Abstract
In recent years, the bidirectional communication between the gut microbiome and the brain has emerged as a factor that influences immunity, metabolism, neurodevelopment and behaviour. Cross-talk between the gut and brain begins early in life immediately following the transition from a sterile in utero environment to one that is exposed to a changing and complex microbial milieu over a lifetime. Once established, communication between the gut and brain integrates information from the autonomic and enteric nervous systems, neuroendocrine and neuroimmune signals, and peripheral immune and metabolic signals. Importantly, the composition and functional potential of the gut microbiome undergoes many transitions that parallel dynamic periods of brain development and maturation for which distinct sex differences have been identified. Here, we discuss the sexually dimorphic development, maturation and maintenance of the gut microbiome–brain axis, and the sex differences therein important in disease risk and resilience throughout the lifespan.
Keywords: development, lifespan, microbiome, sex differences
1. Introduction
The mammalian brain poses an evolutionary paradox: while it has a high metabolic demand, requiring more energy than any other tissue in the body, it contains no energy reserves and is critically dependent on the continual supply of substrates from the periphery [1]. Over the course of evolution, transitions to high-quality and nutrient-dense plant- and animal-based resources have provided access to previously unavailable nutrients, and this considerable expansion in the metabolic pool is considered a key event underlying the rapid enlargement and reorganization of the human brain [1–3]. However, expansion of the human frontal cortex occurred at the cost of other metabolically expensive tissues, such as the gastrointestinal tract, suggesting coevolution of the gut and brain where expansion of the brain resulted in a corresponding reduction in overall size of the gut [1]. Paradoxically, the reduction of the mammalian gut was paralleled by increased capacity to synthesize essential amino acids, ferment complex carbohydrates and more efficiently extract energy, suggesting an essential coevolution between bacterial communities residing within the gut and increased metabolic demands necessary for an energetically expensive brain [4]. This coevolution between host and microbe has been recently suggested to impact the expression of an array of phenotypes across the lifespan, and the evolutionary processes driving these interactions have been the focus of a number of excellent reviews [5–9].
Nevertheless, these communities of microorganisms, including fungi, protozoa, Archaea, viruses and microbiota that reside within our bodies are estimated to be as much as 10 times greater than the total cell number in the human body, and their genetic information is at least 150-fold greater than that of the human genome [10]. This tremendous genetic repertoire of the microbiome provides extensive metabolic, immunological and endocrine potential otherwise unavailable to the host [11]. For instance, early in development, the gut microbiota educate the immune system, fine-tune neural circuits within the gut, induce antimicrobial peptides to ensure rapid clearance of pathogens, metabolize vital dietary components and distribute dietary fat to peripheral tissues [5,6,12–17]. Moreover, animals devoid of any microorganisms, termed germ-free, have contributed to our appreciation of the complex network between the gut microbiome and the brain, and have demonstrated that microbial communities are essential for brain development and function, neural metabolism and mediate a variety of gut–brain axis disorders [18–23].
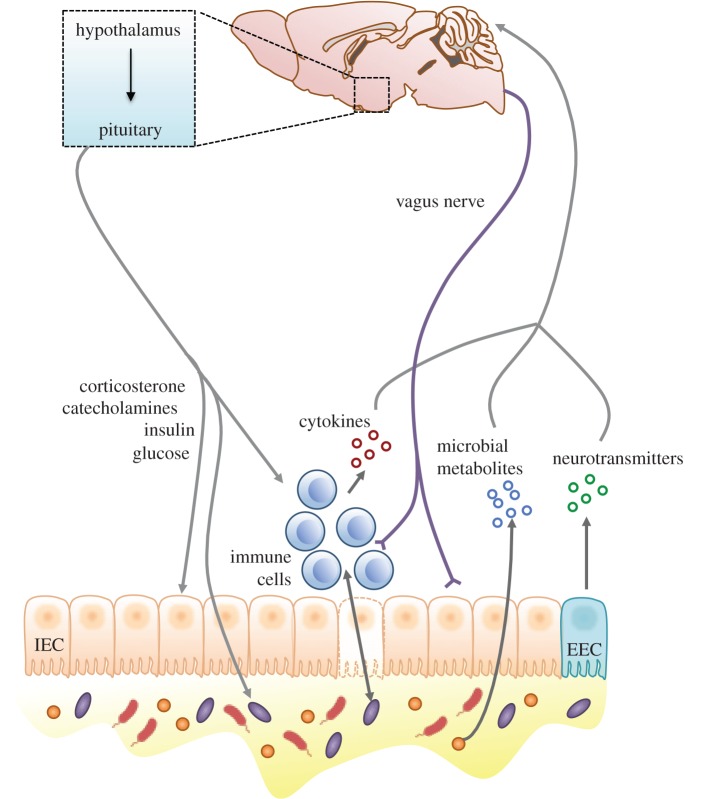
Thus, a critical function of the gut microbiome is to orchestrate the bidirectional communication between the gut and the brain, facilitating the integration of peripheral and central immune, metabolic and endocrine signals [24–27]. This axis starts with the intestinal tract, which continuously monitors and responds to the composition of its content to optimize assimilation of substrates and competitive exclusion of pathogens [28]. Microbial by-products such as short-chain fatty acids (SCFAs) and chemotactic peptides bind to receptors expressed on enteroendocrine cells (EECs) to facilitate secretion of a variety of metabolically related peptides involved in food intake, lipid storage, energy homeostasis, neurotransmission and behaviour [26,29,30]. In turn, EECs release hormones that signal to the intestinal epithelium and immune cells, translocate into the periphery to act at remote sites such as the brain and activate neurons of the enteric nervous system [28]. The signals produced either directly from the microbiota or indirectly through its interaction with lymphocytes, dendritic cells and EECs are relayed to the brain by the enteric nervous system via efferent and afferent fibres as well as interneurons [29]. Centrally, activation of neural circuits, such as those controlling appetite and satiety within the hypothalamus, influence the release of hormones and other peptides that impact the gut by altering the release of cytokines by mucosal immune cells and the composition of microbial communities, giving the signals from the brain the ability to influence overall gut health [24,31–34] (figure 1).
Figure 1.
The gut–brain axis represents a bidirectional communication system that facilitates the integration of peripheral and central immune, metabolic and endocrine signals. The gut and the brain send both direct and indirect signals via immune, neural, endocrine and metabolic pathways in order to influence function of the other tissue. Surrounded by a single layer comprising intestinal epithelial cells (IECs), microbes produce metabolites such as SCFAs and chemotactic peptides that are able to both influence the brain directly as well as bind to receptors expressed on EECs to enable the secretion of metabolically active peptides such as neurotransmitters. Further, microbes interact with immune cells within the gut to alter the number of cytokines, which can also affect brain function and behaviour. Centrally, activation of neural circuits can impact the gut by release of hormones and other peptides that can alter the release of cytokines and the composition of microbial communities within the gut.
The formation of the gut microbiome–brain axis begins very early in life, immediately following colonization by microbial communities that reside within the birth canal [35–39]. The composition of gut microbes, their genetic repertoire and the metabolites they produce are dynamic across the lifespan as they are shaped by many factors, including host genetics, age and sex [40–48]. While the mechanisms involved in the development, maturation and maintenance of the gut microbiome are only beginning to be dissected, recent studies suggest that the composition and functional potential of the microbiome undergoes transitional stages that parallel similar dynamic periods in brain development [49]. This observation further implicates the role of the gut microbiota and the consortium of microbial genes in meeting the metabolic demands of a growing brain during developmental stages.
As males and females exhibit sexually dimorphic patterns in energy and nutritional requirements across the lifespan, sex differences in the gut microbiome–brain axis may be an important biological factor in these processes [2,50–52]. This is supported by a wealth of research highlighting critical sex differences in downstream targets of this axis, including neuroendocrine and neuroimmune systems [53,54]. For instance, female-biased prevalence in functional gastrointestinal disorders, such as irritable bowel syndrome, may result from sex differences in gastrointestinal transit time, visceral sensitivity, central nervous system pain processing and hormone-dependent effects on gut physiology [55–58]. More recent studies in mice demonstrated that the female-biased risk for autoimmune disorders is significantly impacted by sex differences in the gut microbiome, whereby adoptive transfer of male microbiota to recipient females delayed onset and lessened severity of disease [47,59]. Further, sex-specific changes in the composition of the gut microbiome in response to environmental factors, including infection and stress, may represent a component of sex differences in disease risk, as these same environmental factors result in sex-specific alterations in immune function, metabolism, stress responsiveness and behaviours [33,60–62]. Thus, consideration of sex differences in the gut microbiome across the lifespan provides novel mechanistic insight into sex differences in disease prevalence, age of onset and severity, and, may ultimately lead to novel interventions and treatments [63,64] (figure 2).
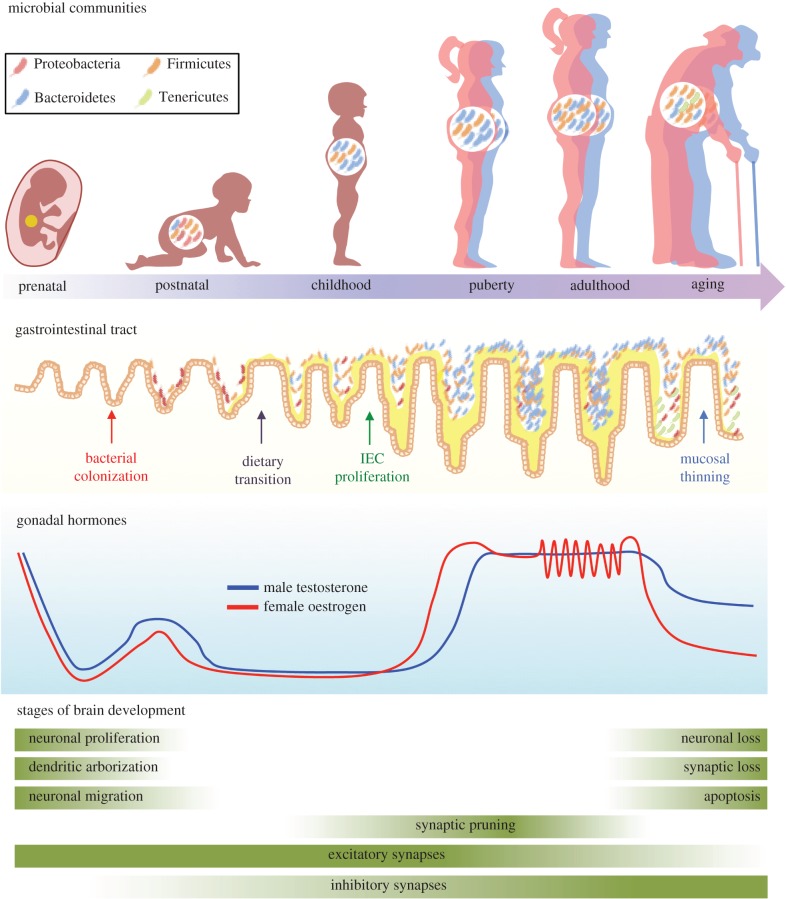
Figure 2.
Timeline showing that critical shifts in maturation of the gut, hormones and the brain occur in parallel, and that sex-specificity in these systems emerges at similar points in development. Across the gut–brain axis, there are several periods of dynamic shifts in tissue development, including infancy, puberty and aging. Here, the microbial communities within the gut, the structure of the gastrointestinal tract and the brain are characterized by dramatic shifts in structure and function, providing windows where perturbations such as stress may result in long-term disruptions of normal function. Sex differences within these systems arise at similar ages, suggesting the potential for sexually dimorphic communication between the gut microbiome and the brain.
In this review, we outline a conceptual framework for sexually dimorphic communication between the gut microbiome and the brain. Specifically, we discuss the parallel development, maturation and maintenance of the gut microbiome and brain across the lifespan, and highlight those known sex differences at each stage (figure 2). Finally, we consider the points in which environmental perturbations such as stress influence or reshape gut–brain signalling, emphasizing potential sex-specific consequences across a number of behavioural domains. We conclude by providing some perspectives on future directions in this area.
2. Sex differences in the gut microbiome–brain axis
Differences between males and females in anatomical, physiological and behavioural traits have been described in nearly all vertebrate species, including humans [65]. Mechanisms through which males and females differ involve a delicate orchestration between the environment, genes, hormones and epigenetic processes—which emerge with different roles during distinct life stages [66,67]. As the gut microbiome is critical in maintaining organismal homeostasis, as evidenced by significant shifts in composition and gene content to accommodate key periods of development and maturation, it is likely an additional mechanism contributing to these important sex differences [52]. Importantly, the nutritional and energetic demands of growth, development and reproduction differ between males and females, suggesting that sex-specific shifts in the ecological structure of the gut microbiome to meet these demands may represent an adaptation by which organisms maintain sex differences in physiology and behaviour throughout life [2,50–52]. During dynamic periods of life, including infancy, puberty and aging, the composition of the microbiota shows high instability and variability that correlate with age- and sex-specific disease risk. Such evidence further underscores the adaptive contribution of the gut microbiome during distinct life stages [49,68–73].
(a). Prenatal
The prenatal period presents both a window of susceptibility and opportunity for intervention with respect to normal brain development and fetal antecedents of disease [74]. As the developing fetus receives all nutrients from the maternal circulation, and as the metabolic demand of male and female fetuses differs during gestation, the capacity of the maternal gut microbiome to mediate maternal energy balance and nutritional status may exhibit significant sex-specific effects on development [75,76]. Emerging evidence suggests that the maternal gut microbiome orchestrates nutrient and metabolite availability in a temporal-specific manner [77]. During the first trimester, the human maternal gut microbiome is overrepresented by communities belonging to the Clostridiales order, a large cluster of bacteria that metabolize fibre to produce SCFAs such as butyrate, propionate and acetate [77,78]. SCFAs cross the placental barrier from maternal serum and enter fetal circulation, suggesting the possibility that maternal-derived microbial substrates can influence neurodevelopment [79]. Indeed, increased availability of butyrate during early pregnancy parallels the dynamic window of blood–brain barrier (BBB) development, and a role of butyrate-producing microbiota in the normal formation of the mouse BBB has been recently demonstrated [80]. Although not included in currently available studies, sex differences in the transport, uptake and downstream effects of maternal microbe-derived substrates on neurodevelopment are exciting areas of research that warrant further study.
Although SCFAs are essential for normal fetal development, prolonged and elevated levels during prenatal development are associated with increased disease risk later in life [81,82]. Late gestation exposure to the microbial metabolites butyrate and propionate is associated with sex-specific delays to reach developmental milestones and altered social behaviour in rodents, which are potential endophenotypes of neurodevelopmental disorders [82,83]. Specifically, exposed males, but not females, exhibited increased anxiety-like behaviour and stereotypy, decreased social interaction and exaggerated stress responsivity. As butyrate and propionate serve a number of epigenetic functions such as balancing histone acetylation and deacetylation activity, exposure to these substrates during critical windows may have important implications for early life programming [84,85]. Interestingly, the human maternal gut microbiome undergoes dynamic remodelling during late gestation characterized by a decrease in SCFA-producing microbiota in exchange for high-energy-yielding microbial communities [77]. While this transition in community composition likely occurs to meet the increased nutritional and metabolic demand associated with rapid offspring development, it may also serve as an adaptation to protect the offspring from prolonged exposure to specific microbial substrates. As microbiome-derived metabolites far exceed the number of metabolites produced by the host, there are likely other microbial metabolites that influence prenatal neurodevelopment that remain to be discovered and their role characterized [86]. Additional avenues for future studies might explore how environmental factors such as infection and stress affect maternal gut microbial composition and metabolite availability, and how those interactions subsequently influence sex-specific neurodevelopment.
(b). Postnatal
During parturition, the neonate ingests the primary inoculum of microbes within the vagina as it passes through the birth canal. The neonatal gut lacks the functional innate and adaptive immune defences that will serve as a ‘demilitarization zone’ between microbes and the intestinal epithelium later in development. In addition, the early neonatal gut allows passive transfer of maternal immunity, and ongoing intimate host–microbe interaction during early life promotes normal progression of gut mucosal immunity and maturation of the intestine [16,87–89]. As a result, the colonizing microbes come in direct contact with the host during these initial stages [87]. Thus, the composition of this pioneer community, as well as its genetic repertoire, is likely to exert critical consequences on long-term health outcomes, including programming of host immunity within the gut, modulating energy balance and homeostasis, and influencing neurodevelopment and behaviour [35,39,49].
In the first few days to weeks of life, a limited consortium of genera, including Bacteroides, Bifidobacterium, Parabacteroides and Escherichia/Shigella, dominate the human neonate gut [90]. Interestingly, Escherichia and Shigella are members of the Proteobacteria phylum, which are Gram-negative bacteria with their outer membranes composed of immune-eliciting lipopolysaccharides (LPS) [88,91]. The role of LPS in guiding sex-specific long-term programming of the nervous system has been the focus of numerous rodent models of early postnatal immune challenge [92]. Although these models have focused on exogenous LPS administration, endogenous sources of LPS derived from the transient colonization of Proteobacteria during early life may be adaptive for the host [88,91]. Proteobacteria-derived LPS may influence neurodevelopment through direct, paracrine or endocrine mechanisms. These include direct communication via vagal afferents, production of microbial metabolites or EEC-derived hormones signalling to the circumventricular organs, induction of peripheral cytokines that cross the BBB or eliciting resident cells that form the BBB to secrete neuroimmune substrates [93]. In addition, more recent evidence suggests that microbial colonization influences immunocompetent cells in the brain such as the microglia, suggesting the possibility that the immunological and metabolic consequences of microbial colonization may influence sex differences in microglia function, and, further may impact long-term programming of brain and behaviour [94].
The advent of modern obstetric practices such as caesarean delivery, accounting for nearly 30% of all births in the United States and nearly 90% in Brazil, has provided a natural human experiment that highlights the importance of maternal–offspring microbial transmission on long-term health outcomes [39,95–99]. As discussed above, vaginal bacteria from the mother initially colonize the intestine of vaginally delivered infants, whereas bacteria from the mother's skin and the local environment (e.g. healthcare workers, hospital and other newborns) colonize infants born via caesarean section [100]. For example, newborns delivered by caesarean section showed delayed colonization by Bacteroides and Bifidobacterium, as well as an increased risk for overgrowth of the enteric pathogen Clostridium difficile. As the Bifidobacterium genome contains a large cluster of genes involved in the utilization and metabolism of maternal breast milk oligosaccharides, delayed colonization by these bacteria may alter nutrient and energy availability necessary for normal growth and development [9,101,102]. The resulting differences in colonizing microbiota for vaginally and caesarean delivered children persist well into childhood and are associated with increased body mass and childhood obesity [103]. In addition, children born by caesarean section exhibit increased risk of allergies, such as allergic rhinoconjunctivitis, and this risk is highest in females born after multiple caesarean sections, paralleling well-established observations of female-biased risk in the development of allergies [104,105]. In addition, a recent meta-analysis reported that caesarean delivery is associated with increased odds of autism spectrum disorder, although a formal test failed to confirm this relationship [106,107].
Disruption to maternal–offspring transmission of microbes during critical windows of development leads to long-term offspring health outcomes [61,71,73,102,108–110]. Maternal insults, including natural disasters, maternal anxiety, immune compromise and maternal diet, have been linked with increased incidence of offspring neurodevelopmental disorders [111–113]. In non-human species, including mice, rats, guinea pigs and non-human primates, prenatal stress increases offspring stress sensitivity, anxiety and depressive-like behaviour, and cognitive deficits [114–124]. Offspring sex is a critical factor in mediating the outcomes of maternal stress, where male offspring are more susceptible to the effects of prenatal stressors, as indicated by altered performance on a variety of tasks, including spatial learning, stress-induced locomotor activity and sucrose preference [117–122,125,126]. Interestingly, the potential involvement of the microbiome in mediating any of these neurodevelopmental programming changes has not previously been considered. Certainly, if the stress exposure during pregnancy were to alter the maternal vaginal microbiome composition, the potential existed for the neonate's gut to also be changed, thereby setting the stage for a progression of developmental programmes that could ultimately result in important differences in the gut microbiome–brain axis.
To directly probe the potential contribution of changes in the maternal vaginal microbiota in offspring programming effects following maternal stress during pregnancy, we used our established mouse model of early prenatal stress (EPS), in which male, but not female, offspring demonstrate significant neurodevelopmental changes in hypothalamic and limbic circuits and in regulation of stress responsivity, cognitive dysfunction and post-pubertal growth, and examined changes in the vaginal microbiota composition and their vertical transmission to offspring at birth [108,117–121]. Consistent with our hypothesis, maternal stress altered proteins related to vaginal immunity and abundance of Lactobacillus, the prominent taxon in the maternal vagina and an important primary colonizer of the neonate gut [108]. These results add to a growing number of studies in rodent models, primates and humans demonstrating that maternal stress decreases Lactobacillus abundance in neonates, suggesting the possibility that the mechanisms by which stress alters Lactobacillus are conserved [71,109,110,127–129]. Further, loss of maternal vaginal Lactobacillus also resulted in decreased transmission of this bacterium to EPS-exposed offspring, which may disrupt the ability of the offspring gut to perform critical functions such as fermenting breast milk lactose and casein, producing lactic acid and maintaining an acidic intestinal environment during early development [130–132]. Interestingly, depletion of Lactobacillus corresponded with a sex-specific and developmentally premature increase of obligate anaerobes, Bacteroides and Clostridium, in EPS-exposed males, but not in EPS-exposed females. This altered microbiota composition in the neonate gut corresponded with sex-specific changes in the availability of nutrients known to influence sex differences in neurodevelopment, such as histidine and glutamate [133,134]. Further, these peripheral shifts in nutrient availability were also associated with sex-specific disruptions of amino acid profiles in the developing paraventricular nucleus of the hypothalamus. Taken together, these results add to accumulating evidence that the maternal vaginal microbiota and subsequent colonization of the neonate gut are important sex-specific factors in prenatal stress programming of both the gut and the brain.
(c). Childhood and puberty
The gut microbiome, following early life colonization, continues to mature throughout childhood and puberty [135,136]. Recent evidence demonstrates that childhood and puberty are critically active and developing phases of gut microbiome compositional and functional changes [137]. For example, bacterial communities during childhood were characterized by both increased complexity and instability relative to adult communities [137]. The compositional differences in children paralleled alterations in the relative abundance of functional pathways previously associated with anti-inflammatory properties such as vitamin B12 synthesis and methane metabolism, while adult communities were more enriched with pathways associated with inflammation, including biosynthesis of immune-eliciting molecules, steroid hormone biosynthesis and oxidative phosphorylation [137]. As childhood is characterized by gonadal hormone quiescence and little sex-specific development, it is not surprising that there were no sex differences in childhood gut microbiome composition [65].
Following the relatively dormant period of sex-specific development in childhood, the transition to puberty is marked by an initiation of sexually dimorphic processes [138]. Time-series studies in which the gut microbiome composition of male and female mice was characterized during puberty, adolescence and adulthood revealed that sex differences in the gut microbiome emerged during puberty and continued to diverge into adulthood [59]. Specifically, the microbial communities of males deviated during puberty and acquired a distinct phenotype during adulthood, whereas the communities of females remained more similar to that of pubertal mice of both sexes [59]. Reduction of testosterone by castration during the pubertal window eliminated sex differences in gut microbiota composition in adult mice, demonstrating the importance of pubertal testosterone in organizing sexually dimorphic microbial communities of males that are maintained in adulthood; however, the mechanism by which testosterone mediates host selection of microbial communities is currently not understood [59]. Transfer of adult male mouse caecal content, which contains the largest number and greatest diversity of bacteria in the intestinal tract of mice, into pubertal female mice masculinized microbiota composition, metabolomic profiles and elevated testosterone levels in the female recipients that persisted into adulthood [47]. Co-administration of adult male caecal content and the androgen receptor antagonist flutamide attenuated all male microbiome-specific changes in female recipients, demonstrating mechanistically that testosterone elevation caused by male microbiome transfer was critical for these downstream phenotypes [47]. While these studies provide a proof-of-concept that microbial communities drive expression of testosterone, it is currently unclear whether transfer of female-specific microbiomes is capable of influencing oestrogen levels in males and warrants further study. Likewise, the capacity of the microbiome to transfer behavioural phenotypes has been recently demonstrated, as germ-free NIH Swiss male mice inoculated with caecal contents from male BALB/c mice, an innately anxious strain of mice, displayed a behavioural phenotype similar to the donor species [21,139]. As sex differences have been observed in anxiety, the possibility that sex-specific microbiome transfers can impact sex differences in behaviour is intriguing and warrants future study. Nevertheless, these results highlight the importance for future studies to examine whether sex-specific microbial transfer influences brain and behaviour through modulation of gonadal hormones. Thus, mounting evidence suggests that microbial communities in the gut may be capable of altering the individual at a phenotypic level, including hormone-driven metabolic and behavioural phenotypes.
Microbial communities may alter host hormones through a variety of mechanisms. Recent bioinformatics analyses of commensal microbes identified large gene clusters that encode hydroxysteroid dehydrogenases, enzymes that regulate the balance between active and inactive steroids, which is consistent with recent metagenomic studies showing that the microbiome exerts steroid hormone synthesizing capacity [137,140]. In addition, enzymatic and kinetic experiments have demonstrated that some microbiota can perform hydrolytic, reductive and oxidative reactions of androgens and oestrogens as well as readily convert glucocorticoids to androgens [141–144]. Importantly, the influence of microbial communities on host hormones stretches far beyond oestrogens and androgens [145–147]. Gut microbiota also produce and respond to neurotransmitters that are critical for normal feedback between the gut and brain, including serotonin, dopamine and norepinephrine [148,149]. For instance, under both normal and germ-free conditions, gut microbiota have been shown to modulate serotonin output, impacting serotonin-related signalling both locally, as evidenced by activity of myenteric neurons and gut motility, as well as peripheral serotonin-related signalling [150]. Reports in germ-free mice demonstrate sex-specific effects of microbiota on serotonin production, whereby the attenuated sex differences in serotonin concentration in germ-free mice were reversed following microbial colonization and resulted in re-establishment of sex differences in hippocampal serotonergic neurocircuitry [23].
Findings such as these are important in expanding our understanding of what makes puberty a time of increased sex-specific risk for stress to precipitate later life affective disturbance, with females particularly vulnerable to disruptions during this period [138]. In parallel to the gut microbiome, the brain is becoming more sex-specific throughout pubertal development. The gut may be linked to brain development indirectly, as the gut provides critical metabolic information that informs the onset of puberty, triggering a cascade of sexually dimorphic processes [51]. However, the gut may also be linked more directly to brain development by changing the gonadal hormone and neurotransmitter environment. Studies have shown that alteration of gonadal hormones during the pubertal window has long-term consequences on sexually dimorphic brain development that cannot be recovered by hormone treatment in adulthood [138]. Thus, any disruption to the gut microbiota that alters hormone or neurotransmitter environment may have significant consequences for brain development, an exciting possibility that will require much future study.
(d). Adulthood and aging
The transition from puberty to adulthood is marked by increased stability and evenness of the gut microbiome that appears better adapted to the continuous ebb and flow of stress, infection, diet and antibiotics [151]. Environmental challenges, such as deprivation from food, water and bedding, decrease the abundance of beneficial bacteria and increase the susceptibility to opportunistic pathogens in mice [152]. Chronic social stressors disrupt intestinal barrier function, alter bacterial composition, increase bacterial translocation into lymphoid tissue and induce immune activation at the intestinal epithelium that facilitates release of chemokines and cytokines into circulation [153–158]. Interestingly, these physiological responses and the composition of microbial communities return to baseline following cessation of stressors, suggesting increased resilience to environmental challenges during adulthood [159].
However, a more detailed examination of the adult microbiome highlights the importance of sex in determining risk for negative symptoms associated with changes in the gut. A recent cross-sectional study demonstrated sex differences in microbial communities, where males exhibited increased abundance of Bacteroides and Prevotella compared with females, suggesting the potential contribution of gonadal hormones in mediating these sex differences in microbial communities [160]. Sex differences are actively maintained in adulthood through differing regulation of the hormonal milieu, with males reaching fairly steady-state testosterone expression and females experiencing a regular cycling of hormone levels [65,161]. In rodent models, microbial communities in females exhibit high variability relative to males, suggesting a possible contribution of the pulsatile nature of oestrogens in the maintenance of a variable female gut microbiome [59]. In particular, periods of declining ovarian hormones are associated with the occurrence and exacerbation of gastrointestinal symptoms, increased risk for infection, hypersensitivity to visceral pain and co-morbidity with affective disorders, including anxiety and depression [56,162–164]. Conversely, these symptoms are frequently alleviated during periods of high ovarian hormone levels, such as pregnancy [165]. The maternal gut microbiome undergoes dynamic remodelling during the first and third trimester of pregnancy, with vast expansion of bacterial diversity between mothers during the third trimester when oestrogens are at maximal peak [77]. Similar to the sex differences in the community structure of the gut microbiome, the sex differences in the adult brain are responsive to fluctuating hormones [166]. Oestrogen and progesterone play important roles in several facets of sex-specific morphology and function of the brain, likely through interactions with numerous neurotransmitter systems, including glutamate, GABA, dopamine and serotonin [166–169]. Brain regions such as the hippocampus, prefrontal cortex and amygdala respond to the presence of oestrogen and progesterone with changes in synaptic density and spine formation [170–172].
In relation to disease risk and resilience, adulthood represents an important period when many of the pathological consequences of early life programming appear in a sex-specific manner [74]. Although the adult gut microbiome appears more resistant to environmental challenge, it is unclear to what extent early life adversity can mediate the magnitude of response to these challenges. For example, the male-specific stress hyper-responsivity in our EPS mouse model emerges only following exposure to an acute stressor, demonstrating that an environmental challenge during adulthood may be required to unmask latent early life reprogramming [117,119–121]. Thus, a critical challenge for early life studies is the necessity to connect alterations in colonization patterns of the neonate gut to long-term health outcomes. Indeed, long-term programming of the offspring gut microbiome has been recently demonstrated in a rat model of late gestation maternal stress exposure [128]. As adults, male rats exposed to prenatal stress showed lower abundance of Streptococcus and Lactobacillus but increased abundance of taxa belonging to the Clostridiales family [128]. Further, changes in the microbial community composition were associated with respiratory instability, hypertension, exaggerated stress response to acute restraint, and a deficit in innervation intensity of the distal colon of exposed adult male rats [128]. Although animal model, type of stressor and window of exposure differ, it is tempting to draw parallels between observed differences in Lactobacillus and Clostridia during colonization and the stable disruption of the same taxa that persist into adulthood, although future series profiling studies are needed to confirm these relationships. Further, as prenatal stress phenotypes typically present with males exhibiting greater vulnerability to disruption, this suggests the possibility of female-biased resilience to prenatal stress [115,117,119,120,124–126,173]. Inclusion of females may reveal a resilience signature of the gut microbiome that, paired with adoptive transfer techniques, could be used to mechanistically assess whether male-biased prenatal stress phenotypes could be rescued via the gut microbiome.
Similar to the dynamic changes that occur to the gut microbiome–brain axis during early life colonization and succession, there are equally notable alterations in aging populations that likely impact signalling between the gut microbiome and brain [151,174]. Slower intestinal transit times that lead to altered nutritional availability and absorption, reduced stability and diversity of microbial communities, thinning of the mucosal lining and subsequent dysfunction of the intestinal barrier, and increased inflammation are all common manifestations of aging in the gastrointestinal tract [175]. Although the number of metagenomic studies of the gut microbiome in aged subjects is severely underrepresented, a hallmark study by the ELDERMET consortium reported age-related changes to microbial communities in relation to a number of health parameters, such as measures of frailty, nutritional status, metabolic profiles and markers of inflammation [176–178]. Microbial community composition and function between young and aged individuals was strikingly distinct. Further, the gut microbiome composition of the older subjects correlated with multiple health measures, including mood, affect, cognition and increased pro-inflammatory cytokine levels. As cytokines can both regulate mood and affect and be affected by gut microbiome function, it is possible that age- and sex-related shifts in microbial communities play a role in the senescent brain [68].
Aging is a particularly dramatic time of hormonal change for women, as the transition into menopause is signalled by a decline in ovarian function and associated hormone levels. While a host of changes occur in behaviour during the perimenopausal transition, it is notable that women experience a high rate of new-onset major depressive episodes during this time [179]. Additionally, perimenopausal and menopausal women are at an increased risk for deficits in several domains of cognitive function, and this has been linked to the change in hormone levels during this window [180,181]. The influence of hormone withdrawal on the gut microbiome in aging population is currently unknown. Additional outstanding questions include the impact of hormone-replacement therapies on the gut microbiome and subsequent sex-specific disease risk [180,181].
3. Conclusion and future directions
Research on the microbiome has certainly undergone its own maturation over the past decade. Seminal reports now exist demonstrating the contribution of microbial communities in various physiological processes in health and disease states. As neuropsychiatric disorders exhibit sex differences in presentation, age of onset, severity and outcome, a critical involvement of a sexually dimorphic microbiome in neurological function and dysfunction provides an exciting avenue for discovery of biomarkers and novel intervention strategies. In order to provide a conceptual framework from which to generate hypotheses and predictions regarding age- and sex-specific periods of vulnerability or resilience, we parallel sex differences in the microbiome across the lifespan to dynamic windows of brain development and maturation. As sex differences in the microbiome parallel immune, metabolic and neural changes, the growing availability of bioinformatic approaches capable of integrating highly dynamic and complex ‘omics datasets will be instrumental in identifying programmatic signatures underlying age- and sex-specific transitions, which, in turn, will provide insight in how these signatures are altered in dysfunctional or disease states.
Acknowledgements
We thank Daniel Beiting, Stefanie Bronson, Jennifer Chan, Christopher Howard, Ana Misic, Bridget Nugent and Ali Rodgers for insightful discussion.
Authors' contributions
E.J., K.E.M. and T.L.B. contributed equally to the conception, execution and revision of the manuscript.
Competing interests
The authors have nothing to declare.
Funding
This work was supported by a pilot award from the PennVet Center for Host-Microbial Interactions at the University of Pennsylvania and by National Institutes of Health grant nos P50-MH099910, MH104184, MH091258, MH087597, MH073030 and MH108286.
References
- 1.Aiello LC, Wheeler P. 1995. The expensive-tissue hypothesis: the brain and the digestive-system in human and primate evolution. Curr. Anthropol. 36, 199–221. ( 10.1086/204350) [DOI] [Google Scholar]
- 2.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185. () [DOI] [Google Scholar]
- 3.Hardy K, Brand-Miller J, Brown KD, Thomas MG, Copeland L. 2015. The importance of dietary carbohydrate in human evolution. Q. Rev. Biol. 90, 251–268. ( 10.1086/682587) [DOI] [PubMed] [Google Scholar]
- 4.Yeoman CJ, et al. 2011. Towards an evolutionary model of animal-associated microbiomes. Entropy 13, 570–594. ( 10.3390/e13030570) [DOI] [Google Scholar]
- 5.Romano-Keeler J, Weitkamp JH. 2015. Maternal influences on fetal microbial colonization and immune development. Pediatr. Res. 77, 189–195. ( 10.1038/pr.2014.163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinde K, Lewis ZT. 2015. Mother's littlest helpers. Science 348, 1427–1428. ( 10.1126/science.aac7436) [DOI] [PubMed] [Google Scholar]
- 7.Alcock J, Maley CC, Aktipis CA. 2014. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. Bioessays 36, 940–949. ( 10.1002/bies.201400071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stilling RM, Bordenstein SR, Dinan TG, Cryan JF. 2014. Friends with social benefits: host-microbe interactions as a driver of brain evolution and development? Front. Cell. Infect. Microbiol. 4, 147 ( 10.3389/fcimb.2014.00147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen-Blevins CR, Sela DA, Hinde K. 2015. Milk bioactives may manipulate microbes to mediate parent-offspring conflict. Evol. Med. Public Health 2015, 106–121. ( 10.1093/emph/eov007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin J, et al. 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. ( 10.1038/nature08821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFall-Ngai M, et al. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110, 3229–3236. ( 10.1073/pnas.1218525110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabouridis PS, Pachnis V. 2015. Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J. Clin. Invest. 125, 956–964. ( 10.1172/JCI76308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bevins CL, Salzman NH. 2011. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9, 356–368. ( 10.1038/nrmicro2546) [DOI] [PubMed] [Google Scholar]
- 14.Vijay-Kumar M, et al. 2010. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231. ( 10.1126/science.1179721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. ( 10.1038/nature05414) [DOI] [PubMed] [Google Scholar]
- 16.Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336, 1268–1273. ( 10.1126/science.1223490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118. ( 10.1016/j.cell.2005.05.007) [DOI] [PubMed] [Google Scholar]
- 18.Neufeld KM, Kang N, Bienenstock J, Foster JA. 2011. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23, 255–264. ( 10.1111/j.1365-2982.2010.01620.x) [DOI] [PubMed] [Google Scholar]
- 19.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA 108, 16 050–16 055. ( 10.1073/pnas.1102999108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. 2011. Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA 108, 3047–3052. ( 10.1073/pnas.1010529108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bercik P, et al. 2011. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609. ( 10.1053/j.gastro.2011.04.052) [DOI] [PubMed] [Google Scholar]
- 22.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. 2014. Microbiota is essential for social development in the mouse. Mol. Psychiatry 19, 146–148. ( 10.1038/mp.2013.65) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. 2013. The microbiome–gut–brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 18, 666–673. ( 10.1038/mp.2012.77) [DOI] [PubMed] [Google Scholar]
- 24.Burokas A, Moloney RD, Dinan TG, Cryan JF. 2015. Microbiota regulation of the mammalian gut-brain axis. Adv. Appl. Microbiol. 91, 1–62. ( 10.1016/bs.aambs.2015.02.001) [DOI] [PubMed] [Google Scholar]
- 25.Mayer EA, Tillisch K, Gupta A. 2015. Gut/brain axis and the microbiota. J. Clin. Invest. 125, 926–938. ( 10.1172/JCI76304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyte M. 2014. Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 817, 3–24. ( 10.1007/978-1-4939-0897-4_1) [DOI] [PubMed] [Google Scholar]
- 27.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. 2012. Host-gut microbiota metabolic interactions. Science 336, 1262–1267. ( 10.1126/science.1223813) [DOI] [PubMed] [Google Scholar]
- 28.Furness JB, Rivera LR, Cho HJ, Bravo DM, Callaghan B. 2013. The gut as a sensory organ. Nat. Rev. Gastro. Hepat. 10, 729–740. ( 10.1038/nrgastro.2013.180) [DOI] [PubMed] [Google Scholar]
- 29.Rhee SH, Pothoulakis C, Mayer EA. 2009. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastro. Hepat. 6, 306–314. ( 10.1038/nrgastro.2009.35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampson TR, Mazmanian SK. 2015. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 17, 565–576. ( 10.1016/j.chom.2015.04.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cryan JF, O'Mahony SM. 2011. The microbiome–gut–brain axis: from bowel to behavior. Neurogastroenterol. Motil. 23, 187–192. ( 10.1111/j.1365-2982.2010.01664.x) [DOI] [PubMed] [Google Scholar]
- 32.Cryan JF, Dinan TG. 2012. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712. ( 10.1038/nrn3346) [DOI] [PubMed] [Google Scholar]
- 33.Dinan TG, Cryan JF. 2012. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37, 1369–1378. ( 10.1016/j.psyneuen.2012.03.007) [DOI] [PubMed] [Google Scholar]
- 34.Stilling RM, Dinan TG, Cryan JF. 2014. Microbial genes, brain and behaviour – epigenetic regulation of the gut-brain axis. Genes Brain Behav. 13, 69–86. ( 10.1111/gbb.12109) [DOI] [PubMed] [Google Scholar]
- 35.Jasarevic E, Rodgers AB, Bale TL. 2015. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiol. Stress 1, 81–88. ( 10.1016/j.ynstr.2014.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. 2015. The infant microbiome development: mom matters. Trends Mol. Med. 21, 109–117. ( 10.1016/j.molmed.2014.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantoja-Feliciano IG, Clemente JC, Costello EK, Perez ME, Blaser MJ, Knight R, Dominguez-Bello MG. 2013. Biphasic assembly of the murine intestinal microbiota during early development. ISME J. 7, 1112–1115. ( 10.1038/ismej.2013.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song SJ, Dominguez-Bello MG, Knight R. 2013. How delivery mode and feeding can shape the bacterial community in the infant gut. CMAJ 185, 373–374. ( 10.1503/cmaj.130147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. 2011. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology 140, 1713–1719. ( 10.1053/j.gastro.2011.02.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. 2009. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697. ( 10.1126/science.1177486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Human Microbiome Project C. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. ( 10.1038/nature11234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu GD, et al. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108. ( 10.1126/science.1208344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David LA, et al. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. ( 10.1038/nature12820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benson AK, et al. 2010. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl Acad. Sci. USA 107, 18 933–18 938. ( 10.1073/pnas.1007028107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schloss PD, Iverson KD, Petrosino JF, Schloss SJ. 2014. The dynamics of a family's gut microbiota reveal variations on a theme. Microbiome 2, 25 ( 10.1186/2049-2618-2-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy EF, et al. 2010. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 59, 1635–1642. ( 10.1136/gut.2010.215665) [DOI] [PubMed] [Google Scholar]
- 47.Markle JG, et al. 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088. ( 10.1126/science.1233521) [DOI] [PubMed] [Google Scholar]
- 48.Gomez A, Luckey D, Yeoman CJ, Marietta EV, Berg Miller ME, Murray JA, White BA, Taneja V. 2012. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS ONE 7, e36095 ( 10.1371/journal.pone.0036095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. 2014. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol. Med. 20, 509–518. ( 10.1016/j.molmed.2014.05.002) [DOI] [PubMed] [Google Scholar]
- 50.Schnorr SL, et al. 2014. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 5, 3654 ( 10.1038/ncomms4654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amato KR, et al. 2014. The role of gut microbes in satisfying the nutritional demands of adult and juvenile wild, black howler monkeys (Alouatta pigra). Am. J. Phys. Anthropol. 155, 652–664. ( 10.1002/ajpa.22621) [DOI] [PubMed] [Google Scholar]
- 52.Bolnick DI, et al. 2014. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat. Commun. 5, 4500 ( 10.1038/ncomms5500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCarthy MM, Pickett LA, VanRyzin JW, Kight KE. 2015. Surprising origins of sex differences in the brain. Horm. Behav. 76, 3–10. ( 10.1016/j.yhbeh.2015.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellis L. 2008. Sex differences: summarizing more than a century of scientific research. New York, NY: Psychology Press. [Google Scholar]
- 55.Gomez A, Luckey D, Taneja V. 2015. The gut microbiome in autoimmunity: sex matters. Clin. Immunol. 159, 154–162. ( 10.1016/j.clim.2015.04.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meleine M, Matricon J. 2014. Gender-related differences in irritable bowel syndrome: potential mechanisms of sex hormones. World J. Gastroenterol. 20, 6725–6743. ( 10.3748/wjg.v20.i22.6725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Labus JS, et al. 2013. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain 154, 2088–2099. ( 10.1016/j.pain.2013.06.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kilpatrick LA, Ornitz E, Ibrahimovic H, Treanor M, Craske M, Nazarian M, Labus JS, Mayer EA, Naliboff BD. 2010. Sex-related differences in prepulse inhibition of startle in irritable bowel syndrome (IBS). Biol. Psychol. 84, 272–278. ( 10.1016/j.biopsycho.2010.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. 2013. Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412. ( 10.1016/j.immuni.2013.08.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moloney RD, Dinan TG, Cryan JF. 2015. Stress and the microbiota–gut–brain axis in visceral pain. Psychoneuroendocrinology 61, 8 ( 10.1016/j.psyneuen.2015.07.408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burokas A, Moloney RD, Dinan TG, Cryan JF. 2015. Targeting the microbiota–gut–brain axis for stress-related disorders: prebiotics selectively reduce anxiety in mice. Eur. Neuropsychopharm. 25, S44–S45. [Google Scholar]
- 62.O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. 2009. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 65, 263–267. ( 10.1016/j.biopsych.2008.06.026) [DOI] [PubMed] [Google Scholar]
- 63.Kessler RC, et al. 2007. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry 6, 168–176. [PMC free article] [PubMed] [Google Scholar]
- 64.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602. ( 10.1001/archpsyc.62.6.593) [DOI] [PubMed] [Google Scholar]
- 65.Ober C, Loisel DA, Gilad Y. 2008. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 9, 911–922. ( 10.1038/nrg2415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maklakov AA, Lummaa V. 2013. Evolution of sex differences in lifespan and aging: causes and constraints. Bioessays 35, 717–724. ( 10.1002/bies.201300021) [DOI] [PubMed] [Google Scholar]
- 67.Geary DC. 2010. Male, female: the evolution of human sex differences, 2nd edn Washington, DC: American Psychological Association. [Google Scholar]
- 68.Prenderville JA, Kennedy PJ, Dinan TG, Cryan JF. 2015. Adding fuel to the fire: the impact of stress on the ageing brain. Trends Neurosci. 38, 13–25. ( 10.1016/j.tins.2014.11.001) [DOI] [PubMed] [Google Scholar]
- 69.Jeffery IB, Lynch DB, O'Toole PW. 2015. Composition and temporal stability of the gut microbiota in older persons. ISME J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Connor EM, O'Herlihy EA, O'Toole PW. 2014. Gut microbiota in older subjects: variation, health consequences and dietary intervention prospects. Proc. Nutr. Soc. 73, 441–451. ( 10.1017/S0029665114000597) [DOI] [PubMed] [Google Scholar]
- 71.Hyland NP, O'Mahony SM, O'Malley D, O'Mahony CM, Dinan TG, Cryan JF. 2015. Early-life stress selectively affects gastrointestinal but not behavioral responses in a genetic model of brain-gut axis dysfunction. Neurogastroenterol. Motil. 27, 105–113. ( 10.1111/nmo.12486) [DOI] [PubMed] [Google Scholar]
- 72.O'Mahony SM, Clarke G, Dinan TG, Cryan JF In press. Early life adversity and brain development: is the microbiome a missing piece of the puzzle? Neuroscience. ( 10.1016/j.neuroscience.2015.09.068) [DOI] [PubMed] [Google Scholar]
- 73.O'Mahony SM, et al. 2014. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 277, 885–901. ( 10.1016/j.neuroscience.2014.07.054) [DOI] [PubMed] [Google Scholar]
- 74.Bale TL, et al. 2010. Early life programming and neurodevelopmental disorders. Biol. Psychiatry 68, 314–319. ( 10.1016/j.biopsych.2010.05.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nugent BM, Bale TL. 2015. The omniscient placenta: metabolic and epigenetic regulation of fetal programming. Front. Neuroendocrinol. 39, 28–37. ( 10.1016/j.yfrne.2015.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bronson SL, Bale TL. 2015. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 41, 207–218. ( 10.1038/npp.2015.231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koren O, et al. 2012. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480. ( 10.1016/j.cell.2012.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macfabe DF. 2012. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb. Ecol. Health Dis. 23, 19 260–19 284. ( 10.3402/mehd.v23i0.19260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagai A, Takebe K, Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T. 2010. Cellular expression of the monocarboxylate transporter (MCT) family in the placenta of mice. Placenta 31, 126–133. ( 10.1016/j.placenta.2009.11.013) [DOI] [PubMed] [Google Scholar]
- 80.Braniste V, et al. 2014. The gut microbiota influences blood–brain barrier permeability in mice. Sci. Transl. Med. 6, 263ra158. ( 10.1126/scitranslmed.3009759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Foley KA, Ossenkopp KP, Kavaliers M, Macfabe DF. 2014. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS ONE 9, e87072 ( 10.1371/journal.pone.0087072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Theije CG, et al. 2014. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav. Immun. 37, 197–206. ( 10.1016/j.bbi.2013.12.005) [DOI] [PubMed] [Google Scholar]
- 83.MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP. 2011. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav. Brain Res. 217, 47–54. ( 10.1016/j.bbr.2010.10.005) [DOI] [PubMed] [Google Scholar]
- 84.Graff J, Tsai LH. 2013. Histone acetylation: molecular mnemonics on the chromatin. Nat. Rev. Neurosci. 14, 97–111. ( 10.1038/nrn3427) [DOI] [PubMed] [Google Scholar]
- 85.McCarthy MM, et al. 2009. The epigenetics of sex differences in the brain. J. Neurosci. 29, 12 815–12 823. ( 10.1523/JNEUROSCI.3331-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ursell LK, Haiser HJ, Van Treuren W, Garg N, Reddivari L, Vanamala J, Dorrestein PC, Turnbaugh PJ, Knight R. 2014. The intestinal metabolome: an intersection between microbiota and host. Gastroenterology 146, 1470–1476. ( 10.1053/j.gastro.2014.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rogier EW, Frantz AL, Bruno ME, Wedlund L, Cohen DA, Stromberg AJ, Kaetzel CS. 2014. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc. Natl Acad. Sci. USA 111, 3074–3079. ( 10.1073/pnas.1315792111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mirpuri J, Raetz M, Sturge CR, Wilhelm CL, Benson A, Savani RC, Hooper LV, Yarovinsky F. 2014. Proteobacteria-specific IgA regulates maturation of the intestinal microbiota. Gut Microb. 5, 28–39. ( 10.4161/gmic.26489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elahi S, et al. 2013. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 504, 158–162. ( 10.1038/nature12675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Backhed F, et al. 2015. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microb. 17, 852 ( 10.1016/j.chom.2015.05.012) [DOI] [PubMed] [Google Scholar]
- 91.Deshmukh HS, et al. 2014. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med. 20, 524–530. ( 10.1038/nm.3542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bilbo SD, Schwarz JM. 2009. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front. Behav. Neurosci. 3, 14 ( 10.3389/neuro.08.014.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quan N, Banks WA. 2007. Brain-immune communication pathways. Brain Behav. Immun. 21, 727–735. ( 10.1016/j.bbi.2007.05.005) [DOI] [PubMed] [Google Scholar]
- 94.Erny D, et al. 2015. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977. ( 10.1038/nn.4030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Betran AP, Merialdi M, Lauer JA, Bing-Shun W, Thomas J, Van Look P, Wagner M. 2007. Rates of caesarean section: analysis of global, regional and national estimates. Paediatr. Perinat. Epidemiol. 21, 98–113. ( 10.1111/j.1365-3016.2007.00786.x) [DOI] [PubMed] [Google Scholar]
- 96.Salminen S, Gibson GR, McCartney AL, Isolauri E. 2004. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut 53, 1388–1389. ( 10.1136/gut.2004.041640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cooperstock M, Riegle L, Woodruff CW, Onderdonk A. 1983. Influence of age, sex, and diet on asymptomatic colonization of infants with Clostridium difficile. J. Clin. Microbiol. 17, 830–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rouphael NG, et al. 2008. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am. J. Obstet. Gynecol. 198 ( 10.1016/j.ajog.2008.01.062) [DOI] [Google Scholar]
- 99.Rousseau C, Levenez F, Fouqueray C, Dore J, Collignon A, Lepage P. 2011. Clostridium difficile colonization in early infancy is accompanied by changes in intestinal microbiota composition. J. Clin. Microbiol. 49, 858–865. ( 10.1128/JCM.01507-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11 971–11 975. ( 10.1073/pnas.1002601107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sela DA, et al. 2008. The genome sequence of Bifidobacterium longum subsp infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl Acad. Sci. USA 105, 18 964–18 969. ( 10.1073/pnas.0809584105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schell MA, et al. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl Acad. Sci. USA 99, 14 422–14 427. ( 10.1073/pnas.212527599) Erratum in Proc. Natl Acad. Sci. USA102, 9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blustein J, Attina T, Liu M, Ryan AM, Cox LM, Blaser MJ, Trasande L. 2013. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int. J. Obes. 37, 900–906. ( 10.1038/ijo.2013.49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roduit C, et al. 2009. Asthma at 8 years of age in children born by caesarean section. Thorax 64, 107–113. ( 10.1136/thx.2008.100875) [DOI] [PubMed] [Google Scholar]
- 105.Renz-Polster H, David MR, Buist AS, Vollmer WM, O'Connor EA, Frazier EA, Wall MA. 2005. Caesarean section delivery and the risk of allergic disorders in childhood. Clin. Exp. Allergy 35, 1466–1472. ( 10.1111/j.1365-2222.2005.02356.x) [DOI] [PubMed] [Google Scholar]
- 106.Curran EA, O'Neill SM, Cryan JF, Kenny LC, Dinan TG, Khashan AS, Kearney PM. 2015. Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J. Child Psychol. Psychiatry 56, 500–508. ( 10.1111/jcpp.12351) [DOI] [PubMed] [Google Scholar]
- 107.Curran EA, Cryan JF, Kenny LC, Dinan TG, Kearney PM, Khashan AS In press. Obstetrical mode of delivery and childhood behavior and psychological development in a British cohort. J. Autism Dev. Disord. ( 10.1007/s10803-015-2616-1) [DOI] [PubMed] [Google Scholar]
- 108.Jasarevic E, Howerton CL, Howard CD, Bale TL. 2015. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 156, 3265–3276. ( 10.1210/en.2015-1177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bailey MT, Coe CL. 1999. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 35, 146–155. () [DOI] [PubMed] [Google Scholar]
- 110.Bailey MT, Lubach GR, Coe CL. 2004. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J. Pediatr. Gastroenterol. Nutr. 38, 414–421. ( 10.1097/00005176-200404000-00009) [DOI] [PubMed] [Google Scholar]
- 111.van Os J, Selten JP. 1998. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br. J. Psychiatry 172, 324–326. ( 10.1192/bjp.172.4.324) [DOI] [PubMed] [Google Scholar]
- 112.Beversdorf DQ, et al. 2005. Timing of prenatal stressors and autism. J. Autism Dev. Disord. 35, 471–478. ( 10.1007/s10803-005-5037-8) [DOI] [PubMed] [Google Scholar]
- 113.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. 2008. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch. Gen. Psychiatry 65, 146–152. ( 10.1001/archgenpsychiatry.2007.20) [DOI] [PubMed] [Google Scholar]
- 114.Weinstock M. 2001. Effects of maternal stress on development and behaviour in rat offspring. Stress 4, 157–167. ( 10.3109/10253890109035015) [DOI] [PubMed] [Google Scholar]
- 115.Lemaire V, Koehl M, Le Moal M, Abrous DN. 2000. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl Acad. Sci. USA 97, 11 032–11 037. ( 10.1073/pnas.97.20.11032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schneider JE, Buckley CA, Blum RM, Zhou D, Szymanski L, Day DE, Bartness TJ. 2002. Metabolic signals, hormones and neuropeptides involved in control of energy balance and reproductive success in hamsters. Eur. J. Neurosci. 16, 377–379. ( 10.1046/j.1460-9568.2002.02118.x) [DOI] [PubMed] [Google Scholar]
- 117.Bronson SL, Bale TL. 2014. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155, 2635–2646. ( 10.1210/en.2014-1040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Howerton CL, Morgan CP, Fischer DB, Bale TL. 2013. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc. Natl Acad. Sci. USA 110, 5169–5174. ( 10.1073/pnas.1300065110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mueller BR, Bale TL. 2006. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol. Behav. 88, 605–614. ( 10.1016/j.physbeh.2006.05.019) [DOI] [PubMed] [Google Scholar]
- 120.Mueller BR, Bale TL. 2007. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol. Behav. 91, 55–65. ( 10.1016/j.physbeh.2007.01.017) [DOI] [PubMed] [Google Scholar]
- 121.Mueller BR, Bale TL. 2008. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 28, 9055–9065. ( 10.1523/JNEUROSCI.1424-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pankevich DE, Mueller BR, Brockel B, Bale TL. 2009. Prenatal stress programming of offspring feeding behavior and energy balance begins early in pregnancy. Physiol. Behav. 98, 94–102. ( 10.1016/j.physbeh.2009.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Darnaudery M, Maccari S. 2008. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res. Rev. 57, 571–585. ( 10.1016/j.brainresrev.2007.11.004) [DOI] [PubMed] [Google Scholar]
- 124.Kapoor A, Kostaki A, Janus C, Matthews SG. 2009. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav. Brain Res. 197, 144–149. ( 10.1016/j.bbr.2008.08.018) [DOI] [PubMed] [Google Scholar]
- 125.Brunton PJ, Sullivan KM, Kerrigan D, Russell JA, Seckl JR, Drake AJ. 2013. Sex-specific effects of prenatal stress on glucose homoeostasis and peripheral metabolism in rats. J. Endocrinol. 217, 161–173. ( 10.1530/JOE-12-0540) [DOI] [PubMed] [Google Scholar]
- 126.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. 2003. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci. Biobehav. Rev. 27, 119–127. ( 10.1016/S0149-7634(03)00014-9) [DOI] [PubMed] [Google Scholar]
- 127.Felice VD, Gibney SM, Gosselin RD, Dinan TG, O'Mahony SM, Cryan JF. 2014. Differential activation of the prefrontal cortex and amygdala following psychological stress and colorectal distension in the maternally separated rat. Neuroscience 267, 252–262. ( 10.1016/j.neuroscience.2014.01.064) [DOI] [PubMed] [Google Scholar]
- 128.Golubeva AV, et al. 2015. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology 60, 58–74. ( 10.1016/j.psyneuen.2015.06.002) [DOI] [PubMed] [Google Scholar]
- 129.Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. 2015. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 53, 233–245. ( 10.1016/j.psyneuen.2015.01.006) [DOI] [PubMed] [Google Scholar]
- 130.Kunji ER, Mierau I, Hagting A, Poolman B, Konings WN. 1996. The proteolytic systems of lactic acid bacteria. Antonie Van Leeuwenhoek 70, 187–221. ( 10.1007/BF00395933) [DOI] [PubMed] [Google Scholar]
- 131.Jiang T, Savaiano DA. 1997. In vitro lactose fermentation by human colonic bacteria is modified by Lactobacillus acidophilus supplementation. J. Nutr. 127, 1489–1495. [DOI] [PubMed] [Google Scholar]
- 132.Soergel KH. 1994. Colonic fermentation: metabolic and clinical implications. Clin. Invest. 72, 742–748. ( 10.1007/BF00180540) [DOI] [PubMed] [Google Scholar]
- 133.McDonald JW, Johnston MV. 1990. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res. Brain Res. Rev. 15, 41–70. ( 10.1016/0165-0173(90)90011-C) [DOI] [PubMed] [Google Scholar]
- 134.McDonald JW, Johnston MV. 1993. Excitatory amino acid neurotoxicity in the developing brain. NIDA Res. Monogr. 133, 185–205. [PubMed] [Google Scholar]
- 135.Yatsunenko T, et al. 2012. Human gut microbiome viewed across age and geography. Nature 486, 222–227. ( 10.1038/nature11053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. 2007. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177 ( 10.1371/journal.pbio.0050177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hollister EB, et al. 2015. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 3, 36 ( 10.1186/s40168-015-0101-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sisk CL, Foster DL. 2004. The neural basis of puberty and adolescence. Nat. Neurosci. 7, 1040–1047. ( 10.1038/nn1326) [DOI] [PubMed] [Google Scholar]
- 139.Collins SM, Kassam Z, Bercik P. 2013. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr. Opin. Microbiol. 16, 240–245. ( 10.1016/j.mib.2013.06.004) [DOI] [PubMed] [Google Scholar]
- 140.Kisiela M, Skarka A, Ebert B, Maser E. 2012. Hydroxysteroid dehydrogenases (HSDs) in bacteria – a bioinformatic perspective. J. Steroid. Biochem. 129, 31–46. ( 10.1016/j.jsbmb.2011.08.002) [DOI] [PubMed] [Google Scholar]
- 141.Bilton RF. 1995. Microbial production of testosterone. Lancet 345, 1186–1187. ( 10.1016/S0140-6736(95)91022-0) [DOI] [PubMed] [Google Scholar]
- 142.Fernandes P, Cruz A, Angelova B, Pinheiro HM, Cabral JMS. 2003. Microbial conversion of steroid compounds: recent developments. Enzyme Microb. Technol. 32, 688–705. ( 10.1016/S0141-0229(03)00029-2) [DOI] [Google Scholar]
- 143.Donova MV, Egorova OV, Nikolayeva VM. 2005. Steroid 17 β-reduction by microorganisms—a review. Process Biochem. 40, 2253–2262. ( 10.1016/j.procbio.2004.09.025) [DOI] [Google Scholar]
- 144.Lombardi P, Goldin B, Boutin E, Gorbach SL. 1978. Metabolism of androgens and estrogens by human fecal microorganisms. J. Steroid Biochem. 9, 795–801. ( 10.1016/0022-4731(78)90203-0) [DOI] [PubMed] [Google Scholar]
- 145.Sandrini S, Aldriwesh M, Alruways M, Freestone P. 2015. Microbial endocrinology: host-bacteria communication within the gut microbiome. J. Endocrinol. 225, R21–R34. ( 10.1530/JOE-14-0615) [DOI] [PubMed] [Google Scholar]
- 146.Neuman H, Debelius JW, Knight R, Koren O. 2015. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 39, 509–521. ( 10.1093/femsre/fuu010) [DOI] [PubMed] [Google Scholar]
- 147.Lyte M. 2013. Microbial endocrinology in the microbiome–gut–brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 9, e1003726 ( 10.1371/journal.ppat.1003726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dinan TG, Stanton C, Cryan JF. 2013. Psychobiotics: a novel class of psychotropic. Biol. Psychiatry 74, 720–726. ( 10.1016/j.biopsych.2013.05.001) [DOI] [PubMed] [Google Scholar]
- 149.O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. 2015. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48. ( 10.1016/j.bbr.2014.07.027) [DOI] [PubMed] [Google Scholar]
- 150.Yano JM, et al. 2015. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276. ( 10.1016/j.cell.2015.02.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Spor A, Koren O, Ley R. 2011. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 9, 279–290. ( 10.1038/nrmicro2540) [DOI] [PubMed] [Google Scholar]
- 152.Tannock GW, Savage DC. 1974. Influences of dietary and environmental stress on microbial-populations in murine gastrointestinal-tract. Infect. Immunol. 9, 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. 2011. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav. Immun. 25, 397–407. ( 10.1016/j.bbi.2010.10.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. 2010. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect. Immun. 78, 1509–1519. ( 10.1128/IAI.00862-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bailey MT, Engler H, Sheridan JF. 2006. Stress induces the translocation of cutaneous and gastrointestinal microflora to secondary lymphoid organs of C57BL/6 mice. J. Neuroimmunol. 171, 29–37. ( 10.1016/j.jneuroim.2005.09.008) [DOI] [PubMed] [Google Scholar]
- 156.Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS, Lyte M, Bailey MT. 2014. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 14, 189 ( 10.1186/1471-2180-14-189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mackos AR, Eubank TD, Parry NMA, Bailey MT. 2013. Probiotic Lactobacillus reuteri attenuates the stressor-enhanced severity of Citrobacter rodentium infection. Infect. Immun. 81, 3253–3263. ( 10.1128/IAI.00278-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tarr AJ, Galley JD, Fisher SE, Chichlowski M, Berg BM, Bailey MT. 2015. The prebiotics 3′sialyllactose and 6′sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: evidence for effects on the gut–brain axis. Brain Behav. Immun. 50, 166–177. ( 10.1016/j.bbi.2015.06.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dethlefsen L, Huse S, Sogin ML, Relman DA. 2008. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 6, e280 ( 10.1371/journal.pbio.0060280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mueller S, et al. 2006. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl. Environ. Microbiol. 72, 1027–1033. ( 10.1128/AEM.72.2.1027-1033.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vina J, Borras C, Gambini J, Sastre J, Pallardo FV. 2005. Why females live longer than males: control of longevity by sex hormones. Sci. Aging Knowledge Environ. 2005, pe17. ( 10.1126/sageke.2005.23.pe17) [DOI] [PubMed] [Google Scholar]
- 162.Mulak A, Tache Y. 2010. Sex difference in irritable bowel syndrome: do gonadal hormones play a role? Gastroenterol. Pol. 17, 89–97. [PMC free article] [PubMed] [Google Scholar]
- 163.Mulak A, Tache Y, Larauche M. 2014. Sex hormones in the modulation of irritable bowel syndrome. World J. Gastroenterol. 20, 2433–2448. ( 10.3748/wjg.v20.i10.2433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK. 2005. Estrogen-related mood disorders - reproductive life cycle factors. Adv. Nurs. Sci. 28, 364–375. ( 10.1097/00012272-200510000-00008) [DOI] [PubMed] [Google Scholar]
- 165.Cullen G, O'Donoghue D. 2007. Constipation and pregnancy. Best Pract. Res. Clin. Gastroenterol. 21, 807–818. ( 10.1016/j.bpg.2007.05.005) [DOI] [PubMed] [Google Scholar]
- 166.Cosgrove KP, Mazure CM, Staley JK. 2007. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry 62, 847–855. ( 10.1016/j.biopsych.2007.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McCarthy MM. 2008. Estradiol and the developing brain. Physiol. Rev. 88, 91–124. ( 10.1152/physrev.00010.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wong M, Thompson TL, Moss RL. 1996. Nongenomic actions of estrogen in the brain: physiological significance and cellular mechanisms. Crit. Rev. Neurobiol. 10, 189–203. ( 10.1615/CritRevNeurobiol.v10.i2.30) [DOI] [PubMed] [Google Scholar]
- 169.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. 2012. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 126, 4–16. ( 10.1037/a0026708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Woolley CS, McEwen BS. 1992. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 12, 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mcewen BS, Woolley CS. 1994. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp. Gerontol. 29, 431–436. ( 10.1016/0531-5565(94)90022-1) [DOI] [PubMed] [Google Scholar]
- 172.Woolley CS, Gould E, Frankfurt M, Mcewen BS. 1990. Naturally-occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 10, 4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Geary D. 2015. Evolution of vulnerability: implications for sex differences in health and development. Boston, MA: Elsevier. [Google Scholar]
- 174.O'Toole PW, Claesson MJ. 2010. Gut microbiota: changes throughout the lifespan from infancy to elderly. Int. Dairy J. 20, 281–291. ( 10.1016/j.idairyj.2009.11.010) [DOI] [Google Scholar]
- 175.Tiihonen K, Ouwehand AC, Rautonen N. 2010. Human intestinal microbiota and healthy ageing. Ageing Res. Rev. 9, 107–116. ( 10.1016/j.arr.2009.10.004) [DOI] [PubMed] [Google Scholar]
- 176.Claesson MJ, et al. 2011. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl Acad. Sci. USA 108(Suppl 1), 4586–4591. ( 10.1073/pnas.1000097107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Claesson MJ, et al. 2012. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184. ( 10.1038/nature11319) [DOI] [PubMed] [Google Scholar]
- 178.Rampelli S, Candela M, Turroni S, Biagi E, Collino S, Franceschi C, O'Toole PW, Brigidi P. 2013. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging-Us 5, 902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cohen GD, Perlstein S, Chapline J, Kelly J, Firth KM, Simmens S. 2006. The impact of professionally conducted cultural programs on the physical health, mental health, and social functioning of older adults. Gerontologist 46, 726–734. ( 10.1093/geront/46.6.726) [DOI] [PubMed] [Google Scholar]
- 180.Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. 2006. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 13, 411–422. ( 10.1097/01.gme.0000189618.48774.7b) [DOI] [PubMed] [Google Scholar]
- 181.Sherwin BB. 2012. Estrogen and cognitive functioning in women: lessons we have learned. Behav. Neurosci. 126, 123–127. ( 10.1037/a0025539) [DOI] [PMC free article] [PubMed] [Google Scholar]