Abstract
The dispersal of larvae and their settlement to suitable habitat is fundamental to the replenishment of marine populations and the communities in which they live. Sound plays an important role in this process because for larvae of various species, it acts as an orientational cue towards suitable settlement habitat. Because marine sounds are largely of biological origin, they not only carry information about the location of potential habitat, but also information about the quality of habitat. While ocean acidification is known to affect a wide range of marine organisms and processes, its effect on marine soundscapes and its reception by navigating oceanic larvae remains unknown. Here, we show that ocean acidification causes a switch in role of present-day soundscapes from attractor to repellent in the auditory preferences in a temperate larval fish. Using natural CO2 vents as analogues of future ocean conditions, we further reveal that ocean acidification can impact marine soundscapes by profoundly diminishing their biological sound production. An altered soundscape poorer in biological cues indirectly penalizes oceanic larvae at settlement stage because both control and CO2-treated fish larvae showed lack of any response to such future soundscapes. These indirect and direct effects of ocean acidification put at risk the complex processes of larval dispersal and settlement.
Keywords: larval dispersal, population replenishment, settlement, sound, snapping shrimps, mulloway
1. Introduction
Climate change has been forecast to substantially change marine ecosystems within this century [1], but the processes through which this will occur are not always obvious. Larval dispersal and settlement are crucial and delicate processes that regulate marine community structuring and connectivity via population replenishment [2]. How this inherently complex process is affected by ocean acidification remains largely unknown.
Oceanic propagules of various marine organisms rely on sound for orientation towards suitable adulthood habitat [3] and this sound is largely derived from biological sources, such as snapping shrimps and fish, which are vulnerable to ocean acidification themselves.
One of the most remarkable effects of ocean acidification on marine animals is its interference with a ubiquitous neurotransmitter, and this has been linked to a range of CO2-driven behavioural alterations [4], which include auditory-mediated behaviour [5]. The findings of these laboratory experiments have been recently confirmed in the field on natural CO2 vents, where fish communities, continuously exposed to elevated CO2, failed to acclimate and showed striking behavioural abnormalities, such as attraction towards predator odour and increased boldness [6,7]. Nevertheless, CO2 vents are not a perfect analogue for the future ocean because they are influenced by larval supply from nearby unaffected populations. The question of how ocean acidification affects population replenishment therefore remains unassessed.
We studied how ocean acidification may affect settlement-stage oceanic larvae that use sound as a cue to find their adult habitat. We tested two potential pathways that could alter successful settlement: direct effects of ocean acidification on larvae via altered behavioural preferences towards useful auditory cues, and indirect effects on larvae via altered quality of biological auditory cues (i.e. the underwater soundscape).
The effect of high CO2 on fish auditory preferences was tested in the laboratory by exposing settlement-stage larvae of a common temperate fish (mulloway, Argyrosomus japonicus) to coastal soundscapes as a potential orientation cue to settlement habitats. Mulloway is a highly valued fish in the Indo-West Pacific, which typically spawns at sea and whose larvae settle in near-shore coastal waters and estuaries on hard substratum and in deep holes [8]. These types of settlement habitat are known to currently experience elevated pCO2 levels [9] due to anthropogenic and natural processes such as eutrophication and coastal upwelling. As these processes will locally exacerbate the effect of ocean acidification, we expect these areas to reach CO2 levels higher than what is expected for the global ocean average [10]. Future projections that take into account the combined effect of ocean acidification and eutrophication in coastal and estuarine hypoxic regions estimate that pCO2 values of 1700–3200 µatm can be easily reached [11]. Mulloway is a soniferous fish with large otoliths [11]. To our knowledge, the hearing range of this species is unknown and this is the first study that investigates auditory responses in this species at the larval stage.
2. Material and methods
(a). Effect of CO2 on larval fish auditory response
Based on future projections for coastal and estuarine hypoxic regions [11], we conservatively exposed our fish larvae to a target pCO2 of approximately 1368 µatm as elevated-CO2 treatment (table 1).
Table 1.
Summary of the water chemistry parameters. Average (±s.e.) temperature (T), pH (National Bureau of Standards), total alkalinity (TA); SW, seawater. pCO2 values were calculated using CO2sys [12].
| treatment | T (°C) | pHNBS | N (T, pH) | TA (µmol kgSW−1) | pCO2 (µatm) | N (TA, pCO2) | salinity | N (sal.) | |
|---|---|---|---|---|---|---|---|---|---|
| CO2 vents (White Island) | control | 17.6 (±0.1) | 8.06 (±0.02) | 21 | 2295.7 (±10.7) | 538.8 (±32.2) | 4 | 35 | 1 |
| elevated | 17.9 (±0.1) | 7.86 (±0.02) | 33 | 2287.2 (±12.1) | 929.6 (±54.1) | 4 | 35 | 1 | |
| mulloway experiment | control | 22.2 (±0.2) | 8.03 (±0.01) | 25 | 2538.4 (±8.5) | 606.5 (±17.6) | 4 | 38.4 (±0.2) | 25 |
| elevated | 22.2 (±0.1) | 7.65 (±0.02) | 25 | 2520.6 (±9.3) | 1368.6 (±145.6) | 4 | 38.3 (±0.2) | 25 |
The response to settlement habitat sound was tested in an auditory choice chamber using an established method [5] and videorecorded for analysis (see electronic supplementary material for an in-depth description).
A total of 128 mulloway larvae at settlement stage (25–28 days old) that had been reared under control and elevated-CO2 conditions (table 1) from the egg stage were used (half in each treatment). Subsequent to the behavioural tests, fish were euthanized using a clove oil overdose and frozen until dissection. Additional details for the behavioural experiments and otolith preparation are provided in the electronic supplementary material.
(b). Study site and soundscape
Natural CO2 vents in temperate waters at White Island (New Zealand) were used to assess the effect of end-of-century business-as-usual levels of CO2 [13] (table 1) on settlement habitat soundscapes. The soundscape data presented in this paper represent a part of a broader study that characterized the effect of ocean acidification on marine soundscapes using three natural CO2 vents (two in Italy and the one in New Zealand presented in this paper) and which verified, with a series of laboratory experiments, that sound-producing behaviour of snapping shrimps is reduced by ocean acidification (T Rossi, I Nagelkerken, SD Connell 2013–2014, unpublished data). Sound recordings from White Island were chosen here as cues for mulloway because they provided a more realistic settlement cue than soundscapes from the Northern Hemisphere. Additional methods for the soundscape analysis are described in the electronic supplementary material.
(c). Statistical analysis
Attraction or deterrence of larval fish towards soundscapes was determined by testing the percentage of time spent in the half section of the choice chamber closest to the active speaker against the threshold for a random response of 50% in each half. Data were not normally distributed, as assessed by a Shapiro–Wilk test (p < 0.05), and therefore a non-parametric one-sample Wilcoxon signed-rank test was used, to test each of the four distributions separately. Differences in fish otolith size were tested using ANOVA.
3. Results
(a). Effect of CO2 on larval fish audition
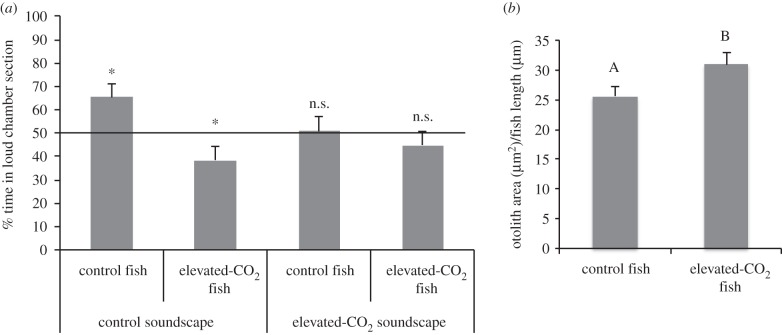
Under present-day ambient seawater pCO2 conditions (approx. 606 µatm), mulloway larvae showed significant attraction towards present-day acoustic habitat cues (Wilcoxon signed-rank test, Z = 2.565, p = 0.010), whereas CO2-treated fish showed significant avoidance towards these cues (Wilcoxon signed-rank test, Z = −2.280, p = 0.023) (figure 1a).
Figure 1.
Effect of ocean acidification on settlement-stage larval fish auditory preferences and otolith (earbone) size. (a) Mean (±s.e.) percentage of time spent by mulloway larvae (N = 64 per treatment) in the half of the choice chamber closest to the broadcasting speaker playing elevated-CO2 or control temperate reef sounds. Stars indicate distributions significantly different (p < 0.05) from a random distribution of 50%. (b) Mean otolith surface area of mulloway (N = 22 per treatment) standardized to larval body length. Different letters indicate statistically significant (p < 0.05) differences. n.s., not significant.
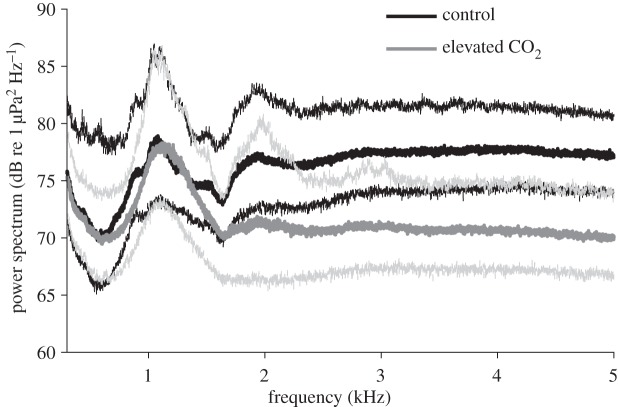
In situ sound recordings in the elevated-CO2 vent areas revealed a marked decline in sound intensity in the frequencies corresponding to snapping shrimp sound as observed by acoustic spectra (figure 2) (above approx. 500 Hz with a peak at approx. 4 KHz). A peak in sound pressure level centred around 1.2 kHz, likely originating from sea urchin rasping sound, did not show differences between treatment sites.
Figure 2.
Acoustic spectra of a current versus a future high-CO2 marine soundscape, based on median (thick lines) ±95% CI (thin lines) acoustic power spectra representing dusk chorus of snapping shrimps (peak frequency approx. 4 kHz) and sea urchins (peak frequency approx. 1.2 kHz) at White Island. Acoustic power was averaged among multiple days of sampling at multiple sites (N = 2 per treatment).
Furthermore, control as well as CO2-treated mulloway larvae lost their responsiveness towards high-CO2-affected soundscapes (respectively, Z = 0.041, p = 0.968 and Z = −0.848, p = 0.396) (figure 1a).
(b). Larval fish otoliths
Mulloway larvae possessed significantly enlarged otoliths (20.9% increase in surface area standardized by body length; ANOVA, F1,42 = 4.3, p = 0.04) under future CO2 conditions (figure 1b).
4. Discussion
The results of our study suggest that ocean acidification could have negative direct and indirect effects on the process of larval orientation and settlement. Biological sounds are recognized as important orientation cues for various marine organisms owing to their long propagation distance and the richness of biological information they carry [3]. Snapping shrimp crackle is certainly the most common biological feature of marine coastal soundscapes and often dominates over background abiotic noise and other biological sound sources. In this study, using field recordings, we found elevated CO2 to reduce snapping shrimp sound intensity, which occupies a substantial part of the sound frequency spectrum (starting at ∼500 Hz), including part of the hearing range of fish [3]. This pattern was also observed at two CO2 vents in the Northern Hemisphere (T Rossi, I Nagelkerken, SD Connell 2013–2014, unpublished data).
We also find that elevated CO2 directly impacts larvae by reversing the innate attraction of settlement-stage fish larvae towards coastal soundscapes. Furthermore, fish larvae showed lack of attraction to future soundscapes whether or not they were treated with elevated CO2. This suggests that fish are able to discern between soundscapes of different qualities and that present-day larvae lose selective attraction towards a high-CO2-degraded future soundscape even when unaffected directly by elevated CO2. Our study therefore indicates that the decrease in sound quality and quantity of marine habitats in a high-CO2 world can result in diminished value of an important biological cue used by marine species for orientation and navigation.
Ocean acidification increases the size of fish earbones (otoliths) [14], which are used by fish for hearing, orientation and balance [3]. Using a modelling approach, it has been hypothesized that enlarged otoliths under future CO2 conditions might increase the hearing range of larval fish [15], but this effect has not been empirically validated. In our study, the mulloway that were raised under future CO2 conditions throughout their larval development showed an enlargement of their otoliths. Nevertheless, these mulloway larvae failed to respond to ecologically relevant habitat sounds from both present-day and future soundscapes. This failure in response suggests that even when ocean acidification leads to enlarged otoliths and potentially increases hearing sensitivity, fish cannot compensate for altered auditory preferences resulting from elevated CO2.
If biological acoustic cues become of lower value for orientation in a future ocean, larvae will have to compensate by using other environmental cues such as chemical. But even other senses such as olfaction are impaired in many fishes by high CO2 [4], as well as larval traits such as swimming speeds and development [16]. A prolonged oceanic life phase, due to delayed settlement, is likely to increase predation risk, delay occupancy of food-rich benthic habitats and undermine population replenishment.
Supplementary Material
Acknowledgements
We thank W. Hutchinson, M. Olmos and M. D. Rutte for invaluable help with this study.
Ethics
Research was carried out under approval of the University of Adelaide animal ethics committee (permit: S-2013-005) and according to the university's Animal Ethics Guidelines.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.4t8c7.
Authors' contributions
All authors contributed to the design of the study, collection of the data and writing of the article. T.R. analysed the data. All authors approve the final version of this manuscript and agree to be held accountable for all aspects of the work performed.
Competing interests
The authors declare no conflict of interests.
Funding
This study was supported by an Australian Research Council (ARC) Future Fellowship to I.N. (grant no. FT120100183). S.D.C. was supported by an ARC Future Fellowship (grant no. FT0991953).
References
- 1.Nagelkerken I, Connell SD. 2015. Global alteration of ocean ecosystem functioning due to increasing human CO2 emissions. Proc. Natl Acad. Sci. USA 112, 13 272–13 277. ( 10.1073/pnas.1510856112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowen R, Paris C, Srinivasan A. 2006. Scaling of connectivity in marine populations. Science 311, 522–527. ( 10.1126/science.1122039) [DOI] [PubMed] [Google Scholar]
- 3.Montgomery JC, Jeffs A, Simpson SD, Meekan M, Tindle C. 2006. Sound as an orientation cue for the pelagic larvae of reef fishes and decapod crustaceans. Adv. Mar. Biol. 51, 143–196. ( 10.1016/S0065-2881(06)51003-X) [DOI] [PubMed] [Google Scholar]
- 4.Nagelkerken I, Munday PL. 2016. Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob. Change Biol. ( 10.1111/gcb.13167) [DOI] [PubMed] [Google Scholar]
- 5.Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY. 2011. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 7, 917–920. ( 10.1098/rsbl.2011.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munday PL, Cheal AJ, Dixson DL, Rummer JL, Fabricius KE. 2014. Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat. Clim. Change 4, 487–492. ( 10.1038/nclimate2195) [DOI] [Google Scholar]
- 7.Nagelkerken I, Russell BD, Gillanders BM, Connell SD. 2015. Ocean acidification alters fish populations indirectly through habitat modification. Nat. Clim. Change 6, 89–93. ( 10.1038/nclimate2757) [DOI] [Google Scholar]
- 8.Silberschneider V, Gray C. 2008. Synopsis of biological, fisheries and aquaculture-related information on mulloway Argyrosomus japonicus (Pisces: Sciaenidae), with particular reference to Australia. J. Appl. Ichthyol. 24, 7–17. ( 10.1111/j.1439-0426.2007.00913.x) [DOI] [Google Scholar]
- 9.Hofmann GE, et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 ( 10.1371/journal.pone.0028983.t002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feely RA, Alin SR, Newton J, Sabine CL, Warner M, Devol A, Krembs C, Maloy C. 2010. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar. Coast. Shelf Sci. 88, 442–449. ( 10.1016/j.ecss.2010.05.004) [DOI] [Google Scholar]
- 11.Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska M, Bange H, Hansen H, Körtzinger A. 2012. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 160, 1875–1888. ( 10.1007/s00227-012-1954-1) [DOI] [Google Scholar]
- 12.Pelletier G, Lewis E, Wallace D. 2005. A calculator for the CO2 system in seawater for Microsoft Excel/VBA. Olympia, WA: Washington State Department of Ecology. [Google Scholar]
- 13.Meinshausen M, et al. 2011. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Change 109, 213–241. ( 10.1007/s10584-011-0156-z) [DOI] [Google Scholar]
- 14.Checkley DM, Dickson AG, Takahashi M, Radich JA, Eisenkolb N, Asch R. 2009. Elevated CO2 enhances otolith growth in young fish. Science 324, 1683–1683. ( 10.1126/science.1169806) [DOI] [PubMed] [Google Scholar]
- 15.Bignami S, Enochs IC, Manzello DP, Sponaugle S, Cowen RK. 2013. Ocean acidification alters the otoliths of a pantropical fish species with implications for sensory function. Proc. Natl Acad. Sci. USA 110, 7366–7370. ( 10.1073/pnas.1301365110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi T, et al. 2015. Ocean acidification boosts larval fish development but reduces the window of opportunity for successful settlement. Proc. R. Soc. B 282, 20151954 ( 10.1098/rspb.2015.1954) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.4t8c7.