Abstract
Hypoxia is a pervasive problem in coastal environments and is predicted to have enduring impacts on aquatic ecosystems. Intraspecific variation in hypoxia tolerance is well documented in fish; however, the factors underlying this variation remain unknown. Here, we investigate the role of the heart in individual hypoxia tolerance of the European sea bass (Dicentrarchus labrax). We found individual whole-animal hypoxia tolerance is a stable trait in sea bass for more than 18 months (duration of study). We next examined in vitro cardiac performance and found myocardial muscle from hypoxia-tolerant individuals generated greater force, with higher rates of contraction and relaxation, than hypoxic-sensitive individuals during hypoxic exposure. Thus, whole-animal hypoxia tolerance is associated with cardiac hypoxia tolerance. As the occurrence of aquatic hypoxia is expected to increase in marine ecosystems, our experimental data suggest that cardiac performance may influence fish survival and distribution.
Keywords: individual variability, climate change, hypoxic dead zones, heart, sea bass
1. Introduction
Oxygen availability is a key driver of animal physiology, behaviour and ecology. Low levels of environmental oxygen (hypoxia) are a growing concern, especially in coastal waters, where the duration, frequency and severity of hypoxic events have increased in recent years [1,2]. Reduced oxygen availability negatively impacts fish, affecting their growth [3], embryonic development [4], sex ratio [5], predator–prey relationships [6], swimming activity [7] and niche utilization [8]. The European sea bass (Dicentrarchus labrax) is known to exhibit pronounced intraspecific variation in hypoxia tolerance which directly relates to survivorship in semi-natural tidal ponds [9]. This suggests that hypoxia tolerance is a determinant of individual fitness in sea bass. However, the factors underpinning intraspecific variation in hypoxia tolerance in this animal remain unclear.
Previous studies have drawn associations between measures of cardiac performance and organismal performance traits in fish [10,11]. For example, the cardiac power output of rainbow trout with exceptional swimming performance is significantly higher than that of poor swimmers [12]. Moreover, cardiac oxygen supply in many fish species, including sea bass, is precarious because they do not have a coronary blood supply but instead rely on the residual oxygen in returning venous blood [13]. Thus, cardiac hypoxia tolerance and whole-animal hypoxia tolerance should be strongly correlated in fish; although this hypothesis has not been directly tested.
Whole-heart contraction and relaxation is dependent on the cycling of Ca2+ in the muscle cells (cardiomyocytes) that constitute the heart. Ca2+ cycling is an energy-consuming process and hypoxia is known to depress contractility due to reduced respiration and adenosine triphosphate (ATP) turnover [14]. It is therefore possible that differences in myocardial Ca2+ handling underlie intraspecific variation in cardiac hypoxia tolerance. Here we test the hypothesis that individual variation in cardiac hypoxia tolerance is associated with individual variation in whole-animal hypoxia tolerance. Our second hypothesis is that superior heart performance during hypoxia is supported mechanistically through enhanced cellular Ca2+ handling, which we investigated pharmacologically.
2. Material and methods
Thirty-four European sea bass (Dicentrarchus labrax) (mass 286 ± 12 g, male) were obtained from a commercial supplier (Aquastream, Ploemeur, France) and maintained at Institut Français de Recherche pour l'Exploitation de la Mer (Ifremer) in Brest, France, where they were implanted with passive integrated transponders (PIT) to identify individual fish (see [9] for details).
Individual hypoxia tolerance was assessed using a hypoxia challenge test (HCT) which has been described in detail [9]. Briefly, nitrogen was used to decrease oxygenation from 100 to 20% air saturation over 90 min and by approximately 2% per hour thereafter. Once fish lost equilibrium, they were identified via PIT tag, and removed to recover in a fully aerated tank. The time taken to lose equilibrium and the corresponding oxygenation level was recorded. HCTs took place on 25th May 2012 (HCT1), 7th June 2012 (HCT2), 15th January 2013 (HCT3) and 12th December 2013 (HCT4). Directly following HCT4, the five most hypoxia-tolerant fish and the five most hypoxia-sensitive fish were used for the functional cardiac analyses.
Fish were killed with a blow to the head in accordance with local animal care protocols. The heart was removed, transferred to physiological saline and four myocardial muscle strip preparations were dissected from the ventricle with a razor blade. Each strip was hung between two vertical clips; the uppermost was attached to a force transducer, while the other provided a stable anchor. The muscle preparations were lowered into individual organ baths containing physiological saline maintained at 12°C, and stimulated to contract (10 ms, 70–85 V, 0.2 Hz). Two of the muscle strips were oxygenated and designated ‘normoxic’ and two were aerated to implement hypoxia. Our hypoxic conditions were designed to mimic the fivefold decrease in blood oxygen known to occur in sea bass exposed to 24 h of hypoxia [15]. See [16], and electronic supplementary material and methods for full experimental details.
All muscle preparations underwent three consecutive force–frequency trials. The first force–frequency trial was conducted in the presence of 1 nM adrenaline [17] and served as the control condition. In the second force frequency trial, one normoxic and one hypoxic preparation were treated with agents (10 µM ryanodine and 2 µM thapsigargin, [16]) that inhibit intracellular Ca2+ cycling through the sarcoplasmic reticulum (SR). The other muscle preparations served as time- and oxygenation-matched controls. For the third force frequency trial, all muscle preparations were exposed to a high dose of adrenaline (1 µM; [18]) which increases extracellular Ca2+ influx across the cell membrane. This drug treatment protocol was designed to test our second hypothesis and establish the role of intracellular Ca2+ (SR) and extracellular Ca2+ cycling in myocardial contractility during hypoxia.
HCT repeatability was assessed with the Pearson product-moment correlation coefficient. The myocardial force and kinetics were calculated as previously described [16]. Linear mixed-effects models (GLM) were used to analyse the relationship between whole-animal hypoxia tolerance and myocardial force of contraction, with force as the fixed factor, heart ID as a random factor and stimulation frequency as a categorical variable. Tukey's post-hoc test was used to determine significance (p < 0.05). All mean values are ±s.e.m.
3. Results
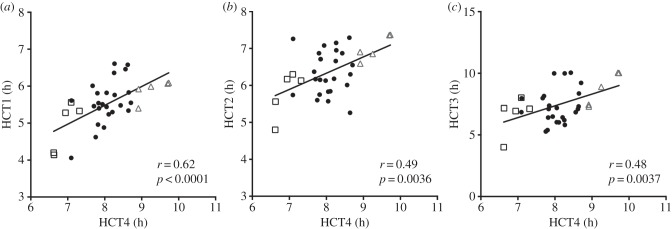
Figure 1 shows individual hypoxia tolerance for each fish in the last HCT (HCT4) plotted against individual hypoxia tolerance in the previous three tests (HCT1–3). Individual hypoxia tolerance was temporally stable for the duration of the study (567 days) with certain fish consistently withstanding hypoxia longer (the ‘hypoxia-tolerant’ fish) and certain fish consistently succumbing to hypoxia earlier (the ‘hypoxia-sensitive fish’). HCT hypoxia-tolerant fish withstood HCT4 for greater than or equal to 8.9 h, whereas all HCT hypoxia-sensitive fish lost equilibrium within less than or equal to 7.5 h. The incipient lethal oxygen saturation for HCT hypoxia-tolerant fish was less than or equal to 3.2% air saturation, while it was greater than or equal to 3.8% air saturation for HCT hypoxia-sensitive fish.
Figure 1.
Individual whole-animal hypoxia tolerance is a stable trait in sea bass. Black squares (n = 5) represent fish subsequently designated HCT hypoxia-sensitive fish and grey triangles (n = 5) represent fish designated as HCT hypoxia-tolerant fish for myocardial preparation experiments. R-values and significance determined by Pearson's correlation coefficient (N = 34). HCT1 (May 2012); HCT2 (June 2012), HCT3 (January 2013) and HCT4 (December 2013).
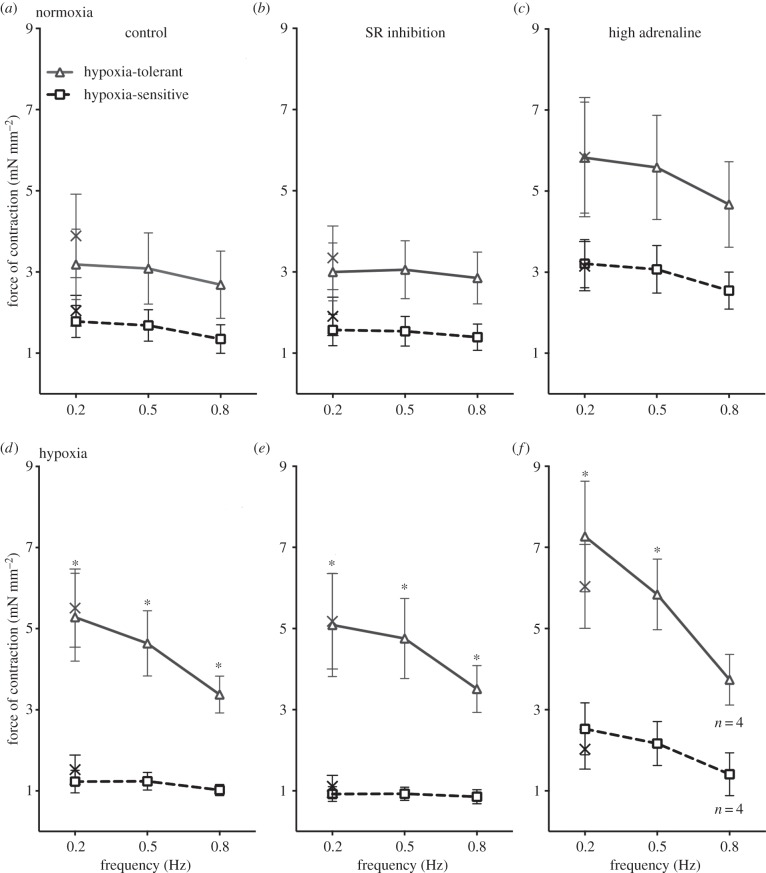
In normoxia, we found no significant difference in the force produced by muscle preparations from HCT hypoxia-tolerant fish and hypoxia-sensitive fish under control conditions (figure 2a), after SR inhibition (figure 2b) or in the presence of high adrenergic stimulation (figure 2c). Likewise, rates of contraction or relaxation did not differ between HCT hypoxia-tolerant and hypoxia-sensitive fish at any frequency or with drug treatments under normoxia (table 1). However, under hypoxia, cardiac muscle preparations from hypoxia-tolerant fish produced significantly more force than hypoxia-sensitive fish under all conditions (control, figure 2d; after SR inhibition, figure 2e; and in the presence of high adrenaline, figure 2f). The difference occurred across all contraction frequencies (0.2, 0.5 and 0.8 Hz) but was most striking at the slow frequencies which are expected to occur in vivo during hypoxic bradycardia [19]. The stronger myocardial contractions under hypoxia in the HCT hypoxia-tolerant fish were accompanied by faster rates of contraction and relaxation than the HCT hypoxia-sensitive fish (table 1).
Figure 2.
Myocardial muscle from hypoxia-tolerant fish outperforms that from hypoxia-sensitive fish under acute hypoxic exposure. Figure shows contractile force of cardiac muscle preparations from HCT hypoxia-tolerant fish and hypoxia-sensitive fish under normoxic conditions (a–c) and hypoxic conditions (d–f). (a,d) Myocardial force across physiologically relevant contraction frequencies under control conditions. (b,e) The effect of inhibiting intracellular Ca2+ cycling. (c,f) The effect of increasing extracellular Ca2+ influx with adrenaline (1 µM). The crosses show contractile force contraction upon return to 0.2 Hz after the frequency challenges. Values are means ± s.e.m. of n = 5 preparations. Asterisks denote difference between hypoxia-tolerant and hypoxia-sensitive fish (GLM).
Table 1.
Contractile kinetics under normoxia and hypoxia in ventricular muscle preparations from European sea bass fish in a given condition. Control saline contained 1 nM adrenaline, SR inhibition was achieved with 10 µM ryanodine and 2 µM thapsigargin and high adrenaline contained 1 µM adrenaline. In total, 0.8 Hz is representative of the in vivo heart rate of sea bass at 12°C and bradycardia (0.2 Hz) occurs during hypoxia. n = 5 in all cases except where marked with a dagger, in which n = 4. Bold typeface and asterisks denote a significant difference between hypoxia-tolerant fish and hypoxia-sensitive fish in a given condition (GLM).
| control |
SR inhibition |
high adrenaline |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hypoxia-tolerant |
hypoxia-sensitive |
hypoxia-tolerant |
hypoxia-sensitive |
hypoxia-tolerant |
hypoxia-sensitive fish |
|||||||
| normoxia | 0.2 Hz | 0.8 Hz | 0.2 Hz | 0.8 Hz | 0.2 Hz | 0.8 Hz | 0.2 Hz | 0.8 Hz | 0.2 Hz | 0.8 Hz | 0.2 Hz | 0.8 Hz |
| rate of contraction (mN mm−2 s−1) | 5.71 | 6.43 | 3.21 | 3.23 | 5.74 | 7.48 | 2.83 | 3.42 | 10.17 | 11.88 | 5.38 | 5.84 |
| (±1.39) | (±1.78) | (±0.64) | (±0.80) | (±1.21) | (±1.48) | (±0.70) | (±0.74) | (±2.18) | (±2.50) | (±1.00) | (±1.01) | |
| rate of relaxation (mN mm−2 s−1) | 6.35 | 6.63 | 3.49 | 3.08 | 6.31 | 7.34 | 3.05 | 3.15 | 11.37 | 10.30 | 6.47 | 5.85 |
| (±1.72) | (±1.99) | (±0.60) | (±0.83) | (±1.53) | (±1.56) | (±0.66) | (±0.68) | (±2.70) | (±2.40) | (±1.03) | (±0.98) | |
| resting tension (mN mm−2) | 2.45 | 2.37 | 1.05 | 1.02 | 2.23 | 2.28 | 0.79 | 0.79 | 2.24 | 2.27 | 0.63 | 0.64 |
| (±0.85) | (±0.83) | (±0.49) | (±0.47) | (±0.84) | (±0.85) | (±0.38) | (±0.36) | (±0.85) | (±0.86) | (±0.36) | (±0.36) | |
| hypoxia | 0.2 Hz | 0.8 Hz | 0.2 Hz | 0.8 Hz | 0.2 Hz | 0.8 Hz | 0.2 Hz | 0.8 Hz | 0.2 Hz | 0.8 Hz† | 0.2 Hz | 0.8 Hz† |
| rate of contraction (mN mm−2 s−1) | 8.59 | 7.78 | 2.16 | 2.68 | 8.89 | 8.97 | 1.71 | 2.15 | 12.19 | 9.24 | 4.22 | 3.38 |
| (±1.76) | (±1.08) | (±0.47)* | (±0.34)* | (±2.14) | (±1.57) | (±0.33)* | (±0.42)* | (±2.29) | (±1.48) | (±0.97)* | (±1.05) | |
| rate of relaxation (mN mm−2 s−1) | 10.54 | 7.98 | 2.77 | 2.71 | 10.24 | 14.88 | 1.59 | 2.09 | 14.88 | 8.57 | 5.49 | 3.44 |
| (±1.85) | (±0.95) | (±0.64)* | (±0.51)* | (±2.49) | (±2.54) | (±0.34)* | (±0.40)* | (±2.54) | (±1.42) | (±1.44)* | (±1.35) | |
| resting tension (mN mm−2) | 1.74 | 1.77 | 0.37 | 0.38 | 1.41 | 1.44 | 0.29 | 0.35 | 1.31 | 1.39 | 0.34 | 0.06 |
| (±0.88) | (±0.90) | (±0.36) | (±0.37) | (±0.83) | (±0.82) | (±0.40) | (±0.41) | (±0.79) | (±1.08) | (±0.37) | (±0.39) | |
4. Discussion
Inter-individual variation in hypoxia tolerance remained stable for the duration of our study (567 days) indicating that acute hypoxia tolerance is a stable trait and a potential target for natural selection [20]. Indeed, fish mass increased fourfold between HCT1 and HCT4, indicating profound growth and phenotypic remodelling, while inter-individual hypoxia tolerance remained consistent. Hypoxia tolerance is a determinant of sea bass ecological performance under field conditions [9] and has been shown to have a genetic basis in other fish species (e.g. Atlantic salmon, [10]). We have shown separately [21] that robust performance under one environmental challenge test does not necessarily correlate with success in another (thermal tolerance and swimming capacity; and also see [9]). Moreover, there was no correlation between HCT performance and swimming performance in a preliminary study conducted on this cohort of sea bass (data not shown). In other words, although not directly tested here, the hypoxia-tolerant fish were not just better ‘all-rounders' than the hypoxia-sensitive fish. Although still to be identified, functional trade-offs with other performance traits are likely, which helps explain why both hypoxia-tolerant and hypoxia-sensitive fish persist over time in a population.
Myocardium from hypoxia-tolerant sea bass performed better under hypoxia than myocardium from hypoxia-sensitive sea bass, linking individual variability in hypoxia tolerance with individual variability in intrinsic cardiac performance. We reasoned that superior contractility under hypoxia would be due to greater involvement of the intracellular Ca2+ stores of the SR because the SR has been implicated in augmenting Ca2+ delivery to the heart during periods of stress [22]. However, we found no support for this hypothesis as inhibiting the SR did not affect force production or contractile kinetics under normoxia or hypoxia. Without Ca2+ cycling through the SR, the sea bass heart would depend wholly on extracellular Ca2+ for contraction. We show that increasing transmembrane Ca2+ flux with adrenergic stimulation (1 µM) increased force production, but did not explain the difference in individual performance during hypoxia.
Because we assessed myocardial performance in vitro, the mechanism underlying superior contractile performance of the hypoxia-tolerant fish in hypoxia must be intrinsic to the heart (i.e. not involving autonomic control). It is possible that variation in cardiac metabolism or myoglobin content may be related to hypoxic performance as fish with a high cardiac myoglobin concentration perform better during hypoxia [10,23].
This study suggests variation in cardiac hypoxia tolerance could, at least in part, underlie variation in whole-animal hypoxia tolerance. Although this finding is based on a small sample size, when it is considered in combination with the finding that hypoxia-tolerant fish have higher survivorship in semi-natural conditions [9], we suggest that the heart may be an important target for natural selection in a future where marine environments are oxygen-stressed. Indeed, we speculate that similar to growing evidence for temperature-tolerance [11], hypoxia tolerance of the fish cardiovascular system may be important in determining fish distribution and survival in the changing oceans.
Supplementary Material
Acknowledgements
We thank the anonymous reviewers for their feedback.
Ethics
All animal procedures were in accordance with the French and EU guidelines for animal research (CGP151019-FISHHEALTH).
Data accessibility
Raw data have been deposited in Dryad.
Authors' contributions
W.J., K.O., G.C. and H.A.S. designed the study; W.J., K.O., F.M., H.O. and G.C. conducted the study; W.J. and K.O. analysed the data and W.J., K.O. and H.A.S. wrote the manuscript. All authors approve the final version and agree to be accountable for the work in the manuscript.
Competing interests
The authors declare they have no competing interests.
Funding
This work was supported by a European Cooperation in Science and Technology (COST) action FA1004 Conservation Physiology of Marine Fishes and via ITOPF R&D award 2012 to G.C. K.O. was funded by an NERC studentship.
References
- 1.Diaz RJ, Rosenberg R. 2008. Spreading dead zones and consequences for marine ecosystems. Science 321, 926–929. ( 10.1126/science.1156401) [DOI] [PubMed] [Google Scholar]
- 2.Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Hansen HP, Körtzinger A. 2013. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 160, 1875–1888. ( 10.1007/s00227-012-1954-1) [DOI] [Google Scholar]
- 3.Chabot D, Dutil J-D. 1999. Reduced growth of Atlantic cod in non-lethal hypoxic conditions. J. Fish Biol. 55, 472–491. ( 10.1111/j.1095-8649.1999.tb00693.x) [DOI] [Google Scholar]
- 4.Robertson CE, Wright PA, Köblitz L, Bernier NJ. 2014. Hypoxia-inducible factor-1 mediates adaptive developmental plasticity of hypoxia tolerance in zebrafish, Danio rerio. Proc. R. Soc. B 281, 20140637 ( 10.1098/rspb.2014.0637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas P, Rahman MS. 2011. Extensive reproductive disruption, ovarian masculinization and aromatase suppression in Atlantic croaker in the northern Gulf of Mexico hypoxic zone. Proc. R. Soc. B 279, 28–38. ( 10.1098/rspb.2011.0529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domenici P, Lefrancois C, Shingles A. 2007. Hypoxia and the antipredator behaviours of fishes. Phil. Trans. R. Soc. B 362, 2105–2121. ( 10.1098/rstb.2007.2103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Killen SS, Marras S, Ryan MR, Domenici P, McKenzie DJ. 2012. A relationship between metabolic rate and risk-taking behaviour is revealed during hypoxia in juvenile European sea bass. Funct. Ecol. 26, 134–143. ( 10.1111/j.1365-2435.2011.01920.x) [DOI] [Google Scholar]
- 8.Chapman LJ, McKenzie DJ. 2009. Behavioural responses and ecological consequences. In Fish physiology, vol. 27 (eds JG Richards, AP Farrell, CJ Brauner), pp. 25–77. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 9.Claireaux G, Théron M, Prineau M, Dussauze M, Merlin FX, Le Floch S. 2013. Effects of oil exposure and dispersant use upon environmental adaptation performance and fitness in the European sea bass, Dicentrarchus labrax. Aquat. Toxicol. 130–131, 160–170. ( 10.1016/j.aquatox.2013.01.004) [DOI] [PubMed] [Google Scholar]
- 10.Anttila K, Dhillon RS, Boulding EG, Farrell AP, Glebe BD, Elliott JAK, Wolters WR, Schulte PM. 2013. Variation in temperature tolerance among families of Atlantic salmon (Salmo salar) is associated with hypoxia tolerance, ventricle size and myoglobin level. J. Exp. Biol. 216, 1183–1190. ( 10.1242/jeb.080556) [DOI] [PubMed] [Google Scholar]
- 11.Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP. 2011. Differences in thermal tolerance among sockeye salmon populations. Science 332, 109–112. ( 10.1126/science.1199158) [DOI] [PubMed] [Google Scholar]
- 12.Claireaux G, McKenzie DJ, Genge AG, Chatelier A, Aubin J, Farrell AP. 2005. Linking swimming performance, cardiac pumping ability and cardiac anatomy in rainbow trout. J. Exp. Biol. 208, 1775–1784. ( 10.1242/jeb.01587) [DOI] [PubMed] [Google Scholar]
- 13.Farrell AP, Axelsson M, Altimiras J, Sandblom E, Claireaux G. 2007. Maximum cardiac performance and adrenergic sensitivity of the sea bass Dicentrarchus labrax at high temperatures. J. Exp. Biol. 210, 1216–1224. ( 10.1242/jeb.002881) [DOI] [PubMed] [Google Scholar]
- 14.Bickler PE, Buck LT. 2007. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu. Rev. Physiol. 69, 145–170. ( 10.1146/annurev.physiol.69.031905.162529) [DOI] [PubMed] [Google Scholar]
- 15.Thomas S, Hughes GM. 1982. Effects of hypoxia on blood gas and acid–base parameters of sea bass. J. Appl. Physiol. 53, 1336–1341. [DOI] [PubMed] [Google Scholar]
- 16.Shiels HA, Freund EV, Farrell AP, Block BA. 1999. The sarcoplasmic reticulum plays a major role in isometric contraction in atrial muscle of yellowfin tuna. J. Exp. Biol. 202, 881–890. [DOI] [PubMed] [Google Scholar]
- 17.Graham MS, Farrell AP. 1989. The effect of temperature acclimation and adrenaline on the performance of a perfused trout heart. Physiol. Zool. 62, 38–61. [Google Scholar]
- 18.Mazeaud MM, Mazeaud F. 1981. Adrenergic responses in fish. In Stress in fish (ed. AD Pickering), pp. 49–75. New York, NY: Academic Press. [Google Scholar]
- 19.Farrell AP. 2007. Tribute to P. L. Lutz: a message from the heart—why hypoxic bradycardia in fishes? J. Exp. Biol. 210, 1715–1725. ( 10.1242/jeb.02781) [DOI] [PubMed] [Google Scholar]
- 20.Dohm MR. 2002. Repeatability estimates do not always set an upper limit to heritability. Funct. Ecol. 16, 273–280. ( 10.1046/j.1365-2435.2002.00621.x) [DOI] [Google Scholar]
- 21.Ozolina K, Shiels HA, Ollivier H, Claireaux G. In press. Intraspecific individual variation of temperature tolerance associated with oxygen demand in the European sea bass (Dicentrarchus labrax). Conserv. Physiol. ( 10.1093/conphys/cov060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cros C, Sallé L, Warren DE, Shiels HA, Brette F. 2014. The calcium stored in the sarcoplasmic reticulum acts as a safety mechanism in rainbow trout heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, 1493–1501. ( 10.1152/ajpregu.00127.2014) [DOI] [Google Scholar]
- 23.Bailey JR, Driedzic WR. 1986. Function of myoglobin in oxygen consumption by isolated perfused fish hearts. Am. J. Physiol. 251, R1144–R1150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data have been deposited in Dryad.