittert G. 2014. The relationship between sleep disorders and testosterone in men. Asian J. Androl. 16, 262–265. ( 10.4103/1008-682X.122586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luboshitzky R, Zabari Z, Shen-Orr Z, Herer P, Lavie P. 2001. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J. Clin. Endocrinol. Metab. 86, 1134–1139. ( 10.1210/jcem.86.3.7296) [DOI] [PubMed] [Google Scholar]
- 68.Schmid SM, Hallschmid M, Jauch-Chara K, Lehnert H, Schultes B. 2012. Sleep timing may modulate the effect of sleep loss on testosterone. Clin. Endocrinol. 77, 749–754. ( 10.1111/j.1365-2265.2012.04419.x) [DOI] [PubMed] [Google Scholar]
- 69.Jauch-Chara K, Schmid SM, Hallschmid M, Oltmanns KM, Schultes B. 2013. Pituitary–gonadal and pituitary–thyroid axis hormone concentrations before and during a hypoglycemic clamp after sleep deprivation in healthy men. PLoS ONE 8, e54209 ( 10.1371/journal.pone.0054209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barrett-Connor E, Dam TT, Stone K, Harrison SL, Redline S, Orwoll E. 2008. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J. Clin. Endocrinol. Metab. 93, 2602–2609. ( 10.1210/jc.2007-2622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu PY, Yee B, Wishart SM, Jimenez M, Jung DG, Grunstein RR, Handelsman DJ. 2003. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J. Clin. Endocrinol. Metab. 88, 3605–3613. ( 10.1210/jc.2003-030236) [DOI] [PubMed] [Google Scholar]
- 72.Venancio DP, Tufik S, Garbuio SA, da Nobrega AC, de Mello MT. 2008. Effects of anabolic androgenic steroids on sleep patterns of individuals practicing resistance exercise. Eur. J. Appl. Physiol. 102, 555–560. ( 10.1007/s00421-007-0621-6) [DOI] [PubMed] [Google Scholar]
- 73.Matsumoto AM, Sandblom RE, Schoene RB, Lee KA, Giblin EC, Pierson DJ, Bremner WJ. 1985. Testosterone replacement in hypogonadal men: effects on obstructive sleep apnoea, respiratory drives, and sleep. Clin. Endocrinol. 22, 713–721. ( 10.1111/j.1365-2265.1985.tb00161.x) [DOI] [PubMed] [Google Scholar]
- 74.Schneider BK, Pickett CK, Zwillich CW, Weil JV, McDermott MT, Santen RJ, Varano LA, White DP. 1986. Influence of testosterone on breathing during sleep. J. Appl. Physiol. (1985) 61, 618–623. [DOI] [PubMed] [Google Scholar]
- 75.Sandblom RE, Matsumoto AM, Schoene RB, Lee KA, Giblin EC, Bremner WJ, Pierson DJ. 1983. Obstructive sleep apnea syndrome induced by testosterone administration. N. Engl. J. Med. 308, 508–510. ( 10.1056/NEJM198303033080908) [DOI] [PubMed] [Google Scholar]
- 76.Berghaus TM, Faul C, Unterer F, Thilo C, von Scheidt W, Schwaiblmair M et al. 2012. Acute pulmonary embolism in patients with obstructive sleep apnoea: does it affect the severity of sleep-disordered breathing? Sleep Breath 16, 1267–1269. ( 10.1007/s11325-011-0633-7) [DOI] [PubMed] [Google Scholar]
- 77.Savard J, Hervouet S, Ivers H. 2013. Prostate cancer treatments and their side effects are associated with increased insomnia. Psychooncology 22, 1381–1388. ( 10.1002/pon.3150) [DOI] [PubMed] [Google Scholar]
- 78.Grunfeld EA, Halliday A, Martin P, Drudge-Coates L. 2012. Andropause syndrome in men treated for metastatic prostate cancer: a qualitative study of the impact of symptoms. Cancer Nurs. 35, 63–69. ( 10.1097/NCC.0b013e318211fa92) [DOI] [PubMed] [Google Scholar]
- 79.Gerber GS, Zagaja GP, Ray PS, Rukstalis DB. 2000. Transdermal estrogen in the treatment of hot flushes in men with prostate cancer. Urology 55, 97–101. ( 10.1016/S0090-4295(99)00370-2) [DOI] [PubMed] [Google Scholar]
- 80.Miller JI, Ahmann FR. 1992. Treatment of castration-induced menopausal symptoms with low dose diethylstilbestrol in men with advanced prostate cancer. Urology 40, 499–502. ( 10.1016/0090-4295(92)90401-H) [DOI] [PubMed] [Google Scholar]
- 81.Sakai H, et al. 2009. Hot flashes during androgen deprivation therapy with luteinizing hormone-releasing hormone agonist combined with steroidal or nonsteroidal antiandrogen for prostate cancer. Urology 73, 635–640. ( 10.1016/j.urology.2008.09.013) [DOI] [PubMed] [Google Scholar]
- 82.Wassersug RJ, Gray R. 2011. The health and well-being of prostate cancer patients and male-to-female transsexuals on androgen deprivation therapy: a qualitative study with comments on expectations and estrogen. Psychol. Health Med. 16, 39–52. ( 10.1080/13548506.2010.516364) [DOI] [PubMed] [Google Scholar]
- 83.Baker FC, Driver HS. 2007. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 8, 613–622. ( 10.1016/j.sleep.2006.09.011) [DOI] [PubMed] [Google Scholar]
- 84.Moline ML, Broch L, Zak R. 2004. Sleep in women across the life cycle from adulthood through menopause. Med. Clin. North Am. 88, 705–7736, ix ( 10.1016/j.mcna.2004.01.009) [DOI] [PubMed] [Google Scholar]
- 85.Lee KA. 1998. Alterations in sleep during pregnancy and postpartum: a review of 30 years of research. Sleep Med. Rev. 2, 231–242. ( 10.1016/S1087-0792(98)90010-7) [DOI] [PubMed] [Google Scholar]
- 86.de Zambotti M, Willoughby AR, Sassoon SA, Colrain IM, Baker FC. 2015. Menstrual cycle-related variation in physiological sleep in women in the early menopausal transition. J. Clin. Endocrinol. Metab. 100, 2918–2926. ( 10.1210/jc.2015-1844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA. 1996. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J. Clin. Endocrinol. Metab. 81, 728–735. [DOI] [PubMed] [Google Scholar]
- 88.Baker FC, Kahan TL, Trinder J, Colrain IM. 2007. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep 30, 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chuong CJ, Kim SR, Taskin O, Karacan I. 1997. Sleep pattern changes in menstrual cycles of women with premenstrual syndrome: a preliminary study. Am. J. Obstet. Gynecol. 177, 554–558. ( 10.1016/S0002-9378(97)70145-5) [DOI] [PubMed] [Google Scholar]
- 90.Baker FC, Driver HS, Paiker J, Rogers GG, Mitchell D. 2002. Acetaminophen does not affect 24-h body temperature or sleep in the luteal phase of the menstrual cycle. J. Appl. Physiol. 92, 1684–1691. ( 10.1152/japplphysiol.00919.2001) [DOI] [PubMed] [Google Scholar]
- 91.Baker FC, Mitchell D, Driver HS. 2001. Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Arch. 442, 729–737. ( 10.1007/s004240100582) [DOI] [PubMed] [Google Scholar]
- 92.Burdick RS, Hoffmann R, Armitage R. 2002. Short note: oral contraceptives and sleep in depressed and healthy women. Sleep 25, 347–349. [PubMed] [Google Scholar]
- 93.Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D. 2001. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J. Physiol. 530, 565–574. ( 10.1111/j.1469-7793.2001.0565k.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ. 2012. Executive summary of the stages of reproductive aging workshop+10: addressing the unfinished agenda of staging reproductive aging. Menopause 19, 387–395. ( 10.1097/gme.0b013e31824d8f40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Polo-Kantola PI. 2008. Dealing with menopausal sleep disturbances. Sleep Med. Clin. 3, 121–131. ( 10.1016/j.jsmc.2007.10.006) [DOI] [Google Scholar]
- 96.Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, Sowers MFR. 2008. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep 31, 979–990. [PMC free article] [PubMed] [Google Scholar]
- 97.Lampio L, Polo-Kantola P, Polo O, Kauko T, Aittokallio J, Saaresranta T. 2014. Sleep in midlife women: effects of menopause, vasomotor symptoms, and depressive symptoms. Menopause 21, 1217–1224. ( 10.1097/GME.0000000000000239) [DOI] [PubMed] [Google Scholar]
- 98.Berecki-Gisolf J, Begum N, Dobson AJ. 2009. Symptoms reported by women in midlife: menopausal transition or aging? Menopause 16, 1021–1029. ( 10.1097/gme.0b013e3181a8c49f) [DOI] [PubMed] [Google Scholar]
- 99.Kravitz HM, Joffe H. 2011. Sleep during the perimenopause: a SWAN story. Obstet. Gynecol. Clin. North Am. 38, 567–586. ( 10.1016/j.ogc.2011.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woods NF, Mitchell ES. 2010. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Sleep 33, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharkey KM, Bearpark HM, Acebo C, Millman RP, Cavallo A, Carskadon MA. 2003. Effects of menopausal status on sleep in midlife women. Behav. Sleep Med. 1, 69–80. ( 10.1207/S15402010BSM0102_1) [DOI] [PubMed] [Google Scholar]
- 102.Shaver J, Giblin E, Lentz M, Lee K. 1988. Sleep patterns and stability in perimenopausal women. Sleep 11, 556–561. [DOI] [PubMed] [Google Scholar]
- 103.Young T, Rabago D, Zgierska A, Austin D, Laurel F. 2003. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep 26, 667–672. [DOI] [PubMed] [Google Scholar]
- 104.Ohayon MM. 2006. Severe hot flashes are associated with chronic insomnia. Arch. Intern. Med. 166, 1262–1268. ( 10.1001/archinte.166.12.1262) [DOI] [PubMed] [Google Scholar]
- 105.Polo-Kantola P. 2011. Sleep problems in midlife and beyond. Maturitas 68, 224–232. ( 10.1016/j.maturitas.2010.12.009) [DOI] [PubMed] [Google Scholar]
- 106.Toth LA, Bhargava P. 2013. Animal models of sleep disorders. Comp. Med. 63, 91–104. [PMC free article] [PubMed] [Google Scholar]
- 107.Franken P, Dudley CA, Estill SJ, Barakat M, Thomason R, O'Hara BF, McKnight SL. 2006. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proc. Natl Acad. Sci. USA 103, 7118–7123. ( 10.1073/pnas.0602006103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cusmano DM, Hadjimarkou MM, Mong JA. 2014. Gonadal steroid modulation of sleep and wakefulness in male and female rats is sexually differentiated and neonatally organized by steroid exposure. Endocrinology 155, 204–214. ( 10.1210/en.2013-1624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Koehl M, Battle S, Meerlo P. 2006. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep 29, 1224–1231. [DOI] [PubMed] [Google Scholar]
- 110.Paul KN, Dugovic C, Turek FW, Laposky AD. 2006. Diurnal sex differences in the sleep–wake cycle of mice are dependent on gonadal function. Sleep 29, 1211–1223. [DOI] [PubMed] [Google Scholar]
- 111.Fang J, Fishbein W. 1996. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 734, 275–285. ( 10.1016/0006-8993(96)00652-X) [DOI] [PubMed] [Google Scholar]
- 112.Ehlen JC, Hesse S, Pinckney L, Paul KN. 2013. Sex chromosomes regulate nighttime sleep propensity during recovery from sleep loss in mice. PLoS ONE 8, e62205 ( 10.1371/journal.pone.0062205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yamaoka S. 1980. Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved. Brain Res. 185, 385–398. ( 10.1016/0006-8993(80)91076-8) [DOI] [PubMed] [Google Scholar]
- 114.Wibowo E, Deurveilher S, Wassersug RJ, Semba K. 2012. Estradiol treatment modulates spontaneous sleep and recovery after sleep deprivation in castrated male rats. Behav. Brain Res. 226, 456–464. ( 10.1016/j.bbr.2011.09.045) [DOI] [PubMed] [Google Scholar]
- 115.Colvin GB, Whitmoyer DI, Lisk RD, Walter DO, Sawyer CH. 1968. Changes in sleep-wakefulness in female rats during circadian and estrous cycles. Brain Res. 7, 173–181. ( 10.1016/0006-8993(68)90095-4) [DOI] [PubMed] [Google Scholar]
- 116.Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA. 2008. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur. J. Neurosci. 27, 1780–1792. ( 10.1111/j.1460-9568.2008.06142.x) [DOI] [PubMed] [Google Scholar]
- 117.Kleinlogel H. 1983. The female rat's sleep during oestrous cycle. Neuropsychobiology 10, 228–237. ( 10.1159/000118016) [DOI] [PubMed] [Google Scholar]
- 118.Schwierin B, Borbely AA, Tobler I. 1998. Sleep homeostasis in the female rat during the estrous cycle. Brain Res. 811, 96–104. ( 10.1016/S0006-8993(98)00991-3) [DOI] [PubMed] [Google Scholar]
- 119.Branchey M, Branchey L, Nadler RD. 1971. Effects of estrogen and progesterone on sleep patterns of female rats. Physiol. Behav. 6, 743–746. ( 10.1016/0031-9384(71)90267-8) [DOI] [PubMed] [Google Scholar]
- 120.Colvin GB, Whitmoyer DI, Sawyer CH. 1969. Circadian sleep–wakefulness patterns in rats after ovariectomy and treatment with estrogen. Exp. Neurol. 25, 616–625. ( 10.1016/0014-4886(69)90104-6) [DOI] [PubMed] [Google Scholar]
- 121.Deurveilher S, Rusak B, Semba K. 2009. Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats. Sleep 32, 865–877. [PMC free article] [PubMed] [Google Scholar]
- 122.Deurveilher S, Rusak B, Semba K. 2011. Female reproductive hormones alter sleep architecture in ovariectomized rats. Sleep 34, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koehl M, Battle SE, Turek FW. 2003. Sleep in female mice: a strain comparison across the estrous cycle. Sleep 26, 267–272. [DOI] [PubMed] [Google Scholar]
- 124.Paul KN, Laposky AD, Turek FW. 2009. Reproductive hormone replacement alters sleep in mice. Neurosci. Lett. 463, 239–243. ( 10.1016/j.neulet.2009.07.081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwartz MD, Mong JA. 2013. Estradiol modulates recovery of REM sleep in a time-of-day-dependent manner. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R271–R280. ( 10.1152/ajpregu.00474.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deurveilher S, Seary ME, Semba K. 2013. Ovarian hormones promote recovery from sleep deprivation by increasing sleep intensity in middle-aged ovariectomized rats. Horm. Behav. 63, 566–576. ( 10.1016/j.yhbeh.2013.02.011) [DOI] [PubMed] [Google Scholar]
- 127.Schwartz MD, Mong JA. 2011. Estradiol suppresses recovery of REM sleep following sleep deprivation in ovariectomized female rats. Physiol. Behav. 104, 962–971. ( 10.1016/j.physbeh.2011.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McCarthy MM, Nugent BM. 2015. At the frontier of epigenetics of brain sex differences. Front. Behav. Neurosci. 9, 221 ( 10.3389/fnbeh.2015.00221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arnold AP. 2009. The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 55, 570–578. ( 10.1016/j.yhbeh.2009.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Branchey L, Branchey M, Nadler RD. 1973. Effects of sex hormones on sleep patterns of male rats gonadectomized in adulthood and in the neonatal period. Physiol. Behav. 11, 609–611. ( 10.1016/0031-9384(73)90244-8) [DOI] [PubMed] [Google Scholar]
- 131.Mong JA, Devidze N, Frail DE, O'Connor LT, Samuel M, Choleris E, Ogawa S, Pfaff DW. 2003. Estradiol differentially regulates lipocalin-type prostaglandin D synthase transcript levels in the rodent brain: evidence from high-density oligonucleotide arrays and in situ hybridization. Proc. Natl Acad. Sci. USA 100, 318–323. ( 10.1073/pnas.262663799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Urade Y, Hayaishi O. 2011. Prostaglandin D2 and sleep/wake regulation. Sleep Med. Rev. 15, 411–418. ( 10.1016/j.smrv.2011.08.003) [DOI] [PubMed] [Google Scholar]
- 133.Cusmano DM, Viechweg S, Mong JA. 2013. Blockade of estrogenic action in the MnPN disrupts sleep/wake behavior in the female rat. Sleep 36, A11. [Google Scholar]
- 134.Silveyra P, Cataldi NI, Lux-Lantos VA, Libertun C. 2010. Role of orexins in the hypothalamic–pituitary–ovarian relationships. Acta Physiol. 198, 355–360. ( 10.1111/j.1748-1716.2009.02049.x) [DOI] [PubMed] [Google Scholar]
- 135.Silveyra P, Cataldi NI, Lux-Lantos V, Libertun C. 2009. Gonadal steroids modulated hypocretin/orexin type-1 receptor expression in a brain region, sex and daytime specific manner. Regul. Pept. 158, 121–126. ( 10.1016/j.regpep.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 136.Silveyra P, Catalano PN, Lux-Lantos V, Libertun C. 2007. Impact of proestrous milieu on expression of orexin receptors and prepro-orexin in rat hypothalamus and hypophysis: actions of Cetrorelix and Nembutal. Am. J. Physiol. Endocrinol. Metab. 292, E820–E828. ( 10.1152/ajpendo.00467.2006) [DOI] [PubMed] [Google Scholar]
- 137.Porkka-Heiskanen T, Kalinchuk A, Alanko L, Huhtaniemi I, Stenberg D. 2004. Orexin A and B levels in the hypothalamus of female rats: the effects of the estrous cycle and age. Eur. J. Endocrinol. 150, 737–742. ( 10.1530/eje.0.1500737) [DOI] [PubMed] [Google Scholar]
- 138.Deurveilher S, Cumyn EM, Peers T, Rusak B, Semba K. 2008. Estradiol replacement enhances sleep deprivation-induced c-Fos immunoreactivity in forebrain arousal regions of ovariectomized rats. Am. J. Physiol. 295, R1328–R1340. ( 10.1152/ajpregu.90576.2008) [DOI] [PubMed] [Google Scholar]
- 139.Saper CB, Cano G, Scammell TE. 2005. Homeostatic, circadian, and emotional regulation of sleep. J. Comp. Neurol. 493, 92–98. ( 10.1002/cne.20770) [DOI] [PubMed] [Google Scholar]
- 140.Yan L, Silver R. 2015. Neuroendocrine underpinnings of sex differences in circadian timing systems. J. Steroid Biochem. Mol. Biol. ( 10.1016/j.jsbmb.2015.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arnold AP. 2004. Sex chromosomes and brain gender. Nat. Rev. Neurosci. 5, 701–708. ( 10.1038/nrn1494) [DOI] [PubMed] [Google Scholar]
- 142.Gimenez S, Romero S, Gich I, Clos S, Grasa E, Rosa-María A, Barbanoj M-J. 2011. Sex differences in sleep after a single oral morning dose of olanzapine in healthy volunteers. Hum. Psychopharmacol. 26, 498–507. ( 10.1002/hup.1232) [DOI] [PubMed] [Google Scholar]