Abstract
Recent evidence suggests that odor-driven responses in the insect antennal lobe (AL) can be modified by associative and nonassociative processes, as has been shown in the vertebrate olfactory bulb. However, the specific network changes that occur in response to olfactory learning remain unknown. To characterize changes in AL network activity during learning, we developed an in vivo protocol in Manduca sexta that allows continuous monitoring of neural ensembles and feeding behavior over the course of olfactory conditioning. Here, we show that Pavlovian conditioning produced a net recruitment of responsive neural units across the AL that persisted after conditioning. Recruitment only occurred when odor reliably predicted food. Conversely, when odor did not predict food, a net loss of responsive units occurred. Simultaneous measures of feeding responses indicated that the treatment-specific patterns of neural recruitment were positively correlated with changes in the insect's behavioral response to odor. In addition to recruitment, conditioning also produced consistent and profound shifts in the temporal responses of 16% of recorded units. These results show that odor representations in the AL are dynamic and related to olfactory memory consolidation. We furthermore provide evidence that the basis of the learning-dependent changes in the AL is not simply an increase in activity in the neural network representing an odorant. Rather, learning produces a restructuring of spatial and temporal components of network responses to odor in the AL.
In the olfactory systems of animals as diverse as insects (1, 2) and vertebrates (3, 4), a large number of odor stimuli can be represented combinatorially by relatively few glomeruli. These systems share a common organization whereby each glomerulus receives primary-afferent input from olfactory receptor cells expressing a specific receptor phenotype (5). A given odor compound activates a spatially distributed ensemble of glomerular projection neurons (PNs), and individual PNs participate in the representation of multiple odor compounds (1, 3, 6–8). Within these ensembles, different features of an odor stimulus are also represented in the temporal structure of odor-driven responses among PNs (1, 3, 6, 7, 9–12).
Like their vertebrate counterparts, foraging insects, such as honeybees, fruit flies, and moths, give modified behavioral responses to an odor stimulus associated with food (13–19). Evidence in vertebrates suggests that the representation of a given odor in the first synaptic relay, the olfactory bulb (OB, the analogue of the insect antennal lobe), can be modified as a result of experience with an odor (6, 20–26). Preliminary studies of learning-dependent changes in the insect antennal lobe (AL), using imaging techniques, also suggest that olfactory responses in this structure might change as a result of experience (27, 28). With an appetitive conditioning paradigm, for example, increased odor-driven calcium signals were observed in honeybee ALs when the odor [conditioned stimulus (CS)] was paired with food [unconditioned stimulus (US)]. Because calcium signals in that study were based on global activity, however, it was not possible to characterize the nature of the changing signal. For example, increased calcium signals simply could have resulted from changes in the responsiveness of the neural network encoding the CS, or alternatively, from a dynamic reorganization of the network, involving the recruitment and/or loss of responsive neurons and possible changes in the temporal relationships among the neurons in the network.
To clarify these issues, we studied experience-dependent plasticity in the AL of the moth Manduca sexta, over the course of appetitive olfactory conditioning. The anatomy and physiology of the AL in Manduca have been studied extensively (7, 9, 11, 12, 29–32). Moreover, this species can be conditioned readily to associate odors with food (19, 33, 34), making this an experimentally favorable model system of olfactory learning. In the current study, we coupled behavioral measures of olfactory learning with neurophysiological measures of odor-evoked ensemble responses in the AL before, during, and after olfactory conditioning (19, 33, 34). We developed a within-animal experimental design that allowed us to correlate, over the course of conditioning, changes in ensemble responses with treatment-dependent changes in behavior.
Experimental Procedures
Details of experimental methods may be found online (see Supporting Text, which is published as supporting information on the PNAS web site). In brief, data were obtained from 21 male M. sexta moths studied under four treatment conditions: forward-paired (FP), discrimination learning, and one- and two-odor CS-only groups. These treatments were selected on the basis of established methods for assessment of associative learning (19, 27, 33, 34). The CS-only groups were used as comparative references for the FP and discrimination-learning treatments. Fully intact moths were prepared identically for all treatments, as shown in Fig. 1a. To measure development of the conditioned feeding behavior, fine-wire electromyographic (EMG) electrodes were implanted in the head of each moth and feeding-muscle responses were recorded. Silicon multichannel electrode arrays (Center for Neural Communication Technology, University of Michigan, Ann Arbor) also were implanted in an AL of each moth for recording of neural-ensemble responses to odor. This procedure allowed simultaneous recording of both behavioral (EMG) and CNS responses to odor stimulation. Both EMG and multichannel recording procedures were matched to standard protocols (refs. 11, 12, and 19 and Supporting Text). Action potentials (spikes) were recorded on a given electrode within the electrode array. Spikes were subsequently sorted into multiple individual “units” by using standard spike-sorting protocols (refs. 11 and 12 and Supporting Text). Each unit is therefore defined by spikes that have statistically identical waveforms. Thus, we assume that each unit is a record of a single neuron. Recording sessions lasted 50 min to 3 h, depending on the experimental treatment and longevity of the preparation. Olfactory stimulation (CS) and sucrose delivery (US) were under computer control as described (19). Histological examination of the brain after recording confirmed placement of the probes (Fig. 1 b and c).
Fig. 1.
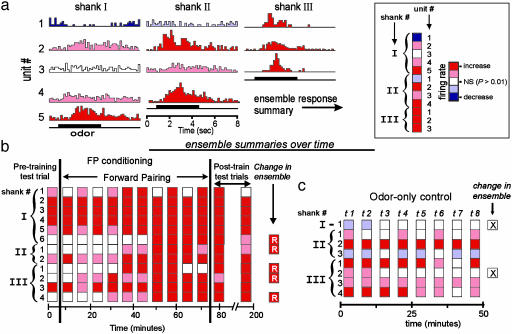
Experimental protocol used to monitor odor-evoked patterns of neural-ensemble activity and feeding behavior in vivo during olfactory conditioning. (a) Moths were restrained in plastic tubes, leaving the antennae and proboscis accessible for stimulation (see Supplemental Text). An electrode recorded electromyographic activity from feeding muscles, providing an indicator of conditioned responses. (b) Multiunit recordings of olfactory responses were obtained across 12 channels of a three-shank silicon microprobe. The shanks were separated by 150 μm(I–III). Recording sites (9 × 12 μm2) were arranged in a linear array on each shank and were spaced 25 μm apart. (c) Laser-scanning confocal micrograph showing a cross-sectional view of the tracks left by the three shanks of the microelectrode array. This example shows that probe III was misplaced into the antennal nerve.
Results
Forward Pairing Modulates AL Ensemble Responses. In the first set of experiments (40 units, 4 moths; Table 1), we used forward pairing to test whether unit responses across the ensemble would be altered when the odor stimulus predicted food. In this FP group, the CS always preceded and partially overlapped presentation of the US (19). A control group of moths, the CS-only group (52 units, 6 moths; Table 1), was presented with only the CS in an identical manner. For each trial, we assessed unit responses to the CS based on a statistically significant change in the peri-stimulus firing rate. Significant changes in spike rate were assigned to one of two categories: excited/increased or inhibited/decreased (P < 0.01; Fig. 2a).
Table 1. Summary of ensemble changes occurring under different experimental conditions.
| Responsive units
|
Statistics
|
Response type
|
|||||
|---|---|---|---|---|---|---|---|
| Experimental condition (no. of moths; no. of units) | Total | At start | At end | Recruited | Lost | Excited | Inhibited |
| Forward-paired (4; 40) | |||||||
| Mean | 10.0 | 5.0 | 8.0 | 3.8 | 0.5 | 7.8 | 0.2 |
| SE | ±2.7 | ±1.6 | ±1.6 | ±0.9 | ±0.6 | ±1.0 | ±0.2 |
| Range | 6-17 | ||||||
| CS only (6; 52) | |||||||
| Mean | 8.7 | 6.0 | 4.0 | 0.2 | 2.0 | 4.0 | 0 |
| SE | ±0.9 | ±0.6 | ±0.7 | ±0.2 | ±0.7 | ±0.8 | |
| Range | 7-13 | ||||||
| Differential conditioning (5; 69) | |||||||
| CS+ | |||||||
| Mean | 16.3 | 7.5 | 8.8 | 3.8 | 2.5 | 6.8 | 2.0 |
| SE | ±3.4 | ±1.4 | ±1.0 | ±1.0 | ±0.9 | ±0.7 | ±0.7 |
| Range | 12-25 | ||||||
| CS- | |||||||
| Mean | 16.3 | 9.8 | 7.8 | 0.8 | 2.8 | 6.2 | 1.6 |
| SE | ±3.4 | ±1.0 | ±1.0 | ±0.3 | ±0.5 | ±0.7 | ±0.6 |
| Range | 12-25 | ||||||
| 2-odor CS only (4; 73) | |||||||
| CS1 | |||||||
| Mean | 18.3 | 10.2 | 8.8 | 0.3 | 1.7 | 5.7 | 3.1 |
| SE | ±6.8 | ±6.1 | ±4.8 | ±0.1 | ±0.4 | ±3.3 | ±2.6 |
| Range | 11-34 | ||||||
| CS2 | |||||||
| Mean | 18.3 | 9.8 | 7.8 | 0.5 | 2.5 | 5.3 | 2.5 |
| SE | ±6.8 | ±5.8 | ±4.5 | ±0.3 | ±0.6 | ±3.6 | ±2.3 |
| Range | 11-34 | ||||||
Summary statistics of recordings from 230 units in 18 moths. The first column lists the conditioning treatment (n, number of moths; number of units). All values in the following columns are the average number of units per ensemble in each of the four experimental categories. Total, the average number of units identified in each ensemble, the standard error, and the range of units across ensembles. Start, the average number of units that responded to the odor stimulus at the beginning of the experiment. End, the average number of units that responded to the odor stimulus at the end of the conditioning experiment. Recruited, the average number of neurons that were unresponsive at the start but began responding during or after conditioning treatment. Lost, the average number of units that initially responded but became statistically unresponsive. Response type, the type of response evoked by the presentation of the odor stimulus. Probabilities reported in the text result from Student's t tests between the indicated mean values.
Fig. 2.
The trial-by-trial statistical assessment of unit/ensemble responsiveness to odor stimulation. (a) Peri-stimulus time histograms (PSTHs) were calculated for each unit in response to each stimulus presentation for all recorded ensembles. Each PSTH was color-coded to reflect statistically significant increases (shades of red) or decreases (shades of blue) in the rate of spiking in response to odor. (Inset) Each odor-evoked response was condensed into a linear “ensemble response summary,” which was then positioned vertically to track changes in the ensemble across successive trials (x axis). (b) Summary of ensemble responses recorded from the AL during FP conditioning. Ensemble responses to the conditioning stimulus (CS = cyclohexanone) were recorded once before conditioning, during each of the 10 FP conditioning trials, and in two odor-only posttests. The two posttests were performed at 6 and 90 min after the last conditioning trial. (c) Summary of ensemble responses recorded from the AL during CS-only trials. Experimental procedures were identical with the FP treatment except that odor was never reinforced, and the duration of the recordings was 50 min. Overall change in unit responses during the course of the experiment are summarized on the right. R, excitatory unit was recruited; X, responsive unit was dropped.
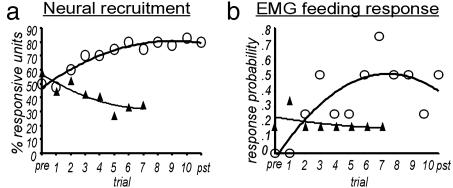
Over the course of successive FP conditioning trials, there was a significant and progressive increase in the number of responsive units (Fig. 2b). In some cases an increase was evident in as few as two conditioning trials. This net increase in responsive units was not observed in the CS-only group (Fig. 2c). Overall, we observed a mean net recruitment of 3.8 responsive units (P < 0.001, relative to the CS-only group). This recruitment amounted to a 60% net increase in responsive units across all ensembles in the FP group. Fig. 3a shows the progression of neural recruitment from trial to trial. This trend is characterized by the embedded regression line, which accounts for 93% of the variance in unit responsiveness across trials. The greatest number of units recruited into any individual ensemble was seven, representing a 100% increase in the number of responding units.
Fig. 3.
The trial-by-trial mean changes in neural and feeding responses to odor stimulation (CS) before (pre), during, and after (pst) conditioning for FP (○) versus CS-only treatment groups (▴). (a) Mean changes in the percentage of recorded units that responded to the CS by trial. (b) Mean changes in conditioned feeding in response by trial for the same animals. Odor-only controls only received eight trials to characterize changes.
Some units showed a gradual reduction in their responsiveness to the CS during successive conditioning trials. In contrast to the recruitment of originally unresponsive units, however, considerably fewer units showed a reduction in response during conditioning. By comparison, loss of unit responsiveness was more prevalent in the CS-only group of moths (P < 0.001; Table 1, CS only). Units that statistically became unresponsive tended to have initially weak excitatory or inhibitory responses. Like recruitment in the FP group, the loss of responsive units was progressive over trials. This trend is characterized in Fig. 3a with a regression line that accounts for 77% of the variance in responsiveness across trials.
Fig. 3b shows the systematic acquisition of a conditioned feeding response (CR) for the same groups of moths used for multichannel recording. Consistent with our observations of recruitment and loss in the FP and CS-only groups, we found associated patterns of acquisition of the odor-driven CR. The regression analysis accounted for 18% and 61% of the variance, respectively, for the FP and CS-only groups.
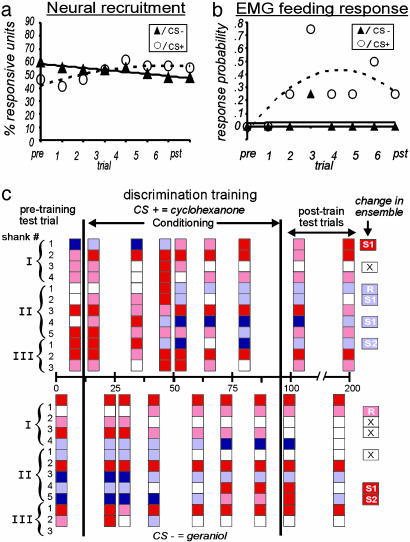
Ensemble Modulation and Reinforcement Context: Differential Conditioning. The results outlined above do not exclude the possibility that a change in the ensemble response could have been due to nonassociative effects of repeated sucrose presentation (e.g., sensitization; refs. 19, 27, and 35). To address this issue, we performed a set of differential-conditioning experiments (65 units, 4 moths; Table 1) during which one of two odor compounds was paired with sucrose reinforcement (CS+, cyclohexanone). The second odor stimulus was presented without reinforcement (CS–, geraniol) for the same number of pseudorandom, interspersed trials. If sensitization were responsible for the recruitment observed in the FP experiments, we would have expected roughly equivalent patterns of recruitment for both the CS+ and CS– odor stimuli. Our results show, however, that the pattern of recruitment depended on the predictive relationship between odor and sucrose reinforcement in the CS+ treatment (Fig. 4).
Fig. 4.
The trial-by-trial mean changes in neural and feeding responses to odor stimulation during differential conditioning. (a) Trial-by-trial mean percentage of units responding to the CS+ (○, cyclohexanone) versus CS– (▴, geraniol) odors. (b) The corresponding mean trial-by-trial acquisition of the EMG feeding response. Second-order polynomial trend lines were used to predict acquisition rates (see Experimental Procedures). (c) Unit response data by trial from a single animal. Results were calculated and color-formatted based on the approach described in Fig. 2. Overall changes in unit responses over the course of the experiment are summarized on the right. Red R, an excitatory unit was recruited; blue R, an inhibitory unit was recruited; Red, responsive unit was dropped; S1, switched response polarity to one odor; S2, switched response polarity to both odors (color in figure denotes response at the first posttest).
Many of the units initially responded to both the CS+ and CS– odor stimuli, but the ensemble patterns evoked by the two odor stimuli were nevertheless distinct (10). During differential conditioning, we observed changes in the unit responses that were consistent with the FP and CS-only treatments with respect to neural recruitment patterns and acquisition of a CR (Fig. 4). When the olfactory stimulus was reinforced (CS+), we observed a progressive mean recruitment of 3.8 units per ensemble (Fig. 4a), which again was associated with the development of the conditioned feeding behavior (Fig. 4b). Net recruitment was lower in the CS+ treatment as compared with the FP treatment, because significantly more units became unresponsive to the CS+ (P < 0.001; Table 1, differential conditioning).
When the odor stimulus was not reinforced (CS–), a progressive net loss of responsive units occurred (Fig. 4a), and we did not observe the development of a CR (Fig. 4b). Results for the CS– odor stimulus were thus consistent with the CS-only group (see Table 1, CS only). Furthermore, net recruitment was significantly greater for the CS+ than for the CS– odor (P < 0.001). Finally, the acquisition of a CR for the CS+ was comparable with that for the FP group, and it was significantly higher than that for the CS– odor (P < 0.001; Table 1, CS only, and Fig. 4b). The fact that different patterns of net recruitment and loss were seen in response to the CS+ and CS– indicates that sensitization alone cannot account for the observed net recruitment in the FP group (27).
We also observed that ≈30% of all units exhibited a pronounced shift in the polarity of the odor-driven response over the course of discrimination conditioning (Table 1, differential conditioning). In each of the discrimination-learning experiments, the response of at least one unit switched over time from inhibitory to excitatory or vice versa. In some cases, a reciprocal switch occurred in response to both odor stimuli. Fig. 4c shows the evolution of the polarity switch in a single animal over the course of the differential conditioning trials. Notice that some units switch responses for both CS+ and CS–, and once switched, these changes persisted for at least 1 h. Re-inspection of the FP and CS-only experiments revealed response switching in 7% of the recorded units, but only in the FP group, suggesting that polarity switching is a property that depends on the reinforcement context.
To account for the unique conditions governing the differential conditioning paradigm, we performed experiments in which two odor stimuli (CS1 and CS2) were presented, but neither was paired with sucrose (73 units in 4 moths; Table 1, two-odor CS only). Results from this experiment corresponded with the CS-only experiments. We observed a net loss of statistically responsive units across trials (Fig. 5a) in parallel with a decrease in EMG response probability over trials (Fig. 5b). When we compared neural recruitment and loss over successive trials, we found, in contrast to the differential conditioning group, no significant difference between the two odor stimuli (Table 1, two-odor CS only; P > 0.10). Furthermore, the frequency of unit recruitment and loss was statistically equivalent to both the CS-only group (Table 1, CS only), and the CS– treatment in the differential conditioning experiments (Table 1, differential conditioning). Again, as the CS-only group, we did not observe switches in response polarity (e.g., Fig. 5c).
Fig. 5.
The trial-by-trial mean changes in neural and feeding responses to odor stimulation for the two-odor CS-only group. (a) Trial-by-trial mean percentage of units responding to the cyclohexanone (□) versus geraniol (▴) odors. (b) The corresponding mean trial-by-trial acquisition of the EMG feeding response. Second-order polynomial regression was used to predict acquisition rates (see Experimental Procedures). (c) Unit response data by trial from a single animal. Results are calculated and formatted based on the approach described in Fig. 2. Overall changes are summarized as in previous figures.
Consistency of Experience-Driven Changes in Ensemble Responses. In both types of treatments in which the CS occurred in the context of a US (i.e., FP and CS+ and CS– discrimination), we observed a characteristic switch in the response polarity of some units. These results suggested that the temporal details of response patterns of individual neurons could change as a result of associative processes. Because we presented only a single stimulation per trial, however, it was impossible to assess the consistency of this effect or to characterize it in detail. To explore this effect further, a group of animals (n = 3 moths and 53 units) was conditioned by using the FP treatment. In this experiment, however, we presented the CS as a sequence of twenty 100-ms pulses spaced 5 s apart, before and after conditioning. Of the 53 units determined to be stable by careful assessment of peak-by-channel-by-time plots (see Supporting Text), we observed distinctive conditioning-dependent changes in the slow temporal dynamics of the odor-driven responses in 9 of them (16%). The examples in Fig. 6 a and b show that the brief excitatory responses evoked by each stimulus pulse before training can be completely absent after olfactory conditioning, or they can shift in latency and/or duration. Other examples in Fig. 6 b and c show that excitatory response epochs can also be recruited as a consequence of conditioning. Interestingly, Fig. 6b shows that more than one type of temporal alteration can be observed in the same unit. These data provide additional evidence for a restructuring of the odor representation at the level of the AL.
Fig. 6.
Examples of learning-dependent changes in slow temporal responses to repeated odor stimulation from 4 units. Data are presented as peri-event rasters showing responses to 20 successive 100-ms odor pulses; each row in the raster plot represents the unit's response before, during (gray bar), and after each pulse. Responses to 20 pulses of odor before conditioning (pre-cond.) are placed above 20 pulses presented post-conditioning (pst-cond.). Arrows point to consistent changes in the slow temporal response in the post-conditioning rasters when compared with pre-conditioning rasters. (a) This unit lost one of two excitatory epochs. (b) Shift in latency and patterning of response. (c) Acquisition of an excitatory response. (d) Loss of an excitatory response. Inhibition followed by bursting is indicative of PN responses (36), which suggest that most of our recorded units were PNs. Other units that changed response to odor displayed a low-latency excitatory response with no evidence of an initial inhibitory phase. This pattern is consistent with intracellular records of LN responses (36).
Discussion
Different types of reinforcement context, including a lack of reinforcement, were found to modulate the spatial and temporal characteristics of the ensemble responses to odors in the AL of Manduca. Presentation of an olfactory stimulus followed by sucrose reinforcement (CS+), in either the FP or discrimination-conditioning groups, resulted in a combination of recruitment and loss of responsive units. This process always yielded a net increase in responsive units. We also observed switches in response polarity, which occurred more frequently during differential conditioning, possibly indicating encoding of information about recently experienced odors that were not associated with food (i.e., the CS– during differential conditioning). When we modified our methods to detail the nature and consistency of these modifications more closely, we found that the FP treatment caused fundamental changes in the temporal patterning of unit responses (Fig. 6), which could account for the polarity switching observed in our initial experiments (Fig. 4).
The meaning of the changes remains unclear, but they show that associative processes lead to a net recruitment and changes in the temporal patterning of responsive units in the AL. Both of these findings demonstrate conclusively that the synaptic circuitry in the AL is not fixed but subject to learning-based plasticity. More importantly, our results demonstrate that the observed changes result from a dramatic restructuring of the neural networks encoding odors in the AL. The output pathways from the AL (PNs) commonly exhibit odor-dependent, temporally complex patterns of inhibition followed by excitation (e.g., ref. 12). Many of the units produced responses that are consistent with results from intracellular recordings from PNs (37). Our findings therefore provide evidence that both the local processing and output codes for odors in the moth AL are dynamic and depend on experience.
It is important to note that an animal's behavioral responses to odors can be modified through both associative and nonassociative processes (19, 35, 36, 38–42). This means that learning can also occur under CS-only conditions. In behavioral experiments CS-only treatment can produce nonassociative effects such as habituation (35, 36, 38, 39) and latent inhibition (40–42), in which subsequent acquisition of a conditioned response is disrupted. The loss of responsive units that we observed with the CS-only treatments may therefore represent nonassociative mechanisms during differential conditioning, but further testing is needed.
Finally, the finding that odor-driven responses within the AL are modified by correlated input from a different sensory modality, in this case a gustatory input, may be explained by the presence of one or more feedback pathways modulating activity in the AL. Identifiable, monoaminergic neurons in both honeybees (43–45) and Manduca (30) arborize in the AL and provide centrifugal input from other brain areas. They thus could provide the basis for a feedback pathway that mediates the learning-related changes we observed. In honeybees, for example, an identified modulatory neuron, called VUMmx1, is one of a group of neurons that, when activated by sucrose-sensitive taste pathways, releases octopamine (44, 45). Stimulation of this pathway in the presence of odor can produce a conditioned response in the absence of sucrose (43). Furthermore, blockade of this pathway can disrupt the acquisition of a conditioned response (46). These neurons may thus relay information about the US to distributed neuropils in the brain, including the AL (45).
Anatomical evidence indicates that similar types of neurons are also present in the moth. For example, an identified serotonin-immunoreactive neuron arborizes throughout the glomeruli of the AL (30), and application of serotonin causes a broadening of action potentials and prolonged firing in AL neurons (47). Several other neurotransmitters/modulators in the AL, including nitric oxide (48, 49), γ-aminobutyric acid (50), and dopamine (51), have been identified and also may be involved in AL plasticity (52). Such mechanisms are now under investigation, but it is also possible that as-yet unidentified feedback pathways (representing other aspects of the learning context, such as visual cues) also could account for some of the changes we observed.
The AL is thus one of the first sites for convergence of the CS (odor) and US (sucrose) processing pathways in the brain. The role of this plasticity in early sensory processing is not clear, although it may be important for olfactory signal enhancement relative to a noisy background (53) and/or olfactory memory consolidation (27, 28). It is known that US-driven plasticity is important for processing in the vertebrate OB and can result in changes in OB output (25). Several studies have revealed that centrifugal noradrenergic fibers modify OB responses related to both birth (22, 54) and suckling (21) in newborn mammals.
In conclusion, we have developed an experimental preparation that uses ensemble recording to monitor the network changes that occur in the AL in a behaving animal undergoing olfactory conditioning. This preparation represents a powerful new tool to study mechanisms of plasticity in olfactory coding in a species with a well characterized olfactory system and robust odor-modulated behaviors. We have shown that AL ensembles undergo substantial reorganization as a result of both associative and nonssociative processes and are correlated with expected changes in behavior. Future experiments, coupling manipulation of these putative modulatory pathways with monitoring of activity in neural ensembles, will help to reveal the functional significance of these centrifugal pathways. The existence of plasticity in odor-evoked ensemble representations in the OB and AL now adds to a growing list of similarities between these phylogenetically divergent species and underscores the need to examine a range of diverse species by using a variety of methods to reveal fundamental mechanisms of olfactory processing that cross phylogenetic boundaries.
Supplementary Material
Acknowledgments
We thank the Center for Neural Communication Technology at the University of Michigan for providing microprobes and Heather Stein, Vincent Pawlowlski, and A. A. Osman for technical assistance. This work was supported by National Institutes of Health Grants RR014166 (to B.H.S.), DC05535 (to K.C.D.), DC02751 (to J.G.H.), and DC05652 (to T.A.C.), and by a contract from Defense Advanced Research Projects Agency (to J.G.H.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AL, antennal lobe; CS, conditioned stimulus; EMG, electromyographic; US, unconditioned stimulus; CR, conditioned response; FP, forward-paired; OB, olfactory bulb; PN, projection neuron.
References
- 1.Laurent, G. (1999) Science 286, 723–728. [DOI] [PubMed] [Google Scholar]
- 2.Galizia, C. G. & Menzel, R. (2001) J. Insect Physiol. 47, 115–130. [DOI] [PubMed] [Google Scholar]
- 3.Mori, K., Nagao, H. & Yoshihara, Y. (1999) Science. 286, 711–715. [DOI] [PubMed] [Google Scholar]
- 4.Xu, F., Greer, C. A. & Shepherd, G. M. (2000) J. Comp. Neurol. 422, 496–509. [DOI] [PubMed] [Google Scholar]
- 5.Vosshall L. B., Wong A. M. & Axel R. (2000) Cell 102, 147–159. [DOI] [PubMed] [Google Scholar]
- 6.Kay, L. M. & Laurent, G. (1999) Nat. Neurosci. 2, 1003–1009. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, T. A. & White, J. E. (2000) in The Neurobiology of Taste and Smell, eds. Finger, T. E., Silver, W. L. & Restrepo, D. (Wiley, New York), pp. 201–232.
- 8.Sachse, S. & Galizia, C. G. (2002) J. Neurophysiol. 87, 1106–1117. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, T. A., Pawlowski, V. M., Lei, H. & Hildebrand, J. G. (2000) Nat. Neurosci. 3, 927–931. [DOI] [PubMed] [Google Scholar]
- 10.Laurent, G., Stopfer, M., Friedrich, R. W., Rabinovich, M. I., Volkovskii, A. & Abarbanel, H. D. (2001) Annu. Rev. Neurosci. 24, 263–297. [DOI] [PubMed] [Google Scholar]
- 11.Lei, H., Christensen, T. A. & Hildebrand, J. G. (2002) Nat. Neurosci. 5, 557–565. [DOI] [PubMed] [Google Scholar]
- 12.Daly, K. C., Wright, G. A. & Smith, B. H. (2004) J. Neurophysiol. 92, 236–254. [DOI] [PubMed] [Google Scholar]
- 13.Bitterman, M. E., Menzel, R., Fietz, A. & Schafer, S. (1983) J. Comp. Physiol. 97, 107–119. [PubMed] [Google Scholar]
- 14.Kuwabara, M. (1957) J. Fac. Sci. Hokkaido Univ. Ser. 6 13, 458–464. [Google Scholar]
- 15.Menzel, R. (1990) in Neurobiology of Comparative Cognition, eds. Kesner, R. P. & Olton, D. S. (Erlbaum, Hillsdale, NJ), pp. 237–292.
- 16.Lofdahl, K. L., Holliday M. & Hirsch, J. (1992) J. Comp. Psychol. 106, 172–183. [DOI] [PubMed] [Google Scholar]
- 17.Hartlieb, E. (1996) Naturwissenschaften 83, 87–88. [Google Scholar]
- 18.Fan, R., Anderson, P. & Hansson, B. (1997) J. Exp. Biol. 200, 2969–2976. [DOI] [PubMed] [Google Scholar]
- 19.Daly, K. C. & Smith, B. H. (2000) J. Exp. Biol. 203, 2025–2038. [DOI] [PubMed] [Google Scholar]
- 20.Wilson, D. A., Sullivan, R. M. & Leon, M. (1987) J. Neurosci. 7, 3154–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan, R. M., Wilson, D. A. & Leon, M. (1989) J. Neurosci. 9, 3998–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kendrick, K. M., Levy, F. & Keverne, E. B. (1992) Science 256, 833–836. [DOI] [PubMed] [Google Scholar]
- 23.Wilson D. A. & Sullivan, R. M. (1994) Behav. Neural Biol. 61, 1–18. [DOI] [PubMed] [Google Scholar]
- 24.Bhalla, U. S. & Bower, J. M. (1997) J. Comput. Neurosci. 4, 221–256. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher, M. L. & Wilson, D. A. (2003) J. Neurosci. 22, RC201 (1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg, S. J. & Moulton, D. G. (1987) Exp. Neurol. 96, 430–442. [DOI] [PubMed] [Google Scholar]
- 27.Faber, T., Joerges, J. & Menzel, R. (1999) Nat. Neurosci. 2, 74–78. [DOI] [PubMed] [Google Scholar]
- 28.Yu, D., Ponormarev, A. & Davis, R. L. (2004) Neuron 42, 437–439. [DOI] [PubMed] [Google Scholar]
- 29.Tolbert, L. P. & Hildebrand, J. G. (1981) J. Neurosci. 3, 1158–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kent, K. S., Hoskins, S. G. & Hildebrand, J. G. (1987) J. Neurobiol. 18, 451–465. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd, G. M. & Hildebrand, J. G. (1997) Annu. Rev. Neurosci. 20, 593–631. [DOI] [PubMed] [Google Scholar]
- 32.Rospars, J. P. & Hildebrand, J. G. (2000) Chem. Senses 25, 119–129. [DOI] [PubMed] [Google Scholar]
- 33.Daly, K. C., Durtschi, M. L. & Smith, B. H. (2001) J. Insect Physiol. 47, 375–384. [DOI] [PubMed] [Google Scholar]
- 34.Daly, K. C., Chandra, S., Durtschi, M. L. & Smith, B. H. (2001) J. Exp. Biol. 204, 3085–3095. [DOI] [PubMed] [Google Scholar]
- 35.Daly, K. C. & Figueredo, A. J. (2000) Physiol. Entomol. 25, 180–190. [Google Scholar]
- 36.Petrinovich, L. (1984) in Habituation, Sensitization and Behaviour, eds. Peeke, H. V. S. & Petrinovich, L. (Academic, New York), pp. 17–55.
- 37.Christensen, T. A., Waldrop, B. R. & Hildebrand, J. G. (1998) J. Neurosci. 18, 5999–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackintosh, N. J. (1983) Conditioning and Associative Learning (Oxford Univ. Press, New York).
- 39.Stopfer, M. & Laurent, G. (1999) Nature 402, 664–668. [DOI] [PubMed] [Google Scholar]
- 40.Lubow, R. E. (1973) Psychol. Bull. 79, 398–407. [DOI] [PubMed] [Google Scholar]
- 41.Lubow, R. E., Weiner, I. & Schnur, P. (1981) in The Psychology of Learning and Motivation, ed. Bower, G. H. (Academic, New York), Vol. 15, pp. 1–49. [Google Scholar]
- 42.Chandra, S., Hosler J. S. & Smith, B. H. (2000) J. Comp. Psychol. 114, 86–97. [DOI] [PubMed] [Google Scholar]
- 43.Hammer, M. (1993) Nature 366, 59–63. [DOI] [PubMed] [Google Scholar]
- 44.Hammer, M. & Menzel, R. (1995) J. Neurosci. 15, 1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammer, M. & Menzel, R. (1998) Learn. Mem. 5, 146–156. [PMC free article] [PubMed] [Google Scholar]
- 46.Farooqui, T., Robinson, K., Vaessin, H. & Smith, B. H. (2003) J. Neurosci. 23, 5370–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mercer, A. R., Hayashi, J. H. & Hildebrand, J. G. (1995) J. Exp. Biol. 198, 613–627. [DOI] [PubMed] [Google Scholar]
- 48.Gibson, N. J. & Nighorn, A. (2000) J. Comp. Neurol. 422, 191–205. [DOI] [PubMed] [Google Scholar]
- 49.Müller, U. & Hildebrandt, H. (2002) J. Neurosci. 22, 8739–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bicker, G. (1999) Microsc. Res. Tech. 45, 174–183. [DOI] [PubMed] [Google Scholar]
- 51.Kokay, I. C., Ebert, P. R., Kirchhof, B. S. & Mercer, A. R. (1999) Micros. Res. Tech. 44, 179–189. [DOI] [PubMed] [Google Scholar]
- 52.Hosler, J. S., Buxton, K. L. & Smith, B. H. (2000) Behav. Neurosci. 114, 514–525. [DOI] [PubMed] [Google Scholar]
- 53.Linster, C. & Smith, B. H. (1997) Behav. Brain Res. 87, 1–14. [DOI] [PubMed] [Google Scholar]
- 54.Kendrick, K. M., Guevara-Guzman, R., Zorrilla, J., Hinton, M. R., Broad, K. D., Mimmack, M. & Ohkura S. (1997) Nature 388, 670–674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.