Abstract
In Australia, large native predators are fatally poisoned when they ingest invasive cane toads (Rhinella marina). As a result, the spread of cane toads has caused catastrophic population declines in these predators. Immediately prior to the arrival of toads at a floodplain in the Kimberley region, we induced conditioned taste aversion in free-ranging varanid lizards (Varanus panoptes), by offering them small cane toads. By the end of the 18-month study, only one of 31 untrained lizards had survived longer than 110 days, compared to more than half (nine of 16) of trained lizards; the maximum known survival of a trained lizard in the presence of toads was 482 days. In situ aversion training (releasing small toads in advance of the main invasion front) offers a logistically simple and feasible way to buffer the impact of invasive toads on apex predators.
Keywords: invasive species, Bufo marinus, taste aversion, conditioned taste aversion, ecological impact, varanid
1. Introduction
Worldwide, one of the most catastrophic impacts of invasive species is to imperil populations of apex predators, thereby inducing trophic cascades that can substantially modify ecosystem function [1]. In most conservation challenges, management solutions focus on the threatening process itself (in this case, controlling the invasive organism); but there is an alternative strategy. We can, instead, manipulate the response of vulnerable native taxa to render them more resilient to the invader's arrival. For example, inoculating African wild dogs against distemper can protect them even if we cannot prevent the disease from spreading [2], and translocating organisms may buffer climate-change impacts on the species involved [3].
We can manipulate animal behaviour in the same way, to achieve conservation outcomes. For example, captive-bred individuals can be trained to recognize predators prior to release into the wild [4], or be taught migration pathways [5]. Additionally, foraging responses can be modified via conditioned taste aversion (CTA, an adaptive learning mechanism that associates a distinctive taste with nausea: [6]). CTA therapy has been used to mitigate human–wildlife conflict (deterring bears from spoiling food stocks, and coyotes from eating sheep [7,8]). More recently, CTA has been used to buffer vulnerable Australian predators from the impact of toxic cane toads (Rhinella marina), by training captive-bred quolls (Dasyurus hallucatus) or wild-caught bluetongue lizards (Tiliqua scincoides intermedia) to associate the sight and scent of cane toads with nausea-inducing chemicals [9,10]. We have taken this work an important further step, inducing long-term toad-avoidance by exposing free-ranging predators (varanid lizards) to small live toads (without using additional chemicals to intensify CTA). The stimulus eliciting CTA thus is one that can readily be applied at a landscape scale, unlike previous methods.
2. Material and methods
The yellow-spotted monitor or floodplain goanna (Varanus panoptes; ‘Gundulla’ to the Balanggarra people in Australia's east Kimberley region) is a large (to 7 kg) terrestrial varanid lizard with a wide distribution across the wet–dry tropics of Australia [11]. These huge reptiles are central to Aboriginal culture and play a keystone ecological role [12,13]. Highly vulnerable to toad toxins, goannas die when they attempt to ingest toads [14]. Their precipitous population declines (more than 90% of individuals) following toad invasion have caused major shifts in abundance of many native vertebrates [12,13,15].
We used radiotelemetry to track the survival of 66 goannas for up to 18 months (November 2013 to May 2015) as cane toads invaded a remote floodplain (12 000 ha in size) in tropical Australia (15°08′34.73″ S, 127°52′36.08″ E; see the electronic supplementary material for detailed field methods). The toad invasion moved south to north across the floodplain over the course of two consecutive wet seasons (November to May each year), with goannas in the southern half of the floodplain exposed to toads in 2013–2014. The southern and northern halves of the floodplain are separated by a large saltpan that is a barrier both to goannas and toads; thus, toad incursion into the northern half of the floodplain was slower (see the electronic supplementary material, figure S1).
Three months prior to the arrival of toads, we began to experimentally expose free-ranging goannas to live juvenile cane toads (size < 25 g, 30–70 mm snout to urostyle length; electronic supplementary material, methods) in the field. Goannas that bit or swallowed a toad were designated as ‘trained’; goannas that did not bite a toad were designated as ‘controls’. We offered small toads multiple times to each goanna while it foraged in the field, and recorded its reactions to toads during these subsequent encounters. To evaluate the effect of CTA training, we compared the survival rates of trained versus control goannas (as ascertained during regular radio-tracking). Toad-induced mortality was readily identified by the contorted posture of the goanna, lack of overt injuries, location close to water, the presence of vomit and the absence of obvious ill-health prior to death.
We used Kaplan–Meier survival analysis [16] to compare rates of survival of trained versus control goannas. The dependent variable was the number of days that each individual was known to have survived after its initial CTA trial. As per Kaplan–Meier methodology, we differentiated individuals that were killed by toads from individuals that left the longitudinal study for other reasons (e.g. transmitter loss, python mortality) or were still alive when the study ended. In Kaplan–Meier terminology this latter group of goannas are termed as being ‘censored’.
We analysed animals from the northern (N = 19) and southern (N = 47) floodplain separately because of differences in the time they were exposed to invading toads. We had a clear a priori expectation that CTA training would enhance goanna survival, so we used one-tailed tests on our data. Overall rates of survival were calculated using Cormack Jolly-Seber models in the program mark (see the electronic supplementary material for methods and results).
3. Results
Of the 66 goannas to which we presented toads, 22 bit the offered toads (and hence, were ‘trained’) and 44 goannas did not. Trained and control goannas were similar in total length (means 116 versus 106 cm; F1,64 = 3.71, p = 0.06) and mass (1.8 versus 1.4 kg; F1,64 = 2.34, p = 0.10). Training was very effective: after a single CTA trial, a goanna was highly unlikely to eat another toad in subsequent trials for the duration of the study (up to 18 months) (χ2 = 47.46, d.f. = 1, p < 0.0001, figure 1).
Figure 1.
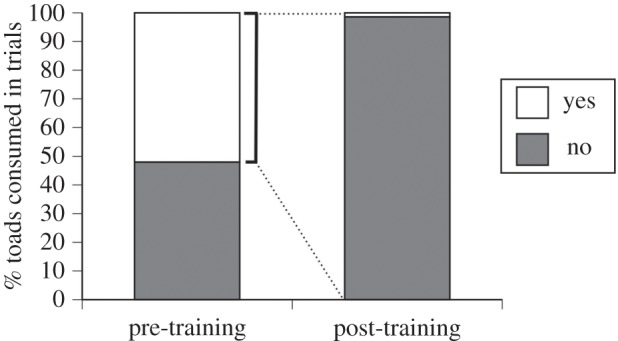
The effect of training on likelihood of a goanna eating an experimentally proffered cane toad. Prior to becoming ‘trained’, goannas ate toads presented to them in 52% of field trials. Post-training, toads were eaten in only 1.4% of subsequent field trials.
In the southern half of the study site, where we studied 47 goannas (trained N = 16, untrained N = 31), CTA-trained animals had higher rates of survival than did controls (χ2 = 4.95, d.f. = 1, one-tailed p = 0.013; figure 2a). In the northern half of the study site, where we studied 19 goannas (trained N = 6, untrained N = 13), toad-induced deaths were rare and training had no significant impact on goanna survival (χ2 = 0.90, d.f. = 1, one-tailed p = 0.38; figure 2b).
Figure 2.
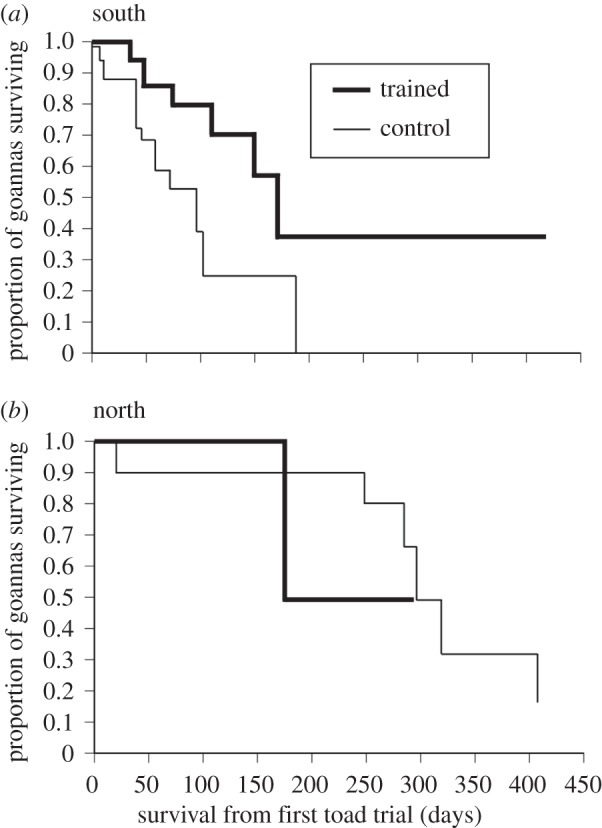
Kaplan–Meier survival curves comparing survival of (‘at risk’) CTA-trained (black line) versus control goannas (grey line) following toad invasion. Data are shown separately for (a) the southern half of the study site, where toads arrived first and in high numbers and (b) the northern part of the study site, where toads arrived later and in smaller numbers.
4. Discussion
Our study provides the first evidence, to our knowledge, that CTA training of free-ranging predators can buffer the impact of invasive cane toads, and that the effect can persist over a long period. Previous work to induce predators to avoid cane toads has relied upon smaller sample sizes of animals trained while in captivity. These studies used nausea-inducing chemicals to intensify CTA learning and monitored subsequent survival over shorter periods (less than 75 days) [9,10]. By contrast, we trained a larger number of free-ranging predators, by exposing them to small toads in the wild without additional chemicals, and monitored subsequent survival over 18 months.
Goannas rapidly learned taste aversion and importantly, this aversion was generalized: goannas avoided adult cane toads (often more than 300 g mass) in the wild after being exposed to juvenile toads (less than 25 g). In the area of the study site with the longest exposure to toads, only one of 31 untrained goannas survived longer than 110 days. This last untrained survivor was eventually killed by a toad on day 183. By contrast, nine of 16 trained animals survived longer than 110 days post-training. At the end of the study, half of the trained animals were still alive, and the maximum known survival of a trained goanna in the presence of toads was 482 days.
Why did some goannas that we trialled not bite or eat a toad, and thus remain untrained? Some goannas were not foraging when approached, and showed no interest in the offered toad. Others were distracted by the presence of the researcher. Regardless, the ‘control’ goannas were similar in size, location and general behaviour to trained individuals. If ‘control’ goannas were less likely to attack and consume wild toads, we would predict they would be at less risk from toads; and yet they died sooner than the trained individuals. In the face of the toad invasion, individual differences in traits such as neophobia among goannas could come under strong selective pressure by conferring a strong survival advantage to individuals wary of novel prey items.
At minimum, then, CTA training allowed many lizards to survive long enough to participate in one or two additional annual breeding seasons. Importantly, cane toads at the invasion front reproduce rarely [17] and thus, juvenile toads are scarce [18]. As a result, predators at the invasion front rarely encounter a toad small enough to elicit CTA; most toads are large adults, fatal if ingested. If even a few trained goannas survive long enough for their progeny to encounter juvenile toads (i.e. after the invasion vanguard has passed through, and toads begin to breed), later generations of predators can learn toad-avoidance on their own.
Our study provides the proof of principle required to set the framework for an innovative method of conservation. Encountering a small live cane toad can change a predator's behaviour in ways that protect it from subsequent encounters with large cane toads. Releasing small toads, then, can offer a simple landscape-scale method to conserve wildlife populations, by giving native predators an opportunity to learn rather than die. Our study provides the first real evidence that this strategy is realistic.
As the main impact on goannas occurs as soon as toads arrive in an area [12], even a short-term protective effect may be enough to buffer predator populations against the initial impact, enabling them to recover far more rapidly than would otherwise be possible. By analogy with physiological immunization, exposure to a small (sublethal) dose of toad toxin can protect an individual from a later exposure to a higher dose that would otherwise be fatal. More generally, managers may be able to buffer invasive species impacts more effectively by targeting the vulnerable native rather than the troublesome alien.
Supplementary Material
Acknowledgements
We thank the Balanggarra traditional owners of the north eastern Kimberley, specifically Cissy Gore-Birch and all who helped in the field; Rowan Clarke and Thomas Grounds played vital roles in the logistics of the project. Melissa Bruton, Miles Bruny, Greg Clarke and Jasper Kruse assisted in the field. The project received financial and logistical support from the Kimberley Science and Conservation Strategy through the Department of Parks and Wildlife (DPaW), Kimberley region. Particular thanks to Corrin Everitt and Daryl Moncrieff. We also thank two anonymous reviewers for suggestions that improved the final manuscript.
Ethics
This study was conducted under Ethics protocol 2013/6034 from the University of Sydney and permit SF010079 from the Department of Parks and Wildlife in Western Australia.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.92n50.
Authors' contributions
G.W.F. designed and executed the study, including the field component, data analysis and manuscript preparation; D.J.P. aided in the facilitation and coordination of the study, participated in the field component; G.P.B. contributed to experimental design, data analysis and manuscript preparation; R.S. conceived the concept, and contributed to experimental design and manuscript preparation; the Balanggarra Rangers provided ecological knowledge, aided in facilitation of the study and participated in the field component. All authors contributed to the revision of this manuscript, gave final approval for publication and agree to be accountable for the content herein.
Competing interests
We have no competing interests.
Funding
This research was funded by DPaW (D.J.P. and G.W.F.), the federal government's National Environmental Research Program (G.W.F. and R.S.) and the Australian Research Council (R.S. and G.P.B., grant no. fl120100074). G.W.F. was supported by an Australian Postgraduate Award, the Val Street scholarship and a grant from the Wildlife Conservation Society.
References
- 1.Ripple WJ, Beschta RL. 2012. Trophic cascades in Yellowstone: the first 15 years after wolf reintroduction. Biol. Conserv. 145, 205–213. ( 10.1016/j.biocon.2011.11.005) [DOI] [Google Scholar]
- 2.Prager KC, Woodroffe R, Cameron A, Haydon DT. 2011. Vaccination strategies to conserve the endangered African wild dog (Lycaon pictus). Biol. Conserva. 144, 1940–1948. ( 10.1016/j.biocon.2011.03.025) [DOI] [Google Scholar]
- 3.Schwartz MW, Martin TG. 2013. Translocation of imperiled species under changing climates. In The year in ecology and conservation biology. Ann. N. Y. Acad. Sci. 1286, 15–28. ( 10.1111/nyas.12050) [DOI] [PubMed] [Google Scholar]
- 4.van Heezik Y, Seddon PJ, Maloney RF. 1999. Helping reintroduced houbara bustards avoid predation: effective anti-predator training and the predictive value of pre-release behaviour. Anim. Conserv. 2, 155–163. ( 10.1111/j.1469-1795.1999.tb00061.x) [DOI] [Google Scholar]
- 5.Sladen WL, Lishman WA, Ellis DH, Shire GG, Rininger DL. 2002. Teaching migration routes to Canada geese and trumpeter swans using ultralight aircraft, 1990–2001. Waterbirds 25, 132–137. [Google Scholar]
- 6.Garcia J, Hankins WG, Rusiniak KW. 1974. Behavioural regulation of milieu in man and rat. Science 185, 824–831. ( 10.1126/science.185.4154.824) [DOI] [PubMed] [Google Scholar]
- 7.Ternent MA, Garshelis DL. 1999. Taste-aversion conditioning to reduce nuisance activity by black bears in a Minnesota military reservation. Wildl. Soc. Bull. 27, 720–728. [Google Scholar]
- 8.Ellins SR, Catalano SM. 1980. Field application of the conditioned taste-aversion paradigm to the control of coyote predation on sheep and turkeys. Behav. Neural Biol. 29, 532–536. ( 10.1016/S0163-1047(80)92882-4) [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell S, Webb JK, Shine R. 2010. Conditioned taste aversion enhances the survival of an endangered predator imperilled by a toxic invader. J. Appl. Ecol. 47, 558–565. ( 10.1111/j.1365-2664.2010.01802.x) [DOI] [Google Scholar]
- 10.Price-Rees SJ, Webb JK, Shine R. 2013. Reducing the impact of a toxic invader by inducing taste aversion in an imperilled native reptile predator. Anim. Conserv. 16, 386–394. ( 10.1111/acv.12004) [DOI] [Google Scholar]
- 11.Cogger HG. 1996. Reptiles and amphibians of Australia. Sydney, Australia: Reed Books. [Google Scholar]
- 12.Brown GP, Ujvari B, Madsen T, Shine R. 2013. Invader impact clarifies the roles of top-down and bottom-up effects on tropical snake populations. Funct. Ecol. 27, 351–361. ( 10.1111/1365-2435.12044) [DOI] [Google Scholar]
- 13.Doody JS, Castellano CM, Rhind D, Green B. 2013. Indirect facilitation of a native mesopredator by an invasive species: are cane toads re-shaping tropical riparian communities? Biol. Invasions 15, 559–568. ( 10.1007/s10530-012-0308-8) [DOI] [Google Scholar]
- 14.Ujvari B, Madsen T. 2009. Increased mortality of naive varanid lizards after the invasion of non-native cane toads (Bufo marinus). Herpetol. Conserv. Biol. 4, 248–251. [Google Scholar]
- 15.Feit B, Letnic M. 2015. Species level traits determine positive and negative population impacts of invasive cane toads on native squamates. Biodivers. Conserv. 24, 1017–1029. ( 10.1007/s10531-014-0850-z) [DOI] [Google Scholar]
- 16.Kaplan EL, Meier P. 1958. Nonparametric estimation from incomplete observations. J. Amer. Stat. Assoc. 53, 457–481. ( 10.1080/01621459.1958.10501452) [DOI] [Google Scholar]
- 17.Hudson CH, Phillips BL, Brown GP, Shine R. 2015. Virgins in the vanguard: low reproductive frequency in invasion-front cane toads. Biol. J. Linnean Soc. 116, 743–747. ( 10.1111/bij.12618) [DOI] [Google Scholar]
- 18.Brown GP, Kelehear C, Shine R. 2013. The early toad gets the worm: cane toads at an invasion front benefit from higher prey availability. J. Anim. Ecol. 82, 854–862. ( 10.1111/1365-2656.12048) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.92n50.


