Abstract
Individual variability in human gender-related behaviour is influenced by many factors, including androgen exposure prenatally, as well as self-socialization and socialization by others postnatally. Many studies have looked at these types of influences in isolation, but little is known about how they work together. Here, we report that girls exposed to high concentrations of androgens prenatally, because they have the genetic condition congenital adrenal hyperplasia, show changes in processes related to self-socialization of gender-related behaviour. Specifically, they are less responsive than other girls to information that particular objects are for girls and they show reduced imitation of female models choosing particular objects. These findings suggest that prenatal androgen exposure may influence subsequent gender-related behaviours, including object (toy) choices, in part by changing processes involved in the self-socialization of gendered behaviour, rather than only by inducing permanent changes in the brain during early development. In addition, the findings suggest that some of the behavioural effects of prenatal androgen exposure might be subject to alteration by postnatal socialization processes. The findings also suggest a previously unknown influence of early androgen exposure on later processes involved in self-socialization of gender-related behaviour, and thus expand understanding of the developmental systems regulating human gender development.
Keywords: androgen, behaviour, gender, congenital adrenal hyperplasia, self-socialization, brain
1. Introduction
Although the behaviour of human males and females is largely similar [1], there are some characteristics that differ on average for men and women or boys and girls [2]. One area of robust gender difference involves children's play behaviour. For example, boys tend to prefer playing with toy vehicles and weapons, whereas girls tend to prefer playing with dolls and tea sets. These gender differences begin to emerge before the age of 2 years [3–7] and grow larger as childhood progresses [8]. In children aged 3–11 years, the differences are large (d > 0.8 [9,10]).
A number of factors, including some typically considered to be in the realm of nature and some typically considered to be in the realm of nurture, have been shown to influence the development of children's gender-related play behaviour, including toy choices. For instance, prenatal androgen exposure, both in normal and atypical ranges, has been related to male-typical toy, playmate and activity preferences [11,12]. These human findings are consistent with evidence from thousands of studies of non-human mammals, where androgen exposure during critical periods of prenatal or neonatal development has been shown to influence later sex-typed behaviour, including juvenile play behaviour and adult reproductive behaviours [13,14]. Androgens during early development also have been found to alter brain structure in non-human mammals [13,15], and these changes in brain structure are thought to underlie the androgen-related behavioural changes. In other words, it is thought that androgen induces brain changes during critical periods of prenatal or neonatal development, and that these early brain changes explain behavioural changes that are seen later in life.
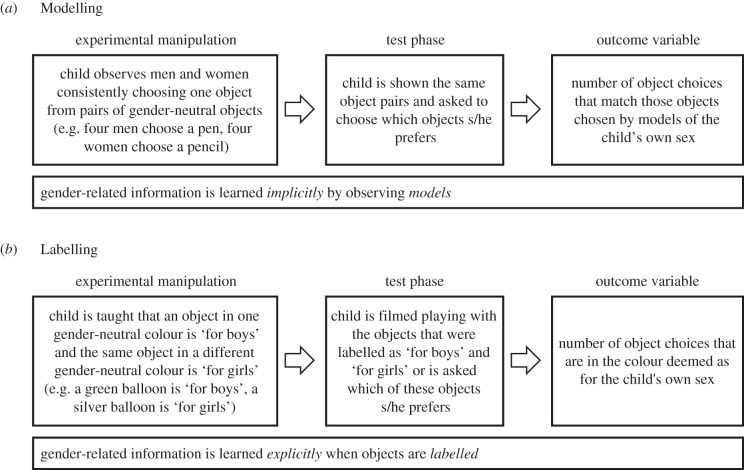
In humans, however, social and cognitive factors also influence gender-related behaviour. Gender-typical behaviour is encouraged by parents, peers and teachers [14,16,17] and, as cognitive development progresses, children socialize themselves with respect to gender-related behaviours [18]. In regard to gender-typed toys, parents, teachers and peers all encourage gender-consistent toy choices more than they encourage gender-atypical choices. Similarly, once children understand that they are girls or boys, they tend to model, or imitate, the object choices and other behaviours of individuals of the same sex more than those of individuals of the other sex [19–21]. They also respond to gender labels, preferring objects, including toys and activities that have been labelled as appropriate for their own sex over those that have been labelled as appropriate for the other sex [22,23]. The effects of gender labels on children's learning appear to be larger than the effects of observing same-sex models [22]. Both models and labels provide information as to which behaviours or object choices are gender-appropriate. Labels, however, may be more effective than models, because they provide explicit, unambiguous cues regarding gender-appropriateness through their clear indication that something is ‘for girls’ or ‘for boys’ (figure 1).
Figure 1.
Experimental manipulations for assessing responses to models of the same gender (Modelling, (a)) and to labels indicating gender-appropriate behaviours (Labelling, (b)).
Theories of human gender development describe developmental systems involving multiple causative factors working together over time [2,24]. However, despite the evidence that hormonal, social and cognitive mechanisms all influence human gender development, there is almost no information on how they work together. It is difficult to obtain this information because the mechanisms typically covary. For instance, most boys are exposed to high concentrations of androgens prenatally, and experience male-typical external socialization and self-socialization of gender-related behaviour. The situation is similarly consistent for most girls.
One exception is girls with the genetic condition classic congenital adrenal hyperplasia (CAH). CAH is characterized by an enzymatic deficiency, typically in 21 hydroxylase, and this deficiency results in a reduced ability to produce cortisol. As a consequence, hormones in the cortisol pathway are shunted into the androgen pathway, and adrenal androgens are increased beginning prenatally [25–27]. This prenatal androgen elevation leads to genital ambiguity at birth in affected girls, followed by rapid diagnosis and treatment to normalize hormones postnatally. Girls with CAH typically are assigned and reared as girls, and their gender identity is almost always female [28]. However, numerous studies have found that girls with CAH show altered toy, playmate and activity preferences [2]. For instance, compared to other girls, they are less likely to play with toys typically chosen by girls and more likely to play with toys typically chosen by boys [9,10,29]. In addition, although most women with CAH have a female gender identity, they are more likely than other women to change to live as men, with about 1–2 in 100 doing so, compared to about 1 in tens of thousands in the general population [2,28,30,31]. In addition, girls and women with CAH report reduced satisfaction with the female gender role compared with other females [32,33], and girls with CAH have been found to have significantly reduced female-typical gender identity [34]. Given this reduced satisfaction with the female gender role and reduced female-typical gender identity, girls with CAH might be expected to show reduced self-socialization of gender-typical behaviour.
In contrast to girls with CAH, boys with CAH do not show increased masculinization of toy, playmate or activity preferences [9,10,35]. Boys and men with CAH also do not show alterations in gender identity [33,34]. The absence of increased male-typical behaviour in boys with CAH may occur because androgen concentrations in boys with CAH are largely similar to those of boys without CAH prenatally [25,26].
In summary, humans, like other mammals, show increased male-typical behaviour following early androgen exposure. In addition, as in the animal literature, the masculinizing effects of prenatal androgen exposure on human behaviour are thought to result from androgen-induced alteration of the fetal brain [36,37]. However, human gender-related behaviour is also influenced by cognitive mechanisms unique to humans—processes of self-socialization related to cognitive understanding of gender. In addition, prenatal androgen exposure appears to influence human gender identity, as evidenced by findings from females with CAH [28,30,33,34]. Here, we investigate the possibility that prenatal androgen exposure influences mechanisms involved in the self-socialization of gender-related behaviour. To do so, we evaluate the responses of children with and without CAH to labels indicating that certain gender-neutral objects are ‘for girls’ or ‘for boys’, as well as to same-sex models expressing consistent preferences for gender-neutral objects. These procedures will test the hypothesis that girls with CAH model the object choices of others of the same sex less than do other children, and that they show reduced positive responses to explicit information labelling objects as for children of their own sex. Boys with and without CAH will also be compared for control purposes. The two groups of boys are not expected to differ, because, as detailed above, prior research generally has not found men or boys with and without CAH to differ in gender-related behaviour.
2. Material and methods
(a). Participants
Children aged 4–11 years (43 girls and 38 boys with CAH; 41 unaffected female and 31 unaffected male relatives) participated in the study. Unaffected relatives were chosen as controls, because they resemble the children with CAH in background characteristics, including familial socio-economic status. All children with CAH had the classic form of the disorder, which is known to cause elevated adrenal androgen production beginning prenatally. Most children with CAH (37 girls and 33 boys) had the salt-wasting form of classic CAH. Only nine (six girls and three boys) had the less severe, simple-virilizing form. All the girls with CAH had been assigned and reared as girls, and treated with hormones postnatally to normalize their cortisol and androgen concentrations. Similarly, all the boys with CAH had been assigned and reared as boys, and they were treated with the same hormones as girls were to normalize postnatal hormone concentrations.
Participants with CAH were recruited through pediatric endocrinologists or through a CAH support group in the UK. Ninety-four per cent of the sample was of white, European descent. Maternal education varied from no secondary education (6%), to some secondary education (54%), to undergraduate (27%) and postgraduate (13%) degrees. Classic CAH is rare, occurring in approximately 1 in 16 000 births [38]. We tested all the children with this rare condition and their unaffected relatives whom we were able to recruit to the study during our period of funding. Data analyses began after all of the data had been collected.
(b). Procedure and materials
Each child completed a 2.5 h battery of assessments. Here we report on the two measures of processes related to self-socialization of gender-related behaviour that were included in the battery. Data related to other types of outcomes are being reported separately.
(i). Self-socialization via gender labels
Responses to gender labels were assessed using a protocol modelled on Masters et al.'s work [22] (figure 1b). Children were shown pictures of four toys: a green balloon, a silver balloon, an orange xylophone and a yellow xylophone, and told that balloons and xylophones of one colour were ‘for girls’, whereas balloons and xylophones of the other colour were ‘for boys’, with random assignment to one of two conditions, counterbalanced for colour. Children were shown pictures of the toys until they correctly labelled each of the toys of each colour as ‘for girls’ or ‘for boys’ on three consecutive trials, to be certain that they had learned the designated gender labels.
Subsequently, children were videotaped for 5 min in a playroom containing the four toys and no other toys. The middle 3 min of the videotapes were scored for the proportion of time spent contacting each of the toys, providing a behavioural preference score. This scoring was carried out by researchers who did not know which children had CAH and which children did not. After the behavioural preference test, children were asked to indicate verbally which of the two balloons and which of the two xylophones they liked better, providing a verbal preference score. Finally, children were asked to indicate which object in each pair was ‘for girls’ and which was ‘for boys’, providing a measure of their memory for the gender labels. This memory measure was obtained for control purposes, to determine whether any other group differences might relate to memory, rather than to the gender-related processes of self-socialization in which we were interested.
(ii). Self-socialization via modelling
Responses to models of the same and of the other sex were assessed using a protocol based on that of Perry & Bussey [19] (figure 1a). Children viewed a video recording showing four adult male models and four adult female models choosing one object from a pair of gender-neutral objects (e.g. a toy cow or a toy horse; a pen or a pencil). For each of 16 such pairs, all four models of each sex chose one object, and all four models of the other sex chose the other object. Professional actors, dressed using gender cues (e.g. neck ties, hair bows) portrayed the models. Children were randomly assigned to view one of two counterbalanced videos.
Subsequently, each child was asked which object from each of the 16 pairs s/he preferred, providing a verbal preference score. After indicating preferences for all object pairs, children were asked to indicate whether they had seen men or women choosing each object, providing a control measure of their memory for the objects modelled by each sex. Learning 16 pairs of objects was difficult for some children, and these children completed half the trials (an 8-trial protocol, N = 34, nine girls with CAH, nine control girls, nine boys with CAH and seven control boys). Analysis using χ2 indicated that children who completed the short, 8-trial protocol were evenly distributed among the four target groups (boys and girls with and without CAH), 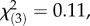 n.s. As would be expected, children who completed the 8-trial protocol were younger on average than those who completed the 16-trial protocol, t142 = −2.05, p < 0.05. Scoring and other procedures for the short protocol were the same as for the longer protocol. There were no protocol-related differences in preference scores or memory scores, t148 = 0.90, p = 0.343 or t148 = −0.94, p = 0.350, respectively. Data from the two protocols, in the form of the proportion scores, were combined for analyses.
n.s. As would be expected, children who completed the 8-trial protocol were younger on average than those who completed the 16-trial protocol, t142 = −2.05, p < 0.05. Scoring and other procedures for the short protocol were the same as for the longer protocol. There were no protocol-related differences in preference scores or memory scores, t148 = 0.90, p = 0.343 or t148 = −0.94, p = 0.350, respectively. Data from the two protocols, in the form of the proportion scores, were combined for analyses.
3. Results
There were no group differences in age (table 1), and age showed a statistically significant correlation with only one outcome variable—memory in the modelling protocol r148 = 0.23, p = 0.005. Data were analysed using two-way (Sex × CAH status) ANOVAs, with the exception of memory in the modelling protocol, where ANCOVA, with age as the covariate, was used, because of the correlation with age. We also conducted planned comparisons of girls with and without CAH, because these were the two groups hypothesized to differ. Boys with and without CAH were also compared for control purposes. The comparison of boys with and without CAH provided some control for the effects of the CAH condition other than elevated androgens, since other CAH-related effects, such as reduced cortisol prenatally and glucocorticoid treatment postnatally, would be expected to influence both boys and girls with CAH. Finally, we used within-sample t-tests to see if children in each of the four groups chose objects that had been labelled as for individuals of their sex, or that had been modelled by individuals of their sex, at levels above chance. We hypothesized that all children, except for girls with CAH, would do so.
Table 1.
Age in girls and boys with and without CAH.
| girls |
boys |
|||
|---|---|---|---|---|
| CAH | control | CAH | control | |
| N = 43 | N = 41 | N = 38 | N = 31 | |
| age (years)a | ||||
| M | 7.13 | 7.59 | 7.15 | 7.81 |
| s.d. | 2.28 | 2.51 | 2.04 | 2.36 |
| range | 4.01–11.90 | 4.00–11.86 | 4.06–11.34 | 4.17–11.93 |
aThere were no significant group differences in age.
(a). Group-wise comparisons for self-socialization based on gender labels
Table 2 shows means, confidence intervals and effect sizes for verbal preferences and observed play preferences in the labelling protocol for each of the four groups of children. The analysis of verbal preferences for objects labelled as gender-appropriate showed significant main effects of sex: F1,142 = 4.59, p = 0.034, and CAH status: F1,142 = 5.52, p = 0.020, and a significant interaction: F1,142 = 6.21, p = 0.014. In addition, girls with CAH showed reduced verbal preferences for objects labelled as for girls compared with control girls, t79 = −3.44, p = 0.001, d = 0.76, whereas boys with and without CAH did not differ. The analysis of observed behavioural preferences for objects labelled as gender-appropriate showed no significant main effects of sex, or CAH status, but there was a significant interaction: F1,137 = 5.48, p = 0.021. In addition, girls with CAH showed reduced behavioural preferences for objects labelled as for girls compared with control girls: t79 = −3.44, p = 0.028, d = 0.54; whereas boys with and without CAH did not differ. The analysis of memory scores showed no significant main effects and no significant interaction. Also, no groups, including girls with and without CAH, differed significantly in memory scores.
Table 2.
Verbal preference for observed play in the playroom with and memory for objects (toys) labelled as gender-appropriate in girls and boys with and without CAH. Observed play is the proportion of time spent playing with objects labelled as gender-appropriate. Probabilities are two-tailed. DVs: (i) verbal preference for objects labelled as gender-appropriate (score ranges from 0 to 2), (ii) observed play: time spent playing in the playroom with objects labelled as gender-appropriate (expressed as a proportion of total time observed playing) and (iii) memory as to which objects were labelled as gender-appropriate (score ranges from 0 to 4).
| girls |
boys |
CAH and control girls difference |
95% CI for difference |
CAH and control boys difference |
95% CI for difference |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| labelling protocol | CAH | control | CAH | control | p | d | mean diff. | upper | lower | p | d | mean diff. | upper | lower |
| verbal preference | ||||||||||||||
| M | 0.83 | 1.38 | 1.36 | 1.34 | 0.001 | 0.76 | −0.55 | −0.87 | −0.23 | 0.918 | −0.03 | 0.02 | 0.33 | −0.30 |
| (s.d.) | (0.73) | (0.71) | (0.59) | (0.67) | ||||||||||
| n | 42 | 39 | 36 | 29 | ||||||||||
| observed play | ||||||||||||||
| M | 0.51 | 0.68 | 0.69 | 0.61 | 0.028 | 0.54 | −0.16 | −0.31 | −0.002 | 0.280 | −0.28 | 0.08 | 0.23 | −0.07 |
| (s.d.) | (0.31) | (0.32) | (0.30) | (0.28) | ||||||||||
| n | 39 | 37 | 36 | 29 | ||||||||||
| memory | ||||||||||||||
| M | 3.31 | 3.58 | 3.37 | 3.21 | 0.245 | 0.26 | −0.27 | 0.23 | −0.73 | 0.586 | −0.13 | 0.17 | 0.77 | −0.44 |
| (s.d.) | (1.18) | (0.83) | (1.17) | (1.24) | ||||||||||
| n | 42 | 38 | 35 | 29 | ||||||||||
(b). Group-wise comparisons for self-socialization through imitation of same-sex models
Table 3 shows means, confidence intervals and effect sizes for verbal preferences in the modelling protocol for each of the four groups of children. The analysis of verbal preferences revealed no significant main effects and no significant interaction. However, girls with CAH showed reduced preference for objects that had been modelled by individuals of the same sex compared with control girls at p < 0.10, t80 = −1.78, p = 0.079, d = 0.41, whereas boys with and without CAH did not differ. The ANCOVA for memory scores with age as the covariate showed neither main effects nor an interaction. Also, no groups, including girls with and without CAH, differed significantly in memory scores.
Table 3.
Self-reported preference and memory for objects modelled by individuals of the same sex in girls and boys with and without CAH.
| girls |
boys |
CAH and control girls diff. |
95% CI for difference |
CAH and control boys diff. |
95% CI for difference |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| modelling protocol | CAH N = 42 | control N = 40 | CAH N = 37 | control N = 29 | p | d | mean diff. | upper | lower | p | d | mean diff. | upper | lower |
| preference | ||||||||||||||
| Ma | 0.51 | 0.60 | 0.58 | 0.63 | 0.079 | 0.41 | −0.09 | −0.18 | 0.01 | 0.351 | −0.25 | −0.05 | 0.05 | −0.15 |
| (s.d.) | (0.21) | (0.23) | (0.19) | (0.22) | ||||||||||
| memory | ||||||||||||||
| Mb | 0.86 | 0.92 | 0.87 | 0.94 | 0.158 | 0.30 | −0.06 | −0.15 | 0.02 | 0.189 | −0.35 | −0.07 | 0.03 | −0.18 |
| (s.d.) | (0.23) | (0.16) | (0.26) | (0.15) | ||||||||||
aMeans represent the proportion of preference for objects modelled by same-sex models.
bMeans represent the proportion of correct memory for objects.
(c). Within-sample t-tests
In addition to group-wise comparisons, we conducted within-sample t-tests to see if children in the four groups showed preferences for sex-appropriate objects at levels above chance. Table 4 shows p-values, confidence intervals, and effects sizes for outcomes for both the labelling and modelling protocols. Girls with CAH scored above chance for memory on both protocols, p < 0.001 for each, d = 2.25 and 3.19, respectively. However, they did not score above chance for any of the measures assessing responses to labels and models. Specifically, they did not report verbal preferences for objects that had been labelled as for girls in the labelling protocol or for objects modelled by females in the modelling protocol in significantly more than 50% of trials (p = 0.146 and 0.785, d = −0.46 and 0.09, respectively). They also did not spend significantly more than 50% of time playing with the objects labelled as for girls in the labelling protocol (p = 0.776, d = 0.09). By contrast, children in each of the other three groups scored above chance on all measures from both the modelling and labelling protocols with effect sizes for verbal and observed behavioural preferences ranging from d = 0.79 to 1.25.
Table 4.
Statistics for one-sample t-tests for performance above chance (50%).
| girls |
boys |
|||
|---|---|---|---|---|
| CAH | control | CAH | control | |
| N = 39–42 | N = 37–40 | N = 35–37 | N = 29 | |
| labelling verbal preferencea | ||||
| p-value | 0.146 | 0.002 | 0.001 | 0.010 |
| effect size d | −0.46 | 1.09 | 1.23 | 1.05 |
| mean difference | −0.083 | 0.192 | 0.181 | 0.172 |
| 95% CI for mean difference | ||||
| upper | −0.200 | 0.077 | 0.080 | 0.045 |
| lower | 0.030 | 0.308 | 0.281 | 0.300 |
| labelling observed playb | ||||
| p-value | 0.776 | 0.002 | 0.001 | 0.047 |
| effect size d | 0.09 | 1.13 | 1.25 | 0.79 |
| mean difference | 0.014 | 0.178 | 0.187 | 0.108 |
| 95% CI for mean difference | ||||
| upper | −0.088 | 0.071 | 0.085 | 0.001 |
| lower | 0.117 | 0.284 | 0.290 | 0.214 |
| labelling memoryc | ||||
| p-value | 0.000 | 0.000 | 0.000 | 0.000 |
| effect size d | 2.25 | 3.87 | 2.39 | 1.99 |
| mean difference | 0.327 | 0.395 | 0.343 | 0.302 |
| 95% CI for mean difference | ||||
| upper | 0.236 | 0.327 | 0.243 | 0.184 |
| lower | 0.419 | 0.463 | 0.443 | 0.419 |
| modelling verbal preferencea | ||||
| p-value | 0.785 | 0.012 | 0.011 | 0.003 |
| effect size d | 0.09 | 0.85 | 0.90 | 1.23 |
| mean difference | 0.009 | 0.095 | 0.084 | 0.132 |
| 95% CI for mean difference | ||||
| upper | −0.057 | 0.022 | 0.021 | 0.049 |
| lower | 0.075 | 0.168 | 0.148 | 0.215 |
| modelling memoryc | ||||
| p-value | 0.000 | 0.000 | 0.000 | 0.000 |
| effect size d | 3.19 | 5.39 | 2.90 | 5.89 |
| mean difference | 0.361 | 0.423 | 0.372 | 0.444 |
| 95% CI for mean difference | ||||
| upper | 0.290 | 0.373 | 0.285 | 0.386 |
| lower | 0.433 | 0.474 | 0.458 | 0.502 |
aScores are the proportion of preferred objects that were labelled as for child's gender, or modelled by same-sex adults.
bScores are the proportion of time spent in contact with objects labelled as for child's gender.
cScores are the proportion correct for memory of which gender model preferred each object (modelling protocol), or for which gender label was given to the object (labelling protocol).
Finally, we computed composite scores for preferences and for memory for same-sex objects by averaging the proportion scores (figure 2). The preferences composite included the proportion preference scores for verbal responses for both the modelling and the labelling tasks as well as observed play for the labelling task. The memory composite included proportion memory scores for both the modelling and the labelling tasks. ANOVA for the preferences composite showed main effects of sex: F1,147 = 5.11, p = 0.025, and CAH status: F1,147 = 6.22, p = 0.014, as well as a Sex × CAH status interaction: F1,147 = 7.32, p = 0.008. Girls with CAH showed reduced preferences for objects modelled by women or labelled as for girls compared with control girls, t82 = −3.82, p < 0.001, d = −0.82. They also responded less to the same gender models and gender-appropriate labels when compared with control boys: t71 = −3.27, p = 0.002, d = −0.75, and boys with CAH: t78 = −3.68, p = 0.001, d = −0.78. There were no other group differences. ANCOVA, with age as the covariate, for the memory composite showed a main effect of age only, F1,146 = 6.40, p = 0.012; r150 = 0.21, p = 0.009, with memory scores increasing with age. There were no other main effects, nor was there an interaction for the memory variable.
Figure 2.
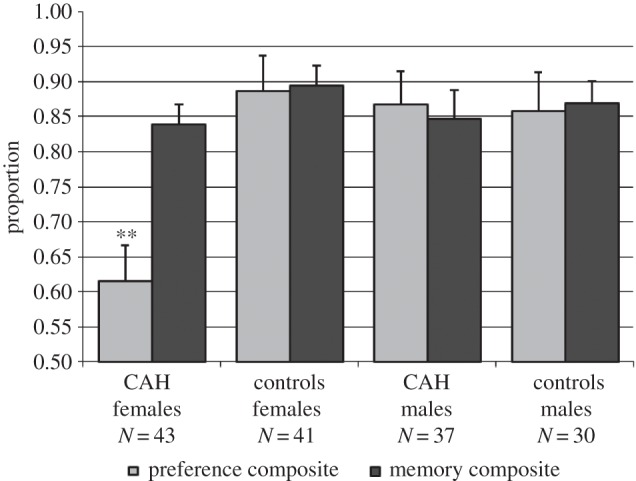
Composite scores for preferences and memory for gender-appropriate objects assessed using labelling and modelling paradigms. The preference composite includes verbal preferences (labelling and modelling) as well as observed play (labelling). The memory composite includes memory scores from the labelling and modelling paradigms. All scores are mean proportions. **p < 0.01, girls with CAH differ compared to control girls, control boys and boys with CAH. There were no other group differences for preference scores, and there were no group differences for memory scores.
4. Discussion
We found that girls with CAH showed reduced positive responses to information indicating that particular toy or object choices were appropriate for girls. This finding was seen using two different protocols, a labelling protocol and a modelling protocol, and, for the labelling protocol, using verbal report as well as behavioural observation. The results did not reflect impaired memory for the information as to what was appropriate for girls, because memory scores for girls with CAH were significantly above chance, and girls with CAH did not differ in memory scores from children in any other group. At the same time, however, and unlike other children, they did not score significantly above chance for interest in objects that had been labelled as being for their own sex (labelling protocol), or in objects that had been chosen by individuals of their own sex (modelling protocol). Consistent with prior research with typically developing children [22], the labelling protocol appeared to produce more marked effects than the modelling protocol. Our findings also resemble results for most other gender-related behavioural outcomes in girls with CAH [10,34,35,37,39,40], in that the responses of the girls with CAH to the models and labels were in between those of unaffected girls and unaffected boys.
These results augment understanding of the mechanisms by which prenatal androgen exposure influences human gender development. As detailed in §1, prior research has shown that children's gender-typed toy preferences relate to prenatal androgen exposure, and that gender-related models and labels also contribute to these toy preferences. Our findings that girls exposed to elevated concentrations of androgens prenatally are less susceptible than are other children to the influences of same-sexed models and of gender labels suggest that these processes are not independent. Instead, early androgen exposure appears to influence later processes of self-socialization.
Our results thus suggest that cognitive processes related to gender identity play a role in the influences of prenatal androgen exposure on later behaviour. Assumedly, however, the effects of prenatal androgen exposure are initiated during fetal development, and include neural changes that begin prenatally. The precise nature of these neural changes is currently unknown. One possibility is that they involve neural systems related to children's object (e.g. toy) preferences. Gender differences in object preferences emerge in infancy, before the age of 2 years [3,5–7], and gender identity usually begins to be measurable somewhat later, beginning at 2–3 years of age [41,42]. Thus, girls with CAH might show altered object preferences early in life, and then, as they grow older and gain a cognitive understanding of gender and gender-related behaviour, these altered object preferences might in turn alter their responses to models of the same sex and to labels indicating that certain objects or activities are for girls. Alternatively, the reduced female-typical gender identity found in girls with CAH [34] might decrease their responses to same-sex models and to gender-related labels. These alterations in processes related to the self-socialization of gendered behaviour could lead to further reductions in female-typical behaviour. Future research, beginning early in development, might usefully evaluate these possibilities.
The results of the present study suggest a previously unexplored mechanism by which early androgen exposure might influence the development of gender-related behaviour in girls with CAH—by reducing self-socialization of gender-related behaviour. Identification of this mechanism has potentially important implications for the interpretation of behavioural changes related to early androgen exposure. For instance, women with CAH have been found to show increased interest in male-typical occupations [37,43,44], and these results are thought to reflect influences of androgen on fetal brain organization. Our findings suggest that these results, and perhaps other behavioural outcomes related to early androgen exposure, could also result in part from alterations in processes involved in self-socialization of gender-typical behaviour, processes that occur postnatally and may be susceptible to social influence. Thus, there may be more plasticity of outcome following early androgen exposure than has been previously thought.
(a). Limitations
We did not manipulate androgens prenatally nor did we assign children at random to receive androgen or placebo treatment. Such rigorous experimental procedures would not be ethical. Reliance on information from a clinical condition could raise concerns about the generalizability of our findings or about interpreting the findings as being caused by prenatal androgen exposure. We used two procedures to try to mitigate these concerns. First, we compared children with CAH to their unaffected relatives, so that socio-economic status and other familial factors were relatively similar in the two groups. Second, we studied boys with CAH as well as girls with CAH. Boys with CAH have not been found to be more male-typical in their behaviour compared with boys without CAH [2,11,34], perhaps because their androgen concentrations are largely similar to those of boys without CAH prenatally [25,26]. Our observation of differences in girls with CAH, but not boys with CAH, increases the likelihood that the effects were caused by elevated androgens, rather than other aspects of the CAH condition, such as cortisol abnormality. Only girls with CAH are exposed to abnormally elevated androgens prenatally, whereas both girls and boys with CAH experience other aspects of the condition, such as cortisol abnormality. So, if cortisol abnormality, or other aspects of CAH that are shared by boys and girls, were responsible for group differences, both boys and girls with CAH would be expected to differ from controls. Of course, the use of relative controls and the inclusion of boys with and without CAH do not fully compensate for the disadvantages of clinical, rather than experimental, research. It is unlikely that it will ever be ethical to conduct rigorous experiments involving androgen manipulations in developing humans, but evidence from other types of studies could increase confidence that the observed effects resulted from prenatal androgen exposure. For instance, it would be useful to know if other conditions causing unusual androgen exposure early in life also influence children's responses to information indicating the gender-appropriateness of behaviour. Similarly, studies relating normal variability in androgen during early development to later responses to gender cues would be of interest, as would studies of these processes in children who show cross gendered behaviour, but have no history of early androgen abnormality.
In summary, girls with CAH are exposed to high concentrations of androgens prenatally, and prior research has shown that they differ from typically developing girls in their gender-typical toy preferences, as well as in some other gender-related behaviours. Our results suggest that processes involved in self-socialization of gender-related behaviour also are altered in girls with CAH. Thus, the influences of prenatal androgen exposure on behaviour across the lifespan may reflect in part the cascading effects of alterations in processes related to the self-socialization of gendered behaviour, as well as neural changes induced by androgen prenatally. Additional studies of other patient groups and of typically developing children could provide convergent evidence that the observed effects are the result of early androgen exposure, rather than other aspects of the CAH condition. Also, further studies of girls with CAH, particularly beginning early in life, might provide additional information on how different types of factors work together to influence gender development, and thus further increase understanding of the developmental systems involved in human gender development.
Supplementary Material
Acknowledgements
We thank all the families whose participation made this study possible. We also thank Sue Elford and the CAH Support Group in the UK for their contributions to the research.
Ethics
Procedures for the study received ethical approval from the National Health Service Health Research Authority, and informed consent and assent, from parents and children, respectively, were obtained prior to participation.
Data accessibility
The dataset supporting this article is included as the electronic supplementary material.
Authors' contributions
M.H. designed the study and prepared the initial draft of the manuscript. V.P. and P.P. analysed the data and assisted with its interpretation. D.S. and S.N. oversaw data acquisition and management. P.C.H., I.A.H. and C.L.A contributed access to some of the participants through clinical resources. All authors inputted and critically evaluated production of the manuscript and approved the final version.
Competing interests
We have no competing interests.
Funding
The study was supported by USPHS grant no. HD24542 to M.H. and by funds from Cambridge University, Cambridge, UK.
References
- 1.Hyde JS. 2005. The gender similarities hypothesis. Amer. Psychol. 60, 581–592. ( 10.1037/0003-066X.60.6.581) [DOI] [PubMed] [Google Scholar]
- 2.Hines M. 2015. Gendered development. In Handbook of child development and developmental science (eds Lerner RM, Lamb ME), pp. 842–887, 7th edn Hoboken, NJ: Wiley. [Google Scholar]
- 3.Alexander GM, Wilcox T, Woods R. 2009. Sex differences in infants’ visual interest in toys. Arch. Sexual Behav. 38, 427–433. ( 10.1007/s10508-008-9430-1) [DOI] [PubMed] [Google Scholar]
- 4.Zosuls KM, Ruble DN, Tamis-LeMonda CS, Shrout PE, Bornstein MH, Greulich FK. 2009. The acquisition of gender labels in infancy: implications for sex-typed play. Dev. Psychol. 45, 688–701. ( 10.1037/a0014053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jadva V, Golombok S, Hines M. 2010. Infants’ preferences for toys, colors and shapes. Arch. Sexual Behav. 39, 1261–1273. ( 10.1007/s10508-010-9618-z) [DOI] [PubMed] [Google Scholar]
- 6.Snow ME, Jacklin CN, Maccoby EE. 1983. Sex of child differences in father-child interaction at one year of age. Child Dev. 54, 227–232. ( 10.2307/1129880) [DOI] [Google Scholar]
- 7.Serbin LA, Poulin-Dubois D, Colbourne KA, Sen MG, Eichstedt JA. 2001. Gender stereotyping in infancy: visual preferences for and knowledge of gender-stereotyped toys in the second year. Int. J. Behav. Dev. 25, 7–15. ( 10.1080/01650250042000078) [DOI] [Google Scholar]
- 8.Pasterski V, Golombok S, Hines M. 2010. Sex differences in social behavior. In The Wiley-Blackwell handbook of childhood social development (eds Smith PK, Hart CH), 2nd edn, pp. 281–298 Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 9.Berenbaum SA, Hines M. 1992. Early androgens are related to childhood sex-typed toy preferences. Psychol. Sci. 3, 203–206. ( 10.1111/j.1467-9280.1992.tb00028.x) [DOI] [Google Scholar]
- 10.Pasterski VL, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M. 2005. Prenatal hormones and postnatal socialization by parents as determinants of male-typical toy play in girls with congenital adrenal hyperplasia. Child Dev. 76, 264–278. ( 10.1111/j.1467-8624.2005.00843.x) [DOI] [PubMed] [Google Scholar]
- 11.Cohen-Bendahan CCC, van de Beek C, Berenbaum SA. 2005. Prenatal sex hormone effects on child and adult sex-typed behavior:methods and findings. Neurosci. Biobehav. Rev. 29, 353–384. ( 10.1016/j.neubiorev.2004.11.004) [DOI] [PubMed] [Google Scholar]
- 12.Hines M. 2011. Gender development and the human brain. Annu. Rev. Neurosci. 34, 67–86. ( 10.1146/annurev-neuro-061010-113654) [DOI] [PubMed] [Google Scholar]
- 13.Arnold AP. 2009. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 55, 570–578. ( 10.1016/j.yhbeh.2009.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hines M. 2004. Brain gender. New York, NY: Oxford University Press. [Google Scholar]
- 15.McCarthy MM, De Vries GJ, Forger NG. 2002. Sexual differentiation of the brain: mode, mechanisms, and meaning. In Hormones, brain and behavior (eds Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT), pp. 1707–1744, 2nd edn San Diego, CA: Academic Press. [Google Scholar]
- 16.Maccoby EE, Jacklin CN. 1974. The psychology of sex differences. Stanford, CA: Stanford University Press. [Google Scholar]
- 17.Ruble D, Martin CL, Berenbaum SA. 2006. Gender development. In Handbook of child psychology (eds Damon W, Lerner RM), 6th edn, pp. 858–932. Hoboken, NJ: Wiley. [Google Scholar]
- 18.Martin CL, Ruble DN, Szkrybalo J. 2002. Cognitive theories of early gender development. Psychol. Bull. 128, 903–933. ( 10.1037/0033-2909.128.6.903) [DOI] [PubMed] [Google Scholar]
- 19.Perry DG, Bussey K. 1979. The social learning theory of sex difference: Imitation is alive and well. J. Pers. Soc. Psychol. 37, 1699–1712. ( 10.1037/0022-3514.37.10.1699) [DOI] [Google Scholar]
- 20.Shutts K, Banaji MR, Spelke ES. 2010. Social categories guide young children's preferences for novel objects. Dev. Sci. 13, 599–610. ( 10.1111/j.1467-7687.2009.00913.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf TM. 1973. Effects of live modeled sex-inappropriate play behavior in a naturalistic setting. Dev. Psychol. 9, 120–123. ( 10.1037/h0035091) [DOI] [Google Scholar]
- 22.Masters JC, Ford ME, Arend R, Grotevant HD, Clark LV. 1979. Modeling and labelling as integrated determinants of children's sex-typed imitative behavior. Child Dev. 50, 364–371. ( 10.2307/1129411) [DOI] [Google Scholar]
- 23.Liebert RM, McCall RB, Hanratty MA. 1971. Effects of sex-typed information on children's toy preferences. J. Genet. Psychol. 119, 133–136. ( 10.1080/00221325.1971.10532635) [DOI] [Google Scholar]
- 24.Martin CL, Ruble DN. 2009. Patterns of gender development. Annu. Rev. Psychol. 61, 353–381. ( 10.1146/annurev.psych.093008.100511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang S, et al. 1980. Amniotic fluid concentrations of delta 5 and delta 4 steroids in fetuses with congenital adrenal hyperplasia due to 21-hydroxylase deficiency and in anencephalic fetuses. J. Clin. Endocrinol. Metab. 51, 223–229. ( 10.1210/jcem-51-2-223) [DOI] [PubMed] [Google Scholar]
- 26.Wudy SA, Dörr HG, Solleder C, Djalali M, Homoki J. 1999. Profiling steroid hormones in amniotic fluid of midpregnancy by routine stable isotope dilution/ gas chromatography-mass spectrometry: reference values and concentrations in fetuses at risk for 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 84, 2724–2728. ( 10.1210/jc.84.8.2724) [DOI] [PubMed] [Google Scholar]
- 27.Merke DP, Bornstein SR. 2005. Congenital adrenal hyperplasia. The Lancet 365, 2125–2136. ( 10.1016/S0140-6736(05)66736-0) [DOI] [PubMed] [Google Scholar]
- 28.Dessens AB, Slijper FME, Drop SLS. 2005. Gender dysphoria and gender change in chromosomal females with congenital adrenal hyperplasia. Arch. Sexual Behav. 34, 389–397. ( 10.1007/s10508-005-4338-5) [DOI] [PubMed] [Google Scholar]
- 29.Nordenstrom A, Servin A, Bohlin G, Larsson A, Wedell A. 2002. Sex-typed toy play behavior correlates with the degree of prenatal androgen exposure assessed by CYP21 genotype in girls with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 87, 5119–5124. ( 10.1210/jc.2001-011531) [DOI] [PubMed] [Google Scholar]
- 30.Meyer-Bahlburg HFL, Gruen RS, New MI, Bell JJ, Morishima A, Shimshi M, Bueno Y, Vargas I, Baker SW. 1996. Gender change from female to male in classical congenital adrenal hyperplasia. Horm. Behav. 30, 319–332. ( 10.1006/hbeh.1996.0039) [DOI] [PubMed] [Google Scholar]
- 31.Zucker KJ, Bradley SJ, Oliver G, Blake J, Fleming S, Hood J. 1996. Psychosexual development of women with congenital adrenal hyperplasia. Horm. Behav. 30, 300–318. ( 10.1006/hbeh.1996.0038) [DOI] [PubMed] [Google Scholar]
- 32.Ehrhardt AA, Epstein R, Money J. 1968. Fetal androgens and female gender identity in the early-treated adrenogenital syndrome. Johns Hopkins Med. J. 122, 160–167. [PubMed] [Google Scholar]
- 33.Hines M, Brook C, Conway GS. 2004. Androgen and psychosexual development: core gender identity, sexual orientation and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH). J. Sex Res. 41, 75–81. ( 10.1080/00224490409552215) [DOI] [PubMed] [Google Scholar]
- 34.Pasterski VL, Zucker KJ, Hindmarsh PC, Hughes IA, Acerini CL, Spencer D, Neufeld S, Hines M. 2015. Increased cross-gender identification independent of gender role behavior in girls with Congenital Adrenal Hyperplasia: results from a standardized assessment of 4–11-year-old children. Arch. Sexual Behav. 44, 1363–1375. ( 10.1007/s10508-014-0385-0) [DOI] [PubMed] [Google Scholar]
- 35.Pasterski VL, Geffner ME, Brain C, Hindmarsh PC, Brook C, Hines M. 2011. Prenatal hormones and childhood sex segregation: playmate and play style preferences in girls with congenital adrenal hyperplasia. Horm. Behav. 59, 549–555. ( 10.1016/j.yhbeh.2011.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baron-Cohen S. 2003. The essential difference: the truth about the male and female brain. New York, NY: Basic Books. [Google Scholar]
- 37.Berenbaum SA. 1999. Effects of early androgens on sex-typed activities and interests in adolescents with congenital adrenal hyperplasia. Horm. Behav. 35, 102–110. ( 10.1006/hbeh.1998.1503) [DOI] [PubMed] [Google Scholar]
- 38.White PC, Speiser PW. 2000. Congenital adrenal hyperplasia due to 21-hydroxylase dficiency. Endocr. Rev. 21, 245–291. [DOI] [PubMed] [Google Scholar]
- 39.Meyer-Bahlburg HFL, Dollard J, Baker SW, Carlson AD, Obeid JS, New MI. 2004. Prenatal androgenization affects gender-related behavior but not gender identity in 5–12-year-old girls with congenital adrenal hyperplasia. Arch. Sexual Behav. 33, 97–104. ( 10.1023/B:ASEB.0000014324.25718.51) [DOI] [PubMed] [Google Scholar]
- 40.Knickmeyer R, Baron-Cohen S, Fane B, Wheelwright S, Mathews GA, Conway GS, Brook CGD, Hines M. 2006. Androgens and autistic traits: a study of individuals with congenital adrenal hyperplasia. Horm. Behav. 50, 148–153. ( 10.1016/j.yhbeh.2006.02.006) [DOI] [PubMed] [Google Scholar]
- 41.Kohlberg L. 1966. A cognitive-developmental analysis of children's sex-role concepts and attitudes. In The development of sex differences. (ed. Maccoby EE.), pp. 82–173. Stanford, CA: Stanford University Press. [Google Scholar]
- 42.Thompson SK. 1975. Gender labels and early sex role development. Child Dev. 46, 339–347. ( 10.2307/1128126) [DOI] [PubMed] [Google Scholar]
- 43.Beltz AM, Swanson JL, Berenbaum SA. 2011. Gendered occupational interests: prenatal androgen effects on psychological orientation to things versus people. Horm. Behav. 60, 313–317. ( 10.1016/j.yhbeh.2011.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frisén J, et al. 2009. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J. Clin. Endocrinol. Metab. 94, 3432–3439. ( 10.1210/jc.2009-0636) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article is included as the electronic supplementary material.