Abstract
The pharmacological exploitation of the galanin receptors as drug targets for treatment of epilepsy, depression, and pain has been hampered by the lack of workable compounds for medicinal chemists from random screening of large chemical libraries. The present work uses the tripeptidomimetic galnon and displays its presumed pharmacophores on a rigid molecular scaffold. The scaffold is related to marine natural products and presents three functional groups near one another in space, in a manner reminiscent of a protein surface. An active compound, Galmic, was identified from a small synthetic library and tested in vitro and in vivo for its affinity and efficacy at galanin receptors. Galmic has micromolar affinity for GalR1 receptors (Ki = 34.2 μM) and virtually no affinity for GalR2 receptors. In vitro, Galmic, like galanin, suppresses long-term potentiation in the dentate gyrus; it blocks status epilepticus when injected intrahippocampally or administered i.p. Galmic applied i.p. shows antidepressant-like effects in the forced-swim test, and it is a potent inhibitor of flinching behavior in the inflammatory pain model induced by formalin injection. These data further implicate brain and spinal cord galanin receptors as drug targets and provide an example of a systemically active compound based on a scaffold that mimics protein surfaces.
Galanin, a 29- to 30-aa-long neuropeptide, has been shown to affect feeding, cognitive, and sexual behavior and to regulate seizure and pain thresholds when applied intraventricularly (1–3). Galanin actions are mediated through three G protein-coupled receptors present in the brain and the peripheral nervous system (4–6). Transgenic mice overexpressing galanin have much higher seizure thresholds (7), and the galanin receptor 1 (GalR1)–/– mice have spontaneous seizures, demonstrating the role of galanin and its receptor in seizure control (8). Despite the wealth of biological information on galanin signaling, no progress has been made in using the galanin-receptor subtypes as drug targets. The lack of progress is largely due to the poor results from random screening at several major pharmaceutical companies (9). More than 4 million compounds were screened, but no workable compounds for medicinal chemical optimization were identified. Both Johnson & Johnson (10) and Schering have published reports of compounds active at high micromolar concentrations that had stability problems, as well as other problems (9). The only nonpeptide galanin receptor ligand available is galnon, a substance intended to display analogs of the three major pharmacophores of galanin, Trp-2, Asn-5, and Tyr-9 (11, 12), on a linear, peptide-like backbone. Galnon is a low-affinity, nonreceptor-subtype-selective agonist that acts at both GalR1- and GalR2-type receptors (13). Despite its serious shortcomings, galnon, within a year of its synthesis, was shown to affect behavioral symptoms and neurochemical correlates in opiate withdrawal (14), pain (15), and seizure (13) and to exert antidepressant-like effects in the forced-swim test (X.L., A.M.B., P. Sanna, J. Lucero, J. Laca, B. Conti, M. M. Behrens, D. Hoyer, G. Bilbe, and T.B., unpublished work).
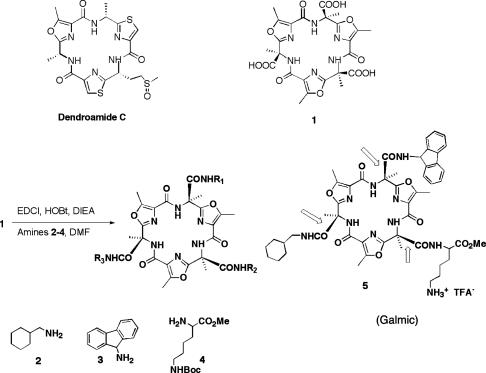
The present work describes the synthesis and characterization of a nonpeptide, systemically active, subtype-selective galanin-receptor agonist. The active compound was obtained from a mixture of triamides derived from the coupling of amines to the triacid (Scheme 1). This triacid was inspired by a number of biologically active and peptide-derived marine natural products such as Dendroamide C (16–20), macrocycles that contain three oxazole or thiazole rings linked by trans-amide bonds. The most favored conformations feature all of the basic oxazole nitrogens and the hydrogens of the secondary amides directed to the center of the macrocycle. This self-complementary array of hydrogen-bond donors and acceptors in the interior of the macrocycle fixes the molecule's conformation; the macrocycle is expected to be rigid and roughly planar. Little space is left within the center of the structure, and only minimal hydration is expected for the inwardly directed N–H bonds. Accordingly, the molecule is a platform that can present three functional groups on the same face. It serves as the core structure for solution-phase combinatorial chemistry.
Scheme 1.
Platform structures that present C3 symmetry. EDCI, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide; HOBt, hydroxybenzotriazole; DIEA, N,N-diisopropylethylamine; DMF, dimethylformamide; TFA–, trifluoroacetate; Boc, t-butoxycarbonyl.
Materials and Methods
Our premise was that the display of the side chains identified as active in galanin or those of the nonpeptide galanin-receptor agonist galnon on a rigid platform should result in recognition by the receptor GalR1. We coupled mixtures of three to four amines to the triacid platform (21–23) and obtained small combinatorial libraries. Active mixtures were obtained (after deblocking with trifluoroacetic acid) from the following amines: cyclohexylmethyl amine, 2; fluorenyl amine, 3; and ε-tBoc-l-lysine methyl ester, 4. This mixture (11 compounds) was fractionated by HPLC, and the most active compound (structure 5, Galmic, or its cyclodiastereomer) was identified by mass spectrometry. The individual molecule was prepared by total synthesis, which will be described elsewhere.
Characterization of Galmic. 1H NMR 300 MHz (CDCl3): 9.01 (s, NH); 8.94 (s, NH); 8.89 (s, NH); 7.97 (s broad, 3H); 7.65 (d, J = 7.5 Hz, 2H); 7.55 (d, J = 7.2 Hz, 1H); 7.42–7.19 (m, 5H); 6.87 (pseudo t, J = 5.4 Hz, 1H); 6.15 (d, J = 8.4 Hz, 1H); 4.55 (m broad, 1H); 3.61 (s, 3H); 3.38 (s broad, 1H); 3.12 (m, 1H); 2.90 (m, 3H); 2.70 (s, 3H); 2.65 (s, 6H); 2.19 (s, 3H); 2.09 (s, 3H); 2.04 (s, 3H); 2.00–1.28 (m, 14H); 1.20–1.04 (m, 3H); 0.92–0.76 (m, 2H).13C NMR 75 MHz (CDCl3): 171.61; 168.81; 167.39; 160.44; 160.41; 160.11; 159.97; 159.17; 156.09; 156.03; 155.91; 143.62; 143.39; 140.50; 140.43; 128.67 (CH); 128.58 (CH); 127.85 (CH); 127.65 (CH); 124.97 (CH); 124.72 (CH); 119.87 (CH); 60.68; 60.45; 59.90; 55.34 (CH); 52.46 (CH3); 52.16 (CH); 46.27 (CH2); 39.55 (CH2); 37.74 (CH); 30.63 (CH2); 26.43 (CH2); 26.23 (CH2); 25.80 (CH2); 21.69 (CH3); 21.50 (CH3); 21.44 (CH3); 11.89 (3 CH3). Matrix-assisted laser desorption ionization–Fourier transform MS [M+H]+: expected, 989.4510; observed, 989.4508.
Cell Lines and Ligand Binding. Stably transfected Chinese hamster ovary cells expressing rat GalR2 and Bowes' melanoma cells that express human GalR1 were cultivated as described earlier (5, 6). They were used to determine the affinity of the components of the chemical library for GalR1 and GalR2 receptors, by using 0.2 nM porcine 125I-galanin [2,200 Ci/mmol (1 Ci = 37 GBq); PerkinElmer] as a tracer. The components of the chemical library were tested as competitors at concentrations 10–8 to 10–4 M. The radioligand binding assay was performed in 150 μl of binding buffer [50 mM Tris·HCl (pH 7.4)/5 mM MgCl2/0.05% BSA, supplemented with peptidase and protease inhibitors: 50 μM leupeptin, 100 μM phenylmethanesulfonyl fluoride, and 2 μg/ml aprotinin]. Incubations were carried out at room temperature for 45 min and terminated by rapid vacuum filtration through glass fiber filters (Packard). The filters were washed three times and counted in a γ counter. The Ki values were determined with prism software (GraphPad, San Diego).
Electrophysiology. The hippocampal slices were prepared from male C57BL/6 mice (4–6 weeks old). After a 1-h equilibrium period, a glass micropipette (1–5 MΩ) filled with artificial cerebrospinal fluid or NaCl was placed in the outer one-third of the molecular layer of the inner blade of the dentate gyrus (DG), and a bipolar tungsten stimulating electrode was placed in the apex of the DG to activate the lateral perforant pathway innervating the outer one-third of the molecular layer. The field potentials were recorded with an AxoClamp 2B (Axon Instruments, Union City, CA) and digitized and processed by using a Digidata and pclamp acquisition software, respectively (both from Axon Instruments). The stability of the field excitatory postsynaptic potential (fEPSP) was established by recording fEPSP responses stimulated at 40–50% of maximum intensity (one per min for 15 min). The input–output curve was generated by stimulating the pathway at three different intensities (threshold, half-maximal, and maximally effective). A series of paired-pulse facilitation (PPF) experiments at 15-, 30-, 50-, 100-, 200-, and 500-ms intervals were carried out before and after baseline fEPSP recordings. The baseline fEPSP recording was conducted 15 min before and 60 min after long-term potentiation (LTP) induction at 40–50% of the maximal amplitude response. LTP was induced by two (20-s interval) trains of high-frequency stimulus at 100 Hz for 1 s at maximally effective stimulus intensity. Ten to 15 min before LTP induction, DMSO, Galmic [dissolved in 30% (vol/vol) DMSO], or galanin(1–29) was applied to the bath at the final concentration of 0.03% for DMSO and 1 μM for Galmic and galanin(1–29), and the application was continued until 1 min after high-frequency trains.
The amplitude of the input–output of fEPSP responses was computed by using clampfit software (Axon Instruments). The initial slope of the fEPSP (between the 0% and 50% points) was calculated and the data were normalized to baseline values. Student's t test was used for statistical analysis.
Self-Sustaining Status Epilepticus (SSSE) and Drug Administration. SSSE was induced in adult male Wistar rats as previously described (24). Briefly, animals were subjected to 30-min perforant path stimulation (PPS) through a stimulating electrode that had been chronically implanted into the angular bundle of perforant path by using stimulator model 8800 (Grass Instruments, Quincy, MA) with the following parameters: 10 s of 20-Hz trains of 1-ms, 30-V pulses delivered every minute, together with the continuous 2-Hz stimulation using the same parameters. Electrographic activity was acquired through a recording electrode-guide cannula (Plastics One, Roanoke, VA), which had been chronically implanted into the DG ipsilateral to PPS, and analyzed off-line by using harmonie software (Stellate Systems, Montreal) configured for automatic detection and saving of seizures and spikes. The following parameters were calculated: SSSE duration, i.e., the time between the end of PPS and the occurrence of the last seizure; time in seizures, i.e., cumulative time spent in software-recognized seizures during SSSE; total number of seizure episodes; and spike duration, i.e., time of the occurrence of the last electrographic spike. Galmic was dissolved in 50% (vol/vol) DMSO/saline and was administered into the DG by using an injection cannula connected to a Hamilton microsyringe and placed into the lumen of the guide cannula. Galmic was injected during the induction phase of SSSE, 10 min after the end of PPS, or during the drug-resistant maintenance phase (25), 60 min after the end of PPS. Control animals were treated with the vehicle (DMSO). Each group included four or five animals. In a separate set of experiments, to test blood–brain permeability, animals received i.p. injections of Galmic, 10 min after PPS (three animals per group). Data were analyzed by using one-way ANOVA, with the Bonferroni post hoc test.
Formalin Test. Male C57BL/6 mice (25–30 g, Harlan–Sprague–Dawley) were used. To quantify formalin paw-injection-induced flinching/licking behavior, an automated sensing system was used (26). Briefly, a C-shaped soft metal band (4.8 mm wide and 8.5 mm long, 0.1 g) was placed on one of the hind paws of an animal. After acclimation for 30 min, animals were gently restrained and 20 μl of 2.5% formalin solution was injected s.c. into the dorsal surface of the banded paw with a 30-gauge needle. Data collection was initiated after the animal was placed inside of the test chamber. Nociceptive behavior was quantified by automatically counting incidences of spontaneous flinching/shaking of the injected paw (26). The flinches were counted over 1-min intervals for 60 min and are presented as total number of flinches per min or per phase (phase 1, 1–9 min; phase 2, 10–60 min; phase 2A, 10–40 min; and phase 2B, 41–60 min). The animals were killed with CO2 immediately after the test. Galmic was dissolved in 30% (vol/vol) DMSO and given i.p. 15 min before formalin paw injection.
Forced-Swim Test. Animals were randomly assigned to either drug treatment group. Rats were injected i.p. either with Galmic (dissolved in 50% DMSO, 15 mg/kg, n = 10) or vehicle (50% DMSO, n = 7), in a volume of 2 ml/kg. Forty minutes after injection, animals were subjected to the forced swim test procedure. Rats were placed singly in a cylindrical glass container (48 cm tall, 21 cm in diameter) that contained tap water (25 ± 1°C) to a depth of 27 cm; this depth was just sufficient for the rat's tail to touch the bottom of the container. Each animal was tested for 10 min. All tests were recorded on videotape and later scored by an experienced observer who was blind to the experimental conditions of the animals. The presence of antidepressant activity in a drug is inferred from its capacity to increase the fraction of time spent in active behavior (27).
Open-Field Test Procedure. One week after the forced-swim test, different groups of rats were tested in the open-field test. Animals were treated with Galmic (15 mg/kg, i.p., n = 10) or vehicle (n = 6), as described above; 40 min after injection, rats were placed in an open-field apparatus. The locomotor activity over a 5-min period was recorded on videotape. Line-crossing behavior (defined as at least three paws in a square) was tallied and compared.
Results
Secondary amides such as peptides are regarded as liabilities in transport across membranes because their hydration, particularly at the N–H bonds, resists partitioning into hydrophobic environments. The activity of Galmic compound in vivo was, therefore, unexpected because it requires transport across membranes including the blood–brain barrier. The secondary amides that are part of the macrocyclic framework are internally solvated because, at best, a single water molecule can be accommodated in the center of the structure. The amides that attach the side chains to the periphery of the scaffold are also capable of internal solvation: the NH donors of the peripheral amides are hindered to external water but can find their complements internally in the N and O acceptors of the oxazole subunits through rotations around the bonds indicated.
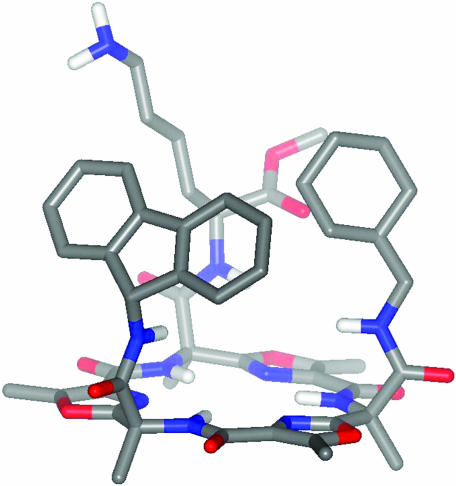
There is evidence for an α-helical structure of galanin in trifluoroethanol (28), but little is known about the conformation of galanin (or, as is the case at hand, of galnon) that is ultimately recognized by galanin receptors. An ingenious terphenyl scaffold devised by Hamilton and colleagues (29, 30) mimics the positions of the i, i + 3, and i + 7 side chains in an α-helix. At first glance, the trioxazole platform does not achieve this presentation of side chains, but rotations about the bonds indicated can shorten and lengthen the distances between the side chains. More importantly, the rigidity of the scaffold ensures that the three side chains all appear on the same face of the structure. A calculated structure is shown in Fig. 1.
Fig. 1.
Calculated structure of Galmic. The side chain amides have been arbitrarily rotated to show the most compact structure. In any conformation, the side chains appear on the same face of the macrocycle.
Receptor Binding. The affinity of Galmic for galanin receptors was determined by competitive equilibrium binding experiments by using membranes prepared from GalR1- or GalR2-expressing cells. Galmic displaces 125I-galanin binding in Bowes cells (hGalR1) with a Ki value of 34.2 μM, whereas in Chinese hamster ovary-GalR2 cells no competition was observed with up to 100 μM Galmic. Compared with galnon, the earlier synthetic galanin receptor nonpeptide ligand, Galmic seems to be more GalR1 selective but posses lower affinity (Table 1).
Table 1. The affinity of Galmic and galnon for galanin receptors.
|
Ki, μM
|
||
|---|---|---|
| Ligand | Bowes cells (hGalR1) | CHO cells (rGalR2) |
| Galmic | 34.2 | >100 |
| Galnon | 11.7 | 34.1 |
Ki values of Galmic and galnon at GalR1 and GalR2 receptors were determined by displacement of 125I-galanin from membranes prepared from human Bowes cells (hGalR1) and stably transfected Chinese hamster ovary (CHO) cells expressing rat GalR2, respectively. 125I-galanin concentration was 0.2 nM, and Galmic and galnon concentrations were between 10-8 M and 10-4 M. Ki values were calculated with prism software.
Effects of Galmic on the Synaptic Transmission and Plasticity in Mouse DG. Administration of DMSO (0.03%, n = 6) or Galmic (1 μM, n = 7) had no significant effect on the fEPSP response of the upper one-third of the molecular layer in DG after single test stimuli at threshold, half-maximal, or maximal intensity (data not shown). Similarly, application of galanin(1–29) (n = 6) had no significant effect on the input–output curves (data not shown). In the PPF test, Galmic (1 μM) caused a significant reduction in the percentage ratio of the fEPSP response of the second pulse over the first one at 500-ms time interval (t test: P < 0.05; data not shown). Administration of galanin(1–29) (1 μM, n = 6) also exhibited a similar effect on PPF at the same time interval. On average, application of galanin(1–29) caused 18.3 ± 4% reduction (data not shown), and Galmic reduced the PPF ratio by 15.4 ± 4%. The effect of Galmic was evident in six experiments of seven. The first pulse fEPSP slope was not affected by galanin(1–29) or Galmic, compared with artificial cerebrospinal fluid-treated response. The slope of the second pulse was reduced significantly, by 19.6 ± 8% and 14.6 ± 5%, following superfusion of galanin(1–29) or Galmic, respectively (t test: P < 0.05). Administration of the 0.03% DMSO used as vehicle had no significant effect on PPF.
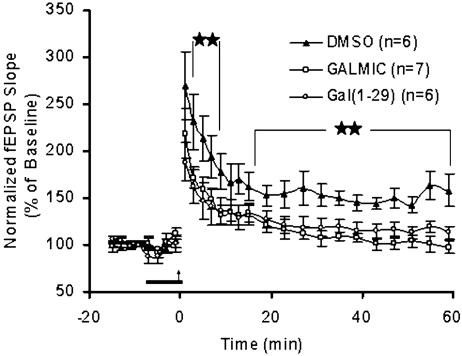
The superfusion of DMSO, Galmic, or galanin(1–29) was started 10–15 min before high-frequency trains and continued until 1 min after LTP induction. At the concentration of 1 μM, neither Galmic nor galanin(1–29) affected the baseline recording obtained before LTP induction compared with DMSO-treated (0.03%) slices (Fig. 2). The level of potentiation was attenuated after application of Galmic or galanin(1–29) in a similar pattern. The level of reduction after application of Galmic was significant at 2–6 min after LTP induction (t test: P < 0.05). Galmic, similar to galanin(1–29), blocked the late phase of LTP-treated slices and, on average (21–61 min after LTP induction), caused a 30 ± 1% (Galmic) reduction in the fEPSP slope compared with DMSO-treated slices (t test: P < 0.005).
Fig. 2.
Effects of Galmic on long-term synaptic plasticity in mouse DG slices. Administration of Galmic (1 μM) for 10–15 min before LTP induction attenuated the early and late phases of LTP, compared with the DMSO-treated (0.03%) slices. The bar illustrates the duration of the vehicle (DMSO) or drug administration, and the arrow indicates the time of stimulation with high-frequency trains (tetanus). The effect of Galmic was significantly different from the effect of DMSO on slices at 2–6 min and 17–60 min after LTP induction. Administration of Galmic (1 μM) caused an effect similar to administration of galanin(1–29) (1 μM) in attenuating the LTP in DG. All values are mean ± SEM (t test: ★★, P < 0.005).
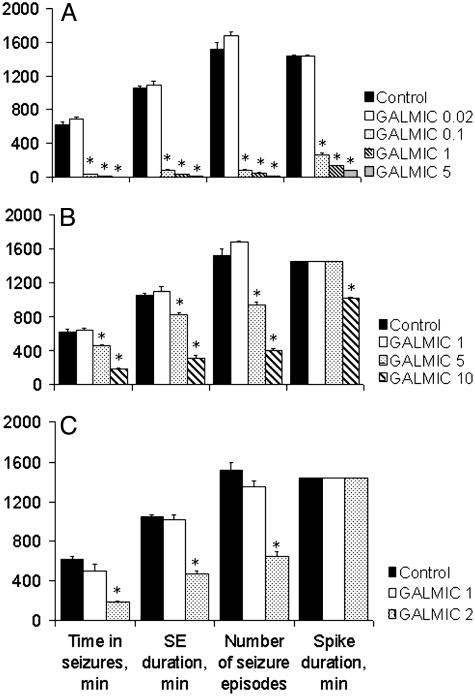
Effects of Galmic in Experimental Status Epilepticus. When injected into DG 10 min after PPS, Galmic attenuated seizures in a dose-dependent manner (Fig. 3A): anticonvulsant effects appeared at 0.1 nmol, when SSSE duration was between 1 and 2 h, versus 10–12 h in control, and became stronger as the dose of the drug was increased to 1 and 5 nmol. At the dose of 5 nmol, the compound irreversibly abolished seizures within 5–10 min and spikes within 2 h after administration. At higher dose-ranges (5 and 10 nmol), Galmic was effective in attenuating SSSE during its maintenance phase (Fig. 3B). Injection of the compound at 10 nM significantly shortened SSSE duration (4–5 h), attenuated its severity (time spent in seizures 20–40 min), and shortened the duration of spikes (under 8 h versus 24 h in controls). Intraperitoneal administration of Galmic at the dose of 2 mg/kg 10 min after PPS significantly attenuated self-sustaining seizures, whereas the 1 mg/kg i.p. dose was without effect (Fig. 3C).
Fig. 3.
Effects of Galmic on SSSE. Galmic was injected into the DG ipsilateral to the stimulation site at 10 min (A) or 60 min (B) after PPS. Dose (in nanomoles) is indicated in the legend to the right of the graphs. SE, status epilepticus. (C) Intraperitoneal administration of Galmic 10 min after PPS attenuated SSSE at 2mg/kg, but not at 1 mg/kg. *, P < 0.05 versus control, one-way ANOVA plus Bonferroni t test.
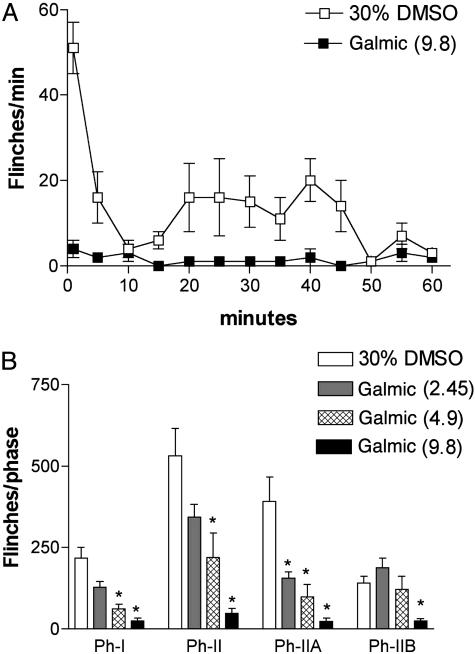
Effects of Galmic in Inflammatory Pain Model. After an injection of 20 μl of 2.5% formalin into the paw, mice displayed two phases of flinching behavior. Phase 1 started with initial intense flinches occurring 1–2 min after injection, followed by a rapid decline at 5–6 min. Phase 2 began after 15–20 min, with the maximal response typically observed around 25–30 min after the formalin injection (Fig. 4). Injection of Galmic into mice (2.45–9.8 μmol/kg) produced a dose-dependent inhibition on both phase 1 and phase 2 (Fig. 4). The ED50 value (μmol/kg, 95% confidence interval) of inhibitory effects of Galmic on phase 1 was 2.9 (2.1–4.1) and on phase 2 was 3.7 (2.6–5.2). Galmic up to the highest doses (9.8 μmol/kg i.p.) did not produce abnormal sensory or motor functions in these animals. Additionally, Galmic produced antinociceptive effects in the rat formalin test with a similar potency as in mouse. In contrast, galnon at equivalent doses had no effect in this test (data not shown).
Fig. 4.
Effects of Galmic on formalin-induced flinching. (A) Time course over 60 min of flinch responses after formalin paw injection in mice with i.p. injection of Galmic (9.8 μmol/kg, n = 5) or vehicle (30% DMSO, 10 μl, n = 8). Each point represents the number of flinches per min. (B) Histograms representing the dose–response effects of Galmic (doses in μmol/kg indicated in brackets, n = 5 each dose) on formalin test phase 1 and phase 2 flinching behavior of mice. The bars represent the total number of flinches in each phase: Ph-I, 1–9 min; Ph-II, 10–60 min; Ph-IIA, 10–40 min; and Ph-IIB, 41–60 min. The data are expressed as mean ± SEM. *, P < 0.05 versus the vehicle group, one-way ANOVA followed by Dunnett's tests.
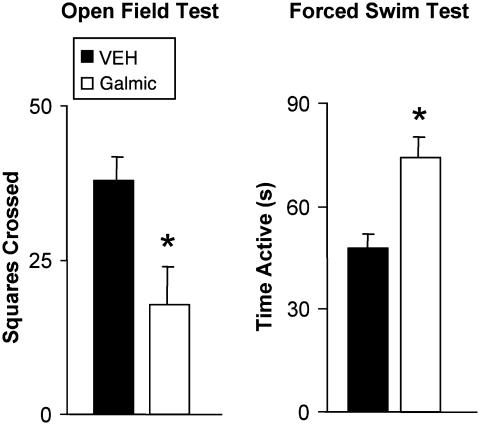
Effects of Galmic in Forced-Swim Test and Open-Field Test. In the forced-swim test, during the first 2 min vehicle-treated rats initially displayed high levels of activity, followed by prolonged periods of immobility interspersed with intermittent short bouts of activity. By contrast, animals treated with Galmic (15 mg/kg i.p.) exhibited a large (55%) increase in activity in the test, similar to other antidepressant compounds (31) and thus consistent with the hypothesis that Galmic displays antidepressant-like properties. The difference in time spent active between the two groups was highly significant (t15 = 3.19, P = 0.006) (Fig. 5). These results are consistent with our observation that galnon produces dose-dependent increases in activity in the forced-swim test (X.L., A.M.B., P. Sanna, J. Lucero, J. Laca, B. Conti, M. M. Behrens, D. Hoyer, G. Bilbe, and T.B., unpublished work).
Fig. 5.
Effects of treatment of rats with Galmic and vehicle (DMSO) in forced-swim and open-field tests. (Right) Activity was measured during a 10-min forced-swim test at 40 min after injection. Animals treated with Galmic (15 mg/kg i.p.) showed a significant increase in activity, compared with those treated with vehicle (50% DMSO), consistent with an antidepressant-like profile. (Left) Galmic (15 mg/kg i.p.) and vehicle were administered in the open-field test. Galmic significantly reduced locomotor activity in this task compared with vehicle-treated animals, without any obvious signs of sedation. Values represent group means (±SEM). Independent means t test: *, P < 0.01 versus control.
In the open-field test, vehicle-treated rats displayed typical thigmotaxic behavior, in which locomotor activity was largely confined to the walled perimeter of the arena and relatively little time was spent in the center. Galmic-treated (15 mg/kg) rats exhibited a similar pattern of behavior, although total levels of activity (measured as the number of squares crossed) was reduced by more than half, a highly significant effect (t14 = 3.66, P = 0.003) (Fig. 5).
Discussion
Finding nonpeptide, systemically active, small ligands that act as agonists at neuropeptide receptors is a difficult task, and, were it not for the example of morphine, many would have given up. The approaches that lead to the development of neuropeptide antagonists have used random screening of large chemical libraries to find molecules that bind to the neuropeptide receptor, followed by painstaking medicinal chemistry work to improve affinity and selectivity and to afford proper pharmacokinetic and metabolic properties. This process requires that there be some compounds in the library that bind with micromolar or better affinity to the neuropeptide receptor. Such “hits” have not been easy to find for the galanin receptors (9), and the two low-affinity compounds that were found were not chemically tractable. Although most neuropeptide-receptor ligands that have reached clinical use are antagonists (32), some neuropeptide-receptor agonists have been developed from peptidomimetics, most often for cyclic peptide ligands such as somatostatin-receptor agonists (33). In the case of galanin receptors, a tripeptide mimetic library based on the pharmacophores of galanin yielded the compound galnon, which, despite its low affinity and poor receptor-subtype selectivity, has rapidly become a tool in our studies on the role of galanin in seizures, cognition, pain, feeding, and opiate withdrawal (13–15).
The present paper describes the structure of Galmic (Scheme 1), which, despite its high molecular weight (984), penetrates the blood–brain barrier reasonably quickly, as evidenced by its effects in suppressing status epilepticus after i.p. injection of a 2 mg/kg dose (Fig. 3C). Under conditions of intrahippocampal administration, Galmic was 6- to 7-fold more potent than galnon (13) in inhibiting SSSE. Similarly, systemically administered Galmic has potent behavioral effects in the forced-swim test, where it exhibits antidepressant-like activity at 15 mg/kg i.p. dose (Fig. 4). Its effects are clearly observable within 40 min of the i.p. injection. The penetration of Galmic into the brain may be passive or may be mediated by some transporter. If the latter, it must have a rather high capacity and velocity, because the compound showed rapid CNS effects and, being a low-affinity ligand at galanin receptors (Table 1), a 10–5 M or higher concentration must be achieved in the brain to exert the observed effects in seizure and behavioral models (Figs. 3, 4, 5) used here. The properties of the scaffold and of the attached side chains in relation to crossing the blood–brain barrier are worth further investigation. The in vitro and in vivo effects of Galmic reported here are congruent with the action of a galanin type-1 receptor agonist and occur at doses similar to those of galnon (2–20 mg/kg i.p.), the tripeptide mimetic (13).
The interpretation of the pharmacologic spectrum of the effects of Galmic is somewhat hampered by its low affinity for the GalR1 type. The Ki value of 34.2 μM requires that high doses of the compound be used in the experiments; thus, we cannot rule out the possibility that Galmic might be able to bind to additional proteins such as receptors, ion channels, and enzymes and thereby contribute to the observed effects. Nevertheless, one must emphasize that the effects we found mimic those of galanin injected intrahippocampally or intracerebroventricularly; accordingly, it is reasonable to ascribe the effects reported in Figs. 2, 3, 4, 5 to the galanin-receptor agonist properties of Galmic.
In conclusion, Galmic represents a second-generation peptide ligand mimic that, through its interactions with galanin receptors, exhibits galanin-agonist-like effects in a variety of animal models upon systemic administration. The rigid scaffold used in Galmic should be useful in the synthesis of other peptidomimetic compounds.
Acknowledgments
This work was supported by National Institutes of Health Grants NS43409 (to A.M.), MH63080-02 (to X.L., H.B.-M., and T.B.), NS41954-02A1 (to X.-Y.H., T.Y., and T.B.), and AI50900 (to J.R.) and the Skaggs Institute for Research.
Abbreviations: DG, dentate gyrus; fEPSP, field excitatory postsynaptic potential; GalR1, galanin receptor 1; LTP, long-term potentiation; PPF, paired-pulse facilitation; PPS, perforant path stimulation; SSSE, self-sustaining status epilepticus.
References
- 1.Vrontakis, M. E. (2002) Curr. Drug Targets CNS Neurol. Disord. 1, 531–541. [DOI] [PubMed] [Google Scholar]
- 2.Mazarati, A., Langel, U. & Bartfai, T. (2001) Neuroscientist 7, 506–517. [DOI] [PubMed] [Google Scholar]
- 3.Bartfai, T., Hökfelt, T. & Langel, U. (1993) Crit. Rev. Neurobiol. 7, 229–274. [PubMed] [Google Scholar]
- 4.Branchek, T. A., Smith, K. E., Gerald, C. & Walker, M. W. (2000) Trends Pharmacol. Sci. 21, 109–117. [DOI] [PubMed] [Google Scholar]
- 5.Wang, S., He, C., Hashemi, T. & Bayne, M. (1997) J. Biol. Chem. 272, 31949–31952. [DOI] [PubMed] [Google Scholar]
- 6.Heuillet, E., Bouaiche, Z., Menager, J., Dugay, P., Munoz, N., Dubois, H., Amiranoff, B., Crespo, A., Lavayre, J., Blanchard, J. C., et al. (1994) Eur. J. Pharmacol. 269, 139–147. [DOI] [PubMed] [Google Scholar]
- 7.Kokaia, M., Holmberg, K., Nanobashvili, A., Xu, Z. Q., Kokaia, Z., Lendahl, U., Hilke, S., Theodorsson, E., Kahl, U., Bartfai, T., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 14006–14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacoby, A. S., Hort, Y. J., Constantinescu, G., Shine, J. & Iismaa, T. P. (2002) Brain Res. Mol. Brain Res. 107, 195–200. [DOI] [PubMed] [Google Scholar]
- 9.Branchek, T., Smith, K. E. & Walker, M. W. (1998) Ann. N.Y. Acad. Sci. 863, 94–107. [DOI] [PubMed] [Google Scholar]
- 10.Scott, M. K., Ross, T. M., Lee, D. H., Wang, H. Y., Shank, R. P., Wild, K. D., Davis, C. B., Crooke, J. J., Potocki, A. C. & Reitz, A. B. (2000) Bioorg. Med. Chem. 8, 1383–1391. [DOI] [PubMed] [Google Scholar]
- 11.Jureus, A., Langel, U. & Bartfai, T. (1997) J. Pept. Res. 49, 195–200. [DOI] [PubMed] [Google Scholar]
- 12.Land, T., Langel, U. & Bartfai, T. (1991) Brain Res. 558, 245–250. [DOI] [PubMed] [Google Scholar]
- 13.Saar, K., Mazarati, A. M., Mahlapuu, R., Hallnemo, G., Soomets, U., Kilk, K., Hellberg, S., Pooga, M., Tolf, B. R., Shi, T. S., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 7136–7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zachariou, V., Brunzell, D. H., Hawes, J., Stedman, D. R., Bartfai, T., Steiner, R. A., Wynick, D., Langel, U. & Picciotto, M. R. (2003) Proc. Natl. Acad. Sci. USA 100, 9028–9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu, W. P., Hao, J. X., Lundstrom, L., Wiesenfeld-Hallin, Z., Langel, U., Bartfai, T. & Xu, X. J. (2003) Eur. J. Pharmacol. 482, 133–137. [DOI] [PubMed] [Google Scholar]
- 16.Faulkner, D. J. (1999) Nat. Prod. Rep. 16, 155–198. [Google Scholar]
- 17.Pettit, G. R. Barkoczy, J., Srirangam, J. K., Singh, S. B., Herald, D. L., Williams, M. D., Kantoci, D., Hogan, F., Wardlaw, T. R. (1994) J. Org. Chem. 59, 2935–2938. [Google Scholar]
- 18.Garson, M. J. (1993) Chem. Rev. (Washington, D.C.) 93, 1699–1733. [Google Scholar]
- 19.Davidson, B. S. (1993) Chem. Rev. (Washington, D.C.) 93, 1771–1791. [Google Scholar]
- 20.Fuesetani, N. & Matsunoga, S. (1993) Chem. Rev. (Washington, D.C.) 93, 1793–1806. [Google Scholar]
- 21.Mink, D., Mecozzi, S. & Rebek, J., Jr. (1998) Tetrahedron Lett. 39, 5709–5712. [Google Scholar]
- 22.Haberhauer, G., Somogyi, L. & Rebek, J., Jr. (2000) Tetrahedron Lett. 41, 5013–5016. [Google Scholar]
- 23.Somogyi, L., Haberhauer, G. & Rebek, J., Jr. (2001) Tetrahedron 57, 1699–1708. [Google Scholar]
- 24.Mazarati, A. M., Liu, H., Soomets, U., Sankar, R., Shin, D., Katsumori, H., Langel, U. & Wasterlain, C. G. (1998) J. Neurosci. 18, 10070–10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasterlain, C. G., Liu, H., Mazarati, A. M., Baldwin, R., Shirasaka, Y., Katsumori, H., Thompson, K. W., Sankar, R., Vasconselos, P. D. & Nehlig, A. (2000) Epilepsia 41, Suppl. 6, S134–S143. [DOI] [PubMed] [Google Scholar]
- 26.Yaksh, T. L., Ozaki, G., McCumber, D., Rathbun, M., Svensson, C., Malkmus, S. & Yaksh, M. C. (2001) J. Appl. Physiol. 90, 2386–2402. [DOI] [PubMed] [Google Scholar]
- 27.Borsini, F. & Meli, A. (1988) Psychopharmacology 94, 147–160. [DOI] [PubMed] [Google Scholar]
- 28.Ohman, A., Lycksell, P. O., Jureus, A., Langel, U., Bartfai, T. & Graslund, A. (1998) Biochemistry 37, 9169–9178. [DOI] [PubMed] [Google Scholar]
- 29.Orner, B. P., Ernst, J. T. & Hamilton, A. D. (2001) J. Am. Chem. Soc. 123, 5382–5383. [DOI] [PubMed] [Google Scholar]
- 30.Ernst, J. T., Kutzki, O., Debnath, A. K., Jiang, S., Lu, H. & Hamilton, A. D. (2002) Angew. Chem. Int. Ed. 41, 278–281. [DOI] [PubMed] [Google Scholar]
- 31.Galea, L. A., Wide, J. K. & Barr, A. M. (2001) Behav. Brain Res. 122, 1–9. [DOI] [PubMed] [Google Scholar]
- 32.Hökfelt, T., Bartfai, T. & Bloom, F. (2003) Lancet Neurol. 2, 463–472. [DOI] [PubMed] [Google Scholar]
- 33.Janecka, A., Zubrzycka, M. & Janecki, T. (2001) J. Pept. Res. 58, 91–107. [DOI] [PubMed] [Google Scholar]