Abstract
The song-control system, a neural circuit that controls the learning and production of birdsong, provided the first example in vertebrates of prominent macro-morphological sex differences in the brain. Forebrain nuclei HVC, robust nucleus of the arcopallium (RA) and area X all exhibit prominent male-biased sex differences in volume in zebra finches and canaries. Subsequent studies compared species that exhibited different degrees of a sex difference in song behaviour and revealed an overall positive correlation between male biases in song behaviour and male biases in the volume of the song nuclei. However, several exceptions have been described in which male biases in HVC and RA are observed even though song behaviour is equal or even female-biased. Other phenotypic measures exhibit lability in both sexes. In the duetting plain-tailed wren (Pheugopedius euophrys), males and females have auditory cells in the song system that are tuned to the joint song the two sexes produce rather than just male or female components. These findings suggest that there may be constraints on the adaptive response of the song system to ecological conditions as assessed by nucleus volume but that other critical variables regulating song can respond so that each sex can modify its song behaviour as needed.
Keywords: sexual selection, song-control system, HVC, canary
1. Introduction: the discovery of the song system and sex differences in the songbird brain
One of the most the most remarkable discoveries in the history of the study of brain sex differences concerns variation in size of key forebrain song-control nuclei that control the learning and production of birdsong ([1], see [2] for a review). In the 1970s Nottebohm described, based primarily on studies in canaries (Serinus canaria), a discrete set of brain nuclei that specifically controlled a complex learned motor behaviour, birdsong (figure 1, see Nottebohm [3] for a review).
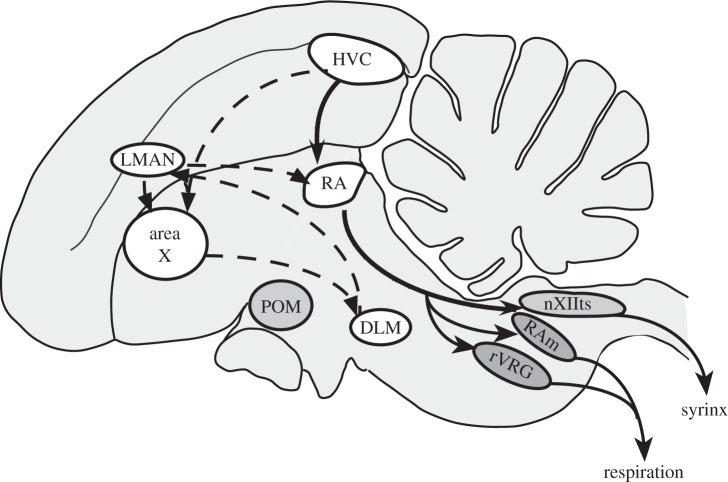
Figure 1.
Schematic of the song-control system of songbirds illustrating the two main pathways connecting nucleus HVC (initially Hyperstriatum Ventrale pars caudale, now acronym used as proper name) to the nucleus robustus arcopallialis (RA). The caudal motor pathway (black arrows) directly connects HVC to RA and then to the motoneurons innervating the syrinx located in the tracheosyringeal part of the XIIth cranial nerve (nXIIts) and to two nuclei controlling respiration, the nucleus retroambigualis (RAm) and the rostral ventral respiratory group of neurons (rVRG). The anterior forebrain pathway (dotted arrows) also connects HVC to RA but via the area X of the striatum, the dorsolateral thalamic nucleus (DLM) and the lateral magnocellular nucleus of the anterior nidopallium (LMAN). The medial preoptic nucleus is also represented; although it does not connect directly to the song system, testosterone action in this nucleus enhances the singing motivation. HVC, RA and X tend to exhibit male-biased sex differences in nucleus volume (see text for discussion).
Initially, Nottebohm assumed like most neuroscientists at the time, that this circuit would be broadly similar in males and females despite the fact that there were marked differences in song behaviour in canaries (see discussion in Nottebohm [4]). He points out that when preparing the canary brain atlas with Stokes & Leonard [5], he and his colleagues used material from males and females inter-changeably on the assumption that there would be no sex difference in overall neural structure [4]. Although the hypothesis was already well accepted that actions of steroid hormones early in ontogeny could set up enduring sex differences in brain function [6], neural sex differences that had been described anatomically were only apparent at the cellular level (e.g. quantitative differences in dendritic spine density) and required a detailed analysis to be detected (e.g. [7]).
The song system is an unusual circuit in that it is cytoarchitectonically discrete and can be linked directly to a specific behaviour, i.e. the regulation of the production and learning of complex vocalizations such as song (see Nottebohm [3] for a review of the early canary studies linking the song system with song behaviour). Zebra finches (Taeniopygia guttata), because of the ease of housing and breeding them in captivity, soon emerged as an even more popular species for the study of the song circuit than canaries [8]. Thus, it is no surprise that the first studies of the neural basis of sex differences in songbirds would focus on these two species. Nottebohm working with canaries and his student Art Arnold working with zebra finches independently observed in Nissl-stained material that the volume of key song nuclei such as HVC (the acronym is the name), the robust nucleus of the arcopallium (RA) and area X of the medium striatum (X) all were markedly larger in volume in males than in females [1]. This discovery immediately had a major effect on the entire field of sex differences in the brain and behaviour among vertebrates, as investigators of the mammalian brain as well as other taxa started looking for differences at this level of organization and the result was that the sexually dimorphic nucleus of the preoptic area in rats was soon discovered by Gorski and colleagues and other examples followed ([9]; see Arnold & Gorski [10] for a review).
2. Species variation in the pattern of sex differences in brain and behaviour
However, there is another important dimension related to the study of sex differences in the song-control system that makes it unusual as compared to other animal and human examples of brain sex differences, and it is a feature that continues to make it an important system for the study of sex differences especially in a functional perspective. This feature is the tremendous potential for comparative studies of sex differences in brain and behaviour. Songbirds, although named after their signature behaviour, are technically a taxonomic group. They are members of the order Passeriformes that consists of approximately 6000 out of the 10 500 extant species of birds [11]. In particular, the term songbirds is thought to correspond to species in the suborder Passeri (also known as oscines), which is sometimes referred to as the ‘true’ songbirds and consists of just under 50% of the 10 500 extant species of birds themselves [12]. A basic feature of oscine species is that they produce learned complex song and have a specialized neural circuit called the song-control system that forms a key aspect of the neural substrate for this behaviour [13,14]. The song system specialization involves the enhancement of existing vocal control circuits that can be observed in non-oscine avian species such as gallinaceous species as well as in suboscines [14,15]. It thus appears that the specialized aspects of the song system that seem to be in common among all oscines (e.g. [16]) is an example of a synapomorphy for oscines. The oscine species exhibit a fascinating diversity in the degree to which there are sex differences in external morphology and behaviour. Studies that followed the original Nottebohm & Arnold work [1] immediately illustrated that there are diverse patterns of sex differences in relation to sex differences in behaviour ([17], figure 2).
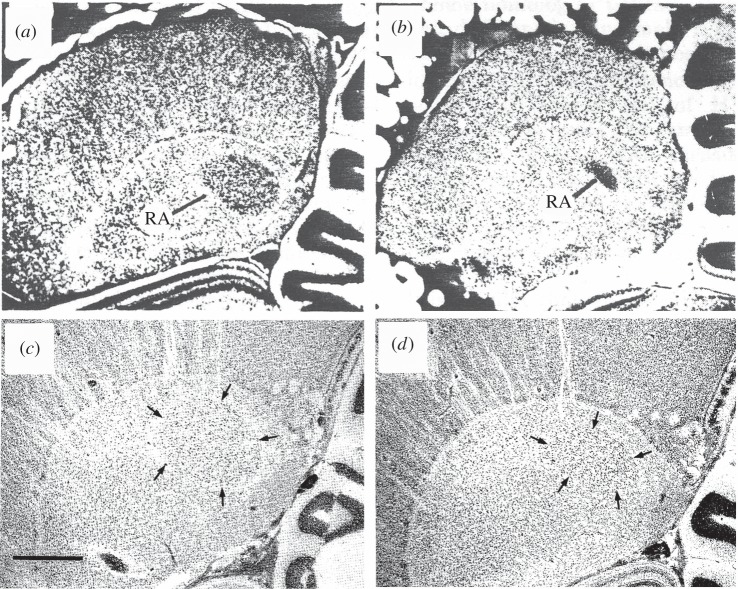
Figure 2.
Photomicrographs illustrating the sex difference in RA in zebra finches (a,b) and European starlings (Sturnus vulgaris) (c,d). Male sections are in the left column (a and c) while females are in the right column (b,d). Note that the sex difference is more extreme in the case of the zebra finch where females do not sing (though they produce calls) than is for starlings where females are known to produce some song though not as frequently or as complex as male song [1 and 17].
Plumage and size variation between males and females varies from being identical to being dimorphic to such a degree that they were thought to be from different species [11]. A recent study on variation in the colour of the plumage among songbirds comprehensively sampled all 6000 living species and found fascinating diversity that the authors linked to sexual selection in many cases (such as when males were clearly brighter than females and engaged in competitive mating behaviours for females), though a surprisingly large number of species exhibited bright coloration in females as well as males, especially in the tropics where intra-sexual competition was relatively high in both males and females and where long-lived sedentary species are competing for mates in both sexes [11]. This comprehensive analysis of factors driving sex differences in plumage among all passerines can also be informative when thinking about the evolution of sex differences in song behaviour and the song system in the same taxa. Studies of structure–function relations related to brain sex differences have been limited in their conclusions in part because of the lack of an ability to perform widespread comparative studies that might illuminate function more clearly [18].
3. Intra- and interspecific variation in the volume of key song nuclei do suggest that attributes of song and the song system have co-evolved
Initial studies of variation in the song system did link species variation in the magnitude of the sex difference in both the brain and in song behaviour to the degree of the operation of sexual selection. For example, the original study by Nottebohm & Arnold [1] included two popular laboratory species, canaries and zebra finches, in which the sex difference in brain nucleus volume was more extreme in the zebra finches, a species in which females do not sing, as compared to canaries where female song has been described albeit to be less complex than male song.
Studies of sex differences in brain structure in relation to behaviour are part of a more general endeavour that tries to relate variation in brain morphology to behaviour based either on different types of intraspecific comparisons (of which the study of sex differences is only one example) or interspecific comparisons. These initial studies on sex differences in brain and behaviour generated a series of studies, many of which focused on males and attempted to relate variation in the size of the song nuclei (generally assessed based on the volume of the nucleus defined with the use of Nissl stains) to variation in male song repertoire and/or song rate based on both intra- and interspecific comparisons (see [19] for a review). Nottebohm and colleagues led the way, conducting studies of intraspecific variation in the volume of HVC in canaries: an analysis of the brains of 46 male and female canaries replicated the significant sex differences previously reported and found that intra-male variation in the volumes of the song nuclei HVC and RA correlated best with repertoire size [20]. It was subsequently found that marsh wrens (Cistothorus palustris) on the west coast of North America exhibit an HVC significantly larger in volume than males on the east coast, and this difference is correlated with geographical variation in male song repertoire size [21]. These studies generated excitement at first because it seemed that active learning would drive intraspecific variation in brain morphology, but experimental studies did not support this contention [22]. At present, correlations between large volumes of HVC and larger repertoire size of songs marks for a potential to learn more songs but does not appear to be driven by learning per se. Positive correlations between intraspecific variation in repertoire size and HVC volume have not always been observed. For example, studies of both red-winged blackbirds and European starlings failed to find such relationships [23,24].
Other studies have tried to link interspecific variation in male song repertoire size with variation in the size of key song nuclei such as HVC and RA (see [19] for review). A study of 41 oscine species did find a strong significant relationship between song repertoire size and HVC volume [25]. An even more ambitious meta-analysis examined inconsistencies in these correlations between song nuclei and different aspects of song behaviour and concluded that there is a strong independent relationship between repertoire size or song rate and HVC volume [19]. A more recent study that investigated correlations among all the nuclei in the song system concluded that the degree of convergence among the nuclei in the song system correlated best with variation in repertoire size [26]. The song system has a hierarchical organization such that HVC codes for syllables and projects to nucleus RA that exhibits a myotopic organization based on the muscles of syrinx, which in turn projects to brainstem nuclei that also correspond to syringeal muscles or coordinate song with respiration. It seems that the ratio of cells in HVC in relation to cells in RA and in turn the ratio of cells in RA in relation the motor nucleus of the XIIth cranial nerve are good predictors of male song repertoire size. Thus, these comparative studies do suggest that variation in the brain may contribute to our understanding of behavioural variation between the sexes both within and between species, though identifying the right variables to assess brain differences is a significant challenge.
4. Studies of interspecific variation in sex differences in brain structures do generally correlate with sex differences in song behaviour, suggesting a coevolutionary process shaped by sexual selection
What sort of species variation is available among songbirds to test ideas about how variation in the volume of the song-control nuclei may have coevolved with the evolution of sex differences in behaviour? Oscines are not equally distributed throughout the planet. Most species are tropical and the ancestral songbird is thought to be tropical. There are many aspects of the natural history of the tropics that are different from the temperate zone (e.g. species tend to be migratory, they lay smaller clutches of eggs, they live longer, etc.). One form of social behaviour is much more common among tropical songbirds than temperate zone species and that is the phenomenon of duetting. In duetting songbird species, males and females will sing a shared song simultaneously and intersperse notes. Although there is evidence that the song serves different functions in males and females (e.g. [27,28]), both males and females defend territories and tend to sing at very high rates. A relatively recent phylogenetic analysis of the evolution of duetting revealed that being sedentary (i.e. not migrating) was a critical life-history variable giving rise to duetting rather than living in the tropics or other aspects of the tropical lifestyle [29]. Thus, duetting seems to have evolved to provide coordinated signals to defend shared resources [29].
Brenowitz et al. [30] compared sex differences in song structure volume in males and females of three neotropical duetting species: white-browed robinchats (Cossypha heuglini), bay wrens (Thryothorus nigricapillus) and buff-breasted wrens (Thryothorus leucotis). They found that the extent of the sex differences in nuclear volume in HVC, RA and area X was correlated with the extent of the sex difference in repertoire size in any given species [30]. This and subsequent studies clearly linked species variation in the degree of sex differences in the song system to variation in parameters associated with song complexity and song production [30,31,32].
These initial studies suggested a coevolution of sex differences in the brain and sex differences in singing behaviour [30]. However, conclusions based on these data are constrained by methodological issues related to across-species comparisons. Because species are not independent data points but are the terminals of a branching lineage, phylogenetic history must be taken into account [33,34]. Thus, although there may be an apparent correlation between two variables (e.g. brain and behaviour) across species, the observed correlation may reflect a confound owing to the inability to control for phylogeny rather than a functional relationship. For example, in the initial observation of a cross-species relationship outlined above, the duetting species with little sex dimorphism in the song-control system were two wren species (family Certhiidae), whereas the extremely dimorphic zebra finch is an estrilid finch (family Passeridae). Thus, the apparent relationship between sex differences in the brain and sex differences in song could have been a product of divergence among different families of songbirds rather than reflecting a functional difference.
To assess a cross-species relationship between two phenotypic variables (brain sex difference and behaviour sex difference) requires some method to control for the effects of phylogeny. Fortunately, evolutionary biologists have developed a number of statistical techniques to do just that [34]. When the phylogenetic history of songbirds, as assessed by molecular genetic data [35], is controlled for, the hypothesis that sex differences in the song-control system have coevolved with sex differences in behaviour does seem to hold [2,36]. Moreover, the development of extreme sex differences in brain and behaviour seems to have evolved multiple times independently. For example, among wrens (family Certhiidae), marsh wren and Carolina wren (Thryothorus ludovicianus) females never sing [21,37], whereas females of other Thryothorus species commonly sing in duet with males. Similarly, in the family Passeridae, females of many species never sing, whereas red-cheeked cordon blue (Uraeginthus bengalus) females commonly sing [38]. In both of these families, the species that have extreme sex differences in behaviour have the most extreme sex differences in the song-control system. In both families, this dimorphism can be as extreme as a complete lack of delineation of the nucleus (as in zebra finches). Thus, the degree that there is a sex difference in song behaviour, and in the song-control system both appear to be evolutionarily labile traits, and to have evolved in tandem.
5. Exceptions that suggest that some morphological sex differences in the song system did not coevolve with sex differences in behaviour
Other studies, especially of duetting species, have raised questions about the extent of the coevolutionary process between morphological variation in the song system and sex differences in behaviour. For example, in African bush shrikes (Laniarius funebris), males and females duet and seem to have comparable song repertoires both in size and complexity. However, male-biased sex differences in the volume of HVC and RA as well as a number of cellular variables such as soma size are still apparent when one compares males and females [39]. These data raise the issue that other behavioural variables besides those related to song production can contribute to variation in the size of the song nuclei (e.g. song perception is an obvious candidate) or perhaps that male-biased sex differences are ancestral and can only change within a limited range of variation in response to selection. In forest weavers (Ploceus bicolor), mated pairs sing a unison duet in which male and females learn to produce identical songs [40]. Despite this similarly, volumes of the song nuclei exhibit a male-biased sex difference (1.5. times larger in males than in females). Interestingly, when the authors measured expression levels of the mRNA of a number of synapse-related proteins in HVC and/or RA they found higher expression levels in females (30–70%) than their male counterparts [40]. This suggests that the volume of the song nuclei may indeed be constrained but females can compensate through other variables to attain the same behavioural goal. Similarly in cooperatively breeding/duetting white-browed sparrow weavers (Plocepasser mahali), male-biased sex differences in the volume of the song-control system are observed despite the fact that there are multiple male-types based on intraspecific variation in song phenotypes (dominant males sing their own song as well as engaging in duets) and females sing song at a similar rate and level of complexity as males [41]. In the corvid group within the oscines, song is rather unusual and hard to identify. In any case, males and females tend to exhibit broad similarities in their vocal behaviour and studies of their neuroanatomy again detect a substantial male-bias in brain nucleus volume [42]. To date only one oscine species has been identified in which females sing at a higher rate than males and in which morphological traits in the song system have been measured, namely the streak-backed oriole (Icterus pustulatus). In this case, substantial male biases in the volume of HVC and area X were reported (but not for RA) in this species where females sing more song and of comparable complexity [43].
6. How labile is the male and female songbird brain? Can sex similarities evolve?
As just reviewed, when one examines variation in the volume of song-control nuclei in males and females as compared to sex differences in song behaviour there are clear limitations. Although one can discern phylogenetic trends suggesting that the degree to which there is a sex difference in brain nucleus volume corresponds to the degree to which there is sex difference in behaviour (e.g. [2,36]) there are constraints on this relationship in that male biases persist even in a species such as the Streak-backed oriole with greater rates of female song than male [43].
Many authors who study the songbird brain argue quite correctly that more refined cellular and molecular measures are appropriate than gross morphological measures such as brain nucleus volume to identify key adaptations that have shaped sex-specific and sexually similar brains. Is there evidence that male and female brains can respond in a similar manner to selection for similarity to enhance cooperation as opposed to specializations as a result of sex-specific patterns of competition?
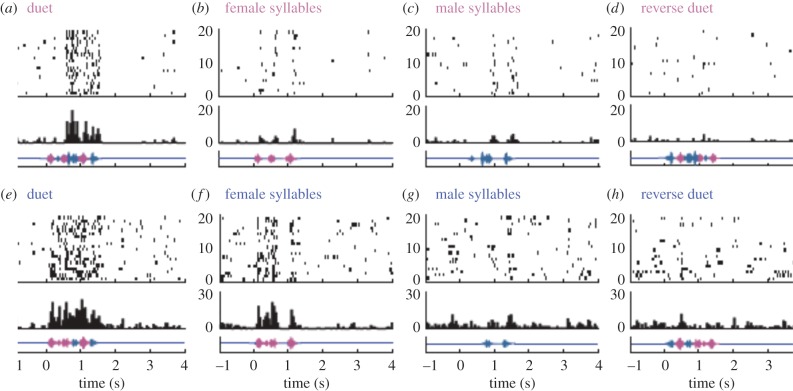
Once again, comparing species with male-biased behaviour to species that engage in cooperative vocal duets is illustrative. One of the most striking discoveries about the physiology of the male songbird brain is that cells in song nuclei such as HVC and LMAN develop a tuning pattern geared specifically towards the bird's own song as the song is learned. This phenomenon was first described in canaries [44] and then in more detail with an extensive characterization in white-crowned sparrows (Zonotrichia leucophyrs) [45,46]. It was subsequently documented comprehensively in zebra finches (see [47] for a review). The identification of cells exhibiting a similar physiological profile in females has been difficult, though administering testosterone to female canaries has helped [48]. Plain-tailed wrens (Pheugopedius euophrys) is a species of neotropical oscines that sing duets in which females and males rapidly alternate syllable production, sounding as if a single bird sang it. To examine how cooperative duet singing is encoded in telencepahlic circuits, birds were captured in the field in Ecuador and then neurophysiological experiments were performed [49]. The question asked was do males and females respond only to their own parts of the song or is something more complicated going on? The surprise was that both females and males exhibited a response profile that was best to the full duet song relative to other stimuli tested for the majority of units (figure 3). The responses to duet stimuli were not simply a sum of responses to female and male syllables, because, in the majority of neurons, response strengths elicited by duet stimuli were significantly greater than the sum of the response strengths to female and male syllables presented alone (figure 3). Thus in these duetting wrens males and females have neural specializations that allow them to develop auditory neurons tuned to the joint male and female song [49]. This is a remarkable example of a neural substrate mediating cooperation between the sexes.
Figure 3.
Example responses in HVC to song stimuli in the duetting species the plain-tailed wren. Responses recorded in Ecuador in a female (a–d) and a male (e–h) wren. The bottom of each panel shows the stimulus oscillogram, at the top are raster plots showing the times of spikes for 20 stimulus repetitions, and in the middle is a histogram (50 ms bins) of the activity. Magenta indicates syllables produced by the female, blue by the male. Stimuli were (a,e) duet song, (b,f) female syllables, (c,g) male syllables and (d,h) reverse duet song. The female recording shown here had a strong preference for duet song over all other stimuli tested. The male recording shown here responded best to the duet song but, nonetheless, had a particularly strong response to the female syllables. Note that males and females respond best to the shared duet song (adapted from [48]).
7. What is the functional basis of species variation in brain and behaviour related to song behaviour and the song system? Adaptation or constraint or both?
When thinking about the ultimate (or evolutionary) causes of sex differences, one can ask are sex differences the result of sexual selection or sex-specific natural selection to represent a neural adaptation to facilitate sex-specific patterns of behaviour? Alternatively, are these sex differences the result of a historical process and some sort of constraint? The song-control system can be viewed as a neural specialization that evolved in the oscine suborder in association with the ability to produced learned birdsong. A key question then is whether it should be viewed as a secondary sexual characteristic [50]? In other words is the song system an adaptation shaped by sexual selection that provides males with an advantage in the competition for mates? Although there is extensive evidence that song or some aspects of song provide males with an advantage for mates either in a competitive or a mate choice situation [51], there is still little evidence specifically linking sex differences in the song-control system to specific aspects of vocal behaviour that provide males with an advantage [52]. For example, in a study in Sedge warblers (Acrocephalus schoenobaenus), males with more complex songs paired with females more successfully than males with simpler songs and this complexity correlated with HVC size [52]. However, the authors could not find a direct relationship between HVC size and male mating success!
There are other evolutionary scenarios to consider. It could be that a basic song system evolved in males and females to support learned vocal behaviour and that the sex differences observed evolved because of male and female specializations in vocal behaviour that are not related to sexual selection per se. For many years it was assumed, based on studies of temperate zone birds, that song was ancestral in males and derived in females and that a similar scenario might explain the evolution of the song system: namely that it was ancestral in males. However, more recent phylogenetic analyses indicate that female song evolved early in the songbird lineage and that widespread song by males and females is ancestral [53]. This would suggest that at least a rudimentary song system was present in males and females in ancestral species early in the origin of songbirds and underwent later evolution. Indeed, a functional song system is very apparent in most avian species studied that clearly is involved in perception as well as production [54]. Even in female zebra finches where the song systems is thought be almost vestigial there is a role for HVC in song perception. In species such as the canary, with a smaller song system than in males based on song nucleus volume, females have a tremendous capacity to produce more male-like song with adult hormone treatment [55]. More recent studies in canaries that have controlled dosage carefully have found that T treatments in females can produce male-like attributes in song and the song system, although it takes longer to occur in females than in males [56]. With this view, more studies need to be conducted on the control of song in males and females in cooperative as well as competitive contexts and direct links should be made between neuroendocrine specializations linked to these different behavioural scenarios.
Studies of sex differences in the control of other behavioural systems regulated by hormones can also be illustrative of ways in which you can get disconnects between sex differences in an important regulatory variable such as the size of a brain area or the magnitude of a hormone signal and sex differences in a behavioural phenotype. For example, some species are ‘sex reversed’ in that females exhibit high levels of male-typical behaviours such as aggression and will compete for access to males. These species raise questions about sex differences in neuroendocrine systems that regulate these behaviours. In African black coucals (Centropus grillii), females are more aggressive than males but have lower circulating concentrations of androgen [57]. However, in one key brain area, nucleus taeniae (homologue of the medial amygdala), androgen receptor mRNA expression was found to be higher in female coucals than in males, indicating that changes in the brain sensitivity to hormone action facilitate such sex role reversals [57]. Indeed, central sensitivity to sex steroids may be the key to understanding the hormonal regulation of female traits that tend to be male-biased. In male but not female dark-eyed juncos (Junco hyemalis) variation in circulating testosterone predicts variation in aggression, but the same is not the case in females [58]. Interestingly, in female and male juncos, mRNA expression for variables influencing the brain response to testosterone such as androgen or oestrogen receptor mRNA as well as the expression of the enzyme aromatase (that converts androgens to oestrogens) do predict intraspecific variation in aggression [58]. These studies stress how important it is to study the signal in hormone-regulated behaviours such as birdsong but also variation in the detector systems such as receptors and metabolizing enzymes that implement hormone action. The key for the future is the study of functional variation in brain morphology, physiology, neurochemistry and cell/molecular biology to sort out how sex differences and similarities are regulated.
8. Possible constraints on the evolution of sex differences in song birds
In conclusion, it will be necessary to identify these functional sex differences in brain but also to be open to basic neural patterns that are subject to constraints. Perhaps the ancestral songbird did have a male-biased song system that may or may not have been related to sexual selection. If that is the case, perhaps development in the female is canalized [59] to some degree preventing her from evolving song nuclei equal to that of males. What might be the physiological basis of this constraint? One possible explanation for the widespread occurrence of male biases in the size of the song nuclei is that males in avian species tend to have higher concentrations of circulating testosterone in the blood than females [60] and these higher concentrations of testosterone tend to promote larger song nuclei (e.g. [55,61]). However, it would also then follow that these effects, though they may promote some morphological changes in the size of song nuclei in males, do not cause functional effects on song behaviour that result in the difference between the male and female song behaviour matching the variation in the brain related to the song system. Dissociations among variables such as high concentrations of testosterone, large song nuclei and high rates of singing have been described in studies of song system plasticity in a seasonal context [61], so this is a plausible hypothesis. For these reasons, neuroscientists may be detecting widespread male-biased sex differences even in situations where an examination of the behavioural ecology relevant to the observed sex roles would not lead one to expect such a difference. The songbird suborder remains an outstanding taxon to sort out these issues of adaptation, sexual selection and developmental constraint in shaping the male and female brains.
Acknowledgements
I thank Margaret McCarthy for valuable discussions concerning these issues. Anonymous reviews provided very helpful suggestions that improved the paper.
Competing interests
I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Nottebohm F, Arnold AP. 1976. Sexual dimorphism in vocal control areas of the songbird brain. Science 194, 211–213. ( 10.1126/science.959852) [DOI] [PubMed] [Google Scholar]
- 2.Ball GF, MacDougall-Shackleton SA. 2001. Sex differences in songbirds 25 years later: what have we learned and where do we go? Microsc. Res. Tech. 54, 327–334. ( 10.1002/jemt.1146) [DOI] [PubMed] [Google Scholar]
- 3.Nottebohm F. 1980. Brain pathways for vocal learning in birds: a review of the first 10 years. In Progress in psychobiology and physiological psychology (eds Sprague JM, Epstein AN), pp. 85–214. New York, NY: Academic Press. [Google Scholar]
- 4.Nottebohm F. 2015. Fernando Nottebohm. In History of neuroscience in autobiography, vol. 8 (ed. Squire L), pp. 324–360. Washington, DC: Society for Neuroscience. [Google Scholar]
- 5.Stokes TM, Leonard CM, Nottebohm F. 1974. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J. Comp. Neurol. 156, 337–374. ( 10.1002/cne.901560305) [DOI] [PubMed] [Google Scholar]
- 6.Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382. ( 10.1210/endo-65-3-369) [DOI] [PubMed] [Google Scholar]
- 7.Raisman G, Field PM. 1971. Sexual dimorphism in the preoptic area of the rat. Science 173, 731–733. ( 10.1126/science.173.3998.731) [DOI] [PubMed] [Google Scholar]
- 8.Fee M, Scharff C. 2010. The songbird as a model for the generation and learning of complex sequential behaviors. ILAR J. 51, 362–377. ( 10.1093/ilar.51.4.362) [DOI] [PubMed] [Google Scholar]
- 9.Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. 1980. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J. Comp. Neurol. 193, 529–539. ( 10.1002/cne.901930214) [DOI] [PubMed] [Google Scholar]
- 10.Arnold AP, Gorski RA. 1984. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 7, 413–442. ( 10.1146/annurev.ne.07.030184.002213) [DOI] [PubMed] [Google Scholar]
- 11.Dale J, Dey CJ, Delhey K, Kempenaers B, Valcu M. 2015. The effects of life history and sexual selection on male and female plumage colouration. Nature 527, 367–370. ( 10.1038/nature15509) [DOI] [PubMed] [Google Scholar]
- 12.Barker FK, Cibois A, Schikler P, Feinstein J, Cracraft J. 2004. Phylogeny and diversification of the largest avian radiation. Proc. Natl Acad. Sci. USA 101, 11 040–11 045. ( 10.1073/pnas.0401892101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenowitz E, Margoliash D, Nordeen KW. 1997. An introduction to birdsong and the avian song system. J. Neurobiol. 33, 495–500. () [DOI] [PubMed] [Google Scholar]
- 14.Farries MA, Perkel DJ. 2008. The songbird brain in comparative perspective. In Neuroscience of birdsong (eds Zeigler HP, Marler P), pp. 63–72. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Liu W-C, Wada K, Jarvis E, Nottebohm F. 2013. Rudimentary substrates for vocal learning in a suboscine. Nat. Commun. 4, 2082 ( 10.1038/ncomms3082) [DOI] [PubMed] [Google Scholar]
- 16.Ball GF. 1994. Neurochemical specializations associated with vocal learning and production in songbirds and budgerigars. Brain Behav. Evol. 44, 234–246. ( 10.1159/000113579) [DOI] [PubMed] [Google Scholar]
- 17.Bernard DJ, Casto JM, Ball GF. 1993. Sexual dimorphism in the volume of song control nuclei in European starlings: assessment by a Nissl stain and autoradiography for muscarinic cholinergic receptors. J. Comp. Neurol. 334, 559–570. ( 10.1002/cne.903340405) [DOI] [PubMed] [Google Scholar]
- 18.De Vries GJ, Sodersten P. 2009. Sex differences in the brain: the relation between structure and function. Horm. Behav. 55, 589–596. ( 10.1016/j.yhbeh.2009.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garamszege LZ, Eens M. 2004. Brain space for a learned task: strong intraspecific evidence for neural correlates of singing behavior in songbirds. Brain Res. Rev. 2044, 187–193. ( 10.1016/j.brainresrev.2003.12.001) [DOI] [PubMed] [Google Scholar]
- 20.Nottebohm F, Kasparian S, Pandazis C. 1981. Brain space for a learned task. Brain Res. 213, 99–109. ( 10.1016/0006-8993(81)91250-6) [DOI] [PubMed] [Google Scholar]
- 21.Canady RA, Kroodsma DE, Nottebohm F. 1984. Population differences in the complexity of a learned skill are correlated with the brain space involved. Proc. Natl Acad. Sci. USA 81, 6232–6234. ( 10.1073/pnas.81.19.6232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenowitz EA, Lent K, Kroodsma DE. 1995. Brain space for learned song in birds develops independently of song learning. J. Neurosci. 9, 6281–6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirn JR, Clower RP, Kroodsma DE, Devoogd TJ. 1989. Song-related brain regions in the red-winged blackbird are affected by sex and season but not repertoire size. J. Neurobiol. 20, 139–163. ( 10.1002/neu.480200304) [DOI] [PubMed] [Google Scholar]
- 24.Bernard DJ, Eens M, Ball GF. 1996. Age- and behavior-related variation in the volume of song control nuclei in male European starlings. J. Neurobiol. 30, 329–339. () [DOI] [PubMed] [Google Scholar]
- 25.DeVoogd TJ, Krebs JR, Healy SD, Purvis A. 1993. Relations between song repertoire size and the volume of brain nuclei related to song: comparative evolutionary analyses amongst oscine birds. Proc. R. Soc. Lond. B 254, 75–82. ( 10.1098/rspb.1993.0129) [DOI] [PubMed] [Google Scholar]
- 26.Moore JM, Szekely T, Buki J, DeVoogd TJ. 2011. Motor pathway convergence predicts syllable repertoire size in oscine birds. Proc. Natl Acad. Sci USA 108, 16 440–16 445. ( 10.1073/pnas.1102077108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levin RN. 1996. Song behavior and reproductive strategies in a duetting wren, Thryothorus migricapillus: I. Removal studies. Anim. Behav. 52, 1093–1106. ( 10.1006/anbe.1996.0257) [DOI] [Google Scholar]
- 28.Levin RN. 1996. Song behavior and reproductive strategies in a duetting wren, Thryothorus nigricapillus; II. Playback studies. Anim. Behav. 52, 1107–1117. ( 10.1006/anbe.1996.0258) [DOI] [Google Scholar]
- 29.Logue DM, Hall ML. 2014. Migration and the evolution of duetting in songbirds. Proc. R. Soc. B 281, 20140103 ( 10.1098/rspb.2014.0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenowitz EA, Arnold AP, Levin RN. 1985. Neural correlates of female song in tropical duetting birds. Brain Res. 343, 104–112. ( 10.1016/0006-8993(85)91163-1) [DOI] [PubMed] [Google Scholar]
- 31.Gurney ME. 1981. Hormonal control of cell form and number in the zebra finch song system. J. Neurosci. 1, 658–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenowitz EA, Arnold AP. 1986. Interspecific comparisons of the size of neural song control regions and song complexity in duetting birds: evolutionary implications. J. Neurosci. 6, 2875–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harvey PH, Pagel MD. 1991. The comparative method in evolutionary biology. Oxford, UK: Oxford University Press. [Google Scholar]
- 34.Martins EP. (ed.). 1996. Phylogenies and the comparative method in animal behavior. Oxford, UK: Oxford University Press. [Google Scholar]
- 35.Sibley CG, Ahlquist JE. 1990. Phylogeny and classification of birds. New Haven, CT: Yale University Press. [Google Scholar]
- 36.MacDougMall-Shackleton SA, Ball GF. 1999. Comparative studies of sex differences in the song-control system of songbirds. Trends Neurosci. 22, 432–436. ( 10.1016/S0166-2236(99)01434-4) [DOI] [PubMed] [Google Scholar]
- 37.Nealen PM, Perkel DJ. 2000. Sexual dimorphism in the song system of the Carolina wren Thryothorus ludovicianus. J. Comp. Neurol. 418, 346–360. () [DOI] [PubMed] [Google Scholar]
- 38.Gahr M, Güttinger H-R. 1986. Functional aspects of singing in male and female Uraeginthus bengalus (Estrildidae). Ethology 72, 123–131. ( 10.1111/j.1439-0310.1986.tb00612.x) [DOI] [Google Scholar]
- 39.Gahr M, Sonnenschein E, Wickler W. 1998. Sex difference in the size of the neural song control regions in a dueting songbird with similar song repertoire size of males and females. J. Neurosci. 18, 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gahr M, Metzdorf R, Schmidl D, Wickler W. 2008. Bi-directional sexual dimorphisms of the song control nucleus HVC in a songbird with unison song. PLoS ONE 3, e3073 ( 10.1371/journal.pone.0003073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voigt C, Gahr M. 2011. Social status affects the degree of sex difference in the songbird brain. PLoS ONE 6, e20723 ( 10.1371/journal.pone.0020723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang R, Sun Y, Zhang X, Zeng S, Xie W, Yu Y, Zhang X, Zuo M. 2009. Song control nuclei in male and female large-billed crows (Corvus macrorhynchos). Zool. Sci. 26, 771–777. ( 10.2108/zsj.26.771) [DOI] [PubMed] [Google Scholar]
- 43.Hall ZJ, MacDougall-Shackleton SA, Osorio-Beristain M, Murphy TG. 2010. Male bias in the song control system despite female bias in song rate in streak-backed orioles (Icterus pustulatus). Brain Behav. Evol. 76, 168–175. ( 10.1159/000320971). [DOI] [PubMed] [Google Scholar]
- 44.McCasland JS, Konishi M. 1981. Interaction between auditory and motor activities in an avian song control nucleus. Proc. Natl Acad. Sci. USA 78, 7815–7819. ( 10.1073/pnas.78.12.7815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Margoliash D. 1983. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J. Neurosci. 3, 1039–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margoliash D, Konishi M. 1985. Auditory representation of autogenous song in the song system of white-crowned sparrows. Proc. Natl Acad. Sci. USA 82, 5997–6000. ( 10.1073/pnas.82.17.5997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prather JF, Mooney R. 2008. Song-selective neurons in the songbird brain: synaptic mechanisms and functional roles. In Neuroscience of birdsong (eds Zeigler HP, Marler P), pp. 174–186. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 48.Diekamp BM, Ball GF, Fortune ES. 2004. Auditory responses in HVc of urethane-anesthetized male and female canaries. Program No. 333.1 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience.
- 49.Fortune ES, Rodriguez C, Li D, Ball GF, Coleman MJ. 2011. Neural mechanisms for the coordination of duet singing in wrens. Science 334, 666–670. ( 10.1126/science.1209867) [DOI] [PubMed] [Google Scholar]
- 50.Ball GF, Balthazart J, McCarthy MM. 2014. Is it useful to view the brain as a secondary sexual characteristic? Neurosci. Biobehav. Rev. 46, 628–638. ( 10.1016/j.neubiorev.2014.08.009) [DOI] [PubMed] [Google Scholar]
- 51.Collins S. 2004. Vocal fighting and flirting: the functions of birdsong. In Nature‘s music: the science of birdsong (eds P Marler, H Slbbekoorn), pp 39–79. Amsterdam, The Netherlands: Elsevier Academic Press. [Google Scholar]
- 52.Airey DC, Buchanan KL, Szekely T, Catchpole CK, DeVoogd TJ. 2000. Song, sexual selection, and a song control nucleus (HVc) in the brains of European sedge warblers. J. Neurobiol. 44, 1–6. () [DOI] [PubMed] [Google Scholar]
- 53.Odom KJ, Hall ML, Riebel K, Omland KE, Langmore NE. 2014. Female song is widespread and ancestral in songbirds. Nat. Commun. 5, 3379 ( 10.1038/ncomms4379) [DOI] [PubMed] [Google Scholar]
- 54.Brenowitz EA. 1991. Altered perception of species-specific song by female birds after lesions of a forebrain nucleus. Science 251, 303–305. ( 10.1126/science.1987645) [DOI] [PubMed] [Google Scholar]
- 55.Nottebohm F. 1980. Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Res. 189, 429–436. ( 10.1016/0006-8993(80)90102-X) [DOI] [PubMed] [Google Scholar]
- 56.Madison FN, Rouse ML, Balthazart J, Ball GF. 2014. Reversing song behavior phenotype: testosterone driven induction of singing and measures of song quality in adult male and female canaries (Serinus canaria). Gen. Comp. Endocrinol. 215, 61–75. ( 10.1016/j.ygcen.2014.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voigt C, Goymann W. 2007. Sex-role reversal is reflected in the brain of African black coucals (Centropus grillii). Dev. Neurobiol. 67, 1560–1573. ( 10.1002/dneu.20528) [DOI] [PubMed] [Google Scholar]
- 58.Rosvall KA, Buergeon Burns CM, Barske J, Goodson JL, Schlinger BA, Sengelaub DR, Ketterson ED. 2012. Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proc. R. Soc. B 279, 3547–3355. ( 10.1098/rspb.2012.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ketterson ED, Nolan V, Sandell M. 2005. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am. Nat. 166, S85–S98. ( 10.1086/444602) [DOI] [PubMed] [Google Scholar]
- 60.McCarthy MM, Pickett LA, Van Ryzin JW, Kight KE. 2015. 2015 Surprising origins of sex differences in the brain. Horm. Behav. 76, 3–10. ( 10.1016/j.yhbeh.2015.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ball GF, Castelino CB, Maney DL, Appeltants D, Balthazart J. 2004. The activation of birdsong by testosterone: multiple sites of action and role of ascending catecholamine projections. In Steroids and the nervous system (eds Panzica GC, Melcangi RC) Annals of the New York Academy of Sciences 1007, pp. 211–231. ( 10.1196/annal.1298.043) [DOI] [PubMed] [Google Scholar]