Abstract
A large number of morphological, physiological and behavioural traits are differentially expressed by males and females in all vertebrates including humans. These sex differences, sometimes, reflect the different hormonal environment of the adults, but they often remain present after subjects of both sexes are placed in the same endocrine conditions following gonadectomy associated or not with hormonal replacement therapy. They are then the result of combined influences of organizational actions of sex steroids acting early during development, or genetic differences between the sexes, or epigenetic mechanisms differentially affecting males and females. Sexual partner preference is a sexually differentiated behavioural trait that is clearly controlled in animals by the same type of mechanisms. This is also probably true in humans, even if critical experiments that would be needed to obtain scientific proof of this assertion are often impossible for pragmatic or ethical reasons. Clinical, epidemiological and correlative studies provide, however, converging evidence strongly suggesting, if not demonstrating, that endocrine, genetic and epigenetic mechanisms acting during the pre- or perinatal life control human sexual orientation, i.e. homosexuality versus heterosexuality. Whether they interact with postnatal psychosexual influences remains, however, unclear at present.
Keywords: sexual orientation, sexual partner preference, homosexuality, organizing effects of steroids, epigenetic controls
1. Introduction
Sexual reproduction implies a specialization of the two sexes, so that one produces large gametes usually in limited numbers (female eggs), whereas the other produces a much larger number of smaller gametes (male sperm). This specialization is by necessity accompanied by major sex differences in reproductive morphology and physiology, such as the presence in vertebrates of ovaries secreting large amounts of oestrogens and progesterone in females and the presence of testes secreting testosterone in males. The action of these sex steroids is, however, not limited to reproduction, and these steroids have now been shown to affect a vast array of physiological and behavioural responses, including, for example, neuronal plasticity, neuroprotection, tumour growth, memory formation and retention, to cite a few [1,2]. Based on the prominent sex differences in production and thus circulating concentrations of sex steroids, it follows that many of the processes influenced by these steroids are themselves associated with sex differences.
Sex differences in brain, behaviour and physiology are thus widespread and not the exception which actually led Larry Cahill (University of California, Irvine) to write that ‘the burden of proof regarding the issue (of sex differences) has shifted from those examining the issue in their investigations generally having to justify why, to those not doing so having to justify why not’ [3,4]. It has also become clear recently that the analysis of the functional significance of these sex differences has become a priority in neurosciences [5].
Another consequence of sexual reproduction is that males are as a rule sexually attracted by females and vice versa. This behavioural difference is usually referred to as the sexual partner preference (for concision, partner preference in the following) or also sexual orientation in humans. Partner preference can be considered as one of the multiple sex differences in behaviour, because males and females present a different target for their sexual attraction. Any deviation from this heterosexual attraction, that is an attraction for the same sex or homosexual attraction, is then considered as a reversed sex difference (see also [6] on this topic). Accepting the idea that partner preference is a sex difference begs the question of the mechanisms that control its development. All behavioural differences in animals and humans develop under two major types of influence: biological factors including mostly genes, their expression and hormones, and environmental factors recovering multiple forms of influences of parents, peers and congeners, in general, associated with various forms of learning.
We shall focus here on the biological aspects that are the topic of this special issue. It must be noted, however, that some scientists, usually with a psychological or sociological background, consider that all behavioural and possibly neural sex differences in humans are culturally constructed [7] and negate biological influences on sex differences [6], a concept known as the gender theory. In §2 we shall first review the mechanisms that control sex differences in brain and behaviour, drawing largely on the literature dedicated to the sexual differentiation of reproductive behaviour in rodents. In §3 we shall then review how these same mechanisms were shown to apply, at least in part, to the sexual differentiation of partner preference in a few animal species in which this process has been studied. Finally, in §4, we shall summarize clinical and epidemiological evidence strongly suggesting that these same biological mechanisms are still at play in the control of human sexual orientation even if data here are still almost exclusively correlational and thus conclusions cannot be presented with the same level of confidence.
2. Sexual differentiation: how do sex differences emerge?
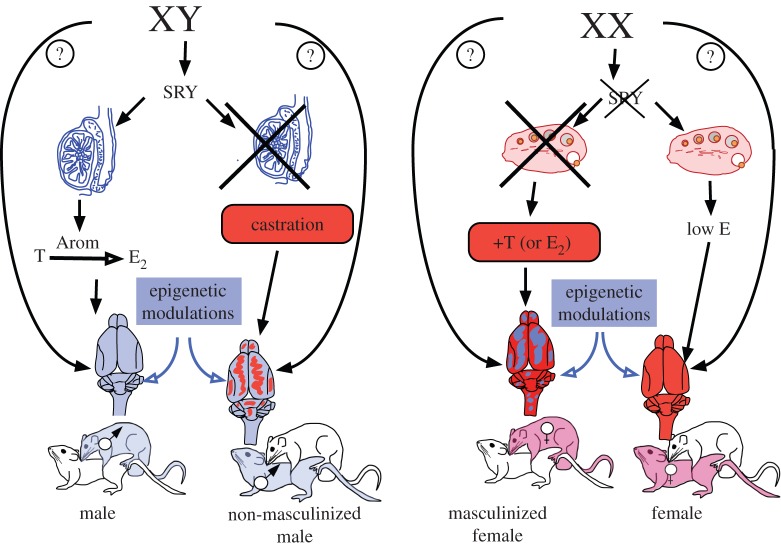
Although multiple forms of sex determination are present in animals (see [8] for a recent review), this process in mammals including humans is controlled almost exclusively by a specialized set of chromosomes, the sex chromosomes, XX in females and XY in males. Schematically, the Y chromosome of males contains a gene called SRY that determines the development of the initially undifferentiated gonad into a testis, whereas in females (XX complement), the absence of SRY will lead to differentiation of the gonadal anlage into an ovary [9,10] (figure 1).
Figure 1.
Schematic of the hormonal, genetic and epigenetic mechanisms controlling sexual differentiation in mammals based mainly on studies of sexual behaviour in rodents. See text §2 for additional explanations.
(a). The organizational effects of hormones
Studies of sex differences in primary sexual characteristics (e.g. penis versus clitoris and vagina, the presence of a uterus in females only) initially led to the formulation of a theory of sexual differentiation explained by the embryonic hormonal secretions of the gonads (for review, see [11]). It was initially believed that differences in reproductive behaviour between males and females resulted from the presence of different hormones in adults of the two sexes: testosterone in males and oestradiol (plus progesterone) in females [12]. However, the seminal work of Young and co-workers [13] in guinea pigs demonstrated that these differences mostly result from the early exposure of embryos to a high concentration of testosterone for males and a much lower (lack of?) exposure to sex steroid in females. These investigators demonstrated that only males exposed to high levels of testosterone in utero exhibit male sexual behaviour in adulthood when they again experience high levels of testosterone. Females artificially exposed to testosterone during development to the same degree and at the same time as males also exhibit male-like sexual behaviours towards other females if supplied with male levels of testosterone when adults. At the same time, these females treated with exogenous testosterone lose the capacity to respond to ovarian hormones in adulthood and thereby lack female sexual behaviour. This interplay between early life hormonal profile and adult responsiveness is referred to as the organizational/activational hypothesis of sexual differentiation and has been shown to apply to a variety of other species including rats, as illustrated in figure 1.
These organizing effects occur early in life, during the embryonic period or just after birth, and are irreversible. Early exposure to testosterone produces a male phenotype: the behavioural characteristics of the male are strengthened (masculinization), and the ability of males to show behaviour typical of females is reduced or lost (defeminization). The female phenotype develops in the apparent absence of hormone action during the embryonic period (or in the presence of very low oestrogen). More recent studies indicate, however, that development of the full female behavioural phenotype requires exposure to oestrogens during ontogeny, but this exposure takes place much later, during the pre-pubertal period rather than in utero [14].
These studies indicated that the type of sexual behaviour (male- or female-typical) displayed by an adult individual is determined by exposure to steroids during the early stages of life. More recent work, however, shows that genes can produce behavioural or physiological differences between males and females in a more direct manner that apparently does not involve sex steroid action.
(b). Gene effects that are not hormone-mediated
The notion of a sexual differentiation that would be independent of early steroid action largely originated in the analysis of a single zebra finch (Taeniopygia guttata) individual that was male on the left side and female on the right side, the well-known gynadromorphic zebra finch [15]. Genetic markers confirmed that this bird had male cells on the right side but female cells on the left side of its brain. Correlatively, the volume of its song control nucleus HVC was much larger on the male than on the female side, despite the fact that both sides had obviously been exposed to the same concentrations of circulating sex steroids. Sex differences in birds are, like in mammals, largely under the control of organizational effects of sex steroids, although modalities of these controls differ markedly (see [16] for review). The morphological difference between left- and right-side HVC in the gynandromorphic subject indicated, however, that this feature was controlled, at least in part, by an action of genes somewhat independent from the organizational action of steroids [15]. Some reframing of the original organizational/activation hypothesis was therefore needed. A few studies had already demonstrated that some phenotypic sex differences [17,18] and sex differences in gene expression [19–21] are observed before the gonads develop and start secreting substantial amounts of sex steroids. These sex differences thus cannot be induced by exposure to a differential hormonal milieu.
To address this question, it is obviously impossible to follow up in the single gynandromorphic zebra finch. Therefore, researchers took advantage of a mutated mouse model in which the SRY gene was no longer functional, so that it could no longer induce the formation of testes (the XYSRY− mouse). In another mouse line, they additionally translocated the SRY gene to an autosome, so that XX females would develop testes during the early embryonic life (XXSRY mouse). Together with control mice (XX and XY), these mice provided four separate genotypes in which the presence of testes (in XY and XXSRY subjects) or ovaries (in XX and XYSRY−subjects) could be disentangled from the presence of an XY or XX genotype; the so-called four core genotype model [22]. In this model, behavioural and neuroanatomical traits directly related to reproduction were usually confirmed to differentiate mostly under the organizing influence of gonadal steroids, but a growing number of other sex differences not directly tied to reproduction have now be shown to differentiate as a function of the chromosome complement independently of the presence of testes or ovaries [23–27]. Interactions between these two processes have also been detected (e.g. in the control of body weight [27,28]).
(c). Epigenetics
Recent studies have added yet another layer of complexity to our understanding of the process of sexual differentiation. It has become clear that a variety of modifications of the DNA itself (mostly methylations) or of the associated histones (acetylations, methylations, etc.) that do not change the primary structure of the DNA markedly affect its transcription. These acquired modifications of DNA and histones, called as a whole epigenetic marks, can even be transmitted to the offspring and in this way influence phenotypic traits in multiple generations [29]. It is, for example, now well established that early stress or early exposure to a high calories/high-fat diet affects stress physiology and energy balance, respectively, in a permanent way in the exposed individual, and also in her offspring (see [29] for review).
These epigenetic effects also extend to the control of behaviour as illustrated by the elegant work of Michael Meaney and co-workers showing that rat mothers providing poor maternal care will transmit this phenotype to their offspring via changes in the methylation of a few key genes, including the gene coding for a glucocorticoid receptor in the hippocampus and the gene of one oestrogen receptor in the medial preoptic area [30,31].
It was also demonstrated that organizing effects of sex steroids on brain and sex behaviour are mediated, to a large extent, by epigenetic mechanisms. Oestradiol, for example, affects the enzymes that control these epigenetic marks such as DNA methyltransferases and histone deacetylases in the brain of neonate rodents, and pharmacological manipulations of these enzymes in neonate rats have been shown to affect very significantly the sexual differentiation of brain and behaviour [32–34]. Oestradiol derived from testosterone aromatization in the brain reduces the activity of DNA methyltransferases in the preoptic area in males. This consequently decreases DNA methylation in subjects exposed to testosterone (males or testosterone-treated females) and releases masculinizing genes from epigenetic repression. Most importantly, experimental manipulation of the DNA methyltransferases (with pharmacological or molecular biology tools) mimicked the effects of testosterone on gene expression and adult behaviour. These data thus quite surprisingly show that the female brain and behaviour are actively maintained by an active suppression of masculinization via DNA methylation, a process that is inhibited by testosterone in males [34]. Recent work also indicates that some of these organizing effects of testosterone on the methylome do not necessarily appear immediately during or after exposure to the steroid but are eventually more pronounced later in life (up to a 20-fold increase) [35]. This observation certainly contributes to explaining the long-lasting (permanent) organizational effects of sex steroids.
Note, however, that not all epigenetic marks that control gene expression are necessarily the result of a differential exposure to steroids, because the expression of many genes is already sexually differentiated on day 10.5 post-coitum of embryonic mice, in advance of gonadal development and differential steroid secretion in males and females [19,20]. The origins of such a differential early expression are not clearly identified at this time but presumably reflect direct genetic effects such as those discussed in §2(a), with a few sex chromosome genes inducing the differential expression of other genes located on autosomes.
In summary, the sexual phenotype of an individual can be affected in a permanent manner by three different types of mechanisms: endocrine, genetic and epigenetic. Importantly, these three types of influences are only partly independent and multiple interactions have been described. In particular, sex steroids do modify epigenetic marks and thus gene expression, and a variety of genes deeply affect hormone secretion and action. Identifying the primary factor(s) responsible for a sex difference is thus often not easy.
3. Sexual differentiation of partner preference in animals
The organizational action of steroids on sexual behaviour patterns (§2a) seems to apply also to the sexual differentiation of partner preference in animals. In most cases, sexual differentiation of different traits is coordinated, and a subject displaying male sexual behaviour patterns will correlatively exhibit a sexual preference for females and vice versa. Sometimes, however, disassociations can occur, presumably under the influence of subtle alterations during limited periods of ontogeny of circulating hormones or of their local hormone action. A genetic male expressing male sexual behaviour can then develop a sexual preference for other males (for review, see [36,37]). This conclusion is supported by studies manipulating the endocrine perinatal environment in a few species and assessing the effects on adult partner preference, and also by the analysis of sexual partner preferences in sheep, a species in which a spontaneous exclusive male homosexual preference is observed in approximately 8% of the rams.
(a). Experimental manipulations of the perinatal endocrine environment
In rats and mice, perinatal manipulations of sex steroid concentrations modify in a permanent manner the partner preferences of the treated subjects. Exposure to testosterone (or its metabolite oestradiol) induces a preference for female over male sex partners (male-typical orientation), whereas in the absence of high concentrations of these steroids, a female pattern of sexual orientation will develop (preference for male partner).
The first set of studies establishing this conclusion were performed in rats at the University of Rotterdam as part of the PhD thesis of Julie Bakker performed under the supervision of Dr Kos Slob. They showed that pharmacological inhibition of aromatase activity during the week before and the week after birth in male rat pups/embryos reverses their adult partner preference, so that the subjects will now prefer to spend time with other males than with sexually receptive females. They will also display female receptive behaviour (lordosis) in the presence of another male and allow these males to mount them [38]. These males with a sex-reversed partner preference also display a neuronal activation, as revealed by expression of the c-fos gene, in nuclei controlling sexual behaviour in response to male urine, whereas control males show such an activation in response to female, but not male, urine [39]. Their sexual orientation and the related neural circuits have thus been profoundly and permanently affected by these neonatal endocrine manipulations.
The same type of endocrine control was demonstrated in females. Treatment of young females during their first three weeks of postnatal life with oestradiol benzoate, a long-acting oestrogen, reversed their adult sexual partner preference, so that after treatment they preferred to interact sexually with other females instead of males [40].
Similar organizational effects of sex steroids on partner preference have been observed in mice, although in this species androgens themselves seem to play a more important role in the sexual differentiation of partner preference than their oestrogenic metabolites produced by aromatization. Specifically, sexual differentiation of partner preference was shown to be affected in testicular feminized mice (tfm) that carry a mutation of the androgen receptor making it non-functional. When adult, males in these mice prefer, like control females, to investigate odours from bedding soiled by control male urine as opposed to female urine [41]. Furthermore, tfm males, like females, show no preference for a partner of one sex or the other, in contrast to control males that show a strong preference for females. Also, there is a strong activation of the preoptic area and nucleus of the stria terminalis of tfm male and of control female mice exposed to bedding soiled by male urine that is not observed in control males. Together, these data show that lack of androgen action in tfm males blocks the masculinization of their partner preference. Additional work in mice also shows that this masculinization can be induced by an early treatment with the non-aromatizable androgen dihydrotestosterone, even if oestrogens are additionally implicated in this process to some extent [42] as they are in rats [43,44].
(b). Homosexual sheep
The studies described in §3(a) concern experimentally induced same-sex partner preference. Spontaneous homosexual behaviour, defined as exclusive same-sex sexual preference, appears to be rare in animal species despite the fact homosexual behaviours (mounting or being mounted by a subject of the same sex) are frequently seen in hundreds of species [45,46] when congeners of the opposite sex are not (easily) available.
One case of spontaneous homosexual preference has, however, been documented in a population of male sheep living in the western part of the USA (Idaho). In this population, an estimated 8% of the rams show little or no sexual reaction to females, but contrary to what had been originally assumed, they are not asexual and they display active mounting behaviour towards other males even if provided with a choice between male and female partners [47].
This behaviour of male-oriented rams (MOR) as termed by the authors of the study is not explained by differences in their rearing conditions or adult endocrine status when compared with female-oriented rams (FOR) (see [48] for review). Analysis of their brain indicates, however, that the ovine sexually dimorphic nucleus (oSDN) of the preoptic area, a structure that is normally three times more voluminous in males than in females, in MOR has the same volume as in females and contains fewer neurons than in FOR. The oSDN of FOR also expresses two to three times more aromatase mRNA than females and MOR as quantified by in situ hybridization [48].
This correlation between the volume of the oSDN and sexual orientation (larger in subjects attracted to females, the FOR, than in subjects attracted to males, the females and the MOR) appears to be the result of a differential exposure to testosterone during embryonic life. Indeed, the volume of the oSDN is already larger in males than in females around day 135 of embryonic life, and treatment of female embryos with testosterone between 30 and 90 days of gestation markedly increases the oSDN volume in these females [49]. These data thus strongly suggest that the volume of the oSDN is determined before birth under the influence of testosterone, in any case well before subjects had an opportunity to express their sexual orientation. The volume of this nucleus is additionally no longer sensitive to changes in testosterone concentrations during adult life. The smaller oSDN of MOR when compared with FOR is thus likely to reflect a lower exposure to androgens during gestation and could, in turn, be responsible for the same-sex attraction characterizing these subjects. It must, indeed, be recalled here that the medial preoptic area is not only a key site of steroid action for the activation of male copulatory behaviour in all vertebrate species investigated so far from fishes to mammals [50], but it also seems to control male sexual orientation. Lesions of this nucleus reverse sexual partner preference in males of several species, including ferrets [51] and rats [52].
In summary, the sex of the preferred sexual partner is markedly influenced if not determined by the early hormonal environment in a manner reminiscent of the early organizational effects of steroids on the sex-specific patterns of reproductive behaviour. There is, however, no experimental material allowing us to assess the possible contribution to this aspect of the adult phenotype of more direct steroid-independent genetic or epigenetic mechanisms, with the exception of studies in fruitflies (Drosophila melanogaster) showing that mutation of the fruitless (fru) gene produces adult males who will court males and females equally [53–55]. These findings do not, however, easily transfer to mammals given the profound differences between vertebrate and insect physiology (see [56] for additional discussion).
4. Sexual orientation in humans
Converging evidence indicates that the three types of mechanism (hormonal, genetic and epigenetic) described in animals are implicated, to some degree at least, in the control of human sexual orientation. However, given the nearly complete impossibility of performing truly causal experiments in humans, this conclusion rests mostly on correlative studies, but these all point in the same direction.
(a). Endocrine influences
It is clear that the sex steroids (testosterone and oestradiol) that organize behaviour in animals are still present in human embryos and adults, and this is also the case for their receptors in the brain. Embryonic testosterone also clearly determines sex differences in human genital morphology [57]. Two types of data, clinical cases and the phenotypic distribution of sexually differentiated characteristics, then suggest that modulations of this early exposure to testosterone influence human sexual orientation. Exposure to a high concentration of testosterone during a critical period of development would predispose to a male-typical attraction to women, whereas a lower embryonic exposure to steroids would lead to a female-typical orientation.
(i). Sexually differentiated characteristics are affected in gays and lesbians
Although it is nearly impossible, for practical reasons, to determine the hormonal milieu to which an individual was exposed during his/her embryonic life, it is possible to gather indirect information about this milieu by studying in adults phenotypic traits that are known to be influenced in a permanent manner by embryonic testosterone. A large number of studies have compared such traits in homo- versus heterosexual populations and found statistically significant differences that strongly suggest that homosexual populations were on average exposed to slightly different endocrine conditions during their early life. These differences concern morphological, physiological and behavioural traits that are too numerous to be reviewed here in detail (see [36,37,56,58,59] for detail and references).
These indicators of exposure to atypical endocrine conditions during early life in homosexual subjects include at the morphological level: (i) the relative length of the index (D2) to the ring finger (D4) (shorter, masculinized ratio in lesbians compared with heterosexual women), (ii) the relative length of long bones in the legs, arms and hands (shorter bones in gays and women who are attracted to men compared with men and lesbians who are attracted by women), and the size of several brain structures including, (iii) the surprachiasmatic nucleus (larger in gays than in heterosexual men), (iv) the anterior commissure (larger in gays than in heterosexual men) and finally (v) the interstitial nucleus of the anterior hypothalamus number 3 (INAH3; two to three times larger in heterosexual men than in gays [60] (figure 2) and having a lower density of neurons in gays than in heterosexual men [61]).
Figure 2.
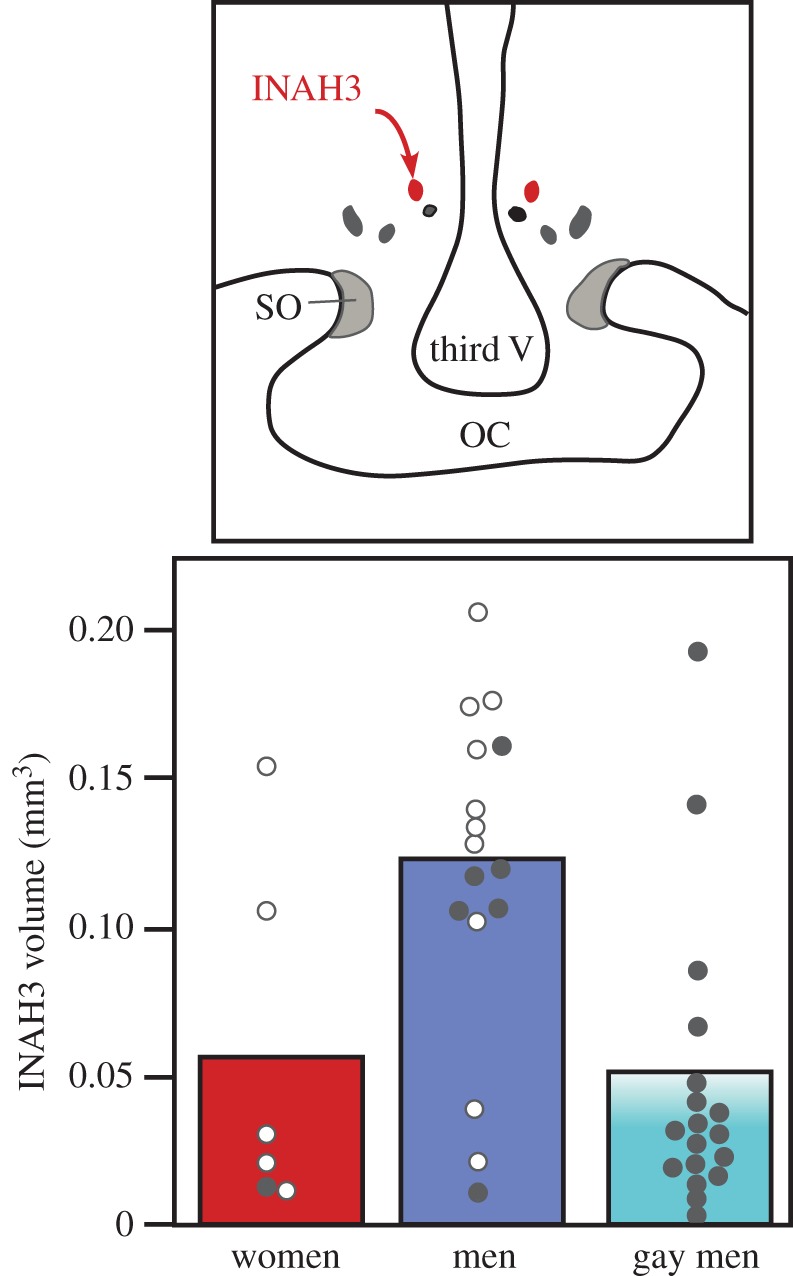
Schematic of the human hypothalamus showing the position of the third interstitial nucleus of the anterior hypothalamus (INAH3; top) and volume of this nucleus (average shown by bars and individual points) measured by histological techniques in a sample of women, men and homosexual (gay) men who had died from AIDS (filled circles) or from another unrelated cause (open circles). Results in heterosexual men do not seem to be affected by whether they died from AIDS or another cause, suggesting that the lower average values in gay men was not a result of their death from AIDS-related factors. Adapted from [60].
Several physiological differences also point to similar modifications of the embryonic exposure to testosterone in homosexual when compared with heterosexual subjects. This is namely the case for (i) aspects of the inner ear physiology, in particular the small noises presumably produced by movements of the tympanic membrane, the so-called otoacoustic emissions (less frequent and of lower amplitude in lesbians compared with heterosexual women; figure 3), (ii) the feedback of steroids on the secretion of luteinizing hormone (presence of a weak positive feedback after injection of a large dose of oestrogens in gays but not in heterosexual men), and (iii) the brain activation as detected by magnetic resonance imaging (MRI) or positron emission tomography (PET) in response to male- or female-typical odours (reaction of gay men to male odours contrary to heterosexual men and lack of reaction of lesbians to male odours contrary to heterosexual women).
Figure 3.
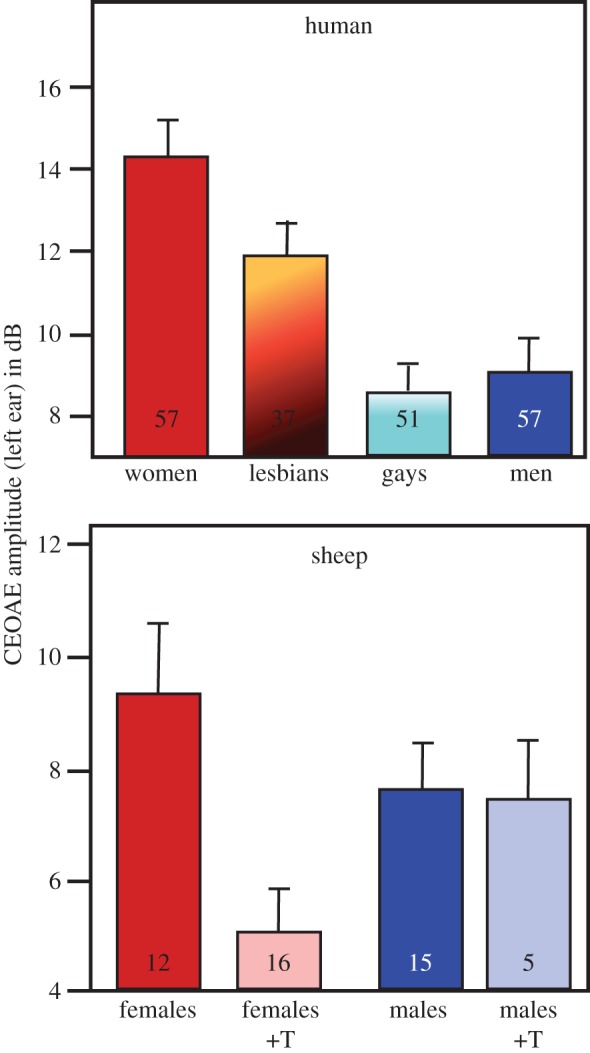
The average amplitude of click-evoked otoacoustic emissions (CEOAE) is larger in women than in men and in female sheep compared with males. In sheep and other animals, the amplitude in females is reduced by a perinatal treatment with testosterone (+T). In homosexual women (lesbians), this amplitude is also significantly lower than in heterosexual women, suggesting that lesbians were exposed to higher concentrations of testosterone during their early life. Adapted from data in [62,63].
There are, in addition, studies reporting average cognitive/behavioural differences between homo- and heterosexual populations in a given sex. Among the most reliably established differences of this type one can cite those concerning: (i) aggressive behaviour (gays less aggressive than heterosexual men), (ii) visuospatial tasks (gay performing poorly compared with heterosexual men), (iii) verbal fluency (gays more fluent than heterosexual men), and (iv) the memorization of object location (gays performing better than heterosexual men).
Interestingly, in all these cases but one (the volume of the suprachismatic nucleus), the modification seen in gays or lesbians makes them more similar to heterosexual subjects of the opposite sex, suggesting that they were exposed to endocrine influences that were typical of the other sex. This approach is, however, bound by certain limitations:
— some of these effects have been reproduced, but others have not and the origin of the discrepancies has not always been identified (different recruitment of study subjects?),
— although statistically significant, the differences observed only explain a part of the variance, and it is clearly impossible to predict the sexual orientation of a subject based on any of these criteria,
— it is sometimes unclear whether the difference observed reflects the signature of a differential early exposure to steroids and is potentially a cause of homosexuality or if it is a consequence of this sexual orientation. This limitation is particularly critical for cognitive/behavioural differences but much less so for morphological or physiological traits. The case of the smaller INAH3 of homosexual men is particularly interesting, because (i) the equivalent (homologous?) sexually dimorphic nucleus of the preoptic area is known to differentiate irreversibly between males and females in response to early endocrine conditions in rats and sheep [64,65], (ii) the volume of INAH3 does not seem to depend significantly on the hormonal status of a man in adulthood [66], (iii) lesions of this nucleus reverse sexual orientation in male rats and ferrets [51,52], and (iv) in sheep, the volume of this nucleus correlates with sexual orientation in rams [48]. The smaller INAH3 of gay men could thus be at the same time the signature of their lower exposure to testosterone in early life and the (partial) cause of their sexual orientation. Owing to the limitations mentioned above, this conclusion remains, however, tentative.
(ii). Clinical studies
A number of human pathologies are associated with significant modifications of the embryonic endocrine environment. Many studies have therefore asked whether these endocrine changes are associated with changes in the incidence of homosexual orientation, and a positive response has been obtained in several cases. Three such clinical conditions are important to mention in this context. First, girls suffering from congenital adrenal hyperplasia are exposed in utero to abnormally high levels of androgens that masculinize their genital structures and a variety of behavioural traits (e.g. aggressive play and toy selection). These girls also display a significantly increased incidence of homosexual (or at least not strictly heterosexual) orientation (up to 40% compared with less than 10% in control populations; [67–69]). Second, girls born from mothers who had been treated with the synthetic oestrogen diethylstilboestrol in the hope of preventing undesired abortions were shown to display a significant increase in non-heterosexual (bi- or homosexual) fantasies and sexual activities [70].
Thirdly, it is also interesting to note here that the sex in which a child is reared does not seem to be able to completely counter the endocrine influences experienced prenatally. This is of course illustrated by the case of John/Joan a young boy who had his penis destroyed during circumcision and was therefore raised as a girl. It turned out that in adulthood he reverted to a male identity and male sexual orientation [71]. This anecdotal story is additionally supported by the systematic study of patients afflicted by cloacal dystrophy, a rare genitourinary malformation resulting in the birth of XY males who, in addition to various malformations of the pelvis, have no penis. These subjects have normal testes and were thus presumably exposed to a male-typical pattern of androgen secretions before birth. Very often, these subjects were submitted to vaginoplasty and raised as girls. Follow-up studies have shown that in about half of the cases, these subjects when adults adopt a male identity, gender role and male-typical sexual orientation, again suggesting a significant influence of their embryonic exposure to androgens [72,73].
Even if alternative explanations can and have been proposed for some of these observations, then the most parsimonious explanation remains that embryonic hormones play a substantial role in the control of adult sexual orientation. Note, however, that changes in sexual orientation as a result of endocrine embryonic disruption always concern a fraction of affected individuals (usually a maximum of 30–40%) so that at least 60–70% of subjects in these conditions still display a heterosexual orientation. Other factors must therefore be involved as described in §4(b,c) following.
(b). Genetic influences
Because hormones obviously influence but do not seem to fully explain sexual orientation, at least in the current stage of knowledge, researchers have considered an alternative group of explanations based on genetic influences. Furthermore, even if embryonic testosterone determines sexual orientation, this raises the question of why testosterone secretion or action was changed during the development of gays and lesbians. A genetic influence would appear in this context as the most likely candidate.
Multiple epidemiological studies have shown that the presence of a gay man in a family is correlated with an increased probability of finding other homosexual men in this family, and in addition this probability is directly correlated with genetic relatedness. For example, if a son is gay, between 20% and 25% of his brothers will share this orientation, compared with 4–6% in the whole population. Similarly, lesbians have a greater probability than heterosexual women of having a homosexual sister [74,75].
Twins studies suggest that this correlation does not reflect the similarity of postnatal experiences (psychosocial factors) but rather genetic similarity. There is indeed a much higher concordance of male sexual orientation in identical (50–65%) than in dizygotic (about 15%) twins who shared the same postnatal environment, but differ in genetic relatedness [74]. Overall, data suggest that in social conditions typical of Western societies, about 50% of the variance in human sexual orientation has a genetic origin.
Although this notion was established many years ago, the genes that might support the phenomenon have so far remained somewhat elusive. Family lineage studies indicate that male homosexuality tends to be transmitted through matriarchal lineage: a gay man has a higher probability of having gay men among his relatives on the maternal side, but not the paternal side. This was originally interpreted as a sign of inheritance through gene(s) located on the X chromosome and one study indeed identified a linkage with markers located in the subtelomeric region of the long arm of the X chromosome, a region called Xq28 [76]. A genomewide scan also identified linkage of male homosexuality to regions of chromosome 7 (7q36) and 8 (8q12) as well as a linkage to chromosome 10 (10q26) resulting from a sharing of maternal alleles only [77].
The association with Xq28 originally detected by Hamer et al. in 1993 [76] was replicated three times by the same and other authors (see [78] for detail), and very recently this association with Xq28 was confirmed in a study based on a much larger sample of over 400 gay brothers [79]. This last study additionally confirmed a significant linkage with a region on chromosome 8 (8q12).
Taken together, these studies leave no doubt about the existence of genetic controls on sexual orientation, but at the same time they show that these controls are likely to be polygenic and very complex. The specific genes implicated in this process remain unknown even if candidate genes located at Xq28, such as the arginine–vasopressin receptor 2, appear as interesting candidates (see [79] for discussion). Whether or not these genes affect sexual orientation by modifying steroid action during ontogeny also remains unknown.
(c). Epigenetic modulation of androgen sensitivity
Although endocrine and genetic factors clearly seem to affect sexual orientation in humans (§4a,b), a significant part of the variance in this trait remains unexplained and a number of important questions remain. Why, for example, is there only 50–60% similarity in orientation of monozygotic twins when they share the same genetic material (see §4b)? It was also noted that even in rats [78] and humans [80,81] which are the best studied, there is during most if not all of the embryonic life some overlap between circulating concentrations of testosterone of males and females, even if males have on average higher concentrations. The sex difference in plasma testosterone concentration is thus an ambiguous signal that cannot by itself explain why there is essentially no overlap between male and female phenotypes. The differentiation of the external genitalia into a male phallus or female vulva takes place, for example, in rats and humans during a period of embryonic life when testosterone concentrations overlap between the sexes [80,82–84]. Yet discordance between genetic sex and sex of the genitalia is extremely rare, clearly indicating that some additional factors are needed to produce this sexually differentiated phenotype. It has been postulated that additional factors upregulate the sensitivity to testosterone in males or downregulate it in females, and multiple mechanisms that mediate such a differential sensitivity have been identified (reviewed in [85]).
This then raises the question of what controls these mechanisms, and evidence has recently accumulated indicating that sex chromosomes independently of sex hormones epigenetically regulate expression of a variety of autosomal genes that may be responsible for the control of sensitivity to sex steroids [86]. There is also evidence that gene expression is sexually differentiated even before the gonads develop [19,20,87] (see also §2b). Actually, XX and XY embryos are differentiated at the stem cell stage of the blastocyst [88] far in advance of androgen production, and epigenetic marks are likely the causal agents of this differentiation.
Importantly, these controls are gene-specific and therefore preferentially modulate particular functional responses. It has, for example, been shown that expression of the 5α-reductase gene coding for the enzyme that catalyses the transformation of testosterone into 5α-dihydrotestosterone is three times higher in the genital structures of male than in female fetuses, and this difference would not be a result of sex steroid action [89]. This transformation critically mediates effects of testosterone on the sexual differentiation of the phallus and probably explains why the sex of the genitalia is usually in agreement with the genetic sex even in the presence of a large overlap between circulating testosterone concentrations in male and female embryos.
Because androgen signalling differs between organs and tissues, namely because the androgen receptors use different co-activators and co-repressors to control transcription, it is conceivable that different epigenetic marks transmitted across generations might affect subsets of sexually dimorphic traits. The androgen-dependent sexual orientation could, for example, be affected in the absence of any effect of the genitalia. A statistical model has been presented demonstrating the feasibility, over a broad range of values for the critical parameters of the model, of a control of sexual orientation based on the inheritance of sexually antagonistic (protecting XX subjects from androgen action) epigenetic marks conditioning androgen sensitivity in a tissue-specific manner (see [85] for a full presentation). Based on whether these marks escape erasure or not in the primordial stem cells and zygote, this model would explain the observed heritability of homosexuality, the failure so far to identify clear genetic markers explaining homosexuality (reviewed in [84,90]), the different degree of concordance of sexual orientation between mono- and dizygotic twins, and also the absence of complete concordance in monozygotic twins.
Note, finally, that the incidence of male homosexuality in a given male subject increases by 33% for each older full brother (born to the same mother) he has. The effect is not related to differences in education or family background and is currently interpreted as the result of accumulation in the mother during successive pregnancies of antibodies against one or more proteins expressed specifically by the male brain. [91]. An epigenetic control of gene expression related to the early interaction of the male fetus with his mother could obviously contribute to explain this phenomenon.
5. Conclusion
Sexual differentiation is clearly the result of an interaction between endocrine, genetic and epigenetic mechanisms, and this conclusion largely applies to the differentiation of sexual orientation in animals and humans. Human sexual orientation, and in particular its less common form homosexuality, is thus not mainly the result of postnatal education but is, to a large extent, determined before birth by multiple biological mechanisms that leave little to no space for personal choice or effects of social interactions.
Our current understanding of these biological mechanisms controlling sexual orientation is admittedly incomplete and will likely remain so, because we are dealing here with a complex behavioural trait and additionally most of the critical experiments that would be needed to reach firm conclusions are obviously unethical. Each of the biological factors identified so far seems to explain homosexuality in only a fraction of individuals, and three non-mutually exclusive reasons potentially explain this limitation. Either different forms of homosexuality (butch/femme in women, hyper-masculine versus feminized in men, any other differential feature) have different origins (endocrine, genetic, epigenetic) or the different biological factors only produce a homosexual phenotype when acting in combination or, finally, the action of these biological factors that predispose to homosexuality must be combined with specific, so far unidentified, psychosocial influences during postnatal life playing an important permissive role. It appears, indeed, likely that genes or hormones do not act specifically on sexual orientation. They rather modify more general behavioural traits, such as cross-gender identification [92,93] or the propensity to be sexually attracted by individuals who are similar or dissimilar to yourself [94], which indirectly predispose or lead to homosexuality.
In addition, relatively recent research in juvenile rats indicates that some aspects of sexual behaviour, including a preference for a same-sex partner, can be conditioned by early experience associated or not with pharmacological manipulations. For example, young female rats allowed to express juvenile play with artificially scented males will in adulthood show a sexual preference for males bearing the same odour over other males [95]. More directly related to the present topic, male rats that were allowed to cohabit three times during 24 h with another almond-scented male immediately after being treated with quinpirole, a D2 dopaminergic agonist, developed a social and sexual preference during later drug-free tests for this scented male over a novel unscented male partner [96] and over a sexually receptive female, but such a preference did not develop if males were injected with saline before the cohabitation periods [97]. Also such preferences do not develop in females even if they are exposed to quinpirole before the cohabitation periods [96]. A similar same-sex socio-sexual preference developed in male rats who cohabitated with an almond-scented male under the influence of oxytocin alone or combined with quinpirole [98].
In another experiment, male rats were first allowed to copulate with a sexually receptive female and were immediately removed from the female compartment to be placed for 1 h during the post-ejaculatory interval (PEI) with another almond-scented male that served as conditioned stimulus. Although this procedure was repeated daily for 10 days in the absence of pharmacological treatment, this cohabitation with a male partner during the PEI that was likely associated with enhanced dopaminergic and oxytocinergic receptor activation in the brain did not induce a same-sex partner preference [99].
Together, these data demonstrate that same-sex partner preference in male rats can, to some extent, be manipulated by cohabitation with another male, provided cohabitation is experienced during pharmacological activation of D2 or oxytocin receptors which presumably enhances the salience of the stimuli or the attention/expectation/reward in experimental subjects [98], but this experience effect does not take place in females or in the absence of pharmacological treatment. These pharmacological treatments facilitate the formation of stimulus–response associations, but it remains to be demonstrated whether the preference for the scented familiar male partner would generalize to other unfamiliar males as opposed to unfamiliar sexually receptive females.
Postnatal effects on sexual partner preference thus seem to be present, but have a limited magnitude in rodents. If these data can be extrapolated, then the same type of limited effects might exist in humans, but presumably do not fully explain the development of exclusive same-sex preference. It seems, therefore, that most if not all human beings do not choose to become homo- or heterosexual. This sexually differentiated behavioural characteristic is largely controlled by the same biological factors as other sexually differentiated traits, and this makes sense in evolutionary terms given the critical importance of sexual orientation for reproductive fitness.
Acknowledgements
I thank Margaret M. McCarthy for her critical reading of an earlier version of this manuscript and for a number of useful comments.
Competing interests
I declare I have no competing interests
Funding
Writing of this review was partially supported by the NIMH grant no. RO1 MH50388 to Gregory F. Ball.
References
- 1.Etgen AM, Pfaff DW. 2009. Molecular mechanisms of hormone actions on behavior. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 2.Pfaff D, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT. 2002. Hormones, brain and behavior. Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 3.Cahill L. 2010. Sex influences on brain and emotional memory: the burden of proof has shifted. Prog. Brain Res. 186, 29–40. ( 10.1016/B978-0-444-53630-3.00003-8) [DOI] [PubMed] [Google Scholar]
- 4.Jazin E, Cahill L. 2010. Sex differences in molecular neuroscience: from fruit flies to humans. Nat. Rev. Neurosci. 11, 9–17. ( 10.1038/nrn2754) [DOI] [PubMed] [Google Scholar]
- 5.Clayton JA, Collins FS. 2014. Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283. ( 10.1038/509282a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan-Young RM. 2010. Brain storm. The flaws in the science of sex differences. Cambridge, MA: Harvard University Press. [Google Scholar]
- 7.Butler J. 1990. Gender trouble: feminism and the subversion of identity. New York, NY: Routledge. [Google Scholar]
- 8.Bachtrog D, et al. 2014. Sex determination: why so many ways of doing it? PLoS Biol. 12, e1001899 ( 10.1371/journal.pbio.1001899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M. 1990. Genetic evidence equating SRY and the testis-determining factor. Nature 348, 448–450. ( 10.1038/348448A0) [DOI] [PubMed] [Google Scholar]
- 10.Sinclair AH, et al. 1990. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, 240–244. ( 10.1038/346240a0) [DOI] [PubMed] [Google Scholar]
- 11.Jost A. 1972. A new look at the mechanisms controlling sex differentiation in mammals. Johns Hopkins Med. J. 130, 38–53. [PubMed] [Google Scholar]
- 12.Beach FA. 1948. Hormones and behavior, pp. 1–368. New York, MA: Paul B. Hoeber. [Google Scholar]
- 13.Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizational action of prenatally administered testosterone propionate on the tissues mediating behavior in the female guinea pig. Endocrinology 65, 369–382. ( 10.1210/endo-65-3-369) [DOI] [PubMed] [Google Scholar]
- 14.Brock O, Baum MJ, Bakker J. 2011. The development of female sexual behavior requires prepubertal estradiol. J. Neurosci. 31, 5574–5578. ( 10.1523/JNEUROSCI.0209-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. 2003. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc. Natl Acad. Sci. USA 100, 4873–4878. ( 10.1073/pnas.0636925100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balthazart J, Arnold AP, Adkins-Regan E. 2009. Sexual differentiation of brain and behavior in birds. In Hormones, brain and behavior (eds Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT), pp. 1745–1787. San Diego, CA: Academic Press. [Google Scholar]
- 17.Reisert I, Pilgrim C. 1991. Sexual differentiation of monoaminergic neurons: genetic or epigenetic? Trends Neurosci. 14, 468–473. ( 10.1016/0166-2236(91)90047-X) [DOI] [PubMed] [Google Scholar]
- 18.Renfree MB, Pask AJ, Shaw G. 2001. Sex down under: the differentiation of sexual dimorphisms during marsupial development. Reprod. Fertil. Dev. 13, 679–690. ( 10.1071/RD01096) [DOI] [PubMed] [Google Scholar]
- 19.Dewing P, Shi T, Horvath S, Vilain E. 2003. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res. Mol. Brain Res. 118, 82–90. ( 10.1016/S0169-328X(03)00339-5) [DOI] [PubMed] [Google Scholar]
- 20.Eakin GS, Hadjantonakis AK. 2006. Sex-specific gene expression in preimplantation mouse embryos. Genome Biol. 7, 205 ( 10.1186/gb-2006-7-2-205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. 2010. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc. Natl Acad. Sci. USA 107, 3394–3399. ( 10.1073/pnas.0913843107). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vries GJ, et al. 2002. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 22, 9005–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du S, Itoh N, Askarinam S, Hill H, Arnold AP, Voskuhl RR. 2014. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 111, 2806–2811. ( 10.1073/pnas.1307091111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gioiosa L, Chen X, Watkins R, Umeda EA, Arnold AP. 2008. Sex chromosome complement affects nociception and analgesia in newborn mice. J. Pain 9, 962–969. ( 10.1016/j.jpain.2008.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold AP, Chen X. 2009. What does the ‘four core genotypes’ mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 30, 1–9. ( 10.1016/j.yfrne.2008.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold AP. 2012. The end of gonad-centric sex determination in mammals. Trends Genet. 28, 55–61. ( 10.1016/j.tig.2011.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold AP. 2014. Conceptual frameworks and mouse models for studying sex differences in physiology and disease: why compensation changes the game. Exp. Neurol. 259, 2–9. ( 10.1016/j.expneurol.2014.01.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold AP, et al. 2016. The importance of having two X chromosomes. Phil. Trans. R. Soc. B 371, 20150113 ( 10.1098/rstb.2015.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bale TL. 2015. Epigenetic and transgenerational reprogramming of brain development. Nat. Rev. Neurosci. 16, 332–344. ( 10.1038/nrn3818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meaney MJ. 2001. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 24, 1161–1192. ( 10.1146/annurev.neuro.24.1.1161) [DOI] [PubMed] [Google Scholar]
- 31.Champagne FA. 2008. Epigenetic mechanisms and the transgenerational effects of maternal care. Front. Neuroendocrinol. 29, 386–397. ( 10.1016/j.yfrne.2008.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M. 2011. Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology 152, 2760–2767. ( 10.1210/en.2011-0193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarthy MM, Nugent BM. 2013. Epigenetic contributions to hormonally-mediated sexual differentiation of the brain. J. Neuroendocrinol. 25, 1133–1140. ( 10.1111/jne.12072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. 2015. Brain feminization requires active repression of masculinization via DNA methylation. Nat. Neurosci. 18, 690–697. ( 10.1038/nn.3988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghahramani NM, et al. 2014. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol. Sex Differ. 5, 8 ( 10.1186/2042-6410-5-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balthazart J. 2011. The biology of homosexuality. New York, NY: Oxford University Press. [Google Scholar]
- 37.Balthazart J, Young LJ. 2014. Mate selection, sexual orientation and pair bonding. In Knobil and Neill's physiology of reproduction (eds Plant TM, Zeleznik AJ), pp. 2157–2210. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 38.Bakker J, Brand T, van Ophemert J, Slob AK. 1993. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav. Neurosci. 107, 480–487. ( 10.1037/0735-7044.107.3.480) [DOI] [PubMed] [Google Scholar]
- 39.Bakker J, Van Ophemert J, Slob AK. 1996. Sexual differentiation of odor and partner preference in the rat. Physiol. Behav. 60, 489–494. ( 10.1016/S0031-9384(96)80023-0) [DOI] [PubMed] [Google Scholar]
- 40.Henley CL, Nunez AA, Clemens LG. 2009. Estrogen treatment during development alters adult partner preference and reproductive behavior in female laboratory rats. Horm. Behav. 55, 68–75. ( 10.1016/j.yhbeh.2008.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bodo C, Rissman EF. 2007. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur. J. Neurosci. 25, 2182–2190. ( 10.1111/j.1460-9568.2007.05484.x) [DOI] [PubMed] [Google Scholar]
- 42.Bodo C, Rissman EF. 2008. The androgen receptor is selectively involved in organization of sexually dimorphic social behaviors in mice. Endocrinology 149, 4142–4150. ( 10.1210/en.2008-0183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakker J, Honda S, Harada N, Balthazart J. 2002. Sexual partner preference requires a functional aromatase (Cyp19) gene in male mice. Horm. Behav. 42, 158–171. ( 10.1006/hbeh.2002.1805) [DOI] [PubMed] [Google Scholar]
- 44.Brock O, Bakker J. 2011. Potential contribution of prenatal estrogens to the sexual differentiation of mate preferences in mice. Horm. Behav. 59, 83–89. ( 10.1016/j.yhbeh.2010.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagemihl B. 1999. Biological exuberance. Animal homosexuality and natural diversity. New York, NY: St. Martin's Press. [Google Scholar]
- 46.Poianni A. 2010. Animal homosexuality. A biological perspective. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 47.Perkins A, Roselli CE. 2007. The ram as a model for behavioral neuroendocrinology. Horm. Behav. 52, 70–77. ( 10.1016/j.yhbeh.2007.03.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roselli CE, Reddy RC, Kaufman KR. 2011. The development of male-oriented behavior in rams. Front. Neuroendocrinol. 32, 164–169. ( 10.1016/j.yfrne.2010.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. 2007. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology 148, 4450–4457. ( 10.1210/en.2007-0454) [DOI] [PubMed] [Google Scholar]
- 50.Balthazart J, Ball GF. 2007. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front. Neuroendocrinol. 28, 161–178. ( 10.1016/j.yfrne.2007.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paredes RG, Baum MJ. 1995. Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area anterior hypothalamus. J. Neurosci. 15, 6619–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paredes RG, Tzschentke T, Nakach N. 1998. Lesions of the medial preoptic area anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Res. 813, 1–8. ( 10.1016/S0006-8993(98)00914-7) [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto D. 2007. The neural and genetic substrates of sexual behavior in Drosophila. Adv. Genet. 59, 39–66. ( 10.1016/S0065-2660(07)59002-4) [DOI] [PubMed] [Google Scholar]
- 54.Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. 1996. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87, 1079–1089. ( 10.1016/S0092-8674(00)81802-4) [DOI] [PubMed] [Google Scholar]
- 55.Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D. 1996. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl Acad. Sci. USA 93, 9687–9692. ( 10.1073/pnas.93.18.9687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LeVay S. 2011. Gay, straight, and the reason why. The science of sexual orientation. New York, NY: Oxford University Press. [Google Scholar]
- 57.Wilson JD, George FW, Griffin JE. 1981. The hormonal control of sexual development. Science 211, 1278–1284. ( 10.1126/science.7010602) [DOI] [PubMed] [Google Scholar]
- 58.Balthazart J. 2011. Minireview: hormones and human sexual orientation. Endocrinology 152, 2937–2947. ( 10.1210/en.2011-0277). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balthazart J. 2012. Brain development and sexual orientation. Charleston, SC: Morgan & Claypool Life Sciences. [Google Scholar]
- 60.LeVay S. 1991. A difference in hypothalamic structure between heterosexual and homosexual men. Science 253, 1034–1037. ( 10.1126/science.1887219) [DOI] [PubMed] [Google Scholar]
- 61.Byne W, Tobet S, Mattiace LA, Lasco MS, Kemether E, Edgar MA, Morgello S, Buchsbaum MS, Jones LB. 2001. The interstitial nuclei of the human anterior hypothalamus: an investigation of variation with sex, sexual orientation, and HIV status. Horm. Behav. 40, 86–92. ( 10.1006/hbeh.2001.1680) [DOI] [PubMed] [Google Scholar]
- 62.McFadden D. 2002. Masculinization effects in the auditory system. Arch. Sex Behav. 31, 99–111. ( 10.1023/A:1014087319682) [DOI] [PubMed] [Google Scholar]
- 63.McFadden D, Pasanen EG, Valero MD, Roberts EK, Lee TM. 2009. Effect of prenatal androgens on click-evoked otoacoustic emissions in male and female sheep (Ovis aries). Horm. Behav. 55, 98–105. ( 10.1016/j.yhbeh.2008.08.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorski RA. 1984. Critical role of the medial preoptic area in the sexual differentiation of the brain. In Sex differences in the brain (eds De Vries GJ, De Bruin JPC, Uylings HBM, Corner MA), pp. 129–146. Amsterdam, The Netherlands: Elsevier. [DOI] [PubMed] [Google Scholar]
- 65.Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. 2004. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology 145, 478–483. ( 10.1210/en.2003-1098) [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Falgueras A, Swaab DF. 2008. A sex difference in the hypothalamic uncinate nucleus: relationship to gender identity. Brain 131, 3132–3146. ( 10.1093/brain/awn276) [DOI] [PubMed] [Google Scholar]
- 67.Money J, Schwartz M, Lewis VG. 1984. Adult erotosexual status and fetal hormonal masculinization and demasculinization: 46,XX congenital virilizing adrenal hyperplasia and 46,XY androgen-insensitivity syndrome compared. Psychoneuroendocrinology 9, 405–414. ( 10.1016/0306-4530(84)90048-9) [DOI] [PubMed] [Google Scholar]
- 68.Zucker KJ, Bradley SJ, Oliver G, Blake J, Fleming S, Hood J. 1996. Psychosexual development of women with congenital adrenal hyperplasia. Horm. Behav. 30, 300–318. ( 10.1006/hbeh.1996.0038) [DOI] [PubMed] [Google Scholar]
- 69.Meyer-Bahlburg HF, Dolezal C, Baker SW, New MI. 2008. Sexual orientation in women with classical or non-classical congenital adrenal hyperplasia as a function of degree of prenatal androgen excess. Arch. Sex Behav. 37, 85–99. ( 10.1007/s10508-007-9265-1). [DOI] [PubMed] [Google Scholar]
- 70.Meyer-Bahlburg HF, Ehrhardt AA, Rosen LR. 1995. Prenatal estrogens and the development of homosexual orientation. Dev. Psychol. S31, 12–21. ( 10.1037/0012-1649.31.1.12) [DOI] [Google Scholar]
- 71.Colapinto J. 2000. As nature made him: the boy that was raised as a girl. New York, NY: Harper Collins. [Google Scholar]
- 72.Reiner WG, Gearhart JP. 2004. Discordant sexual identity in some genetic males with cloacal exstrophy assigned to female sex at birth. N. Engl. J. Med. 350, 333–341. ( 10.1056/NEJMoa022236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meyer-Bahlburg HF. 2005. Gender identity outcome in female-raised 46,XY persons with penile agenesis, cloacal exstrophy of the bladder, or penile ablation. Arch. Sex Behav. 34, 423–438. ( 10.1007/s10508-005-4342-9) [DOI] [PubMed] [Google Scholar]
- 74.Diamond M. 1993. Some genetic considerations in the development of sexual orientation. In The development of sex differences and similarities in behavior (eds Haug M, Whalen RE, Aron C, Olsen KL), pp. 291–309. Dordrecht, The Netherlands: Kluwer Academic. [Google Scholar]
- 75.Rahman Q, Wilson GD. 2003. Born gay? The psychobiology of human sexual orientation. Pers. Ind. Diff. 34, 1337–1382. ( 10.1016/S0191-8869(02)00140-X) [DOI] [Google Scholar]
- 76.Hamer DH, Hu S, Magnuson VL, Hu N, Pattatucci AML. 1993. A linkage between DNA markers on the X chromosome and male sexual orientation. Science 261, 321–327. ( 10.1126/science.8332896) [DOI] [PubMed] [Google Scholar]
- 77.Mustanski BS, Dupree MG, Nievergelt CM, Bocklandt S, Schork NJ, Hamer DH. 2005. A genomewide scan of male sexual orientation. Hum. Genet. 116, 272–278. ( 10.1007/s00439-004-1241-4) [DOI] [PubMed] [Google Scholar]
- 78.Bocklandt S, Vilain E. 2007. Sex differences in brain and behavior: hormones versus genes. Adv. Genet. 59, 245–266. ( 10.1016/S0065-2660(07)59009-7) [DOI] [PubMed] [Google Scholar]
- 79.Sanders AR, et al. 2015. Genome-wide scan demonstrates significant linkage for male sexual orientation. Psychol. Med. 45, 1379–1388. ( 10.1017/S0033291714002451) [DOI] [PubMed] [Google Scholar]
- 80.Reyes FI, Boroditsky RS, Winter JS, Faiman C. 1974. Studies on human sexual development. II. Fetal and maternal serum gonadotropin and sex steroid concentrations. J. Clin. Endocrinol. Metab. 38, 612–617. ( 10.1210/jcem-38-4-612) [DOI] [PubMed] [Google Scholar]
- 81.Perera DM, McGarrigle HH, Lawrence DM, Lucas M. 1987. Amniotic fluid testosterone and testosterone glucuronide levels in the determination of foetal sex. J. Steroid Biochem. 26, 273–277. ( 10.1016/0022-4731(87)90082-3) [DOI] [PubMed] [Google Scholar]
- 82.Weisz J, Ward IL. 1980. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology 106, 306–316. ( 10.1210/endo-106-1-306) [DOI] [PubMed] [Google Scholar]
- 83.Wilson JD, Griffin JE, George FW, Leshin M. 1981. The role of gonadal steroids in sexual differentiation. Recent Prog. Horm. Res 37, 1–39. [DOI] [PubMed] [Google Scholar]
- 84.Ngun TC, Vilain E. 2014. The biological basis of human sexual orientation: is there a role for epigenetics? Adv. Genet. 86, 167–184. ( 10.1016/B978-0-12-800222-3.00008-5) [DOI] [PubMed] [Google Scholar]
- 85.Rice WR, Friberg U, Gavrilets S. 2012. Homosexuality as a consequence of epigenetically canalized sexual development. Q. Rev. Biol. 87, 343–368. ( 10.1086/668167) [DOI] [PubMed] [Google Scholar]
- 86.Wijchers PJ, Festenstein RJ. 2011. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet. 27, 132–140. ( 10.1016/j.tig.2011.01.004) [DOI] [PubMed] [Google Scholar]
- 87.Itoh Y, Arnold AP. 2014. X chromosome regulation of autosomal gene expression in bovine blastocysts. Chromosoma 123, 481–489. ( 10.1007/s00412-014-0461-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gardner DK, Larman MG, Thouas GA. 2010. Sex-related physiology of the preimplantation embryo. Mol. Hum. Reprod. 16, 539–547. ( 10.1093/molehr/gaq042) [DOI] [PubMed] [Google Scholar]
- 89.Boehmer AL, Brinkmann AO, Nijman RM, Verleun-Mooijman MC, de Ruiter P, Niermeijer MF, Drop SL. 2001. Phenotypic variation in a family with partial androgen insensitivity syndrome explained by differences in 5alpha dihydrotestosterone availability. J. Clin. Endocrinol. Metab. 86, 1240–1246. ( 10.1210/jcem.86.3.7333) [DOI] [PubMed] [Google Scholar]
- 90.Ngun TC, Ghahramani N, Sanchez FJ, Bocklandt S, Vilain E. 2011. The genetics of sex differences in brain and behavior. Front. Neuroendocrinol. 32, 227–246. ( 10.1016/j.yfrne.2010.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bogaert AF, Skorska M. 2011. Sexual orientation, fraternal birth order, and the maternal immune hypothesis: a review of evidence. Front. Neuroendocrinol. 32, 247–254. ( 10.1016/j.yfrne.2011.02.004) [DOI] [PubMed] [Google Scholar]
- 92.Wong WI, Hines M. 2015. Preferences for pink and blue: the development of color preferences as a distinct gender-typed behavior in toddlers. Arch. Sex Behav. 44, 1243–1254. ( 10.1007/s10508-015-0489-1) [DOI] [PubMed] [Google Scholar]
- 93.Pasterski V, Zucker KJ, Hindmarsh PC, Hughes IA, Acerini C, Spencer D, Neufeld S, Hines M. 2015. Increased cross-gender identification independent of gender role behavior in girls with congenital adrenal hyperplasia: results from a standardized assessment of 4- to 11-year-old children. Arch. Sex Behav. 44, 1363–1375. ( 10.1007/s10508-014-0385-0) [DOI] [PubMed] [Google Scholar]
- 94.Bem DJ. 2000. Exotic becomes erotic: interpreting the biological correlates of sexual orientation. Arch. Sex Behav. 29, 531–548. ( 10.1023/A:1002050303320) [DOI] [PubMed] [Google Scholar]
- 95.Paredes-Ramos P, Miquel M, Manzo J, Coria-Avila GA. 2011. Juvenile play conditions sexual partner preference in adult female rats. Physiol. Behav. 104, 1016–1023. ( 10.1016/j.physbeh.2011.06.026) [DOI] [PubMed] [Google Scholar]
- 96.Triana-Del Rio R, Montero-Dominguez F, Cibrian-Llanderal T, Tecamachaltzi-Silvaran MB, Garcia LI, Manzo J, Hernandez ME, Coria-Avila GA. 2011. Same-sex cohabitation under the effects of quinpirole induces a conditioned socio-sexual partner preference in males, but not in female rats. Pharmacol. Biochem. Behav. 99, 604–613. ( 10.1016/j.pbb.2011.06.006) [DOI] [PubMed] [Google Scholar]
- 97.Cibrian-Llanderal T, Rosas-Aguilar V, Triana-Del Rio R, Perez CA, Manzo J, Garcia LI, Coria-Avila GA. 2012. Enhaced D2-type receptor activity facilitates the development of conditioned same-sex partner preference in male rats. Pharmacol. Biochem. Behav. 102, 177–183. ( 10.1016/j.pbb.2012.04.007) [DOI] [PubMed] [Google Scholar]
- 98.Triana-Del Rio R, Tecamachaltzi-Silvaran MB, Diaz-Estrada VX, Herrera-Covarrubias D, Corona-Morales AA, Pfaus JG, Coria-Avila GA. 2015. Conditioned same-sex partner preference in male rats is facilitated by oxytocin and dopamine: effect on sexually dimorphic brain nuclei. Behav. Brain Res. 283, 69–77. ( 10.1016/j.bbr.2015.01.019) [DOI] [PubMed] [Google Scholar]
- 99.Cibrian-Llanderal T, Triana-Del Rio R, Tecamachaltzi-Silvaran M, Pfaus JG, Manzo J, Garcia LI, Coria-Avila GA. 2014. Cohabitation between male rats after ejaculation: effects on conditioned partner preference. Physiol. Behav. 128, 303–308. ( 10.1016/j.physbeh.2014.02.016) [DOI] [PubMed] [Google Scholar]