Abstract
Recent years have witnessed an increased attention to studies of sex differences, partly because such differences offer important considerations for personalized medicine. While the presence of sex differences in human behaviour is well documented, our knowledge of their anatomical foundations in the brain is still relatively limited. As a natural gateway to fathom the human mind and behaviour, studies concentrating on the human brain network constitute an important segment of the research effort to investigate sex differences. Using a large sample of healthy young individuals, each assessed with diffusion MRI and a computerized neurocognitive battery, we conducted a comprehensive set of experiments examining sex-related differences in the meso-scale structures of the human connectome and elucidated how these differences may relate to sex differences at the level of behaviour. Our results suggest that behavioural sex differences, which indicate complementarity of males and females, are accompanied by related differences in brain structure across development. When using subnetworks that are defined over functional and behavioural domains, we observed increased structural connectivity related to the motor, sensory and executive function subnetworks in males. In females, subnetworks associated with social motivation, attention and memory tasks had higher connectivity. Males showed higher modularity compared to females, with females having higher inter-modular connectivity. Applying multivariate analysis, we showed an increasing separation between males and females in the course of development, not only in behavioural patterns but also in brain structure. We also showed that these behavioural and structural patterns correlate with each other, establishing a reliable link between brain and behaviour.
Keywords: sex, gender, diffusion imaging, connectome, structural connectivity, behaviour
1. Background
While the presence of sex differences in human behaviour and cognition is well documented [1,2], our knowledge of anatomical foundations for such sex differences, specifically in the brain, is still relatively limited. The enigmatic interplay between brain and behaviour, and its modulation by sex, has intrigued scientists, philosophers and the general public for decades. Behavioural differences include, but are not limited to, enhanced motor [3,4] and spatial skills [5,6] in males and improved memory [7,8] and social cognition [9,10] skills in females [11]. These differences may be attributed to the complementary roles that the sexes play in procreation and social structure; however, increasing evidence suggests the presence of corresponding sex differences in brain structure and function [12–17], as well as the presence of a strong connection between behaviour and brain structure [12,18]. Thus, relying merely on social and cultural effects to explain sex-induced variance in behaviour seems insufficient. Notably, although sex differences in the brain are present even in childhood [16,17], the developmental trajectory of sex differences as related to the brain–behaviour relationship remains relatively unexamined.
With the advent of neuroimaging, both the structure and function of the human brain can be studied in vivo. This capacity facilitates enquiries on large samples and investigation of possible differences between males and females. Several research groups [19–23] including ours [17] have used diffusion tensor imaging (DTI) [24] to study sex-related structural differences in the brain, as characterized by alterations in white matter (WM) microstructure and fibre tracts that connect different grey matter (GM) regions, as well as in overall communication architecture of the brain network. Studying the structural brain network, also known as the structural connectome [25], enables the inference of current and future states of brain connectivity at local, global and intermediate (meso) scales. In a connectome, the identification of network properties pertaining to meso-scale structures such as communities [26] or the communication backbone [27] can reveal how the complex human behavioural repertoire emerges from the simultaneous processes of segregated neuronal clusters and their integration during complicated cognitive tasks [28,29]. In this work, we have investigated sex differences in the meso-scale architecture, specifically in subnetworks, of the structural connectome. A subnetwork is a collection of brain regions (nodes) and their connections (edges) that forms a part of the whole-brain network [30,31].
Previous work on sex differences in brain structure suggested diverse outcomes including microstructural differences demonstrated via changes in DTI-based measures, like fractional anisotropy (FA); namely, increased FA values in major WM regions and tracts in males [19,20,23], and in the corpus callosum in females [22]. Consistent with these findings, we have performed a comprehensive analysis on the structural connectome to elucidate sex differences in terms of individual connections [17], revealing stronger intra-hemispheric connectivity (within both hemispheres) in males and stronger inter-hemispheric connectivity in females. The developmental course of sex differences is a less explored domain [11], mostly due to lack of large datasets spanning age ranges that include the developmental period, with a few exceptions in the case of structural, functional and behavioural modalities [11,16,17,21,32].
Although it is generally tempting to derive (possibly stereotypical) conclusions based on the structural and functional sex differences in the brain, pertaining to the behavioural manifestations, scientifically sound inferences require a multimodal investigation that includes behavioural measurements as a modality. The Philadelphia Neurodevelopmental Cohort (PNC) dataset [33], which is a collection of structural, functional and behavioural modalities, provides a unique opportunity to achieve this investigational purpose. Using a large sample of healthy young individuals from the PNC dataset, each assessed using diffusion MRI and a computerized neurocognitive battery (CNB), we identified structural changes in the subnetworks of the structural connectome and elucidated how these changes may relate to sex differences at the level of behaviour. In our previous work [17], the analyses were mostly limited to individual connections between regions, while in this work, we have mainly studied subnetworks of the brain network in order to establish a reliable link between brain structure and behaviour. Hence, our findings on the structural connectome augment but do not repeat our previously reported findings. The analyses were repeated in different age ranges to render a developmental portrait of sex differences. Our results suggest that sex-related differences in functional and behavioural dimensions are accompanied by related changes in the network properties of the structural connectome, establishing a reliable link between brain structure and behaviour.
2. Results
Presented here are our findings on the network-level sex differences in the human structural connectome, as well as their relation to sex differences in behaviour. First, we identified overall sex-related changes in the network topology of the structural connectome and explored possible differences between male and female groups in terms of alliance of the brain regions, i.e. the way the subnetworks are formed. Then, we identified group differences on these subnetworks by comparing intra- and inter-subnetwork connectivities between males and females. Finally, we established possible links between features of subnetworks and the behavioural measures, with an aim of demonstrating the interplay between brain structure and behaviour. For the developmental analysis, we divided the entire dataset into three age ranges, namely children (aged 8–13.3 years), adolescents (aged 13.4–17 years) and young adults (aged 17.1–22 years). The age ranges were chosen to reflect conventional classification and also to ensure that the samples are balanced in terms of the number of participants.
(a). Network topology of the structural connectome
We first investigated differences in network topology of the structural connectome between males and females. We measured the modularity of the network, a measure of structural/functional segregation that quantifies the degree to which the network can be subdivided into densely interconnected groups of regions [34]. A higher modularity means increased segregation of such groups of regions, as well as increased integration inside the groups, possibly pertaining to the functional specialization of neuronal clusters. In our experiments, males showed significantly higher modularity compared with females, evident in all age ranges (children, p < 0.05; adolescents, p < 0.01; young adults, p < 0.01).
We also compared intra- and inter-modular communications between groups, by first extracting modules of the brain network for each participant using the Louvain community detection algorithm [35]. Then, we calculated the average connectivity within the modules (intra-modular) and between the modules (inter-modular). Consistent with the decreased modularity, females had higher inter-modular connectivity, with significant differences emerging only with the entire dataset (p < 0.05) and in the oldest age range (p < 0.01).
(b). Alliance of brain regions into subnetworks
Before performing further analysis on structurally cohesive subnetworks, using the methodology that is described in §4, we first investigated whether there was a statistically significant difference between males and females in the alliance of the brain regions into subnetworks. The absence of such a difference demonstrated that a common subnetwork portrait could be defined for the entire sample to facilitate a comparison between groups. With a significance level of 0.05, we could not find any statistically significant sex difference between the regional compositions of the subnetworks. We repeated this experiment for different age ranges and with different subnetwork scales (i.e. the number and size of subnetworks), with the same result.
(c). Connectivity within and between subnetworks
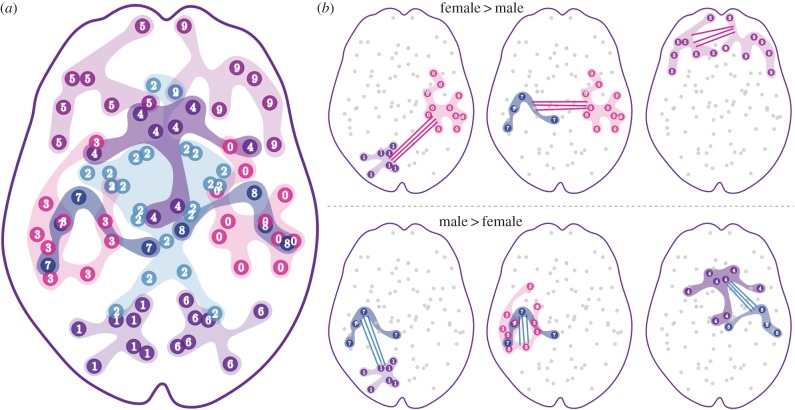
We defined three types of subnetworks (see §4(e) for details). First, we extracted structurally cohesive subnetworks by detecting densely connected brain regions (also known as communities [26]). An illustration of such 10 subnetworks is given in figure 1. We repeated this analysis with different numbers of subnetworks, with similar results. Second, we defined subnetworks based on the functional definitions of regional combinations, adapted from the fMRI literature. Third, we defined subnetworks that are putatively associated with specific behavioural domains, based on neuropsychological literature.
Figure 1.
An illustration of structurally cohesive subnetworks, with 10 subnetworks (a), and a set of example group differences between males and females, with mean connectivity within and between subnetworks being compared (b). Females show higher connectivity in the inter-hemispheric connections, and males have higher connectivity in the intra-hemispheric connections. The complete set of group differences is given in table 1. (Online version in colour.)
Consistent with our previously published edge-wise analysis [17], the connectivity within and between structurally cohesive subnetworks, when the connections were predominantly inter-hemispheric, was higher in females, and was higher in males when connections were predominantly intra-hemispheric. Illustrative results are shown in figure 1, and a complete list of group differences is provided in table 1.
Table 1.
Summary of sex differences on the connectivity within and between structurally cohesive subnetworks. Subnetworks are illustrated in figure 1. FDR, false discovery rate correction.
| mean (s.d.) |
statistical analysis |
|||
|---|---|---|---|---|
| connectivity | male | female | statistic (t) | p-value (FDR) |
| M1–M7 | 128.27 (35.72) | 115.06 (28.87) | 3.15 | 0.016 |
| M3–M7 | 42.92 (12.75) | 36.96 (10.71) | 3.09 | 0.016 |
| M4–M8 | 104.93 (31.24) | 93.57 (24.26) | 2.98 | 0.018 |
| M0–M1 | 0.93 (0.88) | 1.08 (1.01) | −2.69a | 0.037 |
| M0–M2 | 21.43 (4.93) | 21.57 (5.08) | −3.56a | 0.006 |
| M0–M7 | 0.19 (0.20) | 0.27 (0.36) | −3.02a | 0.018 |
| M3–M6 | 1.61 (1.42) | 1.88 (1.68) | −2.68a | 0.037 |
| M3–M9 | 0.03 (0.05) | 0.04 (0.06) | −3.31a | 0.011 |
| M4–M4 | 208.85 (57.44) | 213.76 (54.69) | −3.55a | 0.006 |
| M5–M9 | 16.72 (9.57) | 19.26 (11.61) | −3.56a | 0.006 |
| M7–M9 | 0.09 (0.12) | 0.13 (0.18) | −4.03a | 0.003 |
aHigher connectivity in females.
For the functionally defined subnetworks, the structural connectivity between motor, sensory (auditory and visual) and default mode subnetworks, as well as subnetworks associated with executive control tasks (fronto-parietal and cingulo-opercular) was higher in males. In females, connectivity among subcortical, sensory and attention subnetworks was higher. The results are listed in table 2.
Table 2.
Summary of sex differences on the connectivity within and between functionally defined subnetworks. FDR, false discovery rate correction.
| mean (s.d.) |
statistical analysis |
|||
|---|---|---|---|---|
| connectivity | male | female | statistic (t) | p-value (FDR) |
| motor–auditory | 30.80 (10.27) | 26.20 (9.00) | 4.77 | 0.000 |
| motor–fronto-parietal | 59.11 (14.38) | 52.05 (11.93) | 2.77 | 0.039 |
| cingulo-opercular–dorsal attention | 60.60 (21.56) | 52.21 (17.84) | 3.22 | 0.018 |
| default mode–subcortical | 34.70 (11.45) | 30.68 (10.13) | 2.69 | 0.045 |
| default mode–visual | 34.52 (10.11) | 30.08 (8.78) | 2.91 | 0.034 |
| subcortical–others | 13.17 (3.62) | 13.16 (3.35) | −2.8a | 0.039 |
| subcortical–auditory | 20.88 (7.59) | 22.02 (7.73) | −3.82a | 0.004 |
| dorsal attention–visual | 118.93 (34.18) | 117.62 (31.77) | −2.99a | 0.031 |
| ventral attention–auditory | 7.77 (5.43) | 7.60 (5.23) | −3.28a | 0.018 |
aHigher connectivity in females.
Using the subnetworks that are associated with specific behavioural domains (see table 3 for assignment of nodes to the subnetworks), we observed higher connectivity in males related to the motor, sensory and executive function subnetworks. In reward and memory subnetworks, we observed higher connectivity in females. Table 4 lists all significant group differences.
Table 3.
Assignment of brain regions to subnetworks defined over behavioural domains. Regions from both hemispheres are included, unless otherwise specified in parentheses. Several regions are assigned to multiple behavioural domains. (Online version in colour.)
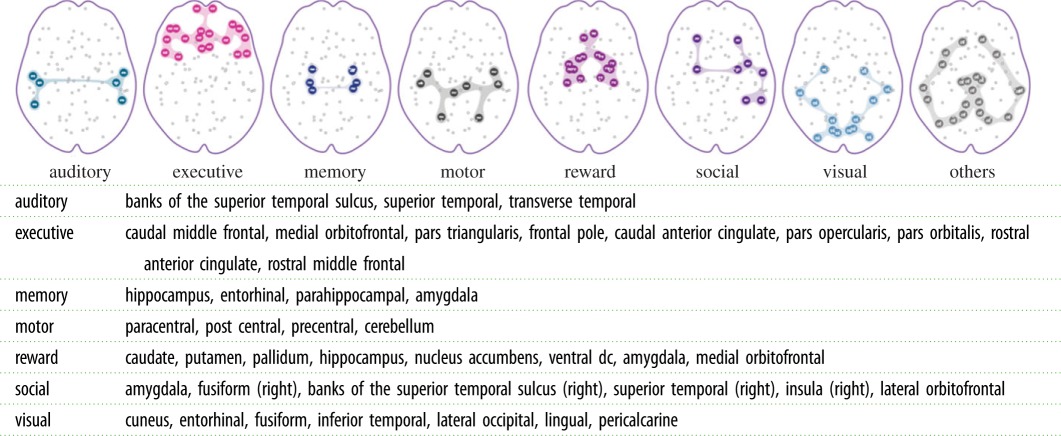 |
Table 4.
Summary of sex differences on the connectivity within and between subnetworks that are defined over behavioural domains. FDR, false discovery rate correction.
| mean (s.d.) |
statistical analysis |
|||
|---|---|---|---|---|
| connectivity | male | female | statistic (t) | p-value (FDR) |
| motor–executive | 27.50 (7.58) | 23.11 (5.93) | 3.75 | 0.003 |
| motor–auditory | 23.13 (7.70) | 19.67 (6.76) | 4.79 | 0.000 |
| reward–auditory | 18.00 (6.36) | 19.00 (6.49) | −3.66a | 0.003 |
| memory–auditory | 22.43 (7.94) | 22.95 (7.58) | −2.82a | 0.045 |
aHigher connectivity in females.
(d). Cognition and motor abilities
We then investigated sex differences in performance on the CNB. Sex differences in cognition and motor abilities in different subsets of the PNC dataset have been repeatedly reported [11,16]. We confirmed these group differences in our subsample of PNC participants. Specifically, in the complex reasoning category of the CNB [11], males were more accurate on spatial and language tasks, while females were faster on non-verbal reasoning (p < 0.05, FDR). In motor and sensorimotor tasks, males performed faster (p < 0.05, FDR). Although females were faster in emotion identification, unlike previously reported results, the difference was not statistically significant (p = 0.26, FDR) in our sample.
(e). Multivariate pattern analysis for classification of sex
The univariate analyses that we have performed thus far help investigate simple structural and behavioural differences between males and females, based on single features such as the mean connectivity in a specific subnetwork or the accuracy of a single cognitive task. Next, we undertook a multivariate analysis to facilitate the investigation of complex interactive patterns of behaviour or connectivity. By training a pattern classifier to distinguish between males and females, we can learn how multiple features (e.g. connectivity of multiple subnetworks) interact and contribute towards the separation of the groups.
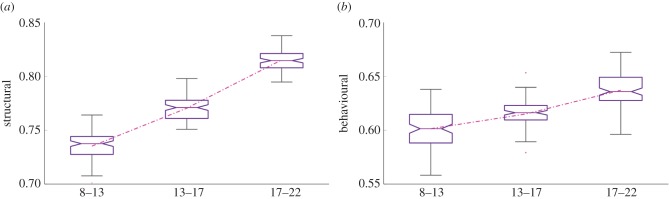
We trained two separate classifiers using structural subnetwork features and behavioural features (see §4(f)). The classification using subnetwork features yielded an overall accuracy of 0.79, while the accuracy of the behavioural classifier was 0.64. Thus, the separation between males and females at the level of brain structure was significantly larger (p < 0.01) compared with the separation at the level of behaviour. For both classifiers, there was a trend of increasing classification accuracies with age, with the highest accuracy achieved when the oldest groups were used; this is illustrated in figure 2.
Figure 2.
Classification accuracy (y-axis) with different age groups in years (x-axis), when using (a) structural connectivity patterns and (b) behavioural patterns. Box plots depict the range of the classification accuracy as estimated using 10-fold cross-validation, repeated by 100 randomization. For both cases, the average classification accuracy increases steadily across development, although the structural scores show a higher increase. (Online version in colour.)
We then assigned each participant a dimensional classification score (for each classifier separately), quantifying the certainty of assignment of the participant to its sex group. This was used to examine how patterns of connectivity and behaviour relate to each other, while being modulated by sex. The correlation between classification scores of structural and behavioural classifiers was statistically significant (p < 0.01), albeit small (0.31). This remained significant even when correlating the classification scores of male and female groups separately (female: 0.12, male: 0.16, p < 0.01), indicating that sex-typical patterns of behaviour and connectivity could be related within a given sex.
3. Discussion
Recent years have witnessed an increased attention to studies of sex differences due to their implications in personalized medicine, and gender-specific medicine has evolved as diseases follow a different aetiology based on gender [36]. As a natural gateway to fathom the human mind and behaviour, studies concentrating on human brain networks constitute an important segment of this sophisticated research effort to understand sex differences. In this work, we studied sex-related differences in brain connectivity by conducting a comprehensive set of experiments on the structural connectome, with an emphasis on its meso-scale structures.
Starting from the global properties of the brain network, we discerned important group differences. We observed higher modularity in males compared with females. This might be due to either increased intra-modular or decreased inter-modular connectivity in males. Simultaneously, females showed increased inter-modular connectivity, possibly consequent to increased inter-hemispheric connectivity [17] (see also table 1 and figure 1) since the modular structure of the human brain is predominantly shaped by the hemispheres. The distribution of connections between intra- and inter-modular communications may provide an insight into how the network manages to orchestrate segregated neuronal clusters, each of which is putatively associated with a different set of functions.
Notably, the reverse (i.e. decreased modularity and increased inter-modular connectivity in males) has been shown previously [16], using resting state fMRI connectivity in another subsample of the PNC dataset. These findings jointly render a complex picture of the simultaneous processes in the brain. The structural basis of the brain network points to a more integrated modular architecture for females beginning in early childhood, and perhaps the increased inter-modular functional connectivity in males may reflect compensation to attain integration at the functional level. Increased inter-modular functional connectivity is possibly achieved by using highly connected functional systems such as the default mode network (the regions of which are part of the structural core of the brain network [37]) that integrates cognitive processes [38]. Our results support such a hypothesis by demonstrating higher structural connectivity in the default mode network in males (table 2).
The alliance of brain regions when forming structurally cohesive subnetworks does not differ significantly between males and females, indicating a fixed reference frame within which we can compare subnetworks between groups without concern for bias by the sex of the sample being used. In other words, we can define a common set of subnetworks for males and females, and then compare them based on their subject-specific manifestation on inter- and intra-subnetwork connectivities. The resting state fMRI study [16] mentioned in §2(c) reported the same conclusion that the regional compositions of subnetworks do not differ between male and female brains. This finding is crucial as it indicates that the network organization of male and female brains does not differ despite the significant difference in volume between the sexes (in our sample, there was a significant difference in volume between the sexes, p < 0.01). As the alliance of individual brain regions is similar in males and females, one may stipulate similar functional associations for the neuronal clusters (subnetworks).
Links between brain structure and behaviour possibly rely on a complex interplay between multiple features of the neurobiological mechanisms in the brain network. Network theoretic studies pertaining to the properties of the structural connectome may provide pioneering insights into these links. Here, we have established sex-related differences in the connectivity of the subnetworks that were defined based on structural characteristics, functional systems and finally behavioural domains. From a structural perspective, when subnetworks were identified as structurally cohesive communities, we observed higher inter-hemispheric connections in females and increased intra-hemispheric connections in males (figure 1 and table 1). Elaborate behavioural inferences based on these differences, however, require direct and rigorous investigations, performed over specific functional systems of the brain.
In order to provide a better association between the structure and function of the brain, we delineated subnetworks based on the definitions of functional systems in the fMRI literature [39]. Analysis of these subnetworks revealed several functionally related differences between male and female groups. Consistent with the behavioural findings on sex differences [3,11], males had increased connectivity between motor and sensory (auditory) systems, along with increased connectivity in the fronto-parietal and cingulo-opercular systems that are traditionally associated with complex reasoning and control (table 2). Furthermore, males had higher connectivity in the integration of the default mode network with subcortical and sensory (visual) systems. Default mode network has been associated with self-related and internal processes such as stimulus-independent thoughts and introspection [40]. It is also believed to play an important role in the integration of cognitive processes [38]. On the other hand, females had increased connectivity with subcortical regions, attention (both dorsal and ventral) systems and sensory (both visual and auditory) systems (table 2). The subcortical regions including amygdala, hypothalamus, hippocampus, dorsal striatum (caudate and putamen), ventral striatum (nucleus accumbens), thalamus and pallidum have been mainly associated with emotion processing, social cognition and motivation [41–43]. In agreement with our findings, improved socially relevant skills have been reported in females [9,10]. We showed that the integration between attention systems and sensory systems was stronger in females, also consistent with behavioural literature [11].
Similar results were observed when we defined subnetworks based on their putative behavioural domains (table 4). Males had higher connectivity between motor, executive functions and sensory (auditory) systems. Females, on the other hand, had higher connectivity between reward, memory and sensory (auditory) systems. Higher memory performance in females has been extensively reported [7,8]. The neuropsychological processes that are managed by the reward circuitry have been traditionally linked to social motivation because anticipation of the rewarding value of a stimulus and then determination of its relevance in other cognitive tasks are critical for engaging and maintaining social interactions [42,44]. Both sets of results regarding functionally defined and behaviourally relevant subnetworks point to an intriguing sex difference in the processing of sensory inputs (auditory and visual). Males had higher connectivity between sensory, motor and default mode systems, while for females higher connectivity was observed between sensory, subcortical, reward and attention systems (tables 2 and 4). This might suggest a better perception–action coordination in males, and better anticipation and subsequent processing of socially and emotionally relevant cues in females.
In our final set of experiments, we identified complex and subtle relationships between behaviour and brain structure, using multivariate analysis techniques. We were able to distinguish males from females using a pattern classifier, with features extracted from both structural connectivity patterns as well as behavioural assessments. The classification accuracy showed an improving trend across development, suggesting an increasing sex difference both in the brain structure and behaviour (figure 2). This indicates an augmented separation between males and females in the course of development, not only in terms of behavioural patterns but also those related to the brain structure. Hence, it is clear that the behavioural differences between males and females are accompanied by related structural differences across their development. The separation at the level of brain structure was higher than that at the level of behaviour, suggesting that not all structural differences manifest themselves in the behavioural measures used in the current battery. We also showed a statistically significant, but relatively small, correlation between the classification scores of individuals, which quantify the certainty of their assignment to one of the groups (males or females), when this score is calculated using structural and behavioural patterns. Overall, these findings may indicate that the causal relationship between brain structure and behaviour, should any exist, may not be linear. It could be affected by numerous nonlinearities contributed by other factors such as environment, culture, even economics or politics.
Importantly, except for the multivariate classification analysis, all our findings are observations on sample averages, i.e. they are not individual level but group differences. In order to conclude structural and behavioural distinctness of an individual compared to the general population, one will need to perform more direct analyses on individual differences. The methodology used in this work paves the way for such future studies, as the features that we used here (e.g. the mean within subnetwork connectivity) are calculated for each individual participant. Thus, one may assign a standardized score to each individual based on the distribution of a feature, quantifying the status of the individual compared to their group. This may also facilitate a function specific analysis, as we were able to define subnetworks based on their functional definitions, with individuals being assigned scores pertaining to each functional subnetwork. More sophisticated analyses can be performed by using multivariate pattern analysis methods trying to predict specific behavioural measurements using structural network features.
Despite their scientific relevance and significance, sex differences in the brain structure are a sensitive topic and a source of much ethical and related controversy. The almost hard-wired nature of structural differences is unfortunately sometimes interpreted by the media and the general public as defining constant, discrete and immutable boundaries between the sexes. Therefore, we need to emphasize that structural differences do not imply aetiology or prognosis, and the current work has several limitations.
First, several practical limitations prevented us from using a functional modality (e.g. fMRI) as an investigative intermediary between structure and behaviour. Instead, we made comparisons to a previously published work that used a subsample of the PNC data and resting state fMRI [16]. Second, in our developmental experiments, we defined three age groups and compared cross-sectional findings of these groups, without using a true longitudinal experimental design. We divided our sample to have an almost equal number of individuals in each group, as in Ingalhalikar et al. [17], without any considerations of puberty or other factors that may be developmentally relevant, such as race or ethnicity. It is expected that one of the primary drivers of sex differences in the brain, especially during puberty, may be the hormonal regulation of the brain. Similarly, ethnicity may also affect sex-related differences both in brain and in behaviour. Thus, future studies that concentrate on developmental aspects of sex differences should consider such factors in order to render a more detailed picture. Such a broader scope was not aimed for in the current study since our sample did not include any data on the hormonal state of participants. It should be noted that when the sample is divided into subsamples following any rationale, there is always a possibility that some other factor affects the results. Our main goal is to demonstrate the synchrony between sex-related differences in the brain network and behaviour, regardless of their underlying causal mechanisms. Hence, the effect of a factor such as puberty that possibly modulates interrelation between brain network and behaviour, thereby having an important role in the underlying causal mechanisms, is beyond the scope of the current work. Third, we did not take into account developmental differences in GM such as differential growth of regions. Detailed investigation of the effect of volumetric changes in GM regions on the creation of the connectome is an active area of ongoing research. We believe that as the field of connectomics evolves, the study of connectivity-based sex differences will gain from new and better ways of creating the connectomes, leading to further insights.
4. Material and methods
(a). Participants
Institutional Review Board approval was obtained from the University of Pennsylvania and the Children's Hospital of Philadelphia. Participants were excluded due to missing cognitive data, poor imaging data quality or a history that suggested potential abnormalities of brain development such as a history of medical problems that might affect brain function, a history of inpatient psychiatric hospitalization, or current use of psychotropic medication. The final study sample included 900 participants. Details on the demographics of the data are given in table 5.
Table 5.
Demographics of our sample.
| no. participants | age mean (s.d.) in years | no. Caucasians | education mean (s.d.) in years | |
|---|---|---|---|---|
| female | 491 | 15.212 (3.454) | 193 | 8.179 (3.317) |
| male | 409 | 14.913 (3.525) | 206 | 7.714 (3.339) |
| total | 900 | 15.076 (3.488) | 399 | 7.968 (3.334) |
(b). Image acquisition and connectome construction
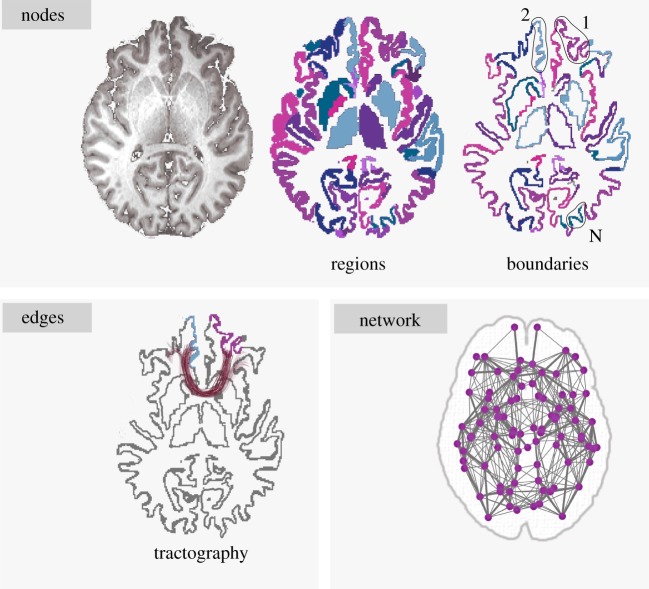
Diffusion weighted magnetic resonance imaging (dMRI) scans were acquired for each individual. Details of the image acquisition, tensor construction and generation of connectome are provided by Ingalhalikar et al. [17]. The connectome construction is illustrated in figure 3.
Figure 3.
Construction of the structural connectome. The nodes of the connectome are the anatomical regions of interest. Edges are generated by seeding probabilistic tractography from WM–GM boundaries of regions. The final network representation defines the connectome. (Online version in colour.)
(c). Computerized neurocognitive battery
The CNB [11,45] was administered to all participants, and consisted of 14 tests that evaluated a broad range of cognitive functions. Each test provided measures of accuracy and speed, with an exception of the motor tests that only measured speed, giving a total of 26 cognitive measures. For the multivariate analysis described in §4(f), we also included five psychiatric evaluation measures related to symptoms of mood disorders, psychosis, externalizing, phobias and overall psychopathology [46].
(d). Alliance of brain regions into subnetworks
We tested whether there is a significant difference in the regional compositions of subnetworks between male and female groups. To this end, we calculated a mean connectome for both male and female groups and detected subnetworks in both, using the Louvain community detection algorithm [35] as implemented in Brain Connectivity Toolbox [34]. We measured the similarity between two sets of subnetworks using the normalized mutual information (NMI) [47]. We then repeated the same measurement 1000 times while permuting gender labels, recalculating mean connectivity matrices and re-detecting subnetworks. This produced a null distribution of NMI values, corresponding to the null hypothesis that the similarity of subnetwork structures between two randomly generated groups would be equal to or lower than the similarity between actual male and female groups. Finally, we compared the actual NMI value calculated using the true male and female groups to the null distribution, to calculate the probability of observing this NMI, or more extreme values, when the null hypothesis is true.
(e). Comparison of subnetwork connectivity
We generated subnetworks of the structural connectome with three different procedures, and compared male and female groups in terms of the mean connectivity within and between subnetworks. First, we formed structurally cohesive subnetworks based on their distinct structural connectivity patterns. We used multi-view spectral clustering [31,48] to decompose the connectome into a common set of subnetworks that are defined by densely connected brain regions. This facilitates comparisons between male and female groups in terms of mean connectivity within and between subnetworks since the subnetworks are common for males and females.
Second, with the aim of linking sex differences in the brain structure and function, we defined functionally defined subnetworks based on the functional associations of the brain regions based on the fMRI literature [39]. We assigned brain regions to 10 functional systems, namely auditory, cingulo-opercular, default mode, dorsal attention, fronto-parietal, motor, subcortical, ventral attention, visual and others, using the definitions from Gu et al. [49].
Third, we defined subnetworks by considering several specific behavioural domains with which they are putatively associated. For this purpose, we compiled seven subnetworks, namely auditory, executive functions, memory, motor, reward, social cognition and visual, based on a literature review on the neuropsychological associations of brain regions. For the motor and sensory (auditory and visual) subnetworks, we used the same definitions as the functionally defined subnetworks. Table 3 gives the region assignments for each of these seven subnetworks.
(f). Multivariate pattern analysis for sex classification
We performed a multivariate analysis using a support vector machine (SVM) classifier [50] to distinguish between males and females using connectivity and behavioural measures. We treated subnetwork and behavioural features (CNB scores) separately to construct two different classifiers, each using only a single set of features. The final unbiased estimate on the classification accuracy of each classifier was calculated using a 10-fold cross-validation procedure where the classifier was trained using 90% of the data and tested on the rest, with the training and testing data being changed for each fold. For the connectivity-based classifier, we compiled the mean connectivity within and between 10 functionally defined subnetworks (auditory, cingulo-opercular, default mode, dorsal attention, fronto-parietal, motor, subcortical, ventral attention, visual and others), resulting in a 55 dimensional feature set. For the behavioural classifier, we used 26 CNB scores and five psychiatric evaluations. We repeated the experiments with different age groups to identify the developmental differences in separation between males and females. Finally, for each classifier, we assigned each participant a classification score, quantifying the certainty of assignment of a participant's pattern of behaviour or connectivity to their sex group, by measuring the distance of the participant's feature vector from the separating hyperplane in SVM. We used these classification scores to calculate the correlation between decisions of two classifiers.
(g). Statistical analysis
When comparing males and females on a single feature (e.g. mean within subnetwork connectivity), we used a two-tailed t-test. Ages of participants were compared between males and females with no significant difference being found. Statistical comparisons were considered significant if corrected p-values were less than 0.05. The false discovery rate (FDR) correction [51] was used for multiple comparisons correction. With comparison on structural connectivity, we used the total brain volume as a covariate because it differed significantly between males and females (p < 0.01). Accuracy of a single multivariate pattern classifier was estimated using K-fold cross-validation, and comparisons between accuracies of two classifiers were performed using K-fold cross-validated paired t-test [52].
Data accessibility
The data reported in this paper have been deposited in the dbGaP database, ww.ncbi.nlm.nih.gov/gap (accession no. phs000607.v1.p1).
Authors' contributions
B.T., B.S. and R.V. designed research; D.P., B.T., B.S., M.A.E., K.R. and M.E.C. performed research; D.P. analysed data; B.T., T.D.S., R.E.G., R.C.G. and R.V. wrote the paper.
Competing interests
We declare that we have no competing interests.
Funding
This work was supported by National Institute of Mental Health (NIMH) grant nos. MH089983, MH089924, MH079938 and MH092862.
References
- 1.Halpern DF, Benbow CP, Geary DC, Gur RC, Hyde JS, Gernsbacher MA. 2007. The science of sex differences in science and mathematics. Psychol. Sci. Public Interest 8, 1–51. ( 10.1111/j.1529-1006.2007.00032.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hines M. 2010. Sex-related variation in human behavior and the brain. Trends Cogn. Sci. 14, 448–456. ( 10.1016/j.tics.2010.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno-Briseño P, Díaz R, Campos-Romo A, Fernandez-Ruiz J. 2010. Sex-related differences in motor learning and performance. Behav. Brain Funct. 6, 74 ( 10.1186/1744-9081-6-74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas JR, French KE. 1985. Gender differences across age in motor performance: a meta-analysis. Psychol. Bull. 98, 260–282. ( 10.1037/0033-2909.98.2.260) [DOI] [PubMed] [Google Scholar]
- 5.Linn MC, Petersen AC. 1985. Emergence and characterization of sex differences in spatial ability: a meta-analysis. Child Dev. 56, 1479–1498. ( 10.2307/1130467) [DOI] [PubMed] [Google Scholar]
- 6.Voyer D, Voyer S, Bryden MP. 1995. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol. Bull. 117, 250–270. ( 10.1037/0033-2909.117.2.250) [DOI] [PubMed] [Google Scholar]
- 7.Hedges LV, Nowell A. 1995. Sex differences in mental test scores, variability, and numbers of high-scoring individuals. Science 269, 41–45. ( 10.1126/science.7604277) [DOI] [PubMed] [Google Scholar]
- 8.Saykin AJ, Gur RC, Gur RE, Shtasel DL, Flannery KA, Mozley LH, Malamut BL, Watson B, Mozley PD. 1995. Normative neuropsychological test performance: effects of age, education, gender and ethnicity. Appl. Neuropsychol. 2, 79–88. ( 10.1207/s15324826an0202_5) [DOI] [PubMed] [Google Scholar]
- 9.Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, Smailis J. 1992. Facial emotion discrimination. I. Task construction and behavioral findings in normal subjects. Psychiatry Res. 42, 231–240. ( 10.1016/0165-1781(92)90115-J) [DOI] [PubMed] [Google Scholar]
- 10.Williams LM, Mathersul D, Palmer DM, Gur RC, Gur RE, Gordon E. 2009. Explicit identification and implicit recognition of facial emotions. I. Age effects in males and females across 10 decades. J. Clin. Exp. Neuropsychol. 31, 257–277. ( 10.1080/13803390802255635) [DOI] [PubMed] [Google Scholar]
- 11.Gur RC, et al. 2012. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology 26, 251–265. ( 10.1037/a0026712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. 1999. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J. Neurosci. 19, 4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W. 2003. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage 18, 880–894. ( 10.1016/S1053-8119(03)00034-X) [DOI] [PubMed] [Google Scholar]
- 14.Szeszko PR, Vogel J, Ashtari M, Malhotra AK, Bates J, Kane JM, Bilder RM, Frevert T, Lim K. 2003. Sex differences in frontal lobe white matter microstructure: a DTI study. Neuroreport 14, 2469–2473. ( 10.1097/01.wnr.0000099475.09597.23) [DOI] [PubMed] [Google Scholar]
- 15.Schmithorst VJ, Holland SK, Dardzinski BJ. 2008. Developmental differences in white matter architecture between boys and girls. Hum. Brain Mapp. 29, 696–710. ( 10.1002/hbm.20431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satterthwaite TD, et al. 2014. Linked sex differences in cognition and functional connectivity in youth. Cereb. Cortex 25, 2383–2394. ( 10.1093/cercor/bhu036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingalhalikar M, et al. 2014. Sex differences in the structural connectome of the human brain. Proc. Natl Acad. Sci. USA 111, 823–828. ( 10.1073/pnas.1316909110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreasen NC, Flaum M, Swayze V, O'Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WT. 1993. Intelligence and brain structure in normal individuals. Am. J. Psychiatry 150, 130–134. ( 10.1176/ajp.150.1.130) [DOI] [PubMed] [Google Scholar]
- 19.Hsu J-L, Leemans A, Bai C-H, Lee C-H, Tsai Y-F, Chiu H-C, Chen W-H. 2008. Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage 39, 566–577. ( 10.1016/j.neuroimage.2007.09.017) [DOI] [PubMed] [Google Scholar]
- 20.Herting MM, Maxwell EC, Irvine C, Nagel BJ. 2011. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb. Cortex 22, 1979–1992. ( 10.1093/cercor/bhr246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bava S, Boucquey V, Goldenberg D, Thayer RE, Ward M, Jacobus J, Tapert SF. 2011. Sex differences in adolescent white matter architecture. Brain Res. 1375, 41–48. ( 10.1016/j.brainres.2010.12.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanaan RA, Allin M, Picchioni M, Barker GJ, Daly E, Shergill SS, Woolley J, McGuire PK. 2012. Gender differences in white matter microstructure. PLoS ONE 7, e38272 ( 10.1371/journal.pone.0038272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clayden JD, Jentschke S, Muñoz M, Cooper JM, Chadwick MJ, Banks T, Clark CA, Vargha-Khadem F. 2012. Normative development of white matter tracts: similarities and differences in relation to age, gender, and intelligence. Cereb. Cortex 22, 1738–1747. ( 10.1093/cercor/bhr243) [DOI] [PubMed] [Google Scholar]
- 24.Basser PJ, Mattiello J, Lebihan D. 1994. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267. ( 10.1016/S0006-3495(94)80775-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sporns O, Tononi G, Kötter R. 2005. The human connectome: a structural description of the human brain. PLoS Comput. Biol. 1, e42 ( 10.1371/journal.pcbi.0010042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girvan M, Newman MEJ. 2002. Community structure in social and biological networks. Proc. Natl Acad. Sci. USA 99, 7821–7826. ( 10.1073/pnas.122653799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van den Heuvel MP, Sporns O. 2011. Rich-club organization of the human connectome. J. Neurosci. 31, 15 775–15 786. ( 10.1523/JNEUROSCI.3539-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz AJ, Gozzi A, Bifone A. 2008. Community structure and modularity in networks of correlated brain activity. Magn. Reson. Imaging 26, 914–920. ( 10.1016/j.mri.2008.01.048) [DOI] [PubMed] [Google Scholar]
- 29.Tononi G, Sporns O, Edelman GM. 1994. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc. Natl Acad. Sci. USA 91, 5033–5037. ( 10.1073/pnas.91.11.5033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghanbari Y, Smith AR, Schultz RT, Verma R. 2014. Identifying group discriminative and age regressive sub-networks from DTI-based connectivity via a unified framework of non-negative matrix factorization and graph embedding. Med. Image Anal. 18, 1337–1348. ( 10.1016/j.media.2014.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tunç B, Shankar V, Parker D, Schultz RT, Verma R. 2015. Towards a quantified network portrait of a population. In Information processing in medical imaging (IPMI) (eds Ourselin S, Alexander DC, Westin C-F, Cardoso MJ), pp. 650–661. Berlin, Germany: Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satterthwaite TD, et al. 2014. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc. Natl Acad. Sci. USA 111, 8643–8648. ( 10.1073/pnas.1400178111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satterthwaite TD, et al. 2014. Neuroimaging of the Philadelphia Neurodevelopmental Cohort. Neuroimage 86, 544–553. ( 10.1016/j.neuroimage.2013.07.064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubinov M, Sporns O. 2010. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. ( 10.1016/j.neuroimage.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 35.Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E. 2008. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 10, P10008 ( 10.1088/1742-5468/2008/10/P10008) [DOI] [Google Scholar]
- 36.Legato MJ. 2009. Principles of gender-specific medicine, 2nd edn New York, NY: Academic Press. [Google Scholar]
- 37.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. 2008. Mapping the structural core of human cerebral cortex. PLoS Biol. 6, e159 ( 10.1371/journal.pbio.0060159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl Acad. Sci. USA 100, 253–258. ( 10.1073/pnas.0135058100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Power JD, et al. 2011. Functional network organization of the human brain. Neuron 72, 665–678. ( 10.1016/j.neuron.2011.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heine L, et al. 2012. Resting state networks and consciousness: alterations of multiple resting state network connectivity in physiological, pharmacological, and pathological consciousness states. Front. Psychol. 3, 295 ( 10.3389/fpsyg.2012.00295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adolphs R. 2009. The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 60, 693–716. ( 10.1146/annurev.psych.60.110707.163514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohls G, et al. 2013. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia 51, 2062–2069. ( 10.1016/j.neuropsychologia.2013.07.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrett LF, Satpute AB. 2013. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol. 23, 361–372. ( 10.1016/j.conb.2012.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. 2012. The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239. ( 10.1016/j.tics.2012.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. 2010. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J. Neurosci. Methods 187, 254–262. ( 10.1016/j.jneumeth.2009.11.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calkins ME, et al. 2014. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry 13, 296–305. ( 10.1002/wps.20152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alexander-Bloch A, Lambiotte R, Roberts B, Giedd J, Gogtay N, Bullmore E. 2012. The discovery of population differences in network community structure: new methods and applications to brain functional networks in schizophrenia. Neuroimage 59, 3889–3900. ( 10.1016/j.neuroimage.2011.11.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A, Rai P, Daume H. 2011. Co-regularized multi-view spectral clustering. In Advances in Neural Information Processing Systems 24: Proc. 25th Annual Conference on Neural Information Processing Systems, 12–15 December 2011, Granada, Spain (eds J Shawe-Taylor, RS Zemel, PL Bartlett, F Pereira, KQ Weinberger), pp. 1413–1421. Red Hook, NY: Curran Associates. [Google Scholar]
- 49.Gu S, Pasqualetti F, Cieslak M, Grafton ST, Bassett DS.2014. Controllability of brain networks. (http://arxiv.org/abs/1406.5197v1. )
- 50.Cortes C, Vapnik VN. 1995. Support-vector networks. Mach. Learn. 20, 273–297. ( 10.1007/BF00994018) [DOI] [Google Scholar]
- 51.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. JR. Stat. Soc. Ser. B 57, 289–300. [Google Scholar]
- 52.Alpaydin E. 2010. Introduction to machine learning, 2nd edn Cambridge, MA: MIT Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data reported in this paper have been deposited in the dbGaP database, ww.ncbi.nlm.nih.gov/gap (accession no. phs000607.v1.p1).