Abstract
Environmentally transmitted, opportunistic bacterial pathogens have a life cycle that alternates between hosts and environmental reservoirs. Resources are often scarce and fluctuating in the outside-host environment, whereas overcoming the host immune system could allow pathogens to establish a new, resource abundant and stable niche within the host. We tested if short-term exposure to different outside-host resource types and concentrations affect Serratia marcescens—(bacterium)'s virulence in Galleria mellonella (moth). As expected, virulence was mostly dictated by the bacterial dose, but we also found a clear increase in virulence when the bacterium had inhabited a low (versus high) resource concentration, or animal-based (versus plant-based) resources for 48 h prior to injection. The results suggest that temporal changes in pathogen's resource environment can induce very rapid changes in virulence and affect infection severity. Such changes could also play an important role in shifts from environmental lifestyle to pathogenicity or switches in host range and have implications for the management of opportunistic pathogens and disease outbreaks.
Keywords: eutrophication, resources, Serratia marcescens, virulence, pathogen, plasticity
1. Introduction
Opportunistic environmentally growing pathogens [1] typically experience two contrasting environments: the resource-poor outside-host environment and the resource-rich host environment. Opportunistic strategy could bring advantages to the pathogens, not only by allowing high virulence (defined as the level of damage caused to the host) without a consequent fitness loss due to reduced host-to-host transmission [2,3], but also by escaping adverse environmental conditions into resource-rich hosts [4–6]. Because resource pulses in the environment could directly select for mechanisms that promote fast resource use when the pulse arrives [7], virulence changes could also be considered as a coincidental consequence of alternating resource conditions. Directly adaptive or not, such selection pressures could have had a role in evolutionary transition of environmentally growing microbes to opportunistic pathogens.
Currently experimental evidence on fast environmentally triggered changes in virulence is very scarce [8–10], and none of the experiments seems to have taken into account the increased number of pathogens due to resource enrichment [10]. Thus, it is not clear if the effects are due to increased encounter rates, pathogen density or environmentally triggered alterations in pathogenicity itself [8,9]. Moreover, we are not aware of studies exploring if the resource quality (e.g. plant-based or animal-based) affects virulence, yet food sources are known to affect expression of potential virulence factors [11,12]. In addition, different levels of resource could cause changes in virulence. For example, it has been shown that nutrient limitation in dense bacterial populations can lead to increased secretion of quorum-sensing molecules which in turn can control the expression of a variety of virulence factors [13,14]. If this were the case, the lower resource levels could cause higher virulence.
To test if bacterial virulence responds to resources in the outside-host environment, we conducted an experiment where we manipulated the growth conditions (high and low concentrations of animal-based and plant-based media) of Serratia marcescens—a bacterium that has the typical characteristics of environmentally growing opportunistic pathogen and can reproduce in many different environments and media [15,16]. Although high bacterial density (i.e. dose) in infection led to higher virulence (measured as the host death rate), the virulence was also increased by previous low resource concentrations and by previous exposure to animal-based resources. These results are, we believe, novel and indicate that immediate exposure to animal-based food sources and food shortages can increase pathogens virulence.
2. Material and methods
Cryopreserved stock of entomopathogenic and environmentally growing S. marcescens db11 was thawed and dilution plated on nutrient broth (NB)–agar plates. After 24 h of growth at 31°C, a single clonal colony was picked with a sterile loop and mixed with 2 ml of liquid 1% NB medium (10 g NB (Difco, Becton & Dickinson, Sparks, MD, USA), 2.5 g yeast extract (Difco) in 1 l de-ionized H2O). Ten microlitre inocula of this solution were added into five centrifuge tubes containing 30 ml high (1%) and low (0.025%) concentrations of NB medium, and five tubes with 30 ml high (2%) and low (1%) concentrations of hay extract medium (2 g cereal grass medium (ScholAR Chemistry, Avon, NY, USA) was boiled for 10 min in 1 l de-ionized H2O and then filtered through a glass fibre filter for the 2% medium). The media were buffered with NaK buffer (50 mM NaH2PO4, 5 mM KCl, 120 mM NaCl, pH 7.4). To remove the growth medium from the injected solution and thus prevent the medium itself having an effect in the host, cultures were centrifuged (900g, 4°C, 10 min), liquid supernatant removed and bacterial pellets washed with 15 ml NaK buffer. This procedure was repeated twice. The solutions were then vortexed, and colony-forming units (CFU) were determined by serial dilutions on NB agar plates. These initial doses of bacteria were then diluted in NaK buffer with 1 : 2, 1 : 5, 1 : 10, 1 : 100, 1 : 1000, 1 : 10 000, 1 : 50 000, 1 : 100 000 and 1 : 200 000 ratios, and the dilutions were then used to infect the wax moth (Galleria mellonella) hosts. Controlling for the cell density had to be done statistically as CFU is not directly known at the time of dilution and optical density (OD)–CFU curves are inaccurate for stationary phase cultures. Note that buffer washing and associated waiting time, prior to injection, lead to depletion of remaining resources. Thus, same generation bacteria that have experienced different media are injected into the insect host. The moth larvae were injected between the abdominal segments six and seven with 2 µl of the dilutions using a Hamilton syringe. Injections were performed in random order regarding resource levels and types. Infected larvae were placed individually on Petri dishes and their survival was monitored at 3 h intervals for 75 h. Survival, i.e. time from injection to death, was analysed with Cox regression (SPSS 20.0, IBM), first fitting a model containing resource quality and concentration and their interaction as categorical covariate. In addition, number of injected cells (CFU ml−1, log transformed, standardized to a mean of zero) was fitted as continuous covariate. Moreover, covariate was also interacted with all treatment levels (both type and concentration) but since evidence for the heterogeneity of slopes was not found (see the electronic supplementary material), the interactions with treatment level and covariate were omitted from final analyses [17]. The analysis was performed on a data range in which CFU from different media overlapped, and thus our results are free from extrapolation errors.
3. Results and discussion
Environmentally induced changes in virulence could play an important role in transition from environmentally growing microbe to an opportunistic pathogen. Low resources are suggested to lead to an increased capability to use resources upon contact with them. Consequently, this could lead to increased virulence [5]. Consistent with this idea, we found that lower resource concentrations led to increased virulence (Exp(b): 1.246; 95% CI: 1.019–1.523; Wald: 4.588, p = 0.032, figure 1a). In both of the resource types, the resource level had a similar effect on virulence (resource level by resource type interaction: Wald: 0.356, p = 0.551). Comparable results have also been found previously ([18,19], but see [20]). The existence of quorum-sensing mechanisms that are upregulated upon nutrient stress could play a role in mediating virulence factor expression [13,14], and at least partly explain the changes in virulence due to different resource concentrations.
Figure 1.
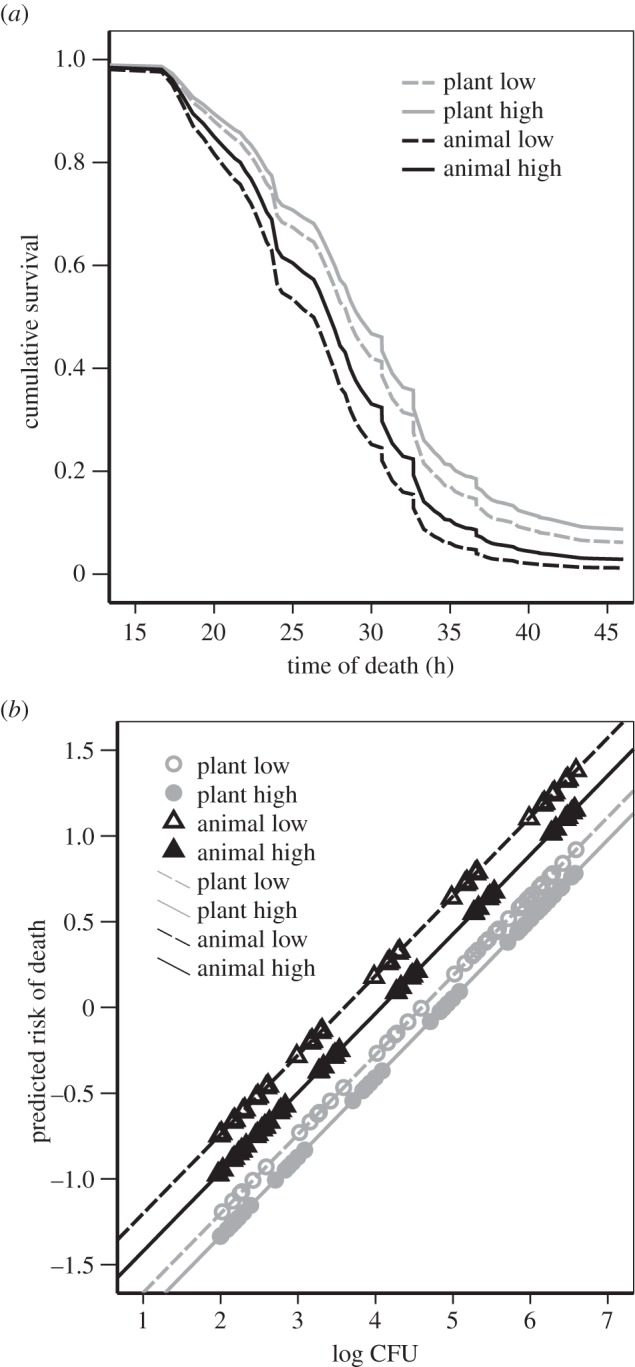
(a) Wax moth (G. mellonella) survival after injecting controlled amounts of S. marcescens bacteria directly into the haemolymph. Prior to injection, bacteria had been grown in high (solid line) or low (dashed line) resource concentrations of plant-based medium (hay extract, grey lines) or animal-based medium (NB, black lines). (b) The estimated effects of dose (CFU) on wax moth risk of death. Markers denote the range of actual bacterial densities in diluted samples.
If the resources were plant-based, the virulence was lower than if the resources were animal-based (Exp(b): 0.688; 95% CI: 0.559–0.846; Wald: 12.505, p < 0.001, figure 1a). Such resource-quality effects on virulence are, we believe, novel findings but not completely unexpected because exposure to different food sources are known to differentially upregulate genes related closely to virulence (e.g. [11,21]). Because bacteria exhibit a lag in growth when resources change, the matching or non-matching resource preference [7] could have notable effects on virulence in the host even if the bacteria are expected to be able to change their growth mode inside the host. Thus, if the bacteria are tuned to make the most of the animal-based resources, they could be more efficient in using the same energy source in the immediate future [7]. This could lead to a situation where previous exposure to animal-based resources, for example through saphrophytism [22], facilitates the pathogen's virulence in an animal host. It is unlikely that 48 h would be a time span long enough for genetic changes to increase to a detectable frequency, and de novo mutations to explain the results. Regardless of the mechanism (plastic, epigenetic or genetic), the finding that environmental conditions can cause rapid changes in virulence is important, indicating that virulence level does not need to be a consequence of persistent long-term selection on virulence itself. In accordance with the general view that eutrophication leads to high pathogen densities and thus higher virulence [10], we found that a larger dose causes faster host mortality (Exp(b): 2.524, 95% CI: 2.275–2.801; Wald: 304.756, p < 0.001, figure 1b).
Note that the infective dose was taken into account in the statistical analysis (i.e. fitted as a covariate) and bacterial cells were washed with buffer to remove remaining growth medium. Thus, the result most likely reflects plastic physiological changes in bacterial cells. Moreover, in a data-analysis where we tested if maximum yield or growth rate attained in different growth media explained the virulence results, it became apparent that these factors were not the causal reasons behind the changes in virulence (electronic supplementary material). Thus, the changes in virulence reflect the properties of food sources and concentrations, rather than simply density or growth rate attained in different media.
Our results show that both quality and quantity of the environmental resources are important in bacterial virulence. The phenomenon could be an important stepping-stone from strictly free-living growth to opportunistic pathogens. The findings also stress the importance of considering the immediate resource environments, as opposed to long-term evolutionary pressures, as important determinants of virulence and as factors that can affect the onset of epidemics.
Supplementary Material
Acknowledgements
We are grateful to J. Mantere and J. Kirvesoja for help in the laboratory.
Data accessibility
Data are archived in Dryad: http://dx.doi.org/10.5061/dryad.316s7.
Authors' contributions
T.K. and L.M. conducted the experiment and analysed the data. T.K., L.M., J.L. and J.M. wrote the manuscript and planned the experiment, accepted the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
The authors declare no conflicts of interest.
Funding
We are grateful for the KONE foundation (T.K., and L.M. via T.K.) and the Academy of Finland for funding (T.K. no. 278751, J.L. no. 1255572 and J.M. SA-252411).
References
- 1.Brown SP, Cornforth DM, Mideo N. 2012. Evolution of virulence in opportunistic pathogens: generalism, plasticity, and control. Trends Microbiol. 20, 336–342. ( 10.1016/j.tim.2012.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walther BA, Ewald PW. 1999. Pathogen survival in the external environment and the evolution of virulence. Biol. Rev. 79, 849–869. ( 10.1017/S1464793104006475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin BR. 1996. The evolution and maintenance of virulence in microparasites. Emerg. Infect. Dis. 2, 93–102. ( 10.3201/eid0202.960203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohmer L, Hocquet D, Miller SI. 2011. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol. 19, 341–348. ( 10.1016/j.tim.2011.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somerville GA, Proctor RA. 2009. At the crossroads of bacterial metabolism and virulence factor synthesis in staphylococci. Microbiol. Mol. Biol. Rev. 73, 233–248. ( 10.1128/MMBR.00005-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouguénec CL, Schouler C. 2011. Sugar metabolism, an additional virulence factor in enterobacteria. Int. J. Med. Microbiol. 301, 1–6. ( 10.1016/j.ijmm.2010.04.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.New AM, Cerulus B, Govers SK, Perez-Samper G, Zhu B, Boogmans S, Xavier JB, Verstrepen KJ. 2014. Different levels of catabolite repression optimize growth in stable and variable environments. PLoS Biol. 12, e1001764 ( 10.1371/journal.pbio.1001764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wedekind C, Gessner MO, Vazquez F, Maerki M, Steiner D. 2010. Elevated resource availability sufficient to turn opportunistic into virulent fish pathogens. Ecology 91, 1251–1256. ( 10.1890/09-1067.1) [DOI] [PubMed] [Google Scholar]
- 9.Johnson PTJ, Chase JM, Dosch KL, Hartson RB, Gross JA, Larson DJ, Sutherland DR, Carpenter SR. 2007. Aquatic eutrophication promotes pathogenic infection in amphibians. Proc. Natl Acad. Sci. USA 104, 15 781–15 786. ( 10.1073/pnas.0707763104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenzie VJ, Townsend AR. 2007. Parasitic and infectious disease responses to changing global nutrient cycles. EcoHealth 4, 384–396. ( 10.1007/s10393-007-0131-3) [DOI] [Google Scholar]
- 11.Tang X, Xiao Y, Zhou J-M. 2006. Regulation of the type III secretion system in phytopathogenic bacteria. Mol. Plant-Microbe Interact. 19, 1159–1166. ( 10.1094/MPMI-19-1159) [DOI] [PubMed] [Google Scholar]
- 12.Aalto SL, Decaestecker E, Pulkkinen K. 2015. A three-way perspective of stoichiometric changes on host–parasite interactions. Trends Parasitol. 31, 333–340. ( 10.1016/j.pt.2015.04.005) [DOI] [PubMed] [Google Scholar]
- 13.Jensen V, Lons D, Zaoui C, Bredenbruch F, Meissner A, Dieterich G, Munch R, Haussler S. 2006. RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and -independent pathways. J. Bacteriol. 188, 8601–8606. ( 10.1128/JB.01378-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornforth DM, Foster KR. 2013. Competition sensing: the social side of bacterial stress responses. Nat. Rev. Genet. 11, 285–293. ( 10.1038/nrmicro2977) [DOI] [PubMed] [Google Scholar]
- 15.Grimont PA, Grimont F. 1978. The genus Serratia. Annu. Rev. Microbiol. 32, 221–248. ( 10.1146/annurev.mi.32.100178.001253) [DOI] [PubMed] [Google Scholar]
- 16.Flyg C, Kenne K, Boman HG. 1980. Insect pathogenic properties of Serratia marcescens: phage-resistant mutants with a decreased resistance to Cecropia immunity and a decreased virulence to Drosophila. J. Gen. Microbiol. 120, 173–181. ( 10.1099/00221287-120-1-173) [DOI] [PubMed] [Google Scholar]
- 17.Hendrix LJ, Carter MW, Scott DT. 1982. Covariance analyses with heterogeneity of slopes in fixed models. Biometrics 38, 641–650. ( 10.2307/2530045) [DOI] [PubMed] [Google Scholar]
- 18.Hammer BK, Swanson MS. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33, 721–731. ( 10.1046/j.1365-2958.1999.01519.x) [DOI] [PubMed] [Google Scholar]
- 19.Sundberg L-R, Kunttu HMT, Valtonen ET. 2014. Starvation can diversify the population structure and virulence strategies of an environmentally transmitting fish pathogen. BMC Microbiol. 14, 67 ( 10.1186/1471-2180-14-67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santander RD, Oliver JD, Biosca EG. 2014. Cellular, physiological, and molecular adaptive responses of Erwinia amylovora to starvation. FEMS Microbiol. Ecol. 88, 258–271. ( 10.1111/1574-6941.12290) [DOI] [PubMed] [Google Scholar]
- 21.Wei ZM, Sneath BJ, Beer SV. 1992. Expression of Erwinia amylovora hrp genes in response to environmental stimuli. J. Bacteriol. 174, 1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunttu HMT, Valtonen ET, Jokinen EI, Suomalainen L-R. 2009. Saprophytism of a fish pathogen as a transmission strategy. Epidemics 1, 96–100. ( 10.1016/j.epidem.2009.04.003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are archived in Dryad: http://dx.doi.org/10.5061/dryad.316s7.


