Abstract
We tested experimentally if photoautotrophic microorganisms are a carbon source for invertebrates in temperate soils. We exposed forest or arable soils to a 13CO2-enriched atmosphere and quantified 13C assimilation by three common animal groups: earthworms (Oligochaeta), springtails (Hexapoda) and slugs (Gastropoda). Endogeic earthworms (Allolobophora chlorotica) and hemiedaphic springtails (Ceratophysella denticulata) were highly 13C enriched when incubated under light, deriving up to 3.0 and 17.0%, respectively, of their body carbon from the microbial source in 7 days. Earthworms assimilated more 13C in undisturbed soil than when the microbial material was mixed into the soil, presumably reflecting selective surface grazing. By contrast, neither adult nor newly hatched terrestrial slugs (Deroceras reticulatum) grazed on algal mats. Non-photosynthetic 13CO2 fixation in the dark was negligible. We conclude from these preliminary laboratory experiments that, in addition to litter and root-derived carbon from vascular plants, photoautotrophic soil surface microorganisms (cyanobacteria, algae) may be an ecologically important carbon input route for temperate soil animals that are traditionally assigned to the decomposer channel in soil food web models and carbon cycling studies.
Keywords: carbon sequestration, energy channels, soil fauna, soil food webs, stable isotope tracer, terrestrial carbon cycle
1. Introduction
Biological soil crusts formed by algae and other photoautotrophic microorganisms are known to be important carbon (C) sources in extreme climatic regions [1,2]. By contrast, few studies have considered such C inputs into soil food webs in temperate regions, focusing on soil organic matter [3] and microorganisms [4]. For soil invertebrate animals, traditional models assumed that vascular plants are the principal C sources, either as living plants (for herbivores such as molluscs) or as plant litter (for detritivores such as earthworms) [5]. However, recent research has shown that C inputs from living roots also need to be considered [6,7]. A third route of C input into temperate soil food webs not yet quantified is via photoautotrophic soil microorganisms. Grazing on and ingestion of soil algae, for example, have been observed in a number of soil animal taxa [8–10], but the functional significance of this C route for animal ecology and soil C dynamics is not known. Here, we report preliminary experiments under controlled laboratory conditions in which we quantified assimilated (rather than ingested) 13C tracer in soil invertebrates derived from atmospheric 13CO2 in the absence of vascular plants.
2. Material and methods
(a). Experimental set-up
Mineral soil (top 5 cm) was collected from a Sitka spruce (Picea sitchensis (Bong.) Carr.) stand in Portlaois, Ireland, and from a cultivated cereal plot at Thornfield, Belfield campus of University College Dublin, Ireland. The forest soil was a clay with 3.8% C, 0.34% N, and the arable soil was a loam with 2.52% C, 0.27% N.
Six large (2.7 l), square glass jars with rubber-seal cliptops (Kilner™, Rayware Group, Liverpool, UK), lying on the side, were filled with a 2 cm layer of laboratory sand (0.1–0.3 mm particle size), onto which a 2 cm layer of sieved (2 mm) forest or arable soil was placed (see the electronic supplementary material, figure S1). The sand drainage layer ensured moist conditions during daytime (high temperatures, high evaporation) and prevented flooding of the soil surface during night-time (lower temperatures and condensation). Soil was kept near water capacity, but never water-logged. To let photoautotrophic microbial surface communities establish, open jars were initially incubated in a plant growth chamber at 20°C for 21 days; light was provided by fluorescent and tungsten lamps at an irradiance of 300 µE in a 12 L/12 D cycle.
Isotopically labelled 13CO2 was produced in 60 ml syringes attached to a three-way valve by releasing 0.15 M HCl onto NaH13CO3 (99 atom% 13C) and injected into the jars through a rubber septum (see the electronic supplementary material, figure S1). Initial nominal 13C enrichment of the CO2 in the jars’ atmosphere was 74 atom% 13C with a concentration of 1300 ppm CO2. Three 13CO2 pulses were administered, after 1, 4 and 8 days. Closed jars (two each with forest and arable soil) were incubated in the same growth chamber at 20°C in the light for 12 days in total. To estimate non-photosynthetic C fixation from 13CO2 in the dark, two additional jars with forest soil were wrapped in aluminium foil and incubated along with the other jars. Replication of jars for the 13C labelling step was kept low (n = 2 per treatment) because variation between jars in 13C uptake efficiency was not of interest and false negative results due to a labelling failure were unlikely with repeated 13CO2 injections.
After 12 days, intact soil cores (8.5 cm diameter) were excised and transferred from the Kilner jars into individual round polypropylene containers (250 ml). The forest soil with the microbial crust was either left intact or mixed manually to simulate soil disturbance that distributes superficial organic matter; the arable soil was left intact. Then, representatives of three important soil animal groups were introduced: earthworms (forest soil) and springtails and slugs (both arable soil), and incubated in the dark at 15°C for 7 days (slugs 3 days).
(b). Soil invertebrates
Adult Allolobophora chlorotica (Savigny 1826) (Annelida: Lumbricidae), a common, endogeic, geophagous earthworm species [11], were collected manually from a vegetable garden in County Dublin. Five adults (mean live weight 268 mg, range 137–406 mg) were introduced per container. There were four containers for light-incubated forest soil (two each with soil left intact and soil mixed) and two containers with dark-incubated, intact forest soil.
Ceratophysella denticulata (Bagnall 1941) (Hexapoda: Collembola) is a common, surface-dwelling (hemiedaphic life form) springtail species that is expected to feed on soil algae [12]. Adults were obtained from own laboratory cultures and 20–25 individuals were introduced into each container (n = 3) with light-incubated, arable soil.
Adult grey field slugs, Deroceras reticulatum (O. F. Müller 1774) (Mollusca: Agriolimacidae) [13], were collected manually from the same cultivated cereal plot at Thornfield. Adults were kept in laboratory culture, and eggs that were laid were incubated until hatching. Four newly hatched D. reticulatum (mean live weight 2 mg) and two freshly collected adult D. reticulatum (mean live weight 294 mg, range 115–601 mg) were introduced into each container (n = 2) with light-incubated, arable soil.
(c). Microbial, isotopic and statistical analysis
Microbial crusts and soil were sampled on the day when invertebrates were introduced. Small samples containing microbial mats were scraped off the soil surface and dispersed in water. Photoautotrophic microorganisms (cyanobacteria, algae) were classified roughly according to Archibald [14] under a light microscope (100× and 400× magnification).
Soil was sampled by scraping the surface layer (about 1 mm) or in bulk after mixing, dried at 50°C and powdered in a ball mill. Invertebrates were starved on moist filter paper for 24 h at 15°C (to allow gut clearance), frozen and freeze-dried for 24 h. Earthworms and adult slugs were ground individually with mortar and pestle before weighing of subsamples (about 1 mg dry weight each) into tin capsules. To achieve sample masses required for mass spectrometry, between 20 and 25 C. denticulata were taken from each container and pooled as one sample per container (0.2–0.3 mg dry weight).
Samples were analysed by continuous flow isotope ratio mass spectrometry (CF-IRMS) as described previously [11]. Isotopic ratios are expressed in conventional delta (‰) notation. Control animals of each species from unlabelled jars or the field (earthworms, large slugs) were analysed and compared statistically to animals that had access to labelled mats by non-parametric Mann–Whitney U-tests (StatView v. 5). Data are shown as scatterplots and medians, as advocated by Weissgerber et al. [15] for such small sample size studies. Data points for the large-bodied species (earthworms and slugs) are shown for individual animals (considered here as replicates) because the main interest was in the variation in 13C assimilation between individuals from the same algal mats (with a known, measured 13C enrichment).
3. Results
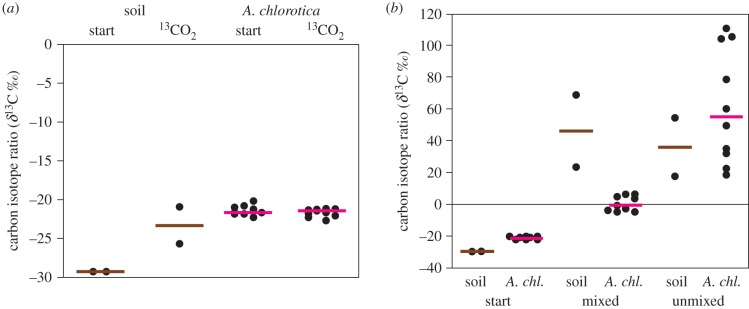
In the dark (figure 1a), non-photosynthetic fixation of 13CO2 in forest soil was negligible and no 13C was assimilated by earthworms (U-test p > 0.10). In the light, significant amounts of 13C were fixed photosynthetically by microorganisms, as evidenced by high 13C values of the bulk soil organic matter (figure 1b) and very high enrichments (δ13C + 3014‰ ± 1045, mean ± s.d., n = 4) of the surface mat samples. This 13C was also assimilated by earthworms, A. chlorotica, especially where the soil had been left intact, but also from mixed soil (figure 1b). All earthworm δ13C comparisons between control, mixed, unmixed treatments were significantly different (U-test p < 0.001, n = 9 or 10).
Figure 1.
Scatterplots showing (a) 13C fixation in soil in the dark (note narrow range of Y-axis scale) and (b) detection of 13C in soil incubated under labelled 13CO2 atmosphere with light, and its assimilation by earthworms (A. chl., Allolobophora chlorotica) from mixed or unmixed forest soil. Coloured bars are medians. (Online version in colour.)
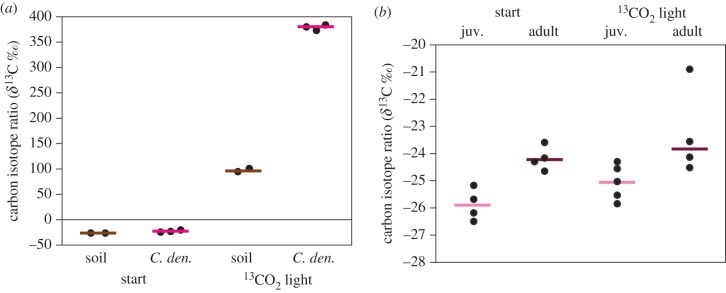
Springtails initially had a median δ13C value of –22.5‰ (n = 3) and were very highly labelled (median δ13C + 380‰, n = 3, U-test p < 0.05) (figure 2a) after 7 days' feeding on soil microbial mats (δ13C + 2360‰ ± 231, mean ± s.d., n = 3), on arable soil. Remarkably, the measured C content of the soil surface crust including microbial mats was only 6.1% C ± 0.6, just over double that of bulk soil (2.5% C ± 0.1).
Figure 2.
(a) Detection of microbially fixed 13C in arable soil and collembolans (C. den., Ceratophysella denticulata) and (b) no evidence for assimilation of microbially fixed 13C by newly hatched (juv.) or adult slugs, Deroceras reticulatum. Coloured bars are medians. (Online version in colour.)
Neither adult nor newly hatched slugs, D. reticulatum, assimilated any labelled C derived from CO2 (figure 2b). Start and end whole-body δ13C values were not significantly different (medians –24.2‰ versus –23.8‰ for adults, –25.9‰ versus –25.0‰ for newly hatched, all n = 4 or 5) (U-test all p > 0.10).
Types of photoautotrophic microorganisms in the soil surface samples included (in order of frequency): Cyanobacteria; unidentified unicellular, coccal cells; Bacillariophyta; Chlorophyta; Euglenophyta.
4. Discussion
C fixed from CO2 by photoautotrophic soil microorganisms was used as a C source by important soil decomposer animals. These results show that a third route of C inputs into temperate soil food webs exists, independently of vascular plants that contribute litter and root-derived inputs [5]. Previous studies have surmised that such C sources must exist, for instance [16] concluded that 20–40% of microbial C sources in a temperate grassland were not derived from vascular plants. Recently, C and nitrogen assimilation by soil microfauna (stylet-bearing nematodes and tardigrades) from cyanobacteria of desert soil crusts was demonstrated [17].
Simple isotope mixing equations suggest that, in just 7 days, earthworms derived 3.0% and 0.7% of their total body C from the autotrophic microbial C source in undisturbed (see the electronic supplementary material, figure S2) and mixed forest soil, respectively, while the proportion of new C on the bulk soil C was 1.8 and 2.1% and just 0.2% in the lower soil layer (table 1). Springtails, which are smaller and have faster tissue turnover than earthworms, derived 17.0% of their body C from the microbial source in unmixed arable soil. These estimates suggest that the microbial C input route could be of ecological importance, representing a small [3,18] but very productive and reactive [1,19] C pool that is grazed selectively by soil animals. As in a study on desert soil crusts [17], the present data cannot distinguish between direct assimilation of photoautotrophs and prior C flow through heterotrophic microorganisms, but the short incubation times tend to support the direct route. Further research is required to establish the significance of such microbial C inputs in natural soils, so that soil food web models [5] and C cycling studies [2] account for this route. Grazing by animals, in turn, probably controls soil photoautotrophic microbial communities [10].
Table 1.
C (% of total) in forest soil and A. chlorotica derived from photoautotrophic microbial sources (mean±s.d.). n.a., not applicable.
| compartment | undisturbed soil | mixed soil |
|---|---|---|
| total soil | 1.8 (0.7) | 2.1 (0.9) |
| lower soil layer | 0.2 (0.2) | n.a. |
| earthworms | 3.0 (0.8) | 0.7 (0.2) |
Having dwelled on highly enriched microbial mats, in the absence of vascular plants, slugs did not assimilate CO2-derived C. Newly hatched D. reticulatum in particular could be expected to graze on algal mats, but the present findings confirm both life stages as herbivores of vascular plants [13].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Charlie Scrimgeour, formerly of the Scottish Crop Research Institute, Dundee, UK, for isotopic analysis.
Ethics
Animals used in experiments are not endangered, nor subject to animal research ethics regulations in the country where the work was conducted.
Data accessibility
Data are available as the electronic supplementary material, dataset S1.
Authors' contributions
O.S. and J.D. conceived and designed the experiment; J.D. carried out the experiment; S.S. handled Collembola and identified algae; O.S. coordinated the study and drafted the manuscript. All authors gave final approval for publication. All authors agree to be held accountable for the work performed.
Competing interests
We declare we have no competing interests.
Funding
J.D. was financially supported by COFORD, the National Council for Forest Research and Development, Dublin, Ireland (CARBiFOR Project).
References
- 1.Belnap J, Lange OL (eds). 2003. Biological soil crusts: structure, function, and management. Berlin, Germany: Springer. [Google Scholar]
- 2.Elbert W, Weber B, Burrows S, Steinkamp J, Budel B, Andreae MO, Poschl U. 2012. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 5, 459–462. ( 10.1038/ngeo1486) [DOI] [Google Scholar]
- 3.Shimmel SM, Darley WM. 1985. Productivity and density of soil algae in an agricultural system. Ecology 66, 1439–1447. ( 10.2307/1938006) [DOI] [Google Scholar]
- 4.Ge TD, et al. 2013. Microbial phototrophic fixation of atmospheric CO2 in China subtropical upland and paddy soils. Geochim. Cosmochim. Acta 113, 70–78. ( 10.1016/j.gca.2013.03.020) [DOI] [Google Scholar]
- 5.DeRuiter PC, Neutel AM, Moore JC. 1995. Energetics, patterns of interaction strengths, and stability in real ecosystems. Science 269, 1257–1260. ( 10.1126/science.269.5228.1257) [DOI] [PubMed] [Google Scholar]
- 6.Pollierer MM, Langel R, Korner C, Maraun M, Scheu S. 2007. The underestimated importance of belowground carbon input for forest soil animal food webs. Ecol. Lett. 10, 729–736. ( 10.1111/j.1461-0248.2007.01064.x) [DOI] [PubMed] [Google Scholar]
- 7.Scheunemann N, Scheu S, Butenschoen O. 2010. Incorporation of decade old soil carbon into the soil animal food web of an arable system. Appl. Soil Ecol. 46, 59–63. ( 10.1016/j.apsoil.2010.06.014) [DOI] [Google Scholar]
- 8.Curry JP, Schmidt O. 2006. The feeding ecology of earthworms: a review. Pedobiologia 50, 463–477. ( 10.1016/j.pedobi.2006.09.001) [DOI] [Google Scholar]
- 9.Shtina EA, Kozlovskaya LS, Nekrasova KA. 1981. Relations of soil oligochaetes and algae. Soviet J. Ecol. 12, 44–48. [Google Scholar]
- 10.Jahnke J, Wehren T, Priefer UB. 2007. In vitro studies of the impact of the naked soil amoeba Thecamoeba similis Greef, feeding on phototrophic soil biofilms. Eur. J. Soil Biol. 43, 14–22. ( 10.1016/j.ejsobi.2006.11.002) [DOI] [Google Scholar]
- 11.Melody C, Schmidt O. 2012. Northward range extension of an endemic soil decomposer with a distinct trophic position. Biol. Lett. 8, 956–959. ( 10.1098/rsbl.2012.0537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buse T, Filser J. 2014. Mucilaginous seeds and algal diets attract soil Collembola in preference tests. Eur. J. Soil Biol. 65, 1–6. ( 10.1016/j.ejsobi.2014.08.005) [DOI] [Google Scholar]
- 13.Pallant D. 1972. Food of grey field slug, Agriolimax reticulatus (Müller), on grassland. J. Anim. Ecol. 41, 761–769. ( 10.2307/3208) [DOI] [Google Scholar]
- 14.Archibald PA. 1990. Soil algae. In Soil biology guide (ed. Dindal DL.), pp. 69–96. New York, NY: John Wiley & Sons. [Google Scholar]
- 15.Weissgerber TL, Milic NM, Winham SJ, Garovic VD. 2015. Beyond bar and line graphs: time for a new data presentation paradigm. PLoS Biol. 13, e1002128 ( 10.1371/journal.pbio.1002128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer C, Gleixner G. 2006. Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biol. Biochem. 38, 3267–3278. ( 10.1016/j.soilbio.2006.04.006) [DOI] [Google Scholar]
- 17.Darby BJ, Neher DA. 2012. Stable isotope composition of microfauna supports the occurrence of biologically fixed nitrogen from cyanobacteria in desert soil food webs. J. Arid Environ. 85, 76–78. ( 10.1016/j.jaridenv.2012.06.006) [DOI] [Google Scholar]
- 18.Jeffery S, Harris JA, Rickson RJ, Ritz K. 2009. The spectral quality of light influences the temporal development of the microbial phenotype at the arable soil surface. Soil Biol. Biochem. 41, 553–560. ( 10.1016/j.soilbio.2008.12.014) [DOI] [Google Scholar]
- 19.Abed RMM, Polerecky L, Al-Habsi A, Oetjen J, Strous M, de Beer D. 2014. Rapid recovery of cyanobacterial pigments in desiccated biological soil crusts following addition of water. PLoS ONE 9, e112372 ( 10.1371/journal.pone.0112372) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as the electronic supplementary material, dataset S1.