Abstract
Female song is an ancestral trait in songbirds, yet extant females generally sing less than males. Here, we examine sex differences in the predation cost of singing behaviour. The superb fairy-wren (Malurus cyaneus) is a Southern Hemisphere songbird; males and females provision the brood and produce solo song year-round. Both sexes had higher song rate during the fertile period and lower song rate during incubation and chick feeding. Females were more likely than males to sing close to or inside the nest. For this reason, female but not male song rate predicted egg and nestling predation. This study identifies a high fitness cost of song when a parent bird attends offspring inside a nest and explains gender differences in singing when there are gender differences in parental care.
Keywords: superb fairy-wren, Maluridae, song rate, predation risk, nest attendance, reproductive cost
1. Introduction
In songbirds, condition-dependent song is generally considered a sexually selected trait used by males to repel rivals and attract females [1,2]. However, there is growing focus on the occurrence [3] and functions of female song [4], mostly using the perspective of life history and social selection theory. Female song is widespread and ancestral in songbirds, and females sing across 71% of extant species spanning 32 families [5]. Many Southern Hemisphere songbirds are sedentary, and females and their pair males sing solo song year-round to defend the territory [6,7]. Females generally sing less than males. The findings by Odom et al. [5] raise questions about why some females have lost or gained song, and why many females currently sing less than males. Singing is a variable behaviour and not a fixed trait, and therefore a songbird may increase or decrease its song rate in relation to how it perceives its surroundings, including social and ecological context [8,9]. One approach to understand gender differences in singing behaviour is to test if there are gender differences in the costs of singing, which is the aim of this study.
Our study system is the superb fairy-wren (Malurus cyaneus), a sedentary long-lived Southern Hemisphere songbird. Male and female fairy-wrens sing solo ‘chatter’ song across the year [6], and both sexes defend the territory against intruders [10]. Male and female superb fairy-wrens differ in patterns of parental care: the female is a uniparental incubator and both sexes feed the chicks [11]. We test if fairy-wren song rate increases nest predation in relation to nesting phase and primary parental care provider. (1) During the fertile period, song rate should be high in both sexes given no nest attendance; (2) during incubation, female song rate should predict egg predation because females are uniparental incubators; (3) during chick attendance, pair song rate should predict chick predation because both sexes feed the young. (4) Both sexes should vocalize away from the nest to reduce predation risk from nest conspicuousness. (5) At artificial nests at which we broadcast female song, we predict higher egg predation when there is a higher rate of song. Prediction (1) could predict either a low song rate at later stages or (4) that both sexes will sing away from the nest. If prediction (4) is correct, then (2) and (3) may not follow; that is, if neither sex sings near the nest, then the song rate of neither sex would matter. If both sexes have the same song rate at the nest, then there should not be an effect of sex-specific song rate on predation.
2. Material and methods
We monitored chatter song rate and nesting outcome at 72 wild superb fairy-wren nests from September to December during 2013 and 2014 at Cleland Wildlife Sanctuary (34°58′ S, 138°41′ E) and Newland Head Conservation Park (35°37′ S, 138°29′ E). One nest was analysed per nesting phase: fertile period (N = 20), incubation (N = 26) and chick feeding (N = 26). In 2014, we measured egg predation at 45 artificial domed nests in relation to experimental broadcast of song rate at Scott Creek Conservation Park (35°05′ S, 138°41′ E).
Male and female fairy-wrens produce a solo chatter song that consists of approximately eight different vocal elements produced approximately 50 times per song for approximately 3 s [10]. Fairy-wrens learn this song as fledglings and produce the song as adults (C.E., S.K. 2015, unpublished data) [12]. We have previously studied incubation calls in this system. Incubation calls are quieter than chatter songs (approx. 60 dB versus approx. 87 dB at 1 m) and are produced by incubating females while inside the nest; the incubation call consists of two vocal elements repeated approximately five times for approximately 1 s [13,14]. In general, songbird songs are learned, have many elements and are produced by adults; calls have few elements and are produced by all age groups [15].
(a). Song rate and predation at natural nests
Territories were monitored every 3 days to record date of first egg, hatching success, predation and vocalization behaviour. We scored the number of chatter songs per nesting phase. The fertile period was considered to begin approximately 5 days before egg-laying and terminate with egg-laying; we scored number of chatter songs per 20 min (multiplied by three to estimate songs per hour) and retrospectively assigned nests after determining date of first egg. All nest observations were done between 07.00 to 10.00. Incubation and nestling phase are each approximately 15 days. We scored number of songs per hour during 1 h of nest observation during either incubation (egg age: 10–12 days) or chick feeding (chick age: 2–4 days). At 12 nests in 2014, we recorded minimum distance (m) of singer to nest and the proportion of nests at which the female produced chatter song inside the nest. For nest observations, the observer was hidden in vegetation (approx. 15 m from the nest). Given the estimate error for birds singing from vegetation near the nest, we used ‘minimum distance of the singer to the nest’ for statistical analysis. We noted egg and chick predation when nest contents were missing during 3-day nest checks; chicks that survived to 10 days were considered to have fledged.
(b). Song rate and predation at artificial nests
From 20 September to 5 October 2014, we experimentally tested the effect of female song rate on egg predation. Artificial domed nests each baited with one quail egg were placed every 30 m along three transects; each transect was separated by 500 m. For 3 h (07.00 to 10.00) at every nest including control nests, we placed a MoshiTM BassBurger rechargeable portable speaker (sensitivity: greater than 80 dB; frequency response: 280 Hz–16 kHz) connected to an Apple iPod (Apple Inc., USA) below the nest. At every second nest, we broadcast female chatter song at low song rate (six calls per hour), and at every third nest we broadcast female chatter song at high song rate (20 calls per hour). We saved the playback stimuli as uncompressed 16 bit 44.1 kHz broadcast wav files using Amadeus Pro v. 1.5; playbacks were 85–88 dB SLP at 1 m, which is within the natural level. We broadcast chatter song every day for 3 days and analysed predation outcome after 3 days (presented here), as well as 14 days (data available from Dryad). Predation was scored if the egg was missing.
Data were analysed with SPSS 20 for Windows (SPSS Inc., Chicago, IL, USA). The variable ‘number of songs per hour’ was log transformed to satisfy requirements of normality for parametric tests. We confirmed homogeneity of variance prior to using ANOVA.
3. Results
(a). Natural nests
The number of pair male and female chatter songs per hour was significantly correlated during the fertile period (r = 0.83, N = 20, p < 0.001), but not during incubation (r = −0.04, N = 26, p = 0.858) or chick feeding (r = 0.33, N = 26, p = 0.099) (figure 1). Song rate differed significantly across the three nesting phases (ANOVA: males: F2,71 = 14.22, p < 0.001, partial η2 = 0.29; females: F2,71 = 6.07, p = 0.004, partial η2 = 0.15). In males, song rate was highest during the fertile period (23.5 ± 4.7) compared with incubation (11.3 ± 3.1) and chick feeding (4.3 ± 0.9). Female song rate was also highest during the fertile period (17.5 ± 3.8) compared with incubation (8.7 ± 1.3) and chick feeding (6.0 ± 1.4). Using paired t-test with log-transformed data, males sang more than females during the fertile phase (t = 2.29, p = 0.034), but song rate in males and females was comparable during incubation and feeding (both p > 0.2). Males had higher song rate during incubation than feeding (independent t-test: t = 2.7, p = 0.010); female song rate was comparable between incubation and feeding (independent t-test: t = 1.68, p = 0.099).
Figure 1.
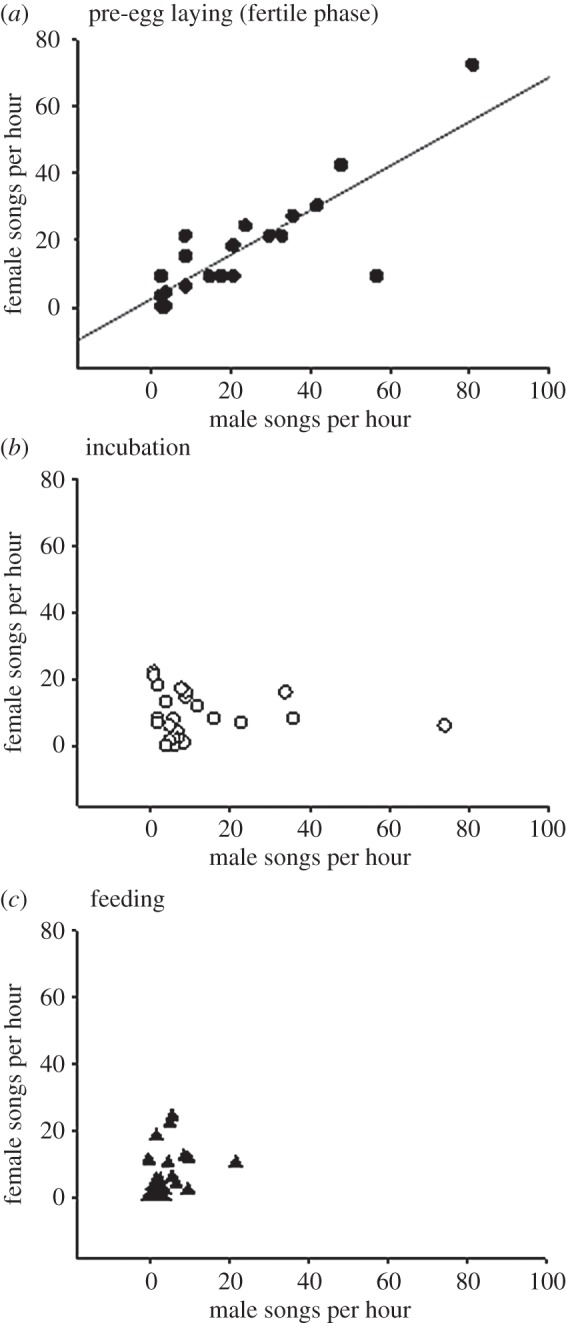
The association between pair male and female superb fairy-wren chatter song rate during the fertile (N = 20 nests), incubation (N = 26 nests) and chick feeding (N = 26 nests) phases. Data are independent per nesting phase.
During incubation, high female song rate predicted egg predation (multiple regression: female song rate: rpart = 0.64, p = 0.001; male song rate: rpart = −0.152, p = 0.467) (histograms in figure 2). During the chick phase, high female song rate predicted chick predation (female song rate: rpart = 0.411, p = 0.041; male song rate: rpart = −0.241, p = 0.246) (histograms in figure 2). Total songs per hour per nest was not significantly associated with egg or chick predation (both p > 0.3). Females sang significantly closer (m) to the nest (0.7 ± 0.3) compared with males (6.3 ± 0.5 m) (paired t-test: t = −10.633, d.f. = 11, p < 0.001). A higher proportion of females (6/12) sang while inside the nest compared with males (0/12) (likelihood ratio = 10.357, p = 0.005, Cramer's V = 0.477). At all six nests with female chatter song inside the nest, the female produced one chatter song.
Figure 2.
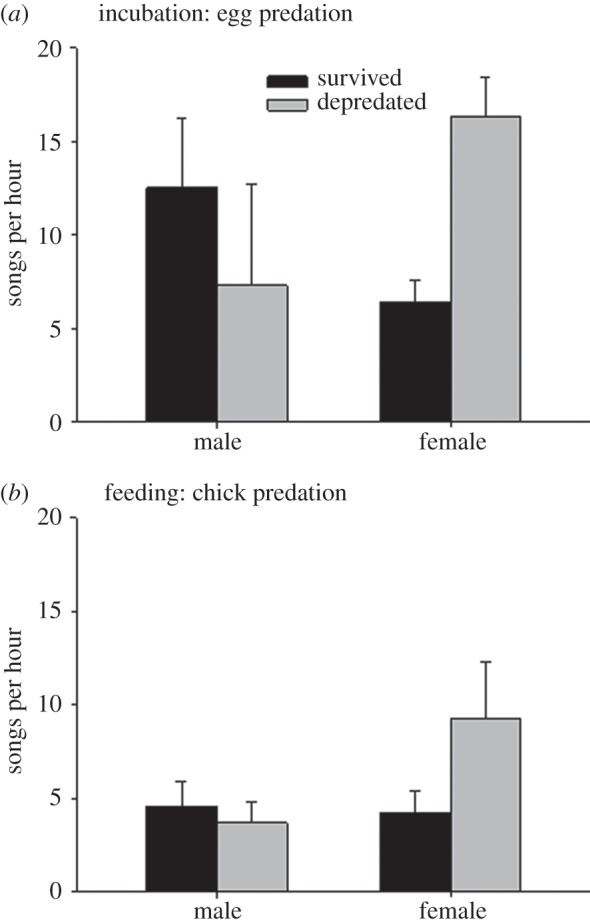
The number of chatter songs (mean ± s.e.) by attending male and female superb fairy-wrens in relation to (a) egg predation and (b) chick predation. Female song rate was significantly higher at depredated nests. Male song rate did not predict predation.
(b). Artificial nests
Egg predation was significantly different across treatment groups (likelihood ratio = 9.834, p = 0.007). Egg predation was lowest at control nests (0%), intermediate at nests with low song rate (20%) and highest at nests with high song rate (40%).
4. Discussion
In this study, we show sex differences in the predation cost to singing, which provides a new perspective to test differences in singing behaviour when both sexes produce solo song. The number of female, but not male, songs per hour predicted egg and chick predation at natural nests. Compared with males, female chatter song is shorter [10], and therefore song characteristics are an unlikely explanation for the observed difference in predation. Females sang significantly closer (m) to the nest than males and were more likely to produce song while inside the nest. Female song likely revealed the nest location to predators [16]. Artificial nests at which we experimentally broadcast higher song rate had more egg predation. While our experimental chatter song rate was within the normal range observed for 15 m near the nest, it was higher than that observed for females inside the nest, which could have exaggerated effect size.
Male and female song rate was positively correlated during the fertile period (see also [17]) but not during incubation and feeding (figure 1). The change in pattern of association (but no significant difference in song rate between the sexes) suggests different mechanisms and/or functions of song in males and females across the nesting phase [18,19]. It remains to be tested if females with lower song rate produce more offspring (silent female hypothesis) or if females that adaptively adjust song rate produce more offspring (adaptive female song rate hypothesis). We did not compare song rate in the same bird across nesting phase and cannot comment on singing consistency [20]. Males and females with eggs and chicks had lower song rate than birds during the fertile phase, and females that sang more incurred more nest predation.
Non-human animals have adaptive risk assessment and attend to aural cues of predators and other brood threats [21–23]. One explanation for different patterns of male and female song rate is that each sex is more likely to encounter different threats while attending the nest. Females have been shown to adjust vocalization behaviour to aural threats. Previously, our group showed increased in-nest incubation call rate by females experimentally exposed to a brood parasite threat [24]; higher incubation call rate resulted in benefits and costs. Fairy-wren embryos exposed to many incubation calls had higher vocal copy accuracy as chicks and received more parental feeds [24], parents had improved discrimination of intruder (cuckoo) chicks that did not learn as embryos [13], but nests with many incubation calls had more egg predation [25]. Here, we found that female chatter song rate, similar to female incubation call rate, increased nest predation. It is unknown if fairy-wrens have a capacity for predator risk assessment that affects song rate—but intriguingly, Fontaine & Martin [9] found that male song rate increased after the experimental removal of nest predators.
Solo chatter song likely has multiple functions in fairy-wrens, including territory defence [4,6,10,20]. What is novel about this study is that 50% of nesting females sang chatter song from inside the nest. Females produced song if they happened to be inside the nest when the male sang upon arrival within 15 m of the nest, but females did not initiate song from inside the nest (S.K. 2014, personal observation). This raises questions about additional functions of female song (e.g. pair-bond, vocal tutoring). Notably, male-only care of eggs occurred in some of the oldest bird lineages (e.g. megapodes, ratites) [26]. Given the high costs of female song under conditions of in-nest parental care, the evolution of avian sociality is creating strong selection on female vocalization behaviour including, we suggest, selection for cognitive capacity to discriminate and assess predation threats during nest attendance.
Ethics
This study was approved by the Animal Welfare Committee of Flinders University (E234–236) and supported by a scientific research permit (Z24699 4).
Data accessibility
Data are deposited in Dryad: http://dx.doi.org/10.5061/dryad.k5f6q.
Authors' contributions
S.K. conceived/designed the study, collected data and wrote the manuscript; C.E. and K.M. collected data and contributed to study design, manuscript preparation and revision.
Competing interests
The authors declare no competing interests. All authors approve the final version and all agree to be held accountable for the content therein.
Funding
This project was funded by Hermon Slade Foundation, Australian Research Council, Birds South Australia and ANZ Holsworth.
References
- 1.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Catchpole CK, Slater PJ. 2003. Bird song: biological themes and variations. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Najar N, Benedict L. 2015. Female song in New World wood warblers. Front. Ecol. Evol. 3, 139 ( 10.3389/fevo.2015.00139) [DOI] [Google Scholar]
- 4.Cain KE, Cockburn AL, Langmore NE. 2015. Female song rates in response to simulated intruder are positively related to reproductive success. Front. Ecol. Evol. 3, 119 ( 10.3389/fevo.2015.00119) [DOI] [Google Scholar]
- 5.Odom KJ, Hall ML, Riebel K, Omland KE, Langmore NE. 2014. Female song is widespread and ancestral in songbirds. Nat. Commun. 5, 3379 ( 10.1038/ncomms4379) [DOI] [PubMed] [Google Scholar]
- 6.Cooney R, Cockburn A. 1995. Territorial defence is the major function of female song in the superb fairy-wren, Malurus cyaneus. Anim. Behav. 49, 1635–1647. ( 10.1016/0003-3472(95)90086-1) [DOI] [Google Scholar]
- 7.Brunton DH, Li X. 2006. The song structure and seasonal patterns of vocal behavior of male and female bellbirds (Anthornis melanura). J. Ethol. 24, 17–25. ( 10.1007/s10164-005-0155-5) [DOI] [Google Scholar]
- 8.Zoratto F, Manzari L, Oddi L, Pinxten R, Eens M, Santucci D, Alleva E, Carere C. 2014. Behavioural response of European starlings exposed to video playback of conspecific flocks: effect of social context and predator threat. Behav. Process. 103, 269–277. ( 10.1016/j.beproc.2014.01.012) [DOI] [PubMed] [Google Scholar]
- 9.Fontaine JJ, Martin TE. 2006. Habitat selection responses of parents to offspring predation risk: an experimental test. Am. Nat. 168, 811–818. ( 10.1086/508297) [DOI] [PubMed] [Google Scholar]
- 10.Kleindorfer S, Evans C, Mihailova M, Colombelli-Negrel D, Hoi H, Griggio M, Mahr K, Robertson J. 2013. When subspecies matter: resident superb fairy-wrens (Malurus cyaneus) distinguish the sex and subspecies of intruder birds. EMU 113, 259–269. ( 10.1071/MU12066) [DOI] [Google Scholar]
- 11.Rowley I, Russell E. 2007. Family Maluridae (fairy-wrens). In Picathartes to tits and chickadees, Handbook of the Birds of the World, vol. 12 (eds J del Hoyo, A Elliott, DA Christie), pp. 490–531. Barcelona: Lynx Edicions. [Google Scholar]
- 12.Greig EI, Taft BN, Pruett-Jones S. 2012. Sons learn songs from their social fathers in a cooperatively breeding bird. Proc. R. Soc. B 279, 3154–3160. ( 10.1098/rspb.2011.2582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombelli-Négrel D, Hauber ME, Robertson J, Sulloway FJ, Hoi H, Griggio M, Kleindorfer S. 2012. Embryonic learning of vocal passwords in superb fairy-wrens reveals intruder cuckoo nestlings. Curr. Biol. 22, 2155–2160. ( 10.106/j.cub.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 14.Colombelli-Négrel D, Hauber ME, Kleindorfer S. 2014. Prenatal learning in an Australian songbird: habituation and individual discrimination in superb fairy-wren embryos. Proc. R. Soc. B 281, 20141154 ( 10.1098/rspb.2014.1154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price PH. 1979. Developmental determinants of structure in zebra finch song. J. Comp. Physiol. Psychol. 93, 260–277. ( 10.1037/h0077553) [DOI] [Google Scholar]
- 16.Magrath RD, Haff TM, Horn AG, Leonard ML. 2010. Calling in the face of danger: predation risk and acoustic communication by parent birds and their offspring. Adv. Study Behav. 41, 187–253. ( 10.1016/S0065-3454(10)41006-2) [DOI] [Google Scholar]
- 17.Hall ML, Peters A. 2008. Coordination between the sexes for territorial defence in a duetting fairy-wren. Anim. Behav. 76, 65–73. ( 10.1016/j.anbehav.2008.01.010) [DOI] [Google Scholar]
- 18.Peters A, Kingma SA, Delhey K. 2013. Seasonal male plumage as a multi-component sexual signal: insights and opportunities. EMU 113, 232–247. ( 10.1071/MU12083) [DOI] [Google Scholar]
- 19.Chiver I, Stutchbury BJ, Morton ES. 2015. The function of seasonal song in a tropical resident species, the red-throated ant-tanager (Habia fuscicauda). J. Ornithol. 156, 55–63. ( 10.1007/s10336-014-1139-4) [DOI] [Google Scholar]
- 20.Cain KE, Langmore NE. 2015. Female and male song rates across breeding stage: testing for sexual and nonsexual functions of female song. Anim. Behav. 109, 65–71. ( 10.1016/j.anbehav.2015.07.034) [DOI] [Google Scholar]
- 21.Kleindorfer S, Evans C, Colombelli-Négrel D, Robertson J, Griggio M, Hoi H. 2013. Host response to cuckoo song is predicted by the future risk of brood parasitism. Front. Zool. 10, 30 ( 10.1186/1742-9994-10-30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumstein DT, Cooley L, Winternitz J, Daniel JC. 2008. Do yellow-bellied marmots respond to predator vocalizations? Behav. Ecol. Sociobiol. 62, 457–468. ( 10.1007/s00265-007-0473-4) [DOI] [Google Scholar]
- 23.Chan AAY-H, Blumstein DT. 2011. Attention, noise, and implications for wildlife conservation and management. Appl. Anim. Behav. Sci. 131, 1–7. ( 10.1016/j.applanim.2011.01.007) [DOI] [Google Scholar]
- 24.Kleindorfer S, Evans C, Colombelli-Négrel D. 2014. Females that experience threat are better teachers. Biol. Lett. 10, 20140046 ( 10.1098/rsbl.2014.0046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleindorfer S, Hoi H, Evans C, Mahr K, Robertson J, Hauber ME, Colombelli-Négrel D. 2014. The cost of teaching embryos in superb fairy-wrens. Behav. Ecol. 25, 1131–1135. ( 10.1093/beheco/aru097) [DOI] [Google Scholar]
- 26.Cockburn A. 2006. Prevalence of different modes of parental care in birds. Proc. R. Soc. B 273, 1375–1383. ( 10.1098/rspb.2005.3458) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are deposited in Dryad: http://dx.doi.org/10.5061/dryad.k5f6q.


