Abstract
Physiological stress may result in short-term benefits to organismal performance, but also long-term costs to health or longevity. Yet, we lack an understanding of the variation in stress hormone levels (i.e. glucocorticoids) that exist within and across species. Here, we present comparative analyses that link the primary stress hormone in most mammals (i.e. cortisol) to metabolic rate. We show that baseline concentrations of plasma cortisol vary with mass-specific metabolic rate among cortisol-dominant mammals, and both baseline and elevated concentrations scale predictably with body mass. The results quantitatively link a classical measure of physiological stress to whole-organism energetics, providing a point of departure for cross-species comparisons of stress levels among mammals.
Keywords: allometry, metabolism, scaling, stressor
1. Background
Studies on physiological stress have provided insights into how animals cope with environmental conditions and respond to disturbance. We have learned how short-term stress may benefit organismal performance [1], and how chronic or high-intensity stress may be detrimental to the behaviour, ecology and physiology of species [2–4]. Over the longer term, factors that cause stress (e.g. social structure, predation; [5,6]) may negatively impact species' health, reproduction and longevity [2,7].
Physiological stress is typically characterized in vertebrates by the concentration of glucocorticoid (GC) hormones (cortisol and/or corticosterone), which are produced by the adrenal cortex along the hypothalamic–pituitary–adrenal axis [8,9]. These hormones serve an important role in the stress response in all major classes of vertebrates [10,11] by signalling the upregulation or downregulation of relevant physiological systems (e.g. immune, metabolic; [7–9,12]). Stress is often assessed by comparing ‘baseline’ to ‘elevated’ concentrations of GC hormones among individuals of a species. The difference in these levels may be as much as an order of magnitude within species and two to three orders of magnitude across species, for reasons that remain unclear [13,14].
Here, we examine the relationship between the dominant GC hormone in mammals, cortisol, and a more general feature of their physiology, metabolic rate. As with cortisol, we know mammals increase metabolic rates (i.e. energy use) upon disturbance or exertion. But unlike cortisol, we have a better understanding of the factors that govern variation in metabolic rates within species at different levels of activity (i.e. metabolic scope) and across species differing in body size [15,16]. Establishing a general relationship between cortisol concentrations and metabolic rate in mammals could therefore serve as a point of departure for understanding intra- and interspecific differences in stress levels.
We hypothesize that cortisol concentration varies proportionally (i.e. linearly) with mass-specific metabolic rate across species, and thus exhibits the same body mass-dependence. We hypothesize that cortisol concentration is related to mass-specific metabolic rate (B/M, in mW g−1) and body mass (M, in g) as:
| 1.1 |
where a is a constant that relates cortisol concentration to the flux of metabolic energy per gram of tissue (ng cortisol (ml plasma)−1 mW−1 (g tissue)−1, and b is a metabolic normalization constant that describes the mass-independent rate of energy flux per gram of tissue (mW g0.25). The above equation predicts cortisol concentrations will scale to the −0.25 power of body mass (i.e. B/M ∞ M−0.25) across species, the same way as mass-specific metabolic rate [17]. We evaluate this model for plasma cortisol levels among mammal species that inhabit different environments, vary by orders of magnitude in mass and exhibit considerable taxonomic diversity (see the electronic supplementary material). Note that to the extent baseline cortisol levels correspond to resting metabolic rate, and elevated levels correspond to active metabolic rate, we expect the two levels to be linearly related to each other and vary similarly with body mass. This is because active metabolic rates are linearly related to resting rates, and both show similar body mass-dependencies [17,18].
2. Methodology
We compiled published data on total plasma cortisol concentrations for placental mammals considered ‘cortisol-dominant’ [13]. We categorized data as representing ‘baseline’ or ‘elevated’ cortisol concentrations based on the original author(s)’ classification or descriptions. Cortisol measures from faecal or saliva samples were not included as these may differ from plasma measures [19].
We evaluated possible relationships of baseline cortisol concentration with metabolic rate and both baseline and elevated concentrations with body mass using Bayesian generalized linear mixed-models (see the electronic supplementary material). In performing these analyses, we explicitly considered the possible effects of six additional factors on cortisol levels (i.e. cortisol collection method, sex, anaesthesia, stressor type, captivity and season) and any non-independence due to shared evolutionary history. The six factors were included as covariates in the models, and a recent phylogeny of mammals was included as a random effect [20]. We then used deviance information criteria (DIC) to select the best models [21].
3. Results
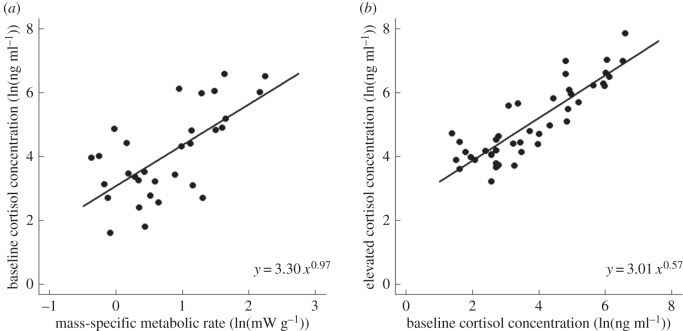
Baseline cortisol concentration was linearly related to resting mass-specific metabolic rate based on the 95% CI of the slope (0.97 (0.36, 1.56); table 1 and figure 1a). Metabolic rate explained 55% of the variation in baseline plasma cortisol concentrations across species of mammals. Elevated cortisol was highly correlated with baseline cortisol, though the slope was significantly less than 1 (slope = 0.57 (0.37, 0.75); table 1 and figure 1b). The best models for these relationships included phylogeny, but excluded covariates.
Table 1.
Outputs from Bayesian generalized linear mixed-models relating plasma cortisol concentrations (ng ml−1) to body mass (g) and resting mass-specific metabolic rate (mW g−1). DIC and conditional R2 are reported, as well as the slopes and intercepts of the relationships with 95% CI.
| model | DIC | slope (95% CI) | intercept (95% CI) | R2 |
|---|---|---|---|---|
| baseline cortisol versus resting metabolic rate | ||||
| metabolic rate + phylogeny | 86.86 | 0.97 (0.36, 1.56) | 3.30 (2.50, 4.18) | 0.55 |
| metabolic rate only | 98.34 | 1.28 (0.75, 1.80) | 3.07 (2.52, 3.62) | 0.49 |
| metabolic rate + covariates | 130.62 | 1.53 (0.63, 2.52) | 5.25 (−0.46,1.18) | 0.49 |
| baseline cortisol versus body mass | ||||
| mass + phylogeny | 162.47 | −0.22 (−0.40, −0.05) | 6.12 (4.19, 7.98) | 0.54 |
| mass only | 164.53 | −0.29 (−0.41, −0.17) | 6.77 (5.56, 8.04) | 0.57 |
| mass + covariates | 185.32 | −0.35 (−0.53, −0.21) | 8.16 (2.45, 10.35) | 0.61 |
| elevated cortisol versus body mass | ||||
| mass + phylogeny | 204.74 | −0.22 (−0.32, −0.12) | 7.26 (6.02, 8.36) | 0.63 |
| mass + covariates | 219.70 | −0.25 (−0.33, −0.18) | 7.72 (5.57, 9.62) | 0.64 |
| mass only | 223.90 | −0.24 (−0.38, −0.15) | 6.99 (4.42, 9.31) | 0.63 |
| elevated cortisol versus baseline cortisol | ||||
| cortisol + phylogeny | 69.39 | 0.57 (0.37, 0.75) | 3.01 (2.04, 3.95) | 0.53 |
| cortisol only | 79.75 | 0.61 (0.53, 0.77) | 2.53 (2.02, 3.02) | 0.47 |
Figure 1.
Relationships in cortisol-dominant mammals (a) between baseline plasma cortisol concentrations (ng ml−1) and resting mass-specific metabolic rate (mW g−1; N = 49) and (b) between elevated plasma cortisol concentrations and baseline cortisol concentrations (N = 43). Data are natural-log transformed.
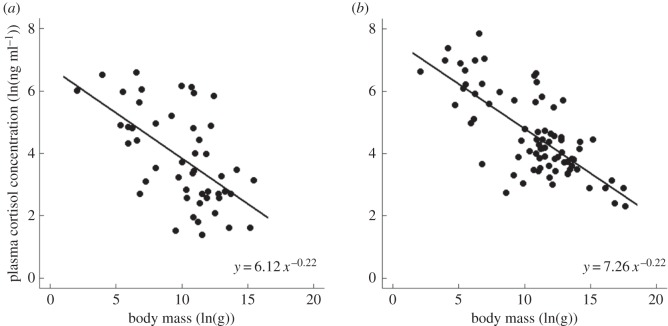
Baseline and elevated cortisol concentrations both showed relationships with body mass that are similar to those observed for mass-specific metabolic rate (table 1 and figure 2), though elevated levels were approximately threefold higher than baseline levels on average. Consistent with equation (1.1), these relationships were well described by power laws, with scaling exponents that were not significantly different from the −0.25 value (i.e. −0.25 fell within CIs; table 1). Body mass explained 54% and 63% of the variation in baseline and elevated cortisol concentrations, respectively. Here, too, the best models included phylogeny, but excluded covariates (table 1).
Figure 2.
Relationships in cortisol-dominant mammals of (a) baseline (N = 49) and (b) elevated plasma cortisol concentrations (ng ml−1) (N = 78) to body mass (g). Data are natural-log transformed.
4. Discussion
These results provide an a priori expectation for how plasma cortisol concentration should vary across species of mammals based on the observed relationships between cortisol, mass-specific metabolic rate and body mass (equation (1.1)). While it has long been understood that GCs play a role in metabolic function by helping to maintain blood glucose concentrations [22], it is still perhaps surprising to observe that the concentration of cortisol is proportional to mass-specific metabolic rate as opposed to production rates of this hormone. This could be achieved if cortisol production showed similar relationships with body mass and metabolic rate, but the turnover of individual molecules of cortisol was independent of these factors.
Perhaps more surprising is our observation that the slopes of the relationships with body mass are similar for both baseline and elevated levels of cortisol, given potential differences in GC-binding proteins and receptor density of stressed animals [22,23]. It is also noteworthy that elevated levels of cortisol were on average threefold greater than baseline levels for a given body mass (table 1), a difference that (perhaps coincidentally) corresponds to the average difference in resting and active metabolic rates reported for mammals [18]. If baseline and elevated levels of cortisol correspond to resting and active metabolic rates, respectively, it would suggest that there exists an approximately constant relationship between cortisol concentration and energy flux across activity levels. It is unclear, however, how such a relationship would correspond to the coordinated activities of GC and mineralocorticoid (MR) receptors during stress. High-affinity MR receptors are thought to play a primary role at low stress levels, or early in the stress response, while low-affinity GC receptors become more important at higher levels of stress, or later in the stress response, as MR receptors become saturated [24].
Unexplained variation about the fitted lines shown in figures 1 and 2 is perhaps explained by the following: first, we did not attempt to account for all of the many factors that may influence cortisol concentration (e.g. environment), including intra-individual variation at baseline or elevated levels [25,26]. Still, at the scale of our analyses, the six covariates we did consider (i.e. cortisol collection method, sex, anaesthesia, stressor type, captivity and season) were not statistically significant. Second, criteria used to categorize cortisol concentrations as ‘baseline’ or ‘elevated’ are more subjective than those used to categorize metabolic rate because they were primarily designed for intraspecific comparisons [27]. Finally, while cortisol was the primary GC hormone considered here, corticosterone may still play a significant role. At present, we lack a clear understanding of how relative concentrations of cortisol and corticosterone vary across species [13,14].
Considering stress in terms of individual energetics may provide insights into the trade-off between its short-term benefits and long-term costs [9]. Assuming that species possess a finite lifetime energy budget, an observation that forms the basis of the ‘rate of living’ hypothesis [28], increases in metabolic rate as part of the acute stress response may result in greater oxidative damage, which affects long-term health or longevity [29,30]. The relationship between stress and individual energetics may also explain why many basic attributes of organisms related to health and longevity have been shown to be negatively correlated with both cortisol concentration and mass-specific metabolic rate (e.g. lifespan; [31]). In this context, chronically high stress hormone levels in smaller mammals should perhaps not be viewed as negatively impacting evolutionary fitness, but rather as reflecting a different rate of living. Future work that relates measures of stress to metabolic energy expenditure may provide further insights into the relationship between the short- and long-term effects of stress.
Supplementary Material
Acknowledgements
We acknowledge Rachel Aaronson for helping with data compilation and Juan Pablo Gomez for guidance with phylogenetic analyses.
Data accessibility
All data used in analyses can be found in the electronic supplementary material.
Authors' contributions
C.G.H., A.K.L. and J.F.G. all designed, analysed, drafted and approved the manuscript and will be held accountable for its accuracy.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Monaghan P. 2014. Organismal stress, telomeres and life histories. J. Exp. Biol. 217, 57–66. ( 10.1242/jeb.090043) [DOI] [PubMed] [Google Scholar]
- 2.von Holst D. 1998. The concept of stress and its relevance for animal behavior. Adv. Stud. Behav. 27, 1–131. ( 10.1016/S0065-3454(08)60362-9) [DOI] [Google Scholar]
- 3.McEwen BS. 2008. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 583, 174–185. ( 10.1016/j.ejphar.2007.11.071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick GL, Shea K, Langkilde T. 2015. How do duration, frequency, and intensity of exogenous CORT elevation affect immune outcomes of stress? Gen. Comp. Endocrinol. 222, 81–87. ( 10.1016/j.ygcen.2015.07.008) [DOI] [PubMed] [Google Scholar]
- 5.Creel S, Dantzer B, Goymann W, Rubenstein DR. 2013. The ecology of stress: effects of the social environment. Funct. Ecol. 27, 66–80. ( 10.1111/j.1365-2435.2012.02029.x) [DOI] [Google Scholar]
- 6.Creel S, Winnie JA, Christianson D. 2009. Glucocorticoid stress hormones and the effect of predation risk on elk reproduction. Proc. Natl Acad. Sci. USA 106, 12 388–12 393. ( 10.1073/pnas.0902235106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero LM. 2004. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255. ( 10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- 8.Wingfield JC, Romero LM. 2010. Adrenocortical responses to stress and their modulation in free-living vertebrates. In Coping with the environment: neural and endocrine mechanisms. Handbook of physiology, vol. IV (eds BS McEwen, HM Goodman), pp. 211–234. New York, NY: Oxford University Press. [Google Scholar]
- 9.Cohen AA, Martin LB, Wingfield JC, McWilliams SR, Dunne JA. 2012. Physiological regulatory networks: ecological roles and evolutionary constraints. Trends Ecol. Evol. 27, 428–435. ( 10.1016/j.tree.2012.04.008) [DOI] [PubMed] [Google Scholar]
- 10.Wack CL, DuRant SE, Hopkins WA, Lovern MB, Feldhoff RC, Woodley SK. 2012. Elevated plasma corticosterone increases metabolic rate in a terrestrial salamander. Comp. Biochem. Phys. A 161, 153–158. ( 10.1016/j.cbpa.2011.10.017) [DOI] [PubMed] [Google Scholar]
- 11.Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD. 2010. Corticosterone, testosterone and life-history strategies of birds. Proc. R. Soc. B 277, 3203–3212. ( 10.1098/rspb.2010.0673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. ( 10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 13.Pfaff DW. 2002. Hormones, brain, and behavior, p. 4393 New York, NY: Academic Press. [Google Scholar]
- 14.Bonier F, Martin PR, Moore IT, Wingfield JC. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642. ( 10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 15.Bishop CM. 1999. The maximum oxygen consumption and aerobic scope of birds and mammals: getting to the heart of the matter. Proc. R. Soc. Lond. B 266, 2275–2281. ( 10.1098/rspb.1999.0919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251. ( 10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 17.Kleiber M. 1932. Body size and metabolism. Hilgardia 6, 315–353. ( 10.3733/hilg.v06n11p315) [DOI] [Google Scholar]
- 18.Ricklefs RE, Konarzewski M, Daan S. 1996. The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. Am. Nat. 147, 1047–1071. ( 10.1086/285892) [DOI] [Google Scholar]
- 19.Palme R, Rettenbacher S, Touma C, El-Bahr SM, Mostl E. 2005. Stress hormones in mammals and birds: comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Trends Comp. Endocr. Neurol. 1040, 162–171. ( 10.1196/annals.1327.021) [DOI] [PubMed] [Google Scholar]
- 20.Bininda-Emonds OR, et al. 2007. The delayed rise of present-day mammals. Nature 446, 507–512. ( 10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- 21.Lunn DJ, Thomas A, Best N, Spiegelhalter D. 2000. WinBUGS - a Bayesian modelling framework: concepts, structure, and extensibility. Stat. Comput. 10, 325–337. ( 10.1023/A:1008929526011) [DOI] [Google Scholar]
- 22.Goulding N, Flower RJ. 2001. Glucocorticoids. In Milestones in drug therapy (eds Parnham MJ, Bruinvels J), p. 205 Basel, Switzerland: Birkhäuser. [Google Scholar]
- 23.Nieuwenhuizen AG, Rutters F. 2008. The hypothalamic–pituitary–adrenal-axis in the regulation of energy balance. Physiol. Behav. 94, 169–177. ( 10.1016/j.physbeh.2007.12.011) [DOI] [PubMed] [Google Scholar]
- 24.de Kloet ER, Marian J, Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–467. ( 10.1038/nrn1683) [DOI] [PubMed] [Google Scholar]
- 25.Lendvai AZ, Giraudeau M, Bokony V, Angelier F, Chastel O. 2015. Within-individual plasticity explains age-related decrease in stress response in a short-lived bird. Biol. Lett. 11, 20150272 ( 10.1098/rsbl.2015.0272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang JQ, Hau M, Bonier F. 2011. Within seasons and among years: when are corticosterone levels repeatable? Horm. Behav. 60, 559–564. ( 10.1016/j.yhbeh.2011.08.004) [DOI] [PubMed] [Google Scholar]
- 27.Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R. 2011. Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166, 869–887. ( 10.1007/s00442-011-1943-y) [DOI] [PubMed] [Google Scholar]
- 28.Pearl R. 1928. The rate of living. London, UK: University of London Press. [Google Scholar]
- 29.Liu JK, Wang XY, Shigenaga MK, Yeo HC, Mori A, Ames BN. 1996. Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats. FASEB J. 10, 1532–1538. ( 10.1096/fj.1530-6860) [DOI] [PubMed] [Google Scholar]
- 30.Finkel T, Holbrook NJ. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247. ( 10.1038/35041687) [DOI] [PubMed] [Google Scholar]
- 31.Sapolsky RM. 2004. Organismal stress and telomeric aging: an unexpected connection. Proc. Natl Acad. Sci. USA 101, 17 323–17 324. ( 10.1073/pnas.0408041101) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in analyses can be found in the electronic supplementary material.