Abstract
In the study of variation in brain structure and function that might relate to sex and gender, language matters because it frames our research questions and methods. In this article, we offer an approach to thinking about variation in brain structure and function that pulls us outside the sex differences formulation. We argue that the existence of differences between the brains of males and females does not unravel the relations between sex and the brain nor is it sufficient to characterize a population of brains. Such characterization is necessary for studying sex effects on the brain as well as for studying brain structure and function in general. Animal studies show that sex interacts with environmental, developmental and genetic factors to affect the brain. Studies of humans further suggest that human brains are better described as belonging to a single heterogeneous population rather than two distinct populations. We discuss the implications of these observations for studies of brain and behaviour in humans and in laboratory animals. We believe that studying sex effects in context and developing or adopting analytical methods that take into account the heterogeneity of the brain are crucial for the advancement of human health and well-being.
Keywords: brain, sex, gender
1. Introduction
In the study of variation in brain structure and function that might relate to sex and gender, language matters. It matters because the choice of words and the meanings behind them frame our research questions and methods [1–4]. And it matters because inconsistent or imprecise use engenders confusion. McCarthy & Konkle [4] made precisely these points in a carefully crafted article in which they distinguished between sex dimorphism and sex difference. In this opinion piece, they argued that we apply the term sexual dimorphism only to those aspects of difference—for example, male and female genitalia or X and Y chromosomes—that truly come (or nearly so [5]) in just two forms [4]. McCarthy & Konkle argued that scientists use care not to refer to male and female brains as dimorphic when actually referring to sex difference, as in most mammals sex-related brain differences consist of overlapping populations with mean differences. Indeed, sexual dimorphism is extremely rare (if it exists at all) in the human brain (e.g. [4,6–9]), including in regions showing very large differences between females and males (e.g. [10–16]).1 For example, in a study by Garcia-Falgueras et al. [14], the intermediate nucleus (InM) of the hypothalamus is on average about twice as large in males compared with females. Nevertheless, in about a third of the males the InM is the size typical of females.
Ten years out from this call for more careful conceptualization of the relationships between sex and the brain, we often remain encumbered by the same imprecise language that McCarthy & Konkle [4] addressed. While some newer scientific work seems to have dropped the use of dimorphism or reference to male versus female brains, instead referring to human brains [8,17], the use of the word dimorphism to describe sex-related brain differences appears in the scientific literature frequently and seemingly without critique (e.g. [18–21]). Matters are far worse in popular renditions of scientific findings. These routinely portray brain differences as dimorphic, uncritically comparing ‘male brains' to ‘female brains' [12–24] (as opposed to comparing brains from males to brains from females).
Even if we were to routinely disentangle the concepts of difference and dimorphism with regard to specific brain features, we would be left with conceptual difficulties. This is because, as explained in §2 below, the existence of differences between the brains of males and the brains of females is insufficient for describing and understanding the relationships between sex and the brain. In this article, we offer an approach to thinking about variation in brain structure and function that pulls us outside the dimorphism-difference formulation. We consider the implications of this approach for future research including both basic and clinical inquiry and in the light of the requirement that sex be explicitly included in all research studies by the United States National Institutes of Health [25,26] and of similar policies by Canada and the European Union [27,28].
2. Developing the mosaic brain hypothesis
Consider the following illustration using a highly simplified ‘brain’ composed of two regions, A and B, each of which can take one of two states, 1 or 2. A ‘state’ in this illustration could be a volume, size, structure, locus of specific gene expression or other functional difference. For argument's sake, the reader might think of A as a hypothalamic nucleus and B as a subcomponent of the hippocampus, and imagine that each of these components may be small (state 1) or large (state 2). Consider further that there is a sex difference in both components, so that in two-thirds of females component A is small (i.e. in state 1), whereas in two-thirds of males component A is large (i.e. in state 2), and the same is true for component B (i.e. it is small (state 1) in two-thirds of females and large (state 2) in two-thirds of males). Is the existence of sex differences sufficient to conclude that this population of ‘brains' is essentially polarized into two types of brain? Moreover, is the existence of sex differences enough to characterize the population? It turns out that the answer to both questions is no.
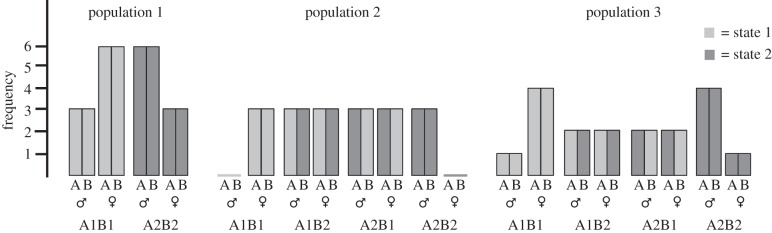
Suppose we were studying 18 brains, nine from males and nine from females. There are several ways to imagine fulfilling the conditions described in the previous paragraph (figure 1). In population 1, all the brains are internally consistent in the form of their components, that is, both components are either in the form prevalent in females (i.e. small), or both are in the form prevalent in males (i.e. large). In this example, the population is indeed split into two types of brains, small brains (A1, B1) and large brains (A2, B2) in equal proportions, with small brains being typical of females (as two-thirds of females have a small brain) and large brains typical of males (as two-thirds of males have a large brain).
Figure 1.
Three hypothetical populations, each with 18 ‘brains', nine from females and nine from males. Each ‘brain’ consists of two components, A and B (left and right bars, respectively), each of which can exist in one of two possible states, 1 and 2 (light and dark grey, respectively). The figure presents the frequency of each ‘brain’ type in females (♀) and males (♂).
In population 2, there are four types of brains: three (A1, B1) brains from females, three (A2, B2) brains from males, six (A1, B2) brains, three from males and three from females, and six (A2, B1) brains, three from males and three from females. Population 3 further complicates the possibilities: here there are five (A1, B1) brains, one from a male and four from females; similarly, there are five (A2, B2) brains, one from a female and four from males; finally (A1, B2) and (A2, B1) brains come in equal number from males and females. In populations 2 and 3, some brain types are equally likely in females and males, (A1, B2) and (A2, B1), whereas some are more common in one sex over the other ((A1, B1) brains are more common in females compared with males, and (A2, B2) brains are more common in males compared with females). Note, however, that the internally consistent brain types ((A1, B1) and (A2, B2)), although more common in one sex over the other, are not typical of that sex, i.e. only a minority of subjects from each sex category has them. So while it is true in all three populations that an (A1, B1) brain is most likely to belong to a female, in populations 2 and 3 it is not true that a female is most likely to have an (A1, B1) brain. In fact, she is just as likely (in population 3) or more likely (population 2), to have a mosaic brain, i.e. a brain that has one component in the form more typical of females (i.e. in state 1) and one component in the form more typical of males (i.e. in state 2).
Are there any data supporting the existence of mosaic brains? Joel [29,30] has recently suggested that such evidence may be found in animal studies reporting that the effects of sex on the brain differ even to the point of opposition under varied environmental conditions and that sex-by-environment interactions may differ for different brain features. For example, Reich et al. [31] found that three weeks of mild stress reversed a sex difference in the density of CB1 receptors in rats' dorsal hippocampus. Thus, what was typical in one sex category under some conditions (i.e. low density of CB1 receptors in non-stressed females and high density of CB1 receptors in non-stressed males) was typical in the other sex category under other conditions (i.e. following three weeks of stress). A different sex-by-environment interaction determined the density of CB1 receptors in the ventral hippocampus, as the same manipulation (three weeks of mild stress) eliminated a sex difference in the density of these receptors in the ventral hippocampus. Thus, even when considering only a single characteristic (the density of CB1 receptors) and only two environmental conditions (no stress versus three weeks of mild stress), the hippocampus can take three forms: low density of CB1 receptors in the dorsal and ventral hippocampus (non-stressed females and stressed males), high receptor density in dorsal and ventral hippocampus (non-stressed males), high receptor density in dorsal hippocampus and low receptor density in ventral hippocampus (stressed females). Stated in terms of our hypothetical example, there are two components (A and B = dorsal and ventral hippocampus) that can assume one of two states (1 and 2 = low and high receptor density), and three types of ‘brains': (A1, B1), (A2, B2) and (A2, B1). Similar findings have been reported following other forms of manipulation (e.g. rearing conditions, pharmacological challenges, acute and chronic stress), for a variety of neural and regional characteristics (e.g. spine density, dendritic arborization, axonal branching, number of neurons, number of glia cells, size of a nucleus), and for many brain regions and neurotransmitter systems [32–54]; thus it is highly likely that brain cells, brain regions, and brains as a whole, are multimorphic rather than dimorphic [29,30].
How does this exercise apply to human brains, for which hundreds of regions have been analysed for sex differences in size, volume or other characteristics? Joel and colleagues [55] recently attempted to answer exactly this question using magnetic resonance imaging (MRI). They analysed volume, cortical thickness, diffusion anisotropy or connectivity in over 1400 human brains from four datasets. In each dataset, they identified a subset of between 7 and 12 brain regions (or connections) that mostly differed between the sexes, and determined for each brain whether the form of each of these regions was at the side of the distribution where females were more prevalent than males (‘female-end’) or at the side of the distribution where males were more prevalent than females (‘male-end’). They found that regardless of the sample, age, type of magnetic resonance imaging, method of analysis, and exact definition of the ‘male-end’ and ‘female-end’ zones, brains that had at least one region with a ‘male-end’ score and one region with a ‘female-end’ score (a condition they have termed substantial variability) were more prevalent than brains that had only ‘male-end’ or only ‘female-end’ scores. For example, defining the ‘male-end’ and ‘female-end’ zones as the scores of the 33% most extreme males and females, respectively, between 23 and 53% of brains (depending on the sample) had at least one region with a ‘male-end’ score and one region with a ‘female-end’ score, whereas the percentage of brains with all ‘male-end’ or all ‘female-end’ scores was between 0 and 8% [55].
As figure 1 demonstrates, the small percentage of internally consistent brains found in Joel and colleagues' study does not result from the overlap between the distribution of females and males in every brain region. If brains were internally consistent, then brains would have only ‘male-end’ features, only ‘female-end’ features, or only ‘intermediate’ features (scores in between the other two zones), with some males and females having brains with only ‘female-end’ features or only ‘male-end’ features, respectively (as in population 1, figure 1). However, a simulation of data from Joel et al. [55], which tested how the chances of obtaining internal consistency versus substantial variability would change under different degrees of random noise in an otherwise internally consistent brain, did not support the possibility that brains are internally consistent in the degree of ‘maleness–femaleness' of each of their elements [55].
Thus, rather than being consistent in their degree of ‘maleness–femaleness', most brains consist of unique ‘mosaics' of features, some more common in females compared with males, some more common in males compared to females, and some common in both females and males. Moreover, this mosaic changes as we experience the world, and, as the animal studies reviewed above demonstrate, some of these changes may be sex-dependent [29].
The animal data reviewed indicate that mosaic brains are not merely a hypothetical construct, while the analysis of internal consistency in the human brain reveals that brain mosaicism is an important characteristic of human brains. Furthermore, the prevalence of mosaicism is at variance with the assumption that sex divides brains into two separate populations. Instead, we can consider the possibility that brains belong to a single highly heterogeneous population in which there may be differences between females and males in the frequencies of specific brain mosaics (e.g. brains with only ‘female-end’ characteristics although rare in the population, are more common in females compared with males). Describing the population of brains as a single highly heterogeneous, one can still account for the existence of group-level sex/gender differences in brain structure as well as the observation that different studies (i.e. using different samples of females and males) report different structural and functional differences between brains of females and brains of males (e.g. [55–58]).
3. Sex and brain function
Even when differences between females and males in brain structure are found, they do not necessarily translate into differences in function. De Vries emphasized the importance of the principle of ‘compensation’ in considering the functionality of a variety of sex effects [59–61]. He and others refer to compensatory sex-dependent processes that act to reduce rather than create differences between females and males. Probably the best-known compensatory mechanism is X inactivation, which occurs only in females, and compensates for the difference between females and males in the number of X chromosomes (i.e. XX versus XY) (for review, see [59,61]). Thus, a truly dimorphic sex-dependent process (i.e. one that occurs in only one sex category) acts to counteract a difference between females and males. In this situation, we could have concluded that males and females not only differ in their chromosomal complement but also in the regulation of gene expression, missing the point that the second difference counteracts the first, thus lessening overall functional differences.
Yet, when scientists and laypeople list differences between females and males in the brain, they often implicitly assume that the more differences there are, the more different are the two sex categories, ignoring the possibility that some—possibly many—differences may compensate for others (for review and examples see [59–61]; see [62] for a detailed example of compensation in the dopaminergic system and [63] for similar considerations in the case of sex effects on gene networks).
Compensation works independently of sexual dimorphism and internal consistency. That is, even if differences between females and males are dimorphic and internally consistent (as in the case of the chromosome complement (XX or XY) and X inactivation (present or absent, respectively)), it is still possible that they are cancelling out each other, thus resulting in two functionally similar systems.
Taken together, the prevalence of brain mosaicism combined with the principle of functional compensation predict that brain function will not usually be characterized by dimorphism. Indeed, direct assessment of brain function using functional imaging techniques most often reveals small, highly overlapping, group-level differences between human females and males. Furthermore, the regions in which such differences are found often differ in different studies (e.g. [56–58]). Similarly, studies of behaviour, in humans and other mammals, reveal overlap in all types of behaviour, including sexual behaviour (e.g. [64–68]). In a study on internal consistency in human behaviour, Joel et al. [55] found that people possess unique mosaics of ‘feminine’ (i.e. more common in females compared to males) and ‘masculine’ (i.e. more common in males compared to females) psychological characteristics. These observations are better explained by the assumption that human brains belong to a single heterogeneous population than by the assumption that they belong to two classes, ‘female brains' and ‘male brains'. Larson et al. [69], for example, suggest that individuals with autism spectrum disorder have an ‘extreme of the typical male brain’ in opposition to a cognitive profile that they describe as an ‘extreme of the typical female brain’ ([69], p. 1). In contrast, under our single heterogeneous mosaic brain assumption, the existence of differences between females and males in the prevalence of specific behaviours and psychopathologies (e.g. extreme physical aggression, autism) is accounted for by the existence of differences between females and males in the frequencies of rare brain mosaics (e.g. brains with only ‘male-end’ characteristics although rare, are more common in males compared with females [55]).
4. The implications of the mosaic approach for future research
We started our discussion with the assertion that the existence of differences between the brains of females and males does not allow us to conclude that brains belong to two types nor to characterize the relationships between sex and the brain. Such characterization is necessary for studying sex effects on the brain as well as for studying brain structure, function and dysfunction in general. This is because if human brains belonged to two distinct populations, then every study of brain structure and function, even if not designed to detect sex effects (e.g. a study of the neural substrates of cognitive functions, psychopathologies, etc.) should include sex category (female, male) as a variable to control for sex-related variability, and studies designed to reveal possible sex effects on the brain would simply list differences between brains of females and brains of males. On the other extreme, if human brains belonged to a single heterogeneous population, then sex category should not be included in studies of brain structure, function and dysfunction, and listing sex differences would not be a useful strategy for studying sex effects on the brain. Future studies may specify when sex category should be included as a variable and when not, and reveal new ways to consider sex as a variable between these two extremes (include/exclude).
Although the data reviewed above do not support the two distinct populations view, they are not sufficient to fully characterize the relationships between sex and the brain (e.g. populations 2 and 3; figure 1). We hope future studies will fill in this gap. Yet, current data are sufficient to outline a general direction for studies of sex effects on the brain and for studies of brain structure, function and dysfunction in general. Thus, given that brains are mosaics of features and that information processing in the brain depends on networks that comprise many brain regions rather than on regions working in isolation, we need to develop or adopt analytical methods that take into account both the variability in the human brain (rather than treating it as noise) and individual differences in the specific composition of the brain mosaic. Analytical methods with the above characteristics have been developed for working with other types of data. For example, with the explosion of large-scale biological data following the sequencing of the human genome, methods for analysis of large-scale genetic variation data have been developed and used for detecting patterns of change that are characteristic of specific disorders (e.g. [70–72]). We hope similar approaches will soon be adapted to or developed for studying the function and dysfunction of the human brain.
But what should one do as we develop such tactics? We would like to distinguish between including sex category as a component of a study's methods and as a component of the results. We recommend using both males and females as study subjects in any experimental design (sex as a component of methods), because such inclusion is crucial for ensuring that we more fully understand species' variability than is possible when only males or only females are studied. In appendix A, we suggest an approach for conducting experiments with both females and males as subjects. With respect to using sex category as a variable in the analysis of the results, we will separate the discussion of humans and laboratory animals.
(a). Should sex category be used as a variable in studies of the human brain?
We believe that current data are better explained by the assumption that human brains belong to a single heterogeneous population rather than to two distinct populations. If this is so, then using sex category as a variable is both unnecessary and misleading. Comparing brains of females to brains of males would be analogous to comparing two samples randomly drawn from a single population of brains, rather than to comparing two samples, one randomly drawn from a population of ‘male brains' and the other from a population of ‘female brains' [29]. As a result, although such comparisons may well yield significant differences between females and males (due to the high heterogeneity of the population), these differences would probably reflect a false-positive finding resulting from a chance difference between the two samples included in a specific study. This concern is emphasized by our and others’ findings that different structural and functional differences between brains of females and brains of males are often found in different samples (e.g. [55–58]). We therefore recommend avoiding the use of sex category as an analytic variable in studies of the structure and function of the human brain (for a similar recommendation, see [3,73,74]).
Since sex category is one of the most salient grouping dimensions of humans, psychological and social variables, such as socio-economic status, stress, type of education and personality characteristics, often correlate with sex category. Therefore, although we do not recommend using sex category as a variable, we do recommend that studies of brain and behaviour include advanced planning about which of these psychological and social variables might be relevant, in order to include them in the initial study design [3]. Krieger [75,76] offers well-developed concepts for the study of what she calls ‘pathways to embodiment’ that are applicable to the study of sex categories and the brain. Psychological and social variables that correlate with sex category should not, however, be integrated into a single measure of gender, because gender is a complex, multilayered system [3,77,78], with psychological, behavioural and social components, each of which is multidimensional and cannot be reduced to a single variable (e.g. [3,77–81]).
One exception to our current recommendation to exclude sex category from analysis might involve studies of brain pathology, in which there are many reports of differences between human females and males (e.g. [82–88]). These differences, which are not categorical (i.e. there is no example of a condition which is evident in only one sex category), could reflect the effects of gender rather than the effects of sex (e.g. [2,89–91]), and as suggested above, might be accounted for by differences between females and males in the frequencies of rare brain mosaics rather than differences between a ‘male brain’ and a ‘female brain’. However, as differences between females and males in pathology and in response to treatment may be of importance in the clinic, including sex category as a variable in these studies could provide a first indication of a possible contribution of sex or of a variable that correlates with sex (e.g. elements of gender). Based on such indications, future studies would be needed to better detect the real variable(s), rather than relying on sex category as a stand-in.
(b). Should sex category be used as a variable in studies of the brain of laboratory animals?
In contrast to humans, genetic, developmental and environmental conditions can be highly controlled in laboratory animals. Thus, the variability of factors that might interact with sex to affect the brain (such as age, stress, housing conditions, nutrition, history of drug exposure; for references and review, see [29,30]) is greatly reduced. Consequently, brains of laboratory animals in a specific experiment are expected to be less heterogeneous compared with brains of humans in a single study. Therefore in laboratory animals, differences between the sex categories may indeed reveal the effects of sex rather than the effects of some chance difference between the sample of females and the sample of males in the study.
On the other hand, the controlled genetic, developmental and environmental conditions limit the results obtained in an animal study to the specific environmental, developmental and genetic conditions under which sex effects were assessed (see many examples for such context-specificity in the field of animal models of psychopathology [92]). Therefore, we should be careful in attempting to generalize from the specific experimental conditions to other conditions and other species, especially humans. Moreover, in order to obtain a better understanding of the effects of sex on the phenomena under study, these effects should be studied using varied environments and on different genetic backgrounds (e.g. using different strains of inbred animals; for a similar position, see [3,74,78]). For example, currently a powerful method, the four core genotypes model is being used to disentangle the genetic and hormonal components of sex effects on brain and behaviour [93]. But as effective as this approach is, as long as different environmental conditions are omitted from experimental designs it could well be that observed sex effects on brain and behaviour will be improperly interpreted. Crews et al. [94] and de Medeiros et al. [95] offer one rigorous approach to manipulating environments by using cross fostering to raise mice in litters with different sibling ratios. This permits the separation of litter effects from in utero hormonal effects (e.g. [96]). In addition to litter composition, there are known interactions of sex with other maternal factors (e.g. maternal deprivation, maternal stress [43,48,52,53]) and method of rearing (number of cage mates, enriched versus standard environments [37,97,98]). What seems to be missing entirely from the rodent work on sex effects on brain development are studies conducted in simulated naturalistic environments. It would surprise us if such studies did not change our understandings of sex effects on sex-related behaviours and brain development in rodents. Embracing the concept of the mosaic brain should lead to multivariate experimental designs in which possible sex category effects are examined under changing environmental and genetic conditions.
Although in animals there is probably no equivalence to gender as a social system, there are still environmental variables that, in addition to physiological variables (e.g. weight), correlate with sex category (e.g. number of animals in the home cage [99]). Studies in laboratory animals that use sex category as a variable should take special care to either control for (physiological) and avoid (environmental) sex differences in these variables, or systematically manipulate them.
5. Concluding remarks
Although comparisons between brains of females and brains of males often reveal differences, the existence of such differences does not unravel the relationships between sex and the brain. Nor is it sufficient to characterize the population of brains. Such characterization is necessary for studying sex effects on the brain as well as for studying brain structure, function and dysfunction in general. This is particularly timely in view of the NIH initiative to consider sex in every study [25,26]. In laboratory animals, in which brain variability is restricted by the tight control of genetic, developmental and environmental conditions, sex category can be used as a variable to reveal sex effects. However, given that sex interacts with other factors to affect the brain, sex effects on the brain must be understood as context-dependent, where context relates to the specific environmental, developmental and genetic conditions under which sex effects were assessed and under which the animals developed. In humans, we believe that the existence of group-level sex/gender differences in brain structure, the observation that different studies report different structural and functional sex differences, as well as the existence of sex differences in the prevalence of specific behaviours and psychopathologies are currently better explained by the assumption that human brains belong to a single heterogeneous population than by the assumption that they belong to two distinct populations. We therefore recommend including equal numbers of males and females in all studies while at the same time avoiding the use of sex category as a variable in studies of brain structure and function. We hope future studies will reveal additional ways to consider sex as a variable or specify when sex category should be included as a variable and when not. We believe that developing or adopting analytical methods that take into account the heterogeneity of the human brain is crucial for the advancement of human health and well-being.
Acknowledgements
We would like to thank Prof. Isaac Meilijson, from the Department of Statistics and Operations Research, Tel-Aviv University, for the mathematical illustration.
Appendix A. An approach for conducting experiments with both females and males as subjects
This appendix describes how to treat sex category in studies of brain and behaviour that do not aim to assess sex effects, for example, in a study assessing the effects of a new treatment of depression in an animal model of this disorder. In such a study, the aim is achieved by including males and females and by looking for interactions between Sex category and Treatment, rather than by looking at the main effect of Sex category, that is, at sex differences. This is because if the effects of an independent variable such as Treatment, do not interact with Sex category, then even if there are sex differences (for example, females are on average more resilient than males in all treatment conditions), this has no relevance for the study's question regarding the effectiveness of the treatment. Obviously, finding such a sex difference, provided that the effect size is large, may form the basis for a new study with a new aim, namely, unraveling the mechanisms that make females more resilient than males in this animal model.
The original study (assessing the effects of a new treatment of depression) should include the following steps:
— Start with n (half females and half males) sufficient to detect the effect of the variable in question (i.e. Treatment), that is, same n one would have used for a study of subjects from only one Sex category. Note that a recent meta-analysis reveals that females and males do not differ in the variability of many behavioural and biological variables [97], so there is no need to change power calculations due to the inclusion of both females and males in the same study.
-
— Look at the actual data to see if they suggest an interaction between Sex category and Treatment, that is, if they suggest that the effects of the treatment are different in females compared with males (look at the actual data not just the statistics, which at this point may not be powered enough to detect a significant Sex category × Treatment interaction). If this is not the case, proceed with the analysis of the results focusing on the effects of Treatment. Sex category may be used as an independent variable in these analyses to decrease within-group variability, but if Sex category does not have an effect, this will only lead to a reduction of degrees of freedom. If the data do suggest a Sex category × Treatment interaction, then double the number of animals (i.e. run a replication of the study with the same n (half males and half females) as in the original study), so that it becomes possible to assess the effects of Treatment in the two sex categories.
The suggested practice is expected to decrease the overall number of animals in research, especially in domains in which animals are bred for a specific study (as in studies involving genetic manipulations) and the use of only one sex category leads to killing of animals of the other sex category.
-
— When reporting the results, always report effect size and not only significance level, and present data using actual distributions (e.g. scatterplots) and not only means and standard deviations or standard errors.
This is good practice in general, as it helps differentiate between significant results and important results, but is especially important when reporting differences between females and males, because of the current tendency to treat every difference between means as a dimorphic difference. Reporting actual data and effect sizes enables scientists to appreciate the extent to which sex category explains (or does not) the variability in their results [78].
Endnote
We use the terms male and female and not man and woman, respectively, to indicate that studies typically assess subjects' sex category (i.e. whether one is male or female) and not gender.
Authors' contributions
D.J. drafted the article and A.F.S. critically revised it.
Competing interests
We have no competing interests.
References
- 1.Fausto-Sterling A. 1992. Myths of gender: biological theories about women and men, 2nd edn New York, NY: Basic Books. [Google Scholar]
- 2.Fausto-Sterling A. 2000. Sexing the body: gender politics and the construction of sexuality. New York, NY: Basic Books. [Google Scholar]
- 3.Jordan-Young R, Rumiati RI. 2012. Hardwired for sexism? Approaches to sex/gender in neuroscience. 5, 305–315. ( 10.1007/s12152-011-9134-4) [DOI] [Google Scholar]
- 4.McCarthy MM, Konkle ATM. 2005. When is a sex difference not a sex difference? Front Neuroendocrinol. 26, 85–102. ( 10.1016/j.yfrne.2005.06.001) [DOI] [PubMed] [Google Scholar]
- 5.Blackless M, Charuvastra A, Derryck A, Fausto-Sterling A, Lauzanne K, Lee E. 2000. How sexually dimorphic are we? Review and synthesis. Am. J. Hum. Biol. 12, 151–166. () [DOI] [PubMed] [Google Scholar]
- 6.Cosgrove KP, Mazure CM, Staley JK. 2007. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry 62, 847–855. ( 10.1016/j.biopsych.2007.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenroot RK, Giedd JN. 2010. Sex differences in the adolescent brain. Brain Cogn. 72, 46–55. ( 10.1016/j.bandc.2009.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. 2014. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 39, 34–50. ( 10.1016/j.neubiorev.2013.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan A, Ma W, Vira A, Marwha D, Eliot L. 2015. The human hippocampus is not sexually-dimorphic: meta-analysis of structural MRI volumes. Neuroimage 124, 350–366. ( 10.1016/j.neuroimage.2015.08.050) [DOI] [PubMed] [Google Scholar]
- 10.Allen LS, Gorski RA. 1990. Sex difference in the bed nucleus of the stria terminalis of the human brain. J. Comp. Neurol. 302, 697–706. ( 10.1002/cne.903020402) [DOI] [PubMed] [Google Scholar]
- 11.Byne W, Lasco MS, Kemether E, Shinwari A, Edgar MA, Morgello S, Jones LB, Tobet S. 2000. The interstitial nuclei of the human anterior hypothalamus: an investigation of sexual variation in volume and cell size, number and density. Brain Res. 856, 254–258. ( 10.1016/S0006-8993(99)02458-0) [DOI] [PubMed] [Google Scholar]
- 12.Chung WCJ, De Vries GJ, Swaab DF. 2002. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J. Neurosci. 22, 1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordero ME, Valenzuela CY, Torres R, Rodriguez A. 2000. Sexual dimorphism in number and proportion of neurons in the human median raphe nucleus. Brain Res. Dev. Brain Res. 124, 43–52. ( 10.1016/S0165-3806(00)00104-8) [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Falgueras A, Ligtenberg L, Kruijver FPM, Swaab DF. 2011. Galanin neurons in the intermediate nucleus (InM) of the human hypothalamus in relation to sex, age, and gender identity. J. Comp. Neurol. 519, 3061–3084. ( 10.1002/cne.22666) [DOI] [PubMed] [Google Scholar]
- 15.Kruijver FP, Zhou JN, Pool CW, Hofman MA, Gooren LJ, Swaab DF. 2000. Male-to-female transsexuals have female neuron numbers in a limbic nucleus. J. Clin. Endocrinol. Metab. 85, 2034–2041. ( 10.1210/jcem.85.5.6564) [DOI] [PubMed] [Google Scholar]
- 16.Swaab DF. 1995. Development of the human hypothalamus. Neurochem. Res. 20, 509–519. ( 10.1007/BF01694533) [DOI] [PubMed] [Google Scholar]
- 17.Kang HJ, et al. 2011. Spatio-temporal transcriptome of the human brain. Nature 478, 483–489. ( 10.1038/nature10523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bejerot S, Eriksson JM, Bonde S, Carlstrom K, Humble MB, Eriksson E. 2012. The extreme male brain revisited: gender coherence in adults with autism spectrum disorder. Br. J. Psychiatry 201, 116–123. ( 10.1192/bjp.bp.111.097899) [DOI] [PubMed] [Google Scholar]
- 19.Giedd JN, Raznahan A, Mills KL, Lenroot RK. 2012. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol. Sex Differ. 3, 19 ( 10.1186/2042-6410-3-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingalhalikar M, et al. 2014. Sex differences in the structural connectome of the human brain. Proc. Natl Acad. Sci. USA 111, 823–828. ( 10.1073/pnas.1316909110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Taylor K, Hackett G, Bullmore ET, Baron-Cohen S. 2012. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J. Neurosci. 32, 674–680. ( 10.1523/JNEUROSCI.4389-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnett D. 2013. Male and female brains: the REAL differences. The Guardian. 4 December 2013.
- 23.Edmonds M. 2008. Do men and women have different brains? See HowStuffWorks.com.
- 24.WEBMD 2015. How male and female brains differ. See http://www.webmd.com/balance/features/how-male-female-brains-differ.
- 25.Clayton JA. In press. Studying both sexes: a guiding principle for biomedicine. FASEB J. ( 10.1096/fj.15-279554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clayton JA, Collins FS. 2014. Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283. ( 10.1038/509282a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson J, Sharman Z, Vissandjee B, Stewart DE. 2014. Does a change in health research funding policy related to the integration of sex and gender have an impact? PLoS ONE 9, e99900 ( 10.1371/journal.pone.0099900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horizon 2020. 2013. Promoting gender equality in research and innovation. See http://www.HowstuffWorks.com.
- 29.Joel D. 2011. Male or female? Brains are intersex. Front. Integr. Neurosci. 5, 57 ( 10.3389/fnint.2011.00057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joel D. 2012. Genetic-gonadal-genitals sex (3G-sex) and the misconception of brain and gender, or, why 3G-males and 3G-females have intersex brain and intersex gender. Biol. Sex Differ. 3, 27 ( 10.1186/2042-6410-3-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reich CG, Taylor ME, McCarthy MM. 2009. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav. Brain Res. 203, 264–269. ( 10.1016/j.bbr.2009.05.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basta-Kaim A, et al. 2015. Prenatal administration of lipopolysaccharide induces sex-dependent changes in glutamic acid decarboxylase and parvalbumin in the adult rat brain. Neuroscience 287, 78–92. ( 10.1016/j.neuroscience.2014.12.013) [DOI] [PubMed] [Google Scholar]
- 33.Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, Papadopoulou-Daifoti Z. 2004. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience 126, 849–857. ( 10.1016/j.neuroscience.2004.04.044) [DOI] [PubMed] [Google Scholar]
- 34.Fumagalli F, Pasini M, Frasca A, Drago F, Racagni G, Riva MA. 2009. Prenatal stress alters glutamatergic system responsiveness in adult rat prefrontal cortex. J. Neurochem. 109, 1733–1744. ( 10.1111/j.1471-4159.2009.06088.x) [DOI] [PubMed] [Google Scholar]
- 35.Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. 1997. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 81, 689–697. ( 10.1016/S0306-4522(97)00233-9) [DOI] [PubMed] [Google Scholar]
- 36.Garrett JE, Wellman CL. 2009. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience 162, 195–207. ( 10.1016/j.neuroscience.2009.04.057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juraska JM. 1991. Sex differences in ‘cognitive’ regions of the rat brain. Psychoneuroendocrinology 16, 105–109. ( 10.1016/0306-4530(91)90073-3) [DOI] [PubMed] [Google Scholar]
- 38.Kuipers SD, Trentani A, van der Zee EA, den Boer JA. 2013. Chronic stress-induced changes in the rat brain: role of sex differences and effects of long-term tianeptine treatment. Neuropharmacology 75, 426–436. ( 10.1016/j.neuropharm.2013.08.018) [DOI] [PubMed] [Google Scholar]
- 39.Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu A, Li X, Westenbroek C. 2009. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb. Cortex 19, 1978–1989. ( 10.1093/cercor/bhn225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCormick CM, Smythe JW, Sharma S, Meaney MJ. 1995. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res. Dev. Brain Res. 84, 55–61. ( 10.1016/0165-3806(94)00153-Q) [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin KJ, Baran SE, Conrad CD. 2009. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol. Neurobiol. 40, 166–182. ( 10.1007/s12035-009-8079-7) [DOI] [PubMed] [Google Scholar]
- 42.Mitsushima D, Masuda J, Kimura F. 2003. Sex differences in the stress-induced release of acetylcholine in the hippocampus and corticosterone from the adrenal cortex in rats. Neuroendocrinology 78, 234–240. ( 10.1159/000073707) [DOI] [PubMed] [Google Scholar]
- 43.Oomen CA, Girardi CE, Cahyadi R, Verbeek EC, Krugers H, Joels M, Lucassen PJ. 2009. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PLoS ONE 4, e3675 ( 10.1371/journal.pone.0003675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhodes ME, Creel TJ, Nord AN. 2009. Sex differences in CNS neurotransmitter influences on behavior. Horm. Brain Behav. 5, 2747–2785. ( 10.1016/B978-008088783-8.00087-5) [DOI] [Google Scholar]
- 45.Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. 2006. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology 147, 2506–2517. ( 10.1210/en.2005-1054) [DOI] [PubMed] [Google Scholar]
- 46.Rothstein S, Simkins T, Nunez JL. 2008. Response to neonatal anesthesia: effect of sex on anatomical and behavioral outcome. Neuroscience 152, 959–969. ( 10.1016/j.neuroscience.2008.01.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shors TJ, Chua C, Falduto J. 2001. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 21, 6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suarez J, Llorente R, Romero-Zerbo SY, Mateos B, Bermudez-Silva FJ, de Fonseca FR, Viveros MP. 2009. Early maternal deprivation induces gender-dependent changes on the expression of hippocampal CB(1) and CB(2) cannabinoid receptors of neonatal rats. Hippocampus 19, 623–632. ( 10.1002/hipo.20537) [DOI] [PubMed] [Google Scholar]
- 49.Vathy I. 2001. Prenatal morphine exposure induces age- and sex-dependent changes in seizure susceptibility. Progress Neuro-Psychopharmacol.Biol. Psychiatry 25, 1203–1226. ( 10.1016/S0278-5846(01)00187-7) [DOI] [PubMed] [Google Scholar]
- 50.Vathy I. 2002. Prenatal opiate exposure: long-term CNS consequences in the stress system of the offspring. Psychoneuroendocrinology 27, 273–283. ( 10.1016/S0306-4530(01)00049-X) [DOI] [PubMed] [Google Scholar]
- 51.Vathy I, Katay L. 1992. Effects of prenatal morphine on adult sexual behavior and brain catecholamines in rats. Brain Res. Dev. Brain Res. 68, 125–131. ( 10.1016/0165-3806(92)90254-T) [DOI] [PubMed] [Google Scholar]
- 52.Viveros MP, Llorente R, Lopez-Gallardo M, Suarez J, Bermudez-Silva F, De la Fuente M, Rodriguez de Fonseca F, Garcia-Segura LM. 2009. Sex-dependent alterations in response to maternal deprivation in rats. Psychoneuroendocrinology 34(Suppl 1), S217–S226. ( 10.1016/j.psyneuen.2009.05.015) [DOI] [PubMed] [Google Scholar]
- 53.Wilber AA, Southwood CJ, Sokoloff G, Steinmetz JE, Wellman CL. 2007. Neonatal maternal separation alters adult eyeblink conditioning and glucocorticoid receptor expression in the interpositus nucleus of the cerebellum. Dev Neurobiol. 67, 1751–1764. ( 10.1002/dneu.20549) [DOI] [PubMed] [Google Scholar]
- 54.Zuena AR, et al. 2008. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS ONE 3, e2170 ( 10.1371/journal.pone.0002170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joel D, et al. 2015. Sex beyond the genitalia: the human brain mosaic. Proc. Natl Acad. Sci. USA 112, 15 468–15 473. ( 10.1073/pnas.1509654112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Domes G, Schulze L, Bottger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC. 2010. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum. Brain Mapp. 31, 758–769. ( 10.1002/hbm.20903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ihnen SKZ, Church JA, Petersen SE, Schlaggar BL. 2009. Lack of generalizability of sex differences in the fMRI BOLD activity associated with language processing in adults. Neuroimage 45, 1020–1032. ( 10.1016/j.neuroimage.2008.12.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaiser A, Haller S, Schmitz S, Nitsch C. 2009. On sex/gender related similarities and differences in fMRI language research. Brain Res. Rev. 61, 49–59. ( 10.1016/j.brainresrev.2009.03.005) [DOI] [PubMed] [Google Scholar]
- 59.De Vries GJ, Boyle PA. 1998. Double duty for sex differences in the brain. Behav. Brain Res. 92, 205–213. ( 10.1016/S0166-4328(97)00192-7) [DOI] [PubMed] [Google Scholar]
- 60.de Vries GJ, Forger NG. 2015. Sex differences in the brain: a whole body perspective. Biol. Sex Differ. 6, 15 ( 10.1186/s13293-015-0032-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Vries GJ, Sodersten P. 2009. Sex differences in the brain: the relation between structure and function. Horm. Behav. 55, 589–596. ( 10.1016/j.yhbeh.2009.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gillies GE, McArthur S. 2010. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol. Rev. 62, 155–198. ( 10.1124/pr.109.002071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arnold AP, Lusis AJ. 2012. Understanding the sexome: measuring and reporting sex differences in gene systems. Endocrinology 153, 2551–2555. ( 10.1210/en.2011-2134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goy RW, Goldfoot DA. 1975. Neuroendocrinology: animal models and problems of human sexuality. Arch. Sex Behav. 4, 405–420. ( 10.1007/BF01541724) [DOI] [PubMed] [Google Scholar]
- 65.Hyde JS. 2005. The gender similarities hypothesis. Am. Psychol. 60, 581–592. ( 10.1037/0003-066X.60.6.581) [DOI] [PubMed] [Google Scholar]
- 66.Hyde JS. 2014. Gender similarities and differences. Annu. Rev. Psychol. 65, 373–398. ( 10.1146/annurev-psych-010213-115057) [DOI] [PubMed] [Google Scholar]
- 67.Whalen RE. 1974. Sexual differentiation: models, methods and mechanisms. In Sex differences in behavior (eds Friedman RC, Riehart RM, Van de Wiele RL), pp. 467–481. New York, NY: John Wiley & Sons. [Google Scholar]
- 68.Zell E, Krizan Z, Teeter SR. 2015. Evaluating gender similarities and differences using metasynthesis. Am. Psychol. 70, 10–20. ( 10.1037/a0038208) [DOI] [PubMed] [Google Scholar]
- 69.Larson FV, et al. 2015. Testing the ‘extreme female brain’ theory of psychosis in adults with autism spectrum disorder with or without co-morbid psychosis. PLoS ONE 10, e0128102 ( 10.1371/journal.pone.0128102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.European Network of National Networks studying Gene-Environment Interactions in Schizophrenia 2014. Identifying gene-environment interactions in schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophrenia Bull. 40, 729–736. ( 10.1093/schbul/sbu069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCarroll SA, Altshuler DM. 2007. Copy-number variation and association studies of human disease. Nat. Genet. 39, S37–S42. ( 10.1038/ng2080). [DOI] [PubMed] [Google Scholar]
- 72.Morris AP, et al. 2012. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 44, 981–990. ( 10.1038/ng.2383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fine C, Fidler F. 2015. Sex and power: why sex/gender neuroscience should motivate statistical reform. In Handbook of neuroethics (eds Clausen J, Levy N), pp. 467–481. Dordrecht, The Netherlands: Springer Science+Business Media. [Google Scholar]
- 74.Springer KW, Mager Stellman J, Jordan-Young RM. 2011. Beyond a catalogue of differences: A theoretical frame and good practice guidelines for researching sex/gender in human health. Soc. Sci. Med. 74, 1817–1824. ( 10.1016/j.socscimed.2011.05.033) [DOI] [PubMed] [Google Scholar]
- 75.Krieger N. 2001. Theories for social epidemiology in the 21st century: an ecosocial perspective. Int. J. Epidemiol. 30, 668–677. ( 10.1093/ije/30.4.668) [DOI] [PubMed] [Google Scholar]
- 76.Krieger N. 2003. Gender, sexes, and health: what are the connections - and why does it matter? Int. J. Epidemiol. 32, 652–657. ( 10.1093/ije/dyg156) [DOI] [PubMed] [Google Scholar]
- 77.Fine C, Jordan-Young R, Kaiser A, Rippon G. 2013. Plasticity, plasticity, plasticity and the rigid problem of sex. Trends Cogn. Sci. 17, 550–551. ( 10.1016/j.tics.2013.08.010) [DOI] [PubMed] [Google Scholar]
- 78.Rippon G, Jordan-Young R, Kaiser A, Fine C. 2014. Recommendations for sex/gender neuroimaging research: key principles and implications for research design, analysis, and interpretation. Front. Hum. Neurosci. 8, 650 ( 10.3389/fnhum.2014.00650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carothers BJ, Reis HT. 2013. Men and women are from Earth: examining the latent structure of gender. J. Pers. Soc. Psychol. 104, 385–407. ( 10.1037/a0030437) [DOI] [PubMed] [Google Scholar]
- 80.Egan SK, Perry DG. 2001. Gender identity: a multidimensional analysis with implications for psychosocial adjustment. Dev. Psychol. 37, 451–463. ( 10.1037/0012-1649.37.4.451) [DOI] [PubMed] [Google Scholar]
- 81.Koestner R, Aube J. 1995. A multifactorial approach to the study of gender characteristics. J. Pers. 63, 681–710. ( 10.1111/j.1467-6494.1995.tb00510.x) [DOI] [PubMed] [Google Scholar]
- 82.Cahill L. 2006. Why sex matters for neuroscience. Nat. Rev. Neurosci. 7, 477–484. ( 10.1038/nrn1909) [DOI] [PubMed] [Google Scholar]
- 83.Fernandez-Guasti A, Fiedler JL, Herrera L, Handa RJ. 2012. Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm. Metab. Res. 44, 607–618. ( 10.1055/s-0032-1312592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Franconi F, Campesi I, Occhioni S, Antonini P, Murphy MF. 2012. Sex and gender in adverse drug events, addiction, and placebo. Handb. Exp. Pharmacol. 214, 107–126. ( 10.1007/978-3-642-30726-3_6) [DOI] [PubMed] [Google Scholar]
- 85.Mathis MAD, Alvarenga PD, Funaro G, Torresan RC, Moraes I, Torres AR, Zilberman ML, Hounie AG. 2011. Gender differences in obsessive-compulsive disorder: a literature review. Rev. Bras. Psiquiatr. 33, 390–399. ( 10.1590/S1516-44462011000400014) [DOI] [PubMed] [Google Scholar]
- 86.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. 2012. Sex differences in the brain: the not so inconvenient truth. J. Neurosci. 32, 2241–2247. ( 10.1523/JNEUROSCI.5372-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mendrek A, Stip E. 2011. Sexual dimorphism in schizophrenia: is there a need for gender-based protocols? Expert Rev. Neurother. 11, 951–959. ( 10.1586/ern.11.78) [DOI] [PubMed] [Google Scholar]
- 88.Vega P, Barbeito S, Ruiz de Azua S, Martinez-Cengotitabengoa M, Gonzalez-Ortega I, Saenz M, Gonzalez-Pinto A. 2011. Bipolar disorder differences between genders: special considerations for women. Womens Health (Lond Engl) 7, 663–674; quiz 675–666 ( 10.2217/whe.11.71) [DOI] [PubMed] [Google Scholar]
- 89.Cheslack-Postava K, Jordan-Young RM. 2012. Autism spectrum disorders: toward a gendered embodiment model. Soc. Sci. Med. 74, 1667–1674. ( 10.1016/j.socscimed.2011.06.013) [DOI] [PubMed] [Google Scholar]
- 90.Hayward C, Sanborn K. 2002. Puberty and the emergence of gender differences in psychopathology. J. Adolesc. Health 30, 49–58. ( 10.1016/S1054-139X(02)00336-1) [DOI] [PubMed] [Google Scholar]
- 91.Nolen-Hoeksema S. 2012. Emotion regulation and psychopathology: the role of gender. Annu. Rev. Clin. Psychol. 8, 161–187. ( 10.1146/annurev-clinpsy-032511-143109). [DOI] [PubMed] [Google Scholar]
- 92.Joel D, Yankelevitch-Yahav R. 2014. Reconceptualizing sex, brain and psychopathology: interaction, interaction, interaction. Br. J. Pharmacol. 171, 4620–4635. ( 10.1111/bph.12732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arnold AP, Chen X. 2009. What does the ‘four core genotypes’ mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 30, 1–9. ( 10.1016/j.yfrne.2008.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crews D, Rushworth D, Gonzalez-Lima F, Ogawa S. 2009. Litter environment affects behavior and brain metabolic activity of adult knockout mice. Front. Behav. Neurosci. 3, 12 ( 10.3389/neuro.08.012.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Medeiros CB, Rees SL, Llinas M, Fleming AS, Crews D. 2010. Deconstructing early life experiences: distinguishing the contributions of prenatal and postnatal factors to adult male sexual behavior in the rat. Psychol. Sci. 21, 1494–1501. ( 10.1177/0956797610382122) [DOI] [PubMed] [Google Scholar]
- 96.Kinsley CH, Konen CM, Miele JL, Ghiraldi L, Svare B. 1986. Intrauterine position modulates maternal behaviors in female mice. Physiol. Behav. 36, 793–799. ( 10.1016/0031-9384(86)90434-8) [DOI] [PubMed] [Google Scholar]
- 97.Prendergast BJ, Onishi KG, Zucker I. 2014. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 40, 1–5. ( 10.1016/j.neubiorev.2014.01.001) [DOI] [PubMed] [Google Scholar]
- 98.Richardson SS, Reiches M, Shattuck-Heidorn H, LaBonte ML, Consoli T. 2015. Opinion: focus on preclinical sex differences will not address women's and men's health disparities. Proc. Natl Acad. Sci. USA 112, 13 419–13 420. ( 10.1073/pnas.1516958112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ritz SA, Antle DM, Cote J, Deroy K, Fraleigh N, Messing K, Parent L, St-Pierre J, Vaillancourt C, Mergler D. 2014. First steps for integrating sex and gender considerations into basic experimental biomedical research. FASEB J. 28, 4–13. ( 10.1096/fj.13-233395) [DOI] [PubMed] [Google Scholar]