Abstract
Scientific studies funded by the United States government must now include both males and females as experimental subjects. This is a welcomed change for those of us who have been reporting on sex differences for decades. That said, there are some issues to consider; I focus on one in this review: females used in animal models of mental illness and health are almost always virgins and yet most adult females around the world, irrespective of species, are not virgins. I am not advocating that all scientists include non-virgin females in laboratory studies, but rather to consider the dynamic nature of the female brain when drawing conclusions through discovery. Stressful life experiences, including those related to sexual aggression and trauma, can have a lasting impact on processes of learning related to mental health and plasticity in the female brain. Her response to stress can change rather dramatically as she emerges from puberty to become pregnant and produce offspring, as she must learn to care for those offspring. The inclusion of females in scientific research has been a long time coming but it comes with a history. Going forward, we should take advantage of that history to generate hypotheses that are both reasonable and meaningful.
Keywords: stress, sexual aggression, neurogenesis, hippocampus, trauma, amygdala
1. Introduction
It was recently mandated that both sexes be included in scientific studies, at least those supported by federal funds provided by the National Institutes of Health (NIH). This is a welcomed development, and one that has been a long time coming. I first began work on sex differences in the mid-1990s. New to the field, I had then referred to them as gender differences, not yet realizing that the word ‘gender’ refers to differences between men and women, often as a result of societal norms and culture. Because my studies were being conducted in laboratory rodent models, I was and have been studying sex, not gender, differences in the brain. However, even the phrase ‘sex differences’ has its own subtle meanings [1], which are important to understand as we move forward to embrace these new regulations set out by the Office of Research on Women's Health and the National Institutes. In this review, I provide a history of similar realizations on a trip down memory lane about sex differences in the brain.
Throughout the 1980s and 1990s, the most accepted ‘mechanism’ for storing memories in the brain was long-term potentiation (LTP). LTP is a long-lasting increase in synaptic efficacy that is evoked after a high-frequency tetanic stimulation to afferent fibres. It was first discovered in the hippocampus by Bliss and Lomo [2], who immediately recognized it as the neurophysiological instantiation of what Donald Hebb had referred to in his famous book: ‘The organization of behavior’ [3]. Hebb had proposed that associations between events could be learned and strengthened by increasing the synaptic connections and/or strength between neurons. Because LTP was discovered in the hippocampus, a structure intimately associated with processes of learning and memory, it was put forth as a putative mechanism whereby new memories are acquired and stored in the mammalian brain [2,4]. Despite its resemblance to the so-called Hebbian synapse, there was little evidence that LTP was necessary or even sufficient for the acquisition or storage of memory [5]. I had been working on LTP for some years when I attended the Learning and Memory Meeting held every few years at the University of California in Irvine, California. There, one of my former laboratory mates, Dr Stephen Maren, told me that females did not express LTP or at least expressed less potentiation when compared with potentiation in male rodents [6]. This report was astounding to me. After all, if LTP were a ‘memory’ mechanism, how could females be left without it (or even less of it)? More astounding to me was the fact that virtually no scientists were testing females in their laboratory experiments. Indeed, the only scientists that I could find who were currently using females in their laboratories were those invested in behavioural processes of animal learning. Females were being used in these studies because they tend to learn faster—not necessarily better—which is of course important when studying processes of learning through performance measures in a laboratory setting. I was also surprised to learn that many of the early studies reporting oestrogen-induced effects on neurophysiological responses in the hippocampus were conducted in male rodents [7]. After these revelations, I went back to my laboratory to study sex differences in learning and their modulation by stressful life experience. That was 20 years ago.
(a). Sex differences in learning and the stress response
Stressful life experience can interfere with processes of learning and memory. However, the effects are not always detrimental and vary according to a number of factors, including the type of stressor, its length, intensity and most importantly, the type of learning process as examined in laboratory settings or appraised under more real-life conditions. In 1992, Shors et al. [8] reported in Science that exposure to an acute stressful event enhanced associative learning in male rats. This report was unexpected, because most scientists at that time considered stress to be detrimental to learning and memory performance and most certainly to have a negative impact on hippocampal function [9]. For our studies, we assessed learning through classical eyeblink conditioning, a procedure that pairs a conditioned stimulus (CS), noise, with subsequent electrical stimulation to the eyelid as an unconditioned stimulus (US). The electrical US stimulation causes the animal to blink its eye, which is recorded through electromyography, or muscle activity. This particular procedure is valuable to scientists, because it allows one to distinguish non-specific effects of stress (of which there are many) on performance variables that are not necessarily indicative of learning, but can interfere with the measurement of learning, thereby leading to erroneous conclusions about the effects of stress on learning. For example, exposure to laboratory stressors such as swim stress or tail stimulation often suppresses motor activity or motivation to forage for and consume food; therefore, stress can induce deficits in performance simply as a matter of change in motor activity and motivation. These effects may still be interesting and/or relevant but they do not necessarily indicate changes in learning, per se. By using a task like classical eyeblink conditioning, one can rule out some non-specific effects of stress. For example, exposure to the stressor did not alter responding to the same stimuli when they were presented one after each other but were not predictable across time. We further determined that the stressor had to be sufficiently intense to enhance learning in males but also had to be uncontrollable—male rats that learned to control the stressor did not express an increase in learning [10,11]. Moreover, the modulation of learning did not extend to memories that had already been acquired. If animals were stressed after they had learned then their responding did not change [12]. We further determined that stress enhanced learning in males through an increase in the release of the stress-related hormones glucocorticoids, at least to the extent that removing the adrenal glands prevented any enhancement of learning [13]. I should note here that in all of these studies, animals were exposed to the stressor 24 h before any training occurred. Therefore, these effects of stress on learning are not directly mediated by the presence of corticosterone, epinephrine or other fear-induced substances, even though these factors may contribute to the long-term mechanisms, which allow changes to be expressed over days. Overall, we were able to document that exposure to an acute uncontrollable stressful and generally noxious environmental experience persistently enhances the acquisition of an associatively learned response in males and does so through mechanisms that include activation of the hypothalamic–pituitary adrenal axis and stress hormone activation.
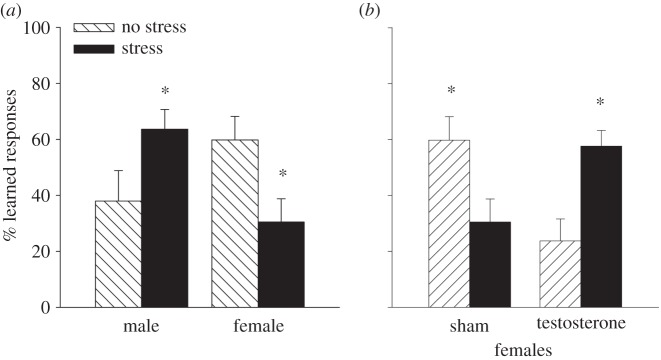
Imagine our surprise when we observed that exposure to an acute stressful event had the opposite effect in females—profoundly suppressing their ability to learn (figure 1a). These results were quite extraordinary, because the male and the female were presented with the same environmental stressor and they were trained on the same learning task, and yet they responded in opposite directions during training [14]. When we first reported this finding, there were several studies indicating sex differences either in learning or in response to stress [15,16]. However, most of these differences were differences in degrees of learning. In other words, males learned better or worse than females or responded more or less to emotional experiences that were stressful. In this case, the effects were not simply a matter of degree but rather reflected a difference in direction. Given these findings, it seemed clear that males and females could respond in opposite directions to the same environmental stressor and as a result, the two sexes must be using different brain structures and/or mechanisms in order to do so. Before discussing their respective brain circuits and mechanisms, it is important to describe the hormonal processes through which these sex differences come to be organized, eventually activated and then expressed so differently in males versus females.
Figure 1.
Learning was assessed during associative learning of the classically conditioned eyeblink response one day after exposure to an acute stressful event. (a) Females learned better under unstressed conditions. However, the percentage of learned responses across training increased after stress in males but decreased in females. (b) After masculinization, females did not learn as well as intact females. However, the percentage of learned responses increased in females that were masculinized at birth with testosterone and exposed to an acute stressor as an adult. Therefore, behavioural responses in masculinized females resembled those expressed by the intact males shown in (a). Asterisks indicate significant difference between adjacent group data (p < 0.05).
(b). Sex hormones
Sex hormones are released from the gonads of males and females to induce many sexual behaviours that we associate with sex differences in the brain. In females, oestrogens and progesterones are released from the ovaries to produce sexual characteristics that we associate with ‘being’ female. As one would expect, ovarian hormones are necessary to suppress learning in females after exposure to a stressful event [14]. Female rodents that were ovariectomized in adulthood did not express a deficit in learning after exposure to the stressor, as they readily learned the classically conditioned response [14]. Therefore, the presence of ovarian hormones is necessary to suppress learning in females after a stressful event.
Every 5 days or so, a female rat cycles through stages of proestrus, oestrus, diestrus I and diestrus II (stages of oestrous are verified by swabbing the vaginal wall for loose cells and conducting histological analyses on their morphology). Proestrus is associated with ovulation and is accompanied by relatively high concentrations of oestradiol. As it turned out, females in proestrus tend to learn faster than females in the other stages and faster than males do—in the absence of a stressful event [17]. However, females in proestrus are also more vulnerable to stress than females in other stages of oestrous. To summarize, female rodents learn especially well during proestrus, when oestrogen concentrations are relatively high and when they are most receptive to sexual activity with males, but they do not learn well if they are exposed to a stressful event during that same time period. Therefore, the stressful experience suppresses the enhanced learning that would normally occur during proestrus. Clearly, the female response to stress is a dynamic one that depends on when a stressful event occurs.
(c). Brain organization theory
Many sex differences in behaviour are organized long before adulthood or even puberty and rather occur in utero or shortly after birth. These effects are referred to as ‘organizational’ because it is presumed that the brain is being organized by the presence of sex hormones during very early stages of brain development. In what are now famous experiments [18–20], it was determined that female rodents were more likely to try to mount other females as adults if they had been exposed to testosterone in utero or immediately after birth. Males, on the other hand, would sometimes express lordosis if they were feminized in utero and then exposed to ovarian hormones in adulthood (note that these behaviours are not absolutely sex-dependent; males can also express lordosis, albeit rarely, and females can try to mount other rodents, again rarely). Most of the early studies focused on sexual behaviour in rodent models as described above or in humans with congenital differences in testosterone exposure or accidental exposure through hormone-related therapies provided to pregnant women. Together, these studies suggested that many sex differences in behaviour, especially sexual behaviour, are not mediated by genetic differences in sex chromosomes, but rather by exposure to reproductive hormones during early development. It is noted that the theory and data supporting brain organization as presented here are more simplistic than sex differences expressed by animal species in their natural environment, especially humans. As pointed out by Jordan-Young [21] in her provocative book on this topic, the scientific community was embracing brain organization theory just as our ideas about sexual activity and femininity/masculinity were changing in our culture. Therefore, many of our preconceived ideas about maleness and femaleness are rooted in our largely subjective experiences in the world. Simply put, we cannot map all sexual activity in humans onto these findings in laboratory models. That said, these early studies did establish gonadal hormones as important mediators of sex differences in sexual behaviour, at least in laboratory rodent models.
Whether brain organization theory can explain sex differences in learning and other cognitive processes is less well established. This is, in part, because sex differences in behaviours unrelated to reproduction are not necessarily robust, especially those related to learning and memory in humans. For example, it is often stated that men are better at learning tasks that involve spatial rotation, whereas women are better at tasks that involve verbal communication [22]. While some studies do support such claims, the data are variable, and there is considerable overlap in distributions [16]. With respect to animal models of learning, a few studies indicate that males can outperform females during spatial navigation learning. For example, Williams, Barnett & Meck [23] reported sex differences in spatial learning while rats were trained to learn a 12-arm radial maze task. Males outperformed females during recall. But, interestingly, females that were masculinized during early development also outperformed intact females, indicating that these sex differences in learning were organized by the presence of gonadal hormones. The researchers went on to demonstrate that males and females were using different strategies to learn the task and those strategies were likewise organized by the presence of gonadal hormones during early development.
Brain organization theory would pose that sex differences in classical conditioning and the opposite effects of stress on learning in males versus females were similarly organized during very early development [24]. To test this hypothesis, female rats were injected with testosterone on the day that they were born. As adults, these females behaved as males typically do. In other words, they did not learn as well as intact females do during proestrus. Moreover, they learned better after exposure to the stressful experience (figure 1b). Therefore, the masculinization of the female brain effectively altered their learning and stress response to that of the male. Importantly, we did not observe a similar transformation in males. Adult males that had been castrated at birth still learned better after exposure to a stressful event. We further examined possible changes before birth but even when the males were exposed to a testosterone antagonist in utero, they did not behave like females in adulthood. However, they were effectively demasculinized because they did not respond like intact males; rather their learning was not altered either way after exposure to the stressful event. Together, these studies indicate that the female responses to stress and learned responses are normally organized as a default system or ‘prototype’ through which learning becomes accelerated in proestrus and suppressed by stress—but only once females reach adulthood. The detrimental effect of stress on learning does not emerge until the female begins to develop an oestrous cycle and dissipates once she reaches menopause [25,26].
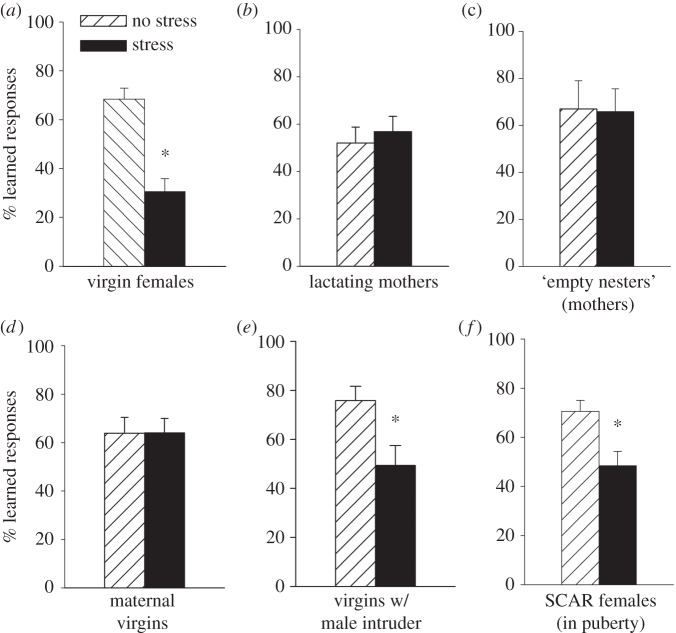
Together, the studies discussed thus far indicate that these effects of stress on learning, although provocative, are expressed under relatively limited stages in the female life, that is, when she is about to ovulate. One would have to wonder why these seemingly maladaptive responses to stress would evolve in the first place. Under naturalistic conditions, female rats in proestrus must move out of their burrows into the open, where they not only encounter males, but also predators. In times of stress and danger, it may be adaptive for the female to reduce exploratory behaviour and opportunities for new learning until the danger has passed; perhaps these responses in laboratory rodents are simply a manifestation of this process or others related to reproduction. It is of course impossible to really know why or how this response to stress evolved in female rodents. That said, one might wonder if these changes in female rodent learning discussed here are meaningful, i.e. do they arise under more naturalistic conditions? To address this, we decided to expose females to a stressor that she might actually encounter in the wild: an adult male rat. During our initial study, an adult virgin female was exposed to an adult male for 30 min [27]. The next day, the female was trained with the classically conditioned eyeblink response, during which learning is typically suppressed after exposure to an acute laboratory stressor such as swim stress. Exposure to the adult male on its own was sufficient to suppress learning in the adult virgin female (figure 2e).
Figure 2.
Learning during classical eyeblink conditioning was assessed in virgin and non-virgin females in different stages of their reproductive lives. Learning is represented and inferred from the percentage of conditioned responses (CRs) during classical eyeblink conditioning on the last day of training. (a) Learning was suppressed in virgin females that were exposed to an acute stressor. (b) If they had delivered offspring and were lactating, stress did not suppress learning. (c) Learning in females that had offspring (mothers), but were no longer taking care of them (‘empty nesters’) was unaffected by stress. Therefore, mothers remained resistant to stress, because they continued to learn well even after exposure to the acute stressor. (d) Learning was not suppressed in adult virgin females that learned to take care of offspring through the process of maternal sensitization. (e) Learning in virgin adults was suppressed after acute social interaction with (w/) an adult male. (f) Learning was suppressed in virgin females that were repeatedly exposed to a sexually experienced adult male throughout puberty, according to a novel model of sexual aggression known as sexual conspecific aggressive response (SCAR). Asterisks indicate significant difference between adjacent group data (p < 0.05).
It is important to note that all types of learning are not suppressed by stress in females. Indeed, females can learn other responses very well and oftentimes better than males when encountering stressful experiences—especially those that involve operant (active movement) responses [28]. Therefore, the classically conditioned responses that I am reviewing here are perhaps unique in the animal kingdom, but, because the male response is so different from the female response, they nonetheless provided us with a useful laboratory model to study robust sex differences in learning and stress-related behaviours.
(d). Motherhood and other changes across the female lifespan
Females are not always vulnerable to stress (figure 2a). For example, females that are taking care of their offspring continue to learn well after exposure to a stressful event (figure 2b; [27]). Even virgin females that learned to care for another female's offspring learned well after the stressful event (figure 2d). Therefore, caring for offspring rather than pregnancy or lactation appears to be sufficient to protect females from the negative effects of stress on associative learning. Most astonishingly, females that had been mothers at some time in their lives were likewise resilient to the negative effects of stress, even when they were no longer taking care of their offspring (long after weaning). In other words, a female rat that had delivered offspring at some point in her life learned well after exposure to the stressor and much better than a virgin female that obviously had never been pregnant nor cared for offspring (figure 2c). These studies indicate that ‘learning’ to be maternal can protect females from some of the negative consequences of stress, but they also illustrate how the female response system changes across her lifetime, especially as she learns to become a mother. Obviously, long-term changes in neuronal plasticity must be engaged to maintain such a response over the course of her lifespan. It is interesting to speculate that the brain is poised to learn maternal behaviours when she is a virgin and once learning occurs, it induces lasting changes in neuronal plasticity, which protect her from the negative consequences of stress and thereby enhance the survival of her offspring.
(e). Sex differences in brain circuits
Sex differences in learning and the stress response are presumably mediated by different brain mechanisms and circuits in males versus females, or minimally through similar mechanisms mediated in opposite ways in the same circuit. One way to determine which of these two possibilities is most parsimonious is to identify the brain regions that are necessary to induce sex differences. If one brain region is necessary in one sex but not in the other, then different mechanisms are indicated. If the same brain regions are critically involved, then similar mechanisms may be modulated in opposite ways. We began with the hippocampus, because it is critical for many types of learning and for regulating the stress response [9,29]. For these experiments, it is important to note that stress modulates learning in both sexes, irrespective of whether or not the learning process itself depends on the hippocampus. For example, in our earliest studies, animals were trained with delay eyeblink conditioning, a task that does not depend on the hippocampus for learning although it does activate many neurons within the structure [14]. Because animals (including humans) can learn delay conditioning without a hippocampus [29], we were able to assess performance with and without an intact hippocampus. Interestingly, stress did not alter learning in males or females that were devoid of hippocampal input. In other words, females with an intact hippocampus did not learn well after stress, whereas females without a hippocampus did. Males with an intact hippocampus learned better after stress, whereas males without one did not learn better. Therefore, the hippocampus is necessary to modify learning after exposure to an acute stressful event [30]. These data were the first to establish that information about stressful life experience and learning intersect within the hippocampus, an idea that was widely accepted but had never been proven.
When an animal is stressed, it automatically learns to recall many of the stimuli associated with that event, including when and where the event occurred as well as the presence of stimuli in the environment that seem to predict it. This type of learning is adaptive, because the animal can then use those memories to avoid potentially dangerous and stressful experience in the future. Although many researchers categorize ‘stress’ differently from ‘fear’ and often study them as separate entities, they are not separate and their neuronal processes overlap to a considerable degree. In our studies, animals were exposed to an acute stressful event and then trained 24 h later in a new context. During the stressor, they learned to fear its context and, indeed, if they are placed back in the context, then the stress-induced effects on learning were reactivated [13]. Therefore, in the absence of ‘testing’ for learned fear, memories of the context are nonetheless acquired; many of these learned fears depend on the amygdala. To determine whether the basolateral nucleus of the amygdala (BLA) was necessary for the modulation of learning after stress, we inactivated the structure during the stressor. Because inactivation only lasts for an hour or so and the training occurs 24 h later, training (and learning) occurred with full participation of the amygdala. For both males and females, inactivation of the amygdala during the stressor prevented any modulation of learning [31]. Again, the manipulation did not ‘change’ the male into a female or vice versa; rather the absence of amygdaloid input during the stressful event simply prevented the enhanced learning in males and similarly prevented the suppressed learning in females. Together with the data discussed above, we can conclude that the amygdala and the hippocampus are both critically involved in the modulation of learning after a stressful event—in both sexes.
(f). Communications between the female amygdala and her prefrontal cortex
Some of the most influential studies on stress and learning were conducted in the 1960s by Maier, Overmier and Seligman [32,33]. They reported that animals that were allowed to learn to control a stressor became immune to its consequences, whereas the animal that did not learn to control the stress or could not control it, became helpless. The phenomenon came to be known as ‘learned helplessness’ and was later promoted by Seligman [34] as an animal model for depression in humans. Maier, on the other hand, continued to work on the neurobiological mechanisms of controllability and helplessness behaviours in animal models. A decade ago now, he and his co-workers reported that neuronal activity in the medial prefrontal cortex (mPFC) is necessary for the expression of controllability because it inhibits activity in brainstem nuclei that would normally produce the behavioural responses observed after uncontrollable stress [35,36]. As mentioned, controllable stress does not have the same impact on associative learning as does uncontrollable stress. Specifically, stress neither enhanced nor impaired learning when the animal had the opportunity to learn to control it [10,11]. Of course, there are many studies to implicate the mPFC in learning about stressful life experiences, in part, because the structure has direct connections to the amygdala and other efferent stress-related pathways to the autonomic nervous system [37,38]. Therefore, it seemed reasonable to hypothesize that the mPFC mediates the effects of uncontrollable stress on classical eyeblink conditioning. To test the hypothesis, we inactivated the mPFC region in males and females [39]. When the mPFC was inactivated during the stressor, females could learn, i.e. the learning deficit was prevented. Similar manipulations in males were of no consequence, i.e. males still learned better after the stressful event. These data indicate that neuronal activity within the mPFC during the stressful event is necessary to suppress learning in females but not necessary to enhance learning in males. We further assessed the infralimbic and prelimbic regions, each of which plays a unique role in processes related to associative learning, at least in males [40]. Neuronal activity within the prelimbic region of the female mPFC was necessary to suppress learning, whereas neuronal activity in the infralimbic region was not [41], but again only in females. Overall, these data indicate that males and females can use different brain regions to learn after exposure to a stressful life experience and one of those regions is the prelimbic region of the mPFC.
Anatomical connections between the mPFC and the amygdala have been implicated in the mechanisms that mediate stress-related mental illnesses in humans as well as in traditional animal models of stress and learning [37,38,42]. To determine whether these anatomical connections were likewise necessary for the stress effects on learning, we conducted a classic disconnection study, similar to that developed by Holland [43]. The method is based on the fact that most (but not all) regions in the rodent brain are connected to one another only on one side of the brain. By lesioning one structure on contralateral sides of the brain, the connections between those structures are disrupted on both sides of the brain (figure 3a,b). As a control procedure, each structure is lesioned on the same side of the brain, thereby only disrupting the connection on one side of the brain while inflicting the same amount of lesion. In our studies, the basolateral nucleus of the amygdala (BLA) was lesioned on one side of the brain and the mPFC on the other with the appropriate controls. Recall that both structures on their own were determined to be necessary to suppress learning in females after stress [30,31] . After disconnecting them on both sides of the brain, females were able to learn as if the stressful event had not occurred (figure 3d). When the two regions were disconnected on just one side of the brain, learning was suppressed after the stressor, and thus the females with unilateral lesions behaved like intact females trained in proestrus (figure 3c). Importantly, learning itself was unaffected by the unilateral or the bilateral lesions, in the absence of the stressor. These data indicate that communications between the mPFC and the amygdala are necessary to suppress learning after a stressful life event in females.
Figure 3.
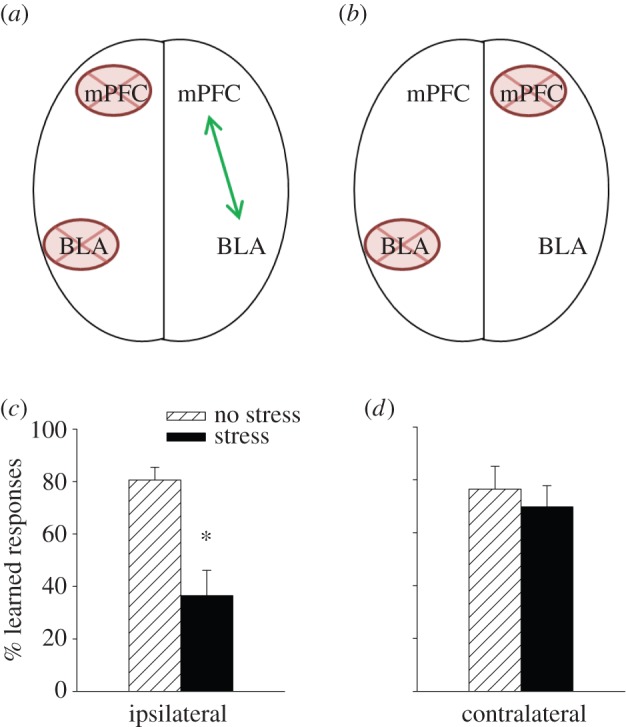
The connections between the medial prefrontal cortex (mPFC) and the basolateral nucleus of the amygdala (BLA) were disrupted. (a) Ipsilateral and unilateral neurotoxic lesions disconnect the mPFC and BLA only on one side of the brain, (b) whereas unilateral lesions on contralateral sides of the brain disrupt connections in both hemispheres. Because most connections between brain regions are unilateral, connections on one side are typically sufficient to maintain behaviours that depend on communications between those structures. (c) Females with communications between the mPFC and BLA did not learn well after stress, (d) whereas females with disrupted communications on both sides learned as well after stress as those that were not stressed. These data indicate that communications between the mPFC and the amygdala are necessary to suppress this type of learning (classical eyeblink conditioning) after a stressful event [39]. Asterisk indicates significant difference between adjacent group data (p < 0.05). (Online version in colour.)
(g). Masculinization of the female's bed nucleus
There have been numerous reports of sex differences in brain anatomy, but most have not stood the test of time, either because they were simply not true or because the techniques were inadequate for observing them (as reviewed in [21]). One region of the brain that does appear to house sex differences is the bed nucleus of the stria terminalis (BNST) [44]. It is one of the principal outputs of the amygdala and is intimately connected to the hypothalamic regions necessary for eliciting the stress response [45]. The BNST has also been implicated in the anticipation of stress, a learned response often referred to as ‘anxiety’. In his now-classic studies, Davis et al. [46] reported that lesions to the BNST prevented the expressions of anxiety-like behaviours in response to stressful and fear-evoking events. It was postulated that mechanisms within the BNST are necessary to maintain a heightened state of vigilance, which in some individuals can be disruptive. It is noted, however, that anxiety in and of itself is an adaptive response because it provides the animal with incentive to remain vigilant when dangerous conditions are forthcoming [47]. Recall that the stress effect on learning in males and females is sustained over days. In one study, stress could still modify learning up to 3 days later. Therefore, the mechanisms that are induced during the stressful event to modify learning must be long-lasting. Because the BNST is implicated in these longer-lasting effects of stress on behavioural processes, we hypothesized that it was necessary for maintaining the stress effects on classical conditioning. As one might expect, inactivation of the BNST during the stressor was of no consequence [48]. In other words, the females still did not learn and the males learned better. However, when the inactivation occurred later during training, the males did not learn better after stress. Together, these data indicate that the stress effect on learning in males is dependent on the neuronal activity within the BNST during training and for some time period after the stressful event has ceased but when vigilance should be sustained. In females, the BNST was unnecessary, irrespective of when the inactivation occurred. Like the mPFC studies, these data indicate that males and females are using different structures to respond to stress, with corresponding changes in associative learning, at least under these laboratory conditions.
These anatomical lesion studies bring us back to the so-called organizational theory of sex differences in the brain. Recall that sex differences for this particular stress response are determined, or perhaps organized, by the presence of gonadal hormones in utero and during early development. Specifically, females that were exposed to testosterone during early development behaved as males did during adulthood and thus learned better after the stressful event [24]. To determine whether sex differences in brain circuits were likewise organized by the presence of testosterone, we masculinized females upon birth and examined whether the BNST would now be necessary to induce the lasting increase in learning (because neuronal activity in the BNST during training is not necessary to suppress learning in an intact female). Consistent with brain organization theory, adult females that are masculinized at birth learned better after stress and their enhanced performance was dependent on neuronal activity within the BNST [49]. Therefore, the stress effects on learning in males versus females are, at least in part, organized during very early development through the presence of gonadal hormones, and these effects are mediated by neuronal activity within the BNST.
(h). Sex differences at the synapse
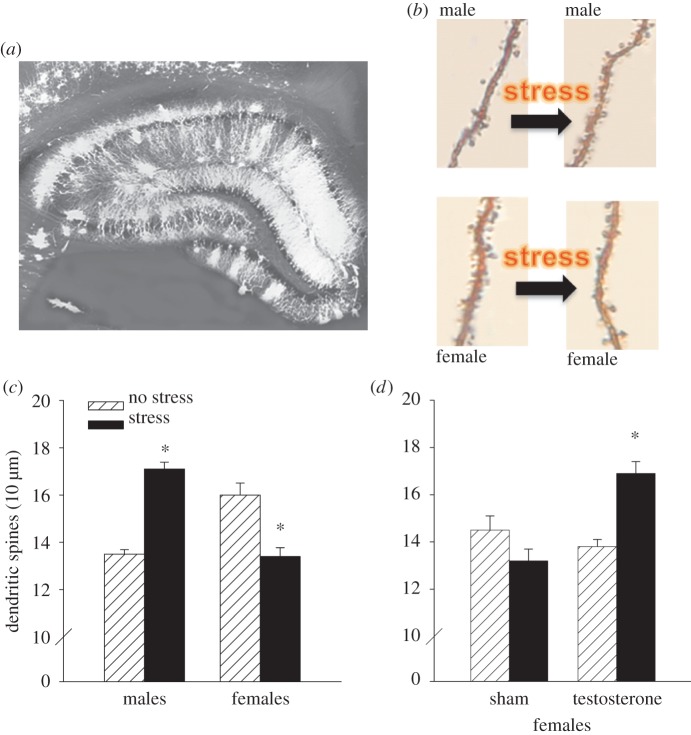
To this point, I have discussed the brain regions that are critically involved in inducing and/or maintaining the opposite effects of stress on associative learning—the amygdala and hippocampus for both sexes, prelimbic cortical regions for females, and the BNST for males. However, the responsible mechanisms within those regions remain largely unknown. We do know that there are changes at the synapse that mimic the stress effects on learning. Dendritic spines, which connect neurons to other neurons, have been implicated in mechanisms of learning for decades (figure 4a,b; [47]). At the beginning of this review, I spoke of sex differences in LTP reported in the 1990s [6], but there were earlier reports of sex hormone-induced changes at the synapse. In 1990, Gould et al. and Woolley et al. [50,51] reported that oestradiol increased spine density on pyramidal cells in area CA1 of the hippocampus. These effects were quite remarkable, with a nearly 30% change in density in response to oestradiol. They went on to report similar changes across the oestrous cycle, with the highest density in proestrus, when oestrogen concentrations are elevated. We were very intrigued by these findings, because in the absence of stress, females learn the classically conditioned eyeblink response faster in proestrus than in other stages [52]. Moreover, associative learning increases the number and density of spines on these same neurons, at least in males [53]. It seemed possible that changes in dendritic spines could play a role in the effects of stress on learning, especially because the hippocampus is necessary for these effects in both sexes [30]. To further examine this idea, we assessed spine densities in males and in females during the various stages of oestrous. As expected, females in proestrus possessed more spines than did males or females in other stages, a profile consistent with previous reports in females and with their learning abilities during stages of oestrous [17,54]. But perhaps more interestingly, exposure to an acute stressor increased spine density in the male hippocampus, whereas exposure to the same stressor decreased spine density in the female hippocampus (figure 4c), mirroring the effects of stress on associative learning (figure 1b). Moreover, the effects of stress on dendritic spines, like those on learning, were organized by the presence of testosterone during early development. Females that were masculinized at birth produced more spines after the stressor rather than fewer spines (figure 4d; [55]). Because these changes in spine density mirror the effects of stress and masculinization on learning in females, one might consider them as anatomical correlates and part of a potential mechanism(s) whereby stress influences learning (figure 1b). Of course, dendritic spines alone cannot fully explain the complex processes involved in the modulation of associative learning, but these data do suggest that their presence may contribute to producing sex differences in learning abilities.
Figure 4.
(a) A photomicrograph of the hippocampus illustrates the presence of dendrites in the hippocampus. Representative examples of dendritic spines in the hippocampus in each sex before and after stress are shown. (b) The numbers of dendritic spines increased after stress in the male hippocampus but decreased in the female hippocampus. (c) These anatomical changes mimic the behavioural changes in learning shown in figure 1. Exposure to the stressor increased the density of dendritic spines in the hippocampus of masculinized females, mirroring the effects of stress on dendritic spines in the hippocampus of intact adult males (d). Asterisks indicate significant difference between adjacent group data (p < 0.05). (Online version in colour.)
(i). Sexual aggression and trauma in females
The World Health Organization estimates that more than 30% of women experience some kind of sexual violence in their lifetimes, much of it during their adolescent years [56]. Another study reports that more than 10% of young women between the ages of 14 and 17 experience sexual assault and/or abuse [57]. The number of women on college campuses who experience sexual violence is staggering, estimated at one in five [58], which translates into upwards of 100 000 incidents a year. We know that many of these statistics are underestimated, because women are reluctant to report what happened. We also know that these experiences, whether reported or not, induce and often go on to produce symptoms of depression, anxiety and post-traumatic stress disorder (PTSD) [59,60]. From animal laboratory studies, some of them discussed here, we know that stressful life experience has a multitude of detrimental effects on neuronal and behavioural outcomes in female rodent models. That said, most of the animal models rely on stressors that are not typically encountered by humans living in modern society (i.e. restraint stress, aversive shocks or swim stress), much less do they represent stressors commonly experienced by young women during their adolescent years. Therefore, we developed an animal model to examine the effects of sexually motivated aggression on the female brain and learned behaviours during puberty. The model is known as SCAR, which stands for sexual conspecific aggressive response [61]. During one session, a pubescent female rodent is paired with a sexually experienced adult male for 30 min. During the encounter, the adult male chases, pins down, smells and tries to mount the female, while she tries to and often escapes. Meanwhile, her adrenal glands release high levels of stress hormones and her ability to learn is suppressed (figure 2f). Perhaps most importantly, most of the females that were exposed to the adult male each day throughout puberty did not learn to express maternal sensitization, a measure of maternal caring behaviour towards offspring. These behaviours are not only necessary for the survival of the offspring, but seemingly important for the survival of newly generated cells in the hippocampus; females that were less likely to express maternal behaviours retained fewer newly generated cells in their hippocampus. Many of these cells die under normal circumstances but if they do survive, the vast majority mature into granule neurons in the dentate gyrus [10,11,62]. Therefore, the act of ‘learning’ to become maternal was apparently sufficient to rescue the newly generated neurons from death and this process was disrupted in females that had stressful and aggressive experiences during puberty and young adulthood. We believe that these findings are especially significant and novel because they point to neuronal mechanisms through which sexual aggression and trauma can impact the female brain to potentially interfere with learned behaviours.
Although provocative, we are not claiming necessarily that exposure to a sexually experienced and aggressive adult male replicates or even resembles what happens to a young woman who experiences sexual aggression and trauma during puberty and young adulthood. Obviously, rodent models of sexual and social behaviour can never fully model the human condition and cannot possibly mimic feelings of horror, shame and guilt, not to mention more positive feelings associated with sex and desire. Instead, these findings and other related ones underscore the need to develop and adopt laboratory models of stress that bear some semblance to conditions that humans experience. For the sake of scientific knowledge, it is of course worthwhile to understand sex differences in the brain and how each sex responds to stressful life experience. But, if our goal is to understand why women are so vulnerable to stress-related mental illnesses such as depression and PTSD, then we do need to adopt realistic models, as flawed or limited in application as they may be. Translational models of sex differences in behavioural and mental processes will facilitate this process [63–66].
2. You have come a long way baby!
In the 1970s, there was a popular advertising campaign designed to entice more women to smoke. It often portrayed a modern woman in a provocative pose smoking a long slim cigarette with the signature saying, ‘You've come a long way, baby’. Given what we now know about cigarettes and their contribution to lung cancer and overall health, this proclamation seems especially sad and unfounded. Along similar lines, I have several points that I think are important to consider as the scientific community embraces both sexes in laboratory models of mental health and wellness.
First, we should try to inhibit the impulse to publish sex differences that are barely detectible, not reproducible or minimally meaningful. As females are integrated into laboratory studies, sex differences in outcome measures will emerge. Frankly, I am surprised that others are surprised by sex differences in the brain. After all, men and women look different and act differently. Therefore, their brains are most assuredly different, at least in some respects. However, those differences will not always be interesting or important to pursue and publish, but that decision is of course an individual one. It is very important to recognize a sex difference for what it is and what it is not. For example, female rodents are typically more physically active than male rodents. Therefore, investigators who rely on measures of activity (such as those measuring conditioning, learned helplessness, fear, etc.) must consider the possibility that observed sex differences in ‘learning’ or ‘depression’ are not necessarily reflecting these complex psychological constructs but are rather attributable to differences in performance. I can give you a good example. Years ago, when I first began working on sex differences, I read a paper that reported that female rodents did not express helplessness behaviour [67], a commonly used animal model of human depression. This report was rather surprising given that women are so much more vulnerable to depression [68]. We re-examined this reported finding and indeed observed that rodent females do not express helplessness behaviour, at least not to the extent the males do [69]. However, this is a performance effect. During phase one of helplessness training, one group of rodents learns to escape an aversive stimulus, whereas another learns that they cannot escape. Upon further training with a more complex task in phase two, those animals that learned that they could not escape do not learn to escape, whereas those that learned previously to escape readily learn. Female rodents, because they are more active than males, are more likely to learn the operant contingencies posed during helplessness training in phase one, which depends on movement to learn. Males are more likely to freeze and therefore, they are less likely to produce an operant response that is necessary to learn the new contingency.
In the end, what may appear as a sex difference in either learning or even ‘depression’ is simply (and I do not mean trivial) difference in inherent characteristics between male and female rodents. A more obvious example relates to gross body weight. Male rodents in adulthood almost always weigh more than females and their brains are substantially bigger. Therefore, one cannot directly compare gross numbers in many outcomes throughout the brain without taking weight and size into account [55]. For example, the absolute number of new neurons in the adult hippocampus of the female are significantly fewer than in males and therefore, measurement differences must be assessed according to density of cells rather than absolute numbers, and even this might not always be a suitable alternative. Furthermore, size and weight differences will often produce differences in performance. As discussed, pairing an adult male rodent with a female rodent produces aggressive behaviours towards the female [61]. The male is able to subjugate the female, at least in part, because he is larger in size. However, the female is also better able to escape, because she is smaller and more agile. Without the sex differences in size, the sex differences in behaviour might not even occur. These are just a few examples but I think that they underscore some of the issues that we must attend to as we test female species in laboratory studies.
The second point that I hope is self-evident is that most adult females in our world, irrespective of species, are not virgins, whereas nearly all females used in laboratory studies are virgins. As discussed, virgin females can respond differently from non-virgin females and certainly differently from females that have become mothers [70,71]. For example, learning was impaired by stress in virgin females, but not in females that had offspring and/or learned to care for offspring at some time in her life—even well after weaning [27,72]. In women, the amount of grey matter in the prefrontal cortex and the amygdala increases during the first month of having a baby and some of those changes relate to positive thoughts about her baby [73]. But motherhood is not the only substantial change that females experience. Female rodents experience stages of life similar to perimenopause and menopause in women, during which their responses to learning and stress can change rather dramatically [26,74]. Obviously, women in perimenopause experience substantial changes as they transition into a stage of life associated with reproductive senescence [75]. These examples notwithstanding, I am not suggesting that researchers investigate all stages of female life in laboratory studies or that we only study non-virgins, but rather that we simply appreciate the dynamic nature of the female brain. Ultimately, we must accept the fact that the female brain (as the male brain) is always changing and it is these processes of change that we may want to understand.
Acknowledgement
I thank Han Yan Michelle Chang for her contributions to manuscript preparation. I am grateful for support from the National Science Foundation and National Institutes of Health for select research reviewed within the manuscript.
Data accessibility
Data presented in this review have been published and are referenced.
Competing interests
I have no competing interests.
Funding
Supported by National Alliance for Research and Schizophrenia (NARSAD) Distinguished Investigator Award from the Brain and Behavior Research Foundation to T.J.S.
References
- 1.McCarthy MM, Arnold AP. 2011. Reframing sexual differentiation of the brain. Nat. Neurosci. 14, 677–683. ( 10.1038/nn.2834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss TV, Lomo T. 1973. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356. ( 10.1113/jphysiol.1973.sp010273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebb D. 1949. The organization of behavior. New York, NY: Wiley & Sons. [Google Scholar]
- 4.Lynch G, Baudry M. 1984. The biochemistry of memory: a new and specific hypothesis. Science 224, 1057–1063. ( 10.1126/science.6144182) [DOI] [PubMed] [Google Scholar]
- 5.Shors TJ, Matzel LD. 1997. Long-term potentiation: what's learning got to do with it? Behav. Brain Sci. 20; 597–614. Discussion 614–55. [DOI] [PubMed] [Google Scholar]
- 6.Maren S, De Oca B, Fanselow MS. 1994. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 661, 25–34. ( 10.1016/0006-8993(94)91176-2) [DOI] [PubMed] [Google Scholar]
- 7.Foy MR, Teyler TJ. 1983. 17-α-estradiol and 17-β-estradiol in hippocampus. Brain Res. Bull. 10, 735–739. ( 10.1016/0361-9230(83)90206-X) [DOI] [PubMed] [Google Scholar]
- 8.Shors TJ, Weiss C, Thompson RF. 1992. Stress-induced facilitation of classical conditioning. Science 257, 537–539. ( 10.1126/science.1636089) [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS, Sapolsky RM. 1995. Stress and cognitive function. Curr. Opin. Neurobiol. 5, 205–216. ( 10.1016/0959-4388(95)80028-X) [DOI] [PubMed] [Google Scholar]
- 10.Leuner B, Mendolia-Loffredo S, Shors TJ. 2004. Males and females respond differently to controllability and antidepressant treatment. Biol. Psychiatry 56, 964–970. ( 10.1016/j.biopsych.2004.09.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leuner B, Mendolia-Loffredo S, Kozoroviskiy Y, Samburg D, Gould E, Shors TJ. 2004. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J. Neurosci. 24, 7477–7481. ( 10.1523/JNEUROSCI.0204-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shors TJ. 2001. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol. Learn. Mem. 75, 10–29. ( 10.1006/nlme.1999.3956) [DOI] [PubMed] [Google Scholar]
- 13.Wood GE, Beylin AV, Shors TJ. 2001. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav. Neurosci. 115, 175–187. ( 10.1037/0735-7044.115.1.175) [DOI] [PubMed] [Google Scholar]
- 14.Wood GE, Shors TJ. 1998. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc. Natl Acad. Sci. USA 95, 4066–4071. ( 10.1073/pnas.95.7.4066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archer J. 1975. Rodent sex differences in emotional and related behavior. Behav Biol. 14, 451–479. ( 10.1016/S0091-6773(75)90636-7) [DOI] [PubMed] [Google Scholar]
- 16.Kimura D. 1999. Sex differences in cognition. Cambridge, MA: MIT Press. [Google Scholar]
- 17.Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. 1998. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport 9, 419–423. ( 10.1097/00001756-199802160-00012) [DOI] [PubMed] [Google Scholar]
- 18.Phoenix HC, Goy RW, Gerall AA, Young WC. 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382. ( 10.1210/endo-65-3-369) [DOI] [PubMed] [Google Scholar]
- 19.Jost A, Edwards RG. 1970. Hormonal factors in the sex differentiation of the mammalian foetus. Phil. Trans. R. Soc. Lond. B 259, 119–130. ( 10.1098/rstb.1970.0052) [DOI] [PubMed] [Google Scholar]
- 20.Gorski RA. 1979. The neuroendocrinology of reproduction: an overview. Biol. Reprod. 20, 111–127. [DOI] [PubMed] [Google Scholar]
- 21.Jordan-Young RM. 2010. Brain storm: the flaws in the science of sex differences. Cambridge, MA: Harvard University Press. [Google Scholar]
- 22.Halpern D. 2000. Sex differences in cognitive abilities. Mahwah, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- 23.Williams CL, Barnett AM, Meck WH. 1990. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav. Neurosci. 104, 84–97. ( 10.1037/0735-7044.104.1.84) [DOI] [PubMed] [Google Scholar]
- 24.Shors TJ, Miesegaes G. 2002. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc. Natl Acad. Sci. USA 99, 13 955–13 960. ( 10.1073/pnas.202199999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodes GE, Shors TJ. 2005. Distinctive stress effects on learning during puberty. Horm. Behav. 48, 163–171. ( 10.1016/j.yhbeh.2005.02.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodes GE, Shors TJ. 2007. Learning during middle age: a resistance to stress? Neurobiol. Aging 28, 1783–1788. ( 10.1016/j.neurobiolaging.2006.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leuner B, Shors TJ. 2006. Learning during motherhood: a resistance to stress. Horm. Behav. 50, 38–51. ( 10.1016/j.yhbeh.2006.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalla C, Shors TJ. 2009. Sex differences in learning processes of classical and operant conditioning. Physiol. Behav. 97, 229–238. ( 10.1016/j.physbeh.2009.02.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beylin A, Gandhi C, Talk A, Zhao M, Matzel LD, Shors TJ. 2001. The role of the hippocampus in trace conditioning: temporal incongruity or task difficulty? Neurobiol. Learn. Mem. 76, 447–461. ( 10.1006/nlme.2001.4039) [DOI] [PubMed] [Google Scholar]
- 30.Bangasser DA, Shors TJ. 2007. The hippocampus is necessary for enhancements and impairments of learning following stress. Nat. Neurosci. 10, 1401–1403. ( 10.1038/nn1973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waddell J, Bangasser DA, Shors TJ. 2008. The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. J. Neurosci. 28, 5290–5294. ( 10.1523/JNEUROSCI.1129-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overmier JB, Seligman ME. 1967. Effects of inescapable shock upon subsequent escape and avoidance responding. J. Comp. Physiol. Psychol. 63, 28–33. ( 10.1037/h0024166) [DOI] [PubMed] [Google Scholar]
- 33.Seligman ME, Maier SF. 1967. Failure to escape traumatic shock. J. Exp. Psychol. 74, 1–9. ( 10.1037/h0024514) [DOI] [PubMed] [Google Scholar]
- 34.Seligman ME. 1972. Learned helplessness. Annu. Rev. Med. 23, 407–412. ( 10.1146/annurev.me.23.020172.002203) [DOI] [PubMed] [Google Scholar]
- 35.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. 2005. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 8, 365–371. ( 10.1038/nn1399) [DOI] [PubMed] [Google Scholar]
- 36.Maier SF, Watkins LR. 2010. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 1355, 52–60. ( 10.1016/j.brainres.2010.08.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilmartin MR, Balderston NL, Helmstetter FJ. 2014. Prefrontal cortical regulation of fear learning. Trends Neurosci. 37, 455–464. ( 10.1016/j.tins.2014.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. 2012. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76, 804–812. ( 10.1016/j.neuron.2012.09.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maeng L, Waddell J, Shors TJ. 2010. The prefrontal cortex communicates with the amygdala to impair learning after acute stress in females but not in males. J. Neurosci. 30, 16 188–16 196. ( 10.1523/JNEUROSCI.2265-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milad MR, Quirk GJ. 2012. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 63, 129–151. ( 10.1146/annurev.psych.121208.131631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeng LY, Shors TJ. 2013. The stressed female brain: neuronal activity in the prelimbic but not infralimbic region of the medial prefrontal cortex suppresses learning after acute stress. Front. Neural Circuits 7, 198 ( 10.3389/fncir.2013.00198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gold PW. 2015. The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry. 20, 32–47. ( 10.1038/mp.2014.163) [DOI] [PubMed] [Google Scholar]
- 43.Holland PC. 2007. Disconnection of the amygdala central nucleus and the substantia innominata/nucleus basalis magnocellularis disrupts performance in a sustained attention task. Behav. Neurosci. 121, 80–89. ( 10.1037/0735-7044.121.1.80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Vries GJ. 2008. Sex differences in vasopressin and oxytocin innervation of the brain. Prog. Brain Res. 170, 17–27. ( 10.1016/S0079-6123(08)00402-0) [DOI] [PubMed] [Google Scholar]
- 45.Dong HW, Petrovich GD, Swanson LW. 2001. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Rev. 38, 192–246. ( 10.1016/S0165-0173(01)00079-0) [DOI] [PubMed] [Google Scholar]
- 46.Davis M, Walker DL, Miles L, Grillon C. 2010. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35, 105–135. ( 10.1038/npp.2009.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leuner B, Shors TJ. 2013. Stress, anxiety, and dendritic spines: what are the connections?. Neuroscience 251, 108–119. ( 10.1016/j.neuroscience.2012.04.021) [DOI] [PubMed] [Google Scholar]
- 48.Bangasser DA, Santollo J, Shors TJ. 2005. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav. Neurosci. 119, 1459–1466. ( 10.1037/0735-7044.119.6.1459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bangasser DA, Shors TJ. 2008. The bed nucleus of the stria terminalis modulates learning after stress in masculinized but not cycling females. J. Neurosci. 28, 6383–6387. ( 10.1523/JNEUROSCI.0831-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gould E, Woolley CS, Frankfurt M, McEwen BS. 1990. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 10, 1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woolley CS, Gould E, Frankfurt M, McEwen BS. 1990. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 10, 4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shors TJ, Lewczyk C, Mathew P, Paczynski M, Pickett J. 1998. Stages of estrus mediate the stress-induced impairment of classical conditioning in females. Neuroreport 9, 419–423. ( 10.1097/00001756-199802160-00012) [DOI] [PubMed] [Google Scholar]
- 53.Leuner B, Falduto J, Shors TJ. 2003. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 23, 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shors TJ, Chua C, Falduto J. 2001. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 21, 6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalla C, Whetstone AS, Hodes GE, Shors TJ. 2009. Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neurosci. Lett. 449, 52–56. ( 10.1016/j.neulet.2008.10.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization. 2013. Global and regional estimates of violence against women: prevalence and health effects of intimate partner violence and non-partner sexual violence. See http://www.who.int/mediacentre/news/releases/2013/violence_against_women_20130620/en/. [Google Scholar]
- 57.Finkelhor D, Turner HA, Shattuck A, Hamby SL. 2013. Violence, crime, and abuse exposure in a national sample of children and youth: an update. JAMA Pediatr. 167, 614–621. ( 10.1001/jamapediatrics.2013.42) [DOI] [PubMed] [Google Scholar]
- 58.Cantor D, Fisher W.2015. Report on the AAU campus climate survey on sexual assault and sexual misconduct assault and sexual misconduct. See http://sexualassaulttaskforce.harvard.edu/files/taskforce/files/final_report_harvard_9.21.15.pdf .
- 59.Briere J, Jordan CE. 2004. Violence against women. Outcome complexity and implications for assessment and treatment. J. Interpers. Violence 19, 1252–1276. ( 10.1177/0886260504269682) [DOI] [PubMed] [Google Scholar]
- 60.Jordan CE, Campbell R, Follingstad D. 2010. Violence and women's mental health: the impact of physical, sexual, and psychological aggression. Annu. Rev. Clin. Psychol. 6, 607–628. ( 10.1146/annurev-clinpsy-090209-151437) [DOI] [PubMed] [Google Scholar]
- 61.Shors TJ, Tobon K, DiFeo G, Durham DM, Chang HYM. 2016 Sexual conspecific aggressive response (SCAR): a model of sexual trauma that disrupts maternal learning and plasticity in the female brain. Sci. Rep. 6, 18960 ( 10.1038/srep18960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shors TJ. 2014. The adult brain makes new neurons and effortful learning keeps them alive. Curr. Dir. Psychol. Sci. 23, 311–318. ( 10.1177/0963721414540167) [DOI] [Google Scholar]
- 63.Haller J, Harold G, Sandi C, Neumann ID. 2014. Effects of adverse early-life events on aggression and anti-social behaviours in animals and humans. J. Neuroendocrinol. 26, 724–738. ( 10.1111/jne.12182) [DOI] [PubMed] [Google Scholar]
- 64.Perry R, Sullivan RM. 2014. Neurobiology of attachment to an abusive caregiver: short-term benefits and long-term costs. Dev. Psychobiol. 56, 1626–1634. ( 10.1002/dev.21219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shors TJ, Olson RL, Bates ME, Selby EA, Alderman BL. 2014. Mental and physical (MAP) training: a neurogenesis-inspired intervention that enhances brain health in humans. Neurobiol. Learn. Mem. 115, 3–9. ( 10.1016/j.nlm.2014.08.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weathington JM, Arnold AR, Cooke BM. 2012. Juvenile social subjugation induces a sex-specific pattern of anxiety and depression-like behaviors in adult rats. Horm. Behav. 61, 91–99. ( 10.1016/j.yhbeh.2011.10.008) [DOI] [PubMed] [Google Scholar]
- 67.Kirk R, Blampied N. 1985. Activity during inescapable shock and subsequent escape avoidance learning: female and male rats compared. N.Z. J. Psychol. 14, 9–14. [Google Scholar]
- 68.Kessler RC. 2003. Epidemiology of women and depression. J. Affect. Disord. 74, 5–13. ( 10.1016/S0165-0327(02)00426-3) [DOI] [PubMed] [Google Scholar]
- 69.Dalla C, Edgecomb C, Whetstone AS, Shors TJ. 2008. Females do not express learned helplessness like males do. Neuropsychopharmacology 33, 1559–1569. ( 10.1038/sj.npp.1301533) [DOI] [PubMed] [Google Scholar]
- 70.Dulac C, O-Connell LA, Wu Z. 2014. Neural control of maternal and paternal behaviors. Science 345, 765–770. ( 10.1126/science.1253291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lonstein JS, Lévy F, Fleming AS. 2015. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Horm. Behav. 73, 156–185. ( 10.1016/j.yhbeh.2015.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maeng L, Shors TJ. 2012. Once a mother, always a mother: motherhood persistently protects females from negative consequences of stress on learning. Behav. Neurosci. 126, 137–141. ( 10.1037/a0026707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. 2010. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav. Neurosci. 124, 695–700. ( 10.1037/a0020884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yin F, et al. 2015. The perimenopausal aging transition in the female rat brain: decline in bioenergetic systems and synaptic plasticity. Neurobiol. Aging 36, 2282–2295. ( 10.1016/j.neurobiolaging.2015.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. 2015. Perimenopause as a neurological transition state. Nat. Rev. Endocrinol. 11, 393–405. ( 10.1038/nrendo.2015.82) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented in this review have been published and are referenced.