Abstract
Zoonotic diseases are a looming threat to global populations, and nearly 75% of emerging infectious diseases can spread among wildlife, domestic animals and humans. A ‘One World, One Health’ perspective offers us an ideal framework for understanding and potentially mitigating the spread of zoonoses, and the island of Madagascar serves as a natural laboratory for conducting these studies. Rapid habitat degradation and climate change on the island are contributing to more frequent contact among humans, livestock and wildlife, increasing the potential for pathogen spillover events. Given Madagascar's long geographical isolation, coupled with recent and repeated introduction of agricultural and invasive species, it is likely that a number of circulating pathogens remain uncharacterized in lemur populations. Thus, it is imperative that new approaches be implemented for de novo pathogen discovery. To this end, we used non-targeted deep sequencing of blood transcriptomes from two species of critically endangered wild lemurs (Indri indri and Propithecus diadema) to characterize blood-borne pathogens. Our results show several undescribed vector-borne parasites circulating within lemurs, some of which may cause disease in wildlife, livestock and humans. We anticipate that advanced methods for de novo identification of unknown pathogens will have broad utility for characterizing other complex disease transmission systems.
Keywords: disease surveillance, metagenomics, next-generation, one health, wildlife, zoonotic
1. Introduction
Traditional methods for surveying pathogens in wild populations largely depend on culturable organisms or a priori knowledge (e.g. PCR primers and microarray probes); however, given the complexity of natural systems, these methods are limited with respect to pathogen discovery. Advanced disease surveillance tools are urgently needed as they provide a more accurate depiction of the disease ecology of natural populations and thus will inform veterinarians and human health professionals in situations where pathogen identity and corresponding genetic signatures are incomplete. Broad implementation of innovative next-generation disease surveillance methodologies [1] for pathogen discovery will greatly advance a ‘One World, One Health’ paradigm that seeks coordinated efforts from wildlife, veterinarian and human health professionals in order to prepare for and combat emerging infectious diseases.
We tested next-generation methods for non-targeted pathogen discovery by examining blood samples of two species of lemurs endemic to the island of Madagascar. Lemurs have evolved in geographical isolation for approximately 60 Myr and are a remarkably diverse radiation of primates, representing perhaps 20% of the world's primate species diversity [2]. Moreover, they are experiencing rapid population declines owing to historical and ongoing destruction of the forests of Madagascar and the hunting of lemurs for bushmeat [3–5]. These pressures are amplified in the context of Madagascar's growing human population and global climate change, resulting in increased contact among wildlife, humans and domesticated animals [6–8]. The demographic effects of these pressures have likely influenced pathogen transmission within wild lemurs and may negatively impact the health and long-term survival of these endangered species, but also alter dynamics of disease transmission between wildlife and humans. Empirical data from Madagascar show elevated parasite densities in several lemur species and spillover of pathogenic enterobacteria and viruses from domesticated species and humans into wild lemurs [4,6,8–10]. Relatively few studies, however, have focused on pathogen discovery in lemurs and none has implemented modern next-generation disease surveillance methods [1,11,12]. Here, we use high-throughput sequencing of total RNA extracted from blood samples (i.e. blood transcriptomics) to identify blood-borne microorganisms. This method is ideally suited for pathogen discovery in wild animal populations, especially endangered species, because it is minimally invasive and, when implemented with metagenomic bioinformatics, can detect multiple blood-borne pathogens [13]. We used this approach to detect vector-borne parasites circulating within two critically endangered species of lemurs in Madagascar, the indri (Indri indri) and diademed sifaka (Propithecus diadema).
2. Material and methods
(a). Molecular methods
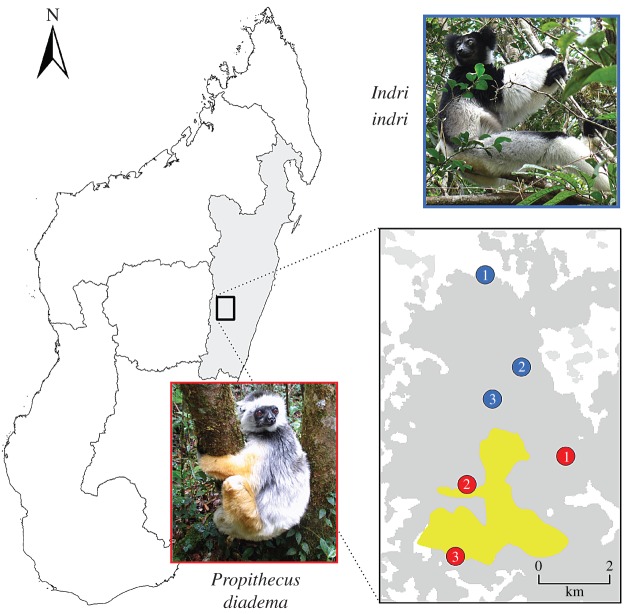
We collected 3 ml of blood from three I. indri and three P. diadema in March 2014. All individuals were sampled from a mid-altitude rainforest consisting of disturbed, transitional and undisturbed primary forest located 80 km northeast of Moramanga, Madagascar (figure 1). Molecular methods are presented in electronic supplementary material. Total RNA was extracted from each blood sample, and RNA samples were barcoded, pooled and sequenced on one Illumina HiSeq 2000 lane (100 bp paired-end). Illumina library preparation and sequencing were performed at the Duke Genome Sequencing Shared Resource (Duke University). All raw data generated for this study have been deposited in the Sequence Read Archive under BioProject number PRJNA293089.
Figure 1.
Map of Madagascar showing sampling localities for three Indri indri and three P. diadema screened for vector-borne pathogens. Grey shading in the country map identifies the Toamasina Province. Inset shows blue (I. indri) and red (P. diadema) sampling localities. Yellow shading defines the approximate boundary of the Ambatovy Minerals nickel mine site, an area where ongoing lemur health evaluations are being conducted by C.V.W., R.E.J., and J.R. Grey shading within inset identifies the extent of transitional and undisturbed primary forest.
Raw reads were quality filtered and mapped to the Microcebus murinus draft genome (GenBank accession: GCA_000165445.1). Unmapped reads were retained for downstream analyses. De novo transcriptome assemblies were performed using Trinity v. 2.0 [14]. Individual de novo blood transcriptome assemblies were imported into Galaxy [15] and were grouped according to taxonomic classification using the megablast tool for preliminary taxonomic identifications (electronic supplementary material). For this study, transcriptome assemblies were screened for putative vector-borne pathogens (e.g. pathogenic tick-borne bacteria and protozoan parasites) and associated sequences were retained for downstream analyses. Final taxonomic identifications consisted of targeted genome mapping to confirm Trinity assembly results and maximum-likelihood (ML) phylogenetic analyses using RAxML v. 8 software (electronic supplementary material) [16]. Contigs used for phylogenetic analyses were submitted to GenBank under the accession numbers KT722781–KT722795.
3. Results
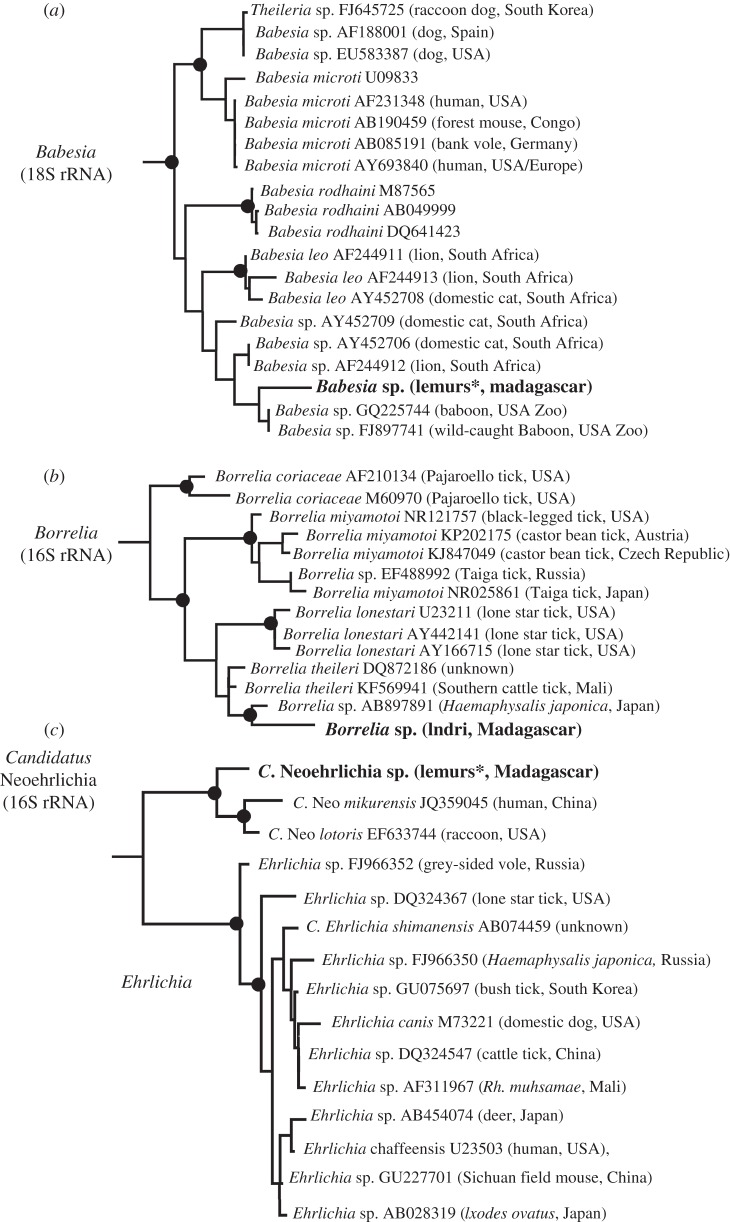
Our blood transcriptome sequencing and metagenomic analyses resulted in the identification of five vector-borne zoonotic pathogens (tick-borne: Babesia, Borrelia, Candidatus Neoehrlichia; and insect-borne: Plasmodium and Trypanosoma) circulating within I. indri and P. diadema in eastern Madagascar (table 1; figures 1 and 2; electronic supplementary material). Phylogenetic analyses of ribosomal and mitochondrial genes assembled from these parasites show the presence of several new strains or unrecognized species in our sample (figure 2 and electronic supplementary material, figure S2).
Table 1.
Blood-borne parasites identified in wild lemur blood transcriptomes. Expressed ribosomal and mitochondrial genes were used for parasite identification and values are numbers of sequenced bases for 18S and 28S (Babesia), 16S and 23S (Borrelia and C. Neoehrlichia), 18S and 28S-Alpha (Trypanosoma) and COI and Cyt-b (Plasmodium) (electronic supplementary material, Supplementary Methods and table S7). Numbers of underlying RNA-Seq reads for these genes appear in parentheses. See figure 1 for geographical sampling localities of I. indri and P. diadema. Phylogenetic analyses are presented in figures 2 and S2.
| Babesia sp. | Borrelia sp. | Candidatus Neoehrlichia sp. | Plasmodium sp. | Trypanosoma sp. | |
|---|---|---|---|---|---|
| I. indri 1 | 1514 bp (3677) | 4476 bp (12 160) | 1396 bp (208) | — | — |
| I. indri 2 | 3250 bp (16 212) | — | — | 2562 bp (994) | 2549 bp (3816) |
| I. indri 3 | 3391 bp (15,686) | — | — | — | 2684 bp (4274) |
| P. diadema 1 | 2723 bp (8184) | — | 2640 bp (3779) | 2562 bp (379) | — |
| P. diadema 2 | 3081 bp (9862) | — | — | 2562 bp (342) | — |
| P. diadema 3 | 3640 bp (25 617) | — | — | 2562 bp (460) | — |
Figure 2.
ML phylogenies of tick-borne parasites (Babesia (a), Borrelia (b) and Candidatus Neoehrlichia (c)) discovered through blood transcriptome sequencing. Taxa in bold were identified in I. indri and P. diadema (table 1; figure 1). Black circles identify statistically supported nodes (>75% bootstrap support). Asterisk indicates that similar genetic strains were found in multiple lemur individuals.
4. Discussion
We discovered several new strains or potentially unrecognized species of Babesia, Borrelia, Candidatus Neoehrlichia, Plasmodium and Trypanosoma circulating in wild lemurs (table 1 and figure 2; electronic supplementary material, figure S2). Of these, Borrelia and Candidatus Neoehrlichia were previously unknown to parasitize lemurs and C. Neoehrlichia represents a new record for Madagascar. Importantly, the tick-borne parasites identified in our survey are closely related to pathogenic strains known to cause disease in humans, domesticated animals and wildlife (e.g. babesiosis, borreliosis, neoehrlichiosis) [17–19]. Phylogenetic analyses of these parasites reveal genetic similarity to species found in domestic cats, cattle and rodents, data that suggest host-spillover events mediated by tick vectors on Madagascar (figure 2; electronic supplementary material, tables S1 and S2). This observation is further supported by the recent discovery of Babesia canis, a species commonly associated with domesticated dogs, circulating in Propithecus verreauxi from western Madagascar [20]. In the light of these results, veterinarians and human health officials working in Madagascar, or with patients who have originated from or travelled to Madagascar, should consider a broader array of tick-borne pathogens when diagnosing illness.
Our findings highlight the remarkable diversity of Plasmodium species circulating in wild lemurs. Collectively, there are at least eight unrecognized Plasmodium lineages on Madagascar, all of which have putatively evolved in isolation for approximately 20 Myr (electronic supplementary material, figure S2) [20,21]. We also have discovered a potentially unique species of trypanosome circulating in lemurs that may shed light on Trypanosoma evolution. The trypanosomes identified in I. indri form a statistically supported sister relationship to an undescribed Australian species (electronic supplementary material, figure S2). Multiple hypotheses exist regarding the forces underlying the cosmopolitan distribution of trypanosomes and the Australian Trypanosoma sp. has been at the centre of this debate [22,23]. Recent evidence suggests that many trypanosomes were likely distributed by bats [23], and our results generally support a dispersal hypothesis given the relatively low genetic distance value separating Australian and Madagascar trypanosomes (approx. 1.7%) and that the maximum time of origin hypothesized for the Australian trypanosome is approximately 20 Myr [24], a value younger than the formation of the island of Madagascar (approx. 80 Myr).
We provide empirical evidence of multiple blood-borne parasites circulating within wild lemurs. It is likely that several of these parasites represent novel species, and additional research focused on describing this diversity is warranted. Moreover, our phylogenetic analyses indicate that the tick-borne parasites Babesia and Borrelia, identified herein, most likely did not evolve in isolation on Madagascar and instead were imported to the island alongside domesticated species. Although parasites are natural components of healthy ecosystems, lemur conservationists must consider non-native parasitic zoonoses when examining the health of wild species. From a One Health perspective, we recommend screening for symptoms of babesiosis, borreliosis and neoehrlichiosis in Madagascar's wildlife, domesticated animals and human population. These findings show the utility of next-generation disease surveillance approaches for pathogen discovery.
Supplementary Material
Supplementary Material
Acknowledgements
The authors are grateful to Dr Karine Mahefarisoa and Dr Tsiky Rajoanarivelo for assisting with sample collection and acknowledge the efforts of Ambatovy Biocamp Agents in locating and tracking lemurs sampled for this study. Justin B. Lack kindly provided the sequence alignment used in his 2012 Piroplasmida publication. We appreciate the assistance of the Duke GCB Genome Sequencing Shared Resource staff and we wish to acknowledge the support of Duke Research Computing and the Duke Data Commons (NIH 1S10OD018164-01). This is Duke Lemur Center publication 1312.
Ethics
Samples were collected by veterinarians of the Duke Lemur Center, Durham, North Carolina and the Columbus Zoo and Aquarium, Columbus, Ohio. Animal procedures were approved by the Duke University IACUC (protocol A028-14-02).
Data accessibility
Raw Illumina RNA-Seq data have been made available on the NCBI Sequence Read Archive under BioProject number PRJNA293089. Contigs used for parasite identification were uploaded to the Dryad repository (doi:10.5061/dryad.44p35) and the NCBI GenBank repository under accession numbers KT722781–KT722795.
Authors' contributions
P.A.L and C.V.W conceived and designed the study. P.A.L. and C.E.H. preformed molecular and bioinformatics work. C.V.W., R.E.J, J.R., V.M. and H.R. coordinated fieldwork and assisted with field logistics. P.A.L., C.V.W. and A.D.Y. wrote the article with assistance from all authors. P.A.L., C.E.H., C.V.W., R.E.J., J.R., V.M., H.R. and A.D.Y. gave final approval of the version to be published and agree to be held accountable for its content.
Competing interests
We have no competing interests.
Funding
Funding was provided by Ambatovy Minerals S.A., Madagascar and the Duke Lemur Center, Durham, NC.
References
- 1.Vayssier-Taussat M, Moutailler S, Michelet L, Devillers E, Bonnet S, Cheval J, Hébert C, Eloit M. 2013. Next generation sequencing uncovers unexpected bacterial pathogens in ticks in western Europe. PLoS ONE 8, e81439 ( 10.1371/journal.pone.0081439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittermeier R, et al. 2010. Lemurs of Madagascar, 3rd edn, Tropical Field Guide Series Arlington, VA: Conservation International. [Google Scholar]
- 3.Jenkins RK, Keane A, Rakotoarivelo AR, Rakotomboavonjy V, Randrianandrianina FH, Razafimanahaka HJ, Ralaiarimalala SR, Jones JP. 2011. Analysis of patterns of bushmeat consumption reveals extensive exploitation of protected species in eastern Madagascar. PLoS ONE 6, e27570 ( 10.1371/journal.pone.0027570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett MA, Brown JL, Junge RE, Yoder AD. 2013. Climate change, predictive modeling and lemur health: assessing impacts of changing climate on health and conservation in Madagascar. Biol. Conserv. 157, 409–422. ( 10.1016/j.biocon.2012.09.003) [DOI] [Google Scholar]
- 5.Schwitzer C, et al. 2014. Averting lemur extinctions amid Madagascar's political crisis. Science 343, 842–843. ( 10.1126/science.1245783) [DOI] [PubMed] [Google Scholar]
- 6.Junge RE. 2007. Overview on the health and disease ecology of wild lemurs: conservation implications. In Lemurs (eds Lisa Gould, Michelle L. Sauther), pp. 423–440. Berlin, Germany: Springer. [Google Scholar]
- 7.Brown JL, Yoder AD. 2015. Shifting ranges and conservation challenges for lemurs in the face of climate change. Ecol. Evol. 5, 1131–1142. ( 10.1002/ece3.1418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bublitz DC, Wright PC, Rasambainarivo FT, Arrigo-Nelson SJ, Bodager JR, Gillespie TR. 2014. Pathogenic enterobacteria in lemurs associated with anthropogenic disturbance. Am. J. Primatol. 77, 330–337. ( 10.1002/ajp.22348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junge RE, Barrett MA, Yoder AD. 2011. Effects of anthropogenic disturbance on indri (Indri indri) health in Madagascar. Am. J. Primatol. 73, 632–642. ( 10.1002/ajp.20938) [DOI] [PubMed] [Google Scholar]
- 10.Zohdy S, Grossman MK, Fried IR, Rasambainarivo FT, Wright PC, Gillespie TR. 2015. Diversity and prevalence of diarrhea-associated viruses in the lemur community and associated human population of Ranomafana National Park, Madagascar. Int. J. Primatol. 36, 143–153. ( 10.1007/s10764-015-9817-5) [DOI] [Google Scholar]
- 11.Lipkin WI. 2013. The changing face of pathogen discovery and surveillance. Nat. Rev. Microbiol. 11, 133–141. ( 10.1038/nrmicro2949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radford AD, Chapman D, Dixon L, Chantrey J, Darby AC, Hall N.. 2012. Application of next-generation sequencing technologies in virology. J. Gen. Virol. 93, 1853–1868. ( 10.1099/vir.0.043182-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey KG, Herrera-Galeano JE, Redden CL, Luu TV, Servetas SL, Mateczun AJ, Mokashi VP, Bishop-Lilly KA. 2014. Comparison of three next-generation sequencing platforms for metagenomic sequencing and identification of pathogens in blood. BMC Genomics 15, 96 ( 10.1186/1471-2164-15-96) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q.. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644–652. ( 10.1038/nbt.1883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giardine B, Riemer C, Hardison RC, Burhans R, Elnitski L, Shah P, Zhang Y, Blankenberg D, Albert I, Taylor J.. 2005. Galaxy: a platform for interactive large-scale genome analysis. Genome Res. 15, 1451–1455. ( 10.1101/gr.4086505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. ( 10.1093/bioinformatics/btu033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy BN, Maïga O, Schwan TG. 2014. Detection of Borrelia theileri in Rhipicephalus geigyi from Mali. Ticks Tick-borne Dis. 5, 401–403. ( 10.1016/j.ttbdis.2014.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homer MJ, Aguilar-Delfin I, Telford SR, Krause PJ, Persing DH. 2000. Babesiosis. Clin. Microbiol. Rev. 13, 451–469. ( 10.1128/CMR.13.3.451-469.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rar V, Golovljova I.. 2011. Anaplasma, Ehrlichia, and ‘Candidatus Neoehrlichia’ bacteria: pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 11, 1842–1861. ( 10.1016/j.meegid.2011.09.019) [DOI] [PubMed] [Google Scholar]
- 20.Springer A, Fichtel C, Calvignac-Spencer S, Leendertz FH, Kappeler PM. 2015. Hemoparasites in a wild primate: infection patterns suggest interaction of Plasmodium and Babesia in a lemur species. Int. J. Parasitol. Parasites Wildl. 4, 385–395. ( 10.1016/j.ijppaw.2015.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacheco MA, et al. 2011. Timing the origin of human malarias: the lemur puzzle. BMC Evol. Biol. 11, 299 ( 10.1186/1471-2148-11-299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens J, Noyes H, Dover G, Gibson W.. 1999. The ancient and divergent origins of the human pathogenic trypanosomes, Trypanosoma brucei and T. cruzi. Parasitology 118, 107–116. ( 10.1017/S0031182098003473) [DOI] [PubMed] [Google Scholar]
- 23.Hamilton PB, Teixeira MM, Stevens JR. 2012. The evolution of Trypanosoma cruzi: the ‘bat seeding’ hypothesis. Trends Parasitol. 28, 136–141. ( 10.1016/j.pt.2012.01.006) [DOI] [PubMed] [Google Scholar]
- 24.Stevens J, Rambaut A. 2001. Evolutionary rate differences in trypanosomes. Infect. Genet. Evol. 1, 143–150. ( 10.1016/S1567-1348(01)00018-1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw Illumina RNA-Seq data have been made available on the NCBI Sequence Read Archive under BioProject number PRJNA293089. Contigs used for parasite identification were uploaded to the Dryad repository (doi:10.5061/dryad.44p35) and the NCBI GenBank repository under accession numbers KT722781–KT722795.