Abstract
Personal success often necessitates expending greater effort for greater reward but, equally important, also requires judicious use of our limited cognitive resources (e.g., attention). Previous animal models have shown that the prelimbic (PL) and infralimbic (IL) regions of the prefrontal cortex (PFC) are not involved in (physical) effort-based choice, whereas human studies have demonstrated PFC contributions to (mental) effort. Here, we utilize the rat Cognitive Effort Task (rCET) to probe PFC's role in effort-based decision making. In the rCET, animals can choose either an easy trial, where the attentional demand is low but the reward (sugar) is small or a difficult trial on which both the attentional demand and reward are greater. Temporary inactivation of PL and IL decreased all animals' willingness to expend mental effort and increased animals' distractibility; PL inactivations more substantially affected performance (i.e., attention), whereas IL inactivations increased motor impulsivity. These data imply that the PFC contributes to attentional resources, and when these resources are diminished, animals shift their choice (via other brain regions) accordingly. Thus, one novel therapeutic approach to deficits in effort expenditure may be to focus on the resources that such decision making requires, rather than the decision-making process per se.
Keywords: animal model, cortico-limbic-striatal circuits, infralimbic cortex, mental effort, prelimbic cortex
Introduction
Successful decision making frequently requires weighing a given option's costs against its associated benefits, and perturbations in this cost/benefit decision making are observed in a number of marginalized groups, including those who suffer from mental illness or live below the poverty line (Gleichgerrcht et al. 2010; Goschke 2014; Haushofer and Fehr 2014). The origin of such decision-making studies resides in economics, wherein individuals are identified as “irrational” if they fail to optimize their financial returns on a given task (Tversky and Kahneman 1981). Indeed, the so-called optimal decision making is better than measures of IQ at predicting future “life success,” including emotional coping, SAT scores, educational attainment, and body/mass index (Mischel et al. 1989, 2011; Schlam et al. 2013).
However, personal success also requires judicious use of our limited neurobiological resources, such as those underlying attention; the sensation of exerting “effort” reflects a strain on these limited resources (Kahneman 1973). The specific mechanisms that effort represents is currently unclear (Kurzban et al. 2013), but nevertheless individuals appear to have finite mental capacities to apply at any one time. Furthermore, a substantial body of literature contends that economic models of rationality are often too narrow in their timescale and index of optimality (for a review, see Brase 2014) and do not take individuals' biological limitations into account (Gigerenzer and Selten 2001). As such, conditions can be met wherein seemingly suboptimal choices may in fact be driven by a rational strategy (McGuire and Kable 2013).
A key neurobiological locus of both attention and effort is the prefrontal cortex (PFC); here, we define PFC as separate from the neighboring anterior cingulate cortex (ACC), due to its dissociable anatomy and function (Bissonette et al. 2013). In rodent physiological studies, PFC activity appears to track sustained attention across the session, and reducing the effort required also reduces PFC activity (Passetti et al. 2000; Dalley et al. 2001). Human imaging studies suggest that the PFC is involved in mental effort exertion (Schmidt et al. 2012), and individual variation in both lateral and medial PFC functioning correlates to individual differences in effortful choice (McGuire and Botvinick 2010; Treadway, Buckholtz et al. 2012). Related to this, prefrontal dysfunction is observed in many mental illnesses as well as stress (Rogers et al. 2004; Arnsten 2011), and individuals suffering from these conditions also demonstrate both impaired attentional performance (Vedhara et al. 2000; Paelecke-Habermann et al. 2005; Hong et al. 2011) and suboptimal decision making with effort costs (Shafiei et al. 2012; Treadway, Bossaller et al. 2012; Barch et al. 2014). One intriguing possibility is therefore that these individuals' aberrant choice strategies are not solely the result of compromised decision-making processes per se but rather because individuals are more judiciously applying their diminished attentional capacities.
Here, we utilize the rat Cognitive Effort Task (rCET) to directly examine the relationship between PFC dysfunction, attention, and effort. The rCET is adapted from the five-choice serial reaction-time task (5CSRTT), a well-established model of both visuospatial attention and motor impulsivity (Robbins 2002), and allows animals the choice to expend lesser or greater attention for lesser or greater reward, respectively. To obtain a within-subjects comparison of effortful choice with and without intact PFC functioning, we used temporary inactivations of the 2 sub-regions of the rat medial PFC, the prelimbic (PL) and infralimbic (IL) cortices. When connectivity, function, and neuromodulatory presence are considered together, there is some consensus that the rat PL and IL are less differentiated than their human counterparts but that they share features of human PFC (Uylings et al. 2003; Seamans et al. 2008). These regions' unique contributions to behavior have been demonstrated via selective inactivations and electrophysiological recordings (Seamans et al. 1995; Burgos-Robles et al. 2013). As lesions to PL and IL previously impaired rats' attentional performance on the 5CSRTT (Muir et al. 1996; Passetti et al. 2002), we therefore hypothesized that animals would decrease their choice of high-effort, high-reward trials as their performance declined.
Materials and Methods
Subjects
Subjects were 32 male Long-Evans rats (Charles River Laboratories), each weighing 275–300 g at the beginning of the experiment. Animals were maintained at ∼85% of their free-feeding weight and food restricted to 14 g rat chow per day. Water was available ad libitum. Animals were pair-housed in a climate-controlled colony room on a 12-h reverse light-dark cycle (lights off: 8 : 00 am; temperature: 21°C). All housing and testing was in accordance with the Canadian Council of Animal Care, and all procedures were approved by the UBC Animal Care Committee.
Behavioral Testing
All testing took place within 16 standard five-hole operant chambers, each supplemented with 2 retractable response levers and enclosed in a ventilated, sound-attenuating cabinet (Med Associates, Inc.). The chambers were controlled by software written in Med-PC by CAW, running on an IBM-compatible computer.
Habituation and Pretask Training
All animals were habituated and trained for the rCET as previously described (see Cocker, Hosking et al. 2012, including Supplementary methods). In brief, and as per five-choice serial reaction-time task (5CSRTT) training (Winstanley et al. 2010), animals learned to make a nosepoke response in an illuminated aperture within 5 s to obtain a sucrose pellet reward (Bioserv, 45 mg). In subsequent sessions, animals were trained to respond on both of the levers at a fixed ratio 1 (FR1) schedule for reward. Animals were then trained on a forced-choice variant of the rCET (60 sessions), wherein only a single lever extended, before the standard free-choice program.
The Rat Cognitive Effort Task (rCET)
The rCET has been previously described in detail (Cocker, Hosking et al. 2012), and a schematic of the trial structure and subsequent reinforcement is presented in Figure 1. Briefly, animals were tested 4–5 days per week in 30-min sessions of no fixed trial limit. At the outset of training, the levers were permanently designated to initiate either low-effort/low-reward (LR) or high-effort/high-reward (HR) trials, and these designations were evenly counterbalanced across subjects.
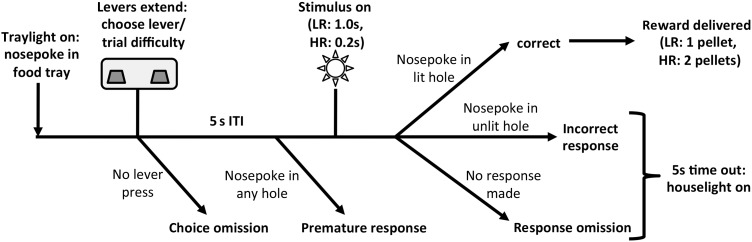
Figure 1.
Schematic diagram showing the trial structure of the rCET. Trials began when the food-tray light illuminated. A nosepoke response in the food-tray extinguished the light and extended the levers. Each lever was permanently designated to initiate either low-effort/low-reward (LR) or high-effort/high-reward (HR) trials. When animals pressed one of the levers, both levers retracted and a 5-s ITI began. Following the ITI, 1 of the 5 stimulus lights briefly illuminated, 1.0 s for an LR trial and 0.2 s for an HR trial. If animals nosepoked in the previously illuminated aperture within 5 s (a correct response), they were rewarded 1 sugar pellet for an LR and 2 sugar pellets for an HR trial. A number of behaviors led to a 5-s timeout, signaled by house-light illumination: failure to make a lever response (choice omission), failure to withhold responding during the ITI (premature response), nosepoke in an unlit hole following the stimulus (incorrect response), and failure to make a nosepoke response following the stimulus (response omission). Figure reprinted with permission from Cocker, Hosking et al. (2012).
New rCET trials were available when the food-tray light was illuminated. A nosepoke in the food tray extinguished the light and extended the levers. Animals would then press one of the levers, thereby choosing an LR or HR trial, and this would cause both levers to retract and a 5-s intertrial interval (ITI) to commence. After the ITI, 1 of the 5 stimulus lights briefly illuminated, with a stimulus duration of 1.0 s for an LR trial and 0.2 s for an HR trial. Animals were rewarded if they nosepoked the previously illuminated aperture within 5 s (a correct response) and received 1 sugar pellet for an LR trial and 2 sugar pellets for an HR trial. Upon reward delivery, the tray light again illuminated, thus signaling the opportunity to begin the next trial.
Trials went unrewarded for a number of reasons: if animals failed to make a lever response within 10 s (a choice omission), if animals nosepoked during the ITI (a premature response, a well-established measure of motor impulsivity; Robbins 2002), if animals nosepoked in any aperture other than the one that was illuminated (an incorrect response), and if animals failed to nosepoke at the array within 5 s after stimulus-light illumination (a response omission). All such behaviors were punished with a 5-s timeout period, accompanied by illumination of the house light. During the timeout, new trials could not be initiated and thus reward could not be earned. Following the timeout, the house light extinguished and the tray light illuminated to signal that the rat could begin the next trial.
Behavioral Measurements
Percent choice, rather than the absolute number of choices, was used to determine preference for lever/trial type, in order to minimize the influence of variation in the number of trials completed. Percent choice was calculated as follows: (number of choices of a particular lever/total number of choices) * 100. When baseline performance on the rCET was deemed statistically stable (i.e., no effect of session on repeated-measures ANOVA for choice, accuracy, and premature responding over the last 3 sessions; see “Data Analysis” below), the mean choice of the HR option was 68%. Animals were grouped as “workers” if they chose HR for >70% of trials (n = 16) and as “slackers” if they chose HR for ≤70% of trials (n = 15); 1 animal was excluded prior to surgery due to unrelated health complications. This worker/slacker subdivision was based on the mean split from the original rCET paper (Cocker, Hosking et al. 2012), where workers and slackers were categorized based on their preference for greater than or less than the average of 70% HR trials. To maintain consistency when discussing individual differences and to avoid arbitrary categorization, we therefore held the worker/slacker distinction at 70% HR trials for this study.
The following variables were also analyzed separately for LR and HR trials: percent accuracy {[number of correct responses/number of correct and incorrect responses] * 100}; percent premature responses {[number of premature responses/total number of trials initiated] * 100}; lever choice latency (average latency to choose between the LR and HR levers); correct latency (average latency to correctly nosepoke in the illuminated aperture); collection latency (average latency to collect reward); and percent response omissions {[number of trials omitted/number of correct, incorrect, and omitted trials] * 100}. Choice omissions (number of failures to choose a lever at the beginning of the trial) and the total number of completed trials were also analyzed.
Guide Cannulae Surgery
Surgical methods were based on a previous study (Hosking, Cocker et al. 2014). When baseline performance was deemed statistically stable (40 sessions), animals were implanted with 22-gauge stainless steel guide cannulae (Plastics One) bilaterally into the medial prefrontal cortex (mPFC) using standard stereotaxic techniques. Animals were anesthetized with 2% isoflurane in O2 and implanted at the following coordinates: AP = +2.7 mm, ML =±0.75 mm from bregma, DV = −2.3 mm from dura (Paxinos and Watson 1998). Guide cannulae were fixed to the skull via 3 stainless steel screws and dental acrylic, and obdurators with dust caps were inserted and extended flush with the end of the cannulae. Animals were given at least 1 week of recovery in their home cages before subsequent testing. Five animals were excluded due to poor recovery.
Microinfusion
Following recovery, animals performed 10 free-choice sessions, after which all individuals displayed stable behavior. Animals were then habituated to the microinfusion process with 2 mock infusions, wherein the 30-gauge injectors with tips extending 1 mm beyond the guide cannulae were inserted for 2 min but no infusion was performed, followed by a testing session. Infusions adhered to a 3-day cycle starting with a baseline session, followed by a drug or saline injection session, and then by a non-testing day. The PL and IL cortices were each inactivated by a mixture of the GABAB agonist baclofen and the GABAA agonist muscimol (Sigma–Aldrich), prepared separately at 0.5 µg/µL in saline, and mixed together in equal volumes to form a 0.25 µg/µL solution. 0.5 µL per hemisphere injections of saline or baclofen/muscimol (i.e., 0.125 µg of drug per hemisphere) were administered bilaterally at a rate of 0.4 µL/min, and injectors were left in place for an additional minute to allow diffusion. Injectors were then removed, obdurators were replaced, and animals were returned to their home cages for 10 min before being placed in the operant chambers and performing the rCET. Animals underwent 4 infusion sessions in total: On the first infusion day, half of the rats received saline infusions to the PL (via injectors with +1 mm tips) whereas the other half received baclofen/muscimol to the PL; these administrations were reversed on the second infusion day, allowing for a within-subjects comparison; on the third infusion day, half of the rats received saline infusions to the IL (via injectors with +2 mm tips) whereas the other half received baclofen/muscimol; and again these administrations were reversed on the fourth infusion day. Animals were given a minimum of 1 week drug-free testing between PL and IL inactivations, to minimize any carryover effects. PL inactivation caused severe behavioral disruption in 1 animal, whereas IL inactivation caused behavioral disruption in 2 animals, such that these animals no longer completed rCET trials, and thus, these animals were removed from each respective analysis.
Histology
Following completion of all behavioral testing, animals were anesthetized with isoflurane and sacrificed by carbon dioxide exposure. Brains were removed and fixed in 4% formaldehyde for at least 24 h, transferred to a 30% sucrose solution, and then frozen and cut via cryostat into 40-µm coronal sections. These sections were stained with Cresyl violet for visualization, and the projected locations of the injector tips protruding from the guide cannulae were mapped onto standard sections from Paxinos and Watson (1998).
Data Analysis
All data were analyzed using within-subjects repeated-measures ANOVA in SPSS (version 16.0; SPSS/IBM), with choice (2 levels: LR or HR) and inactivation (2 levels: saline or drug) as repeated-measures factors. All percentages were arcsine transformed to minimize artificial ceiling effects (Winstanley et al. 2003; Zeeb et al. 2009) prior to analysis. As discussed earlier, animals were categorized as workers and slackers at baseline, and group (2 levels: worker or slacker) was used as a between-subjects factor in all analyses. Similarly to previous reports (Cocker, Hosking et al. 2012; Hosking, Cocker et al. 2014), groups proved stable across the experiment: Choice preferences did not change from baseline to postsurgery and throughout all saline conditions for inactivations (session: F3,57 = 0.446, NS), and workers chose a significantly greater percentage of HR trials than slackers (all Fs > 19.005, P < 0.001). Any significant effects were further analyzed via post hoc one-way ANOVA. Any P-values of >0.05 but of <0.075 were reported as a statistical trend.
Results
Cannula Placements
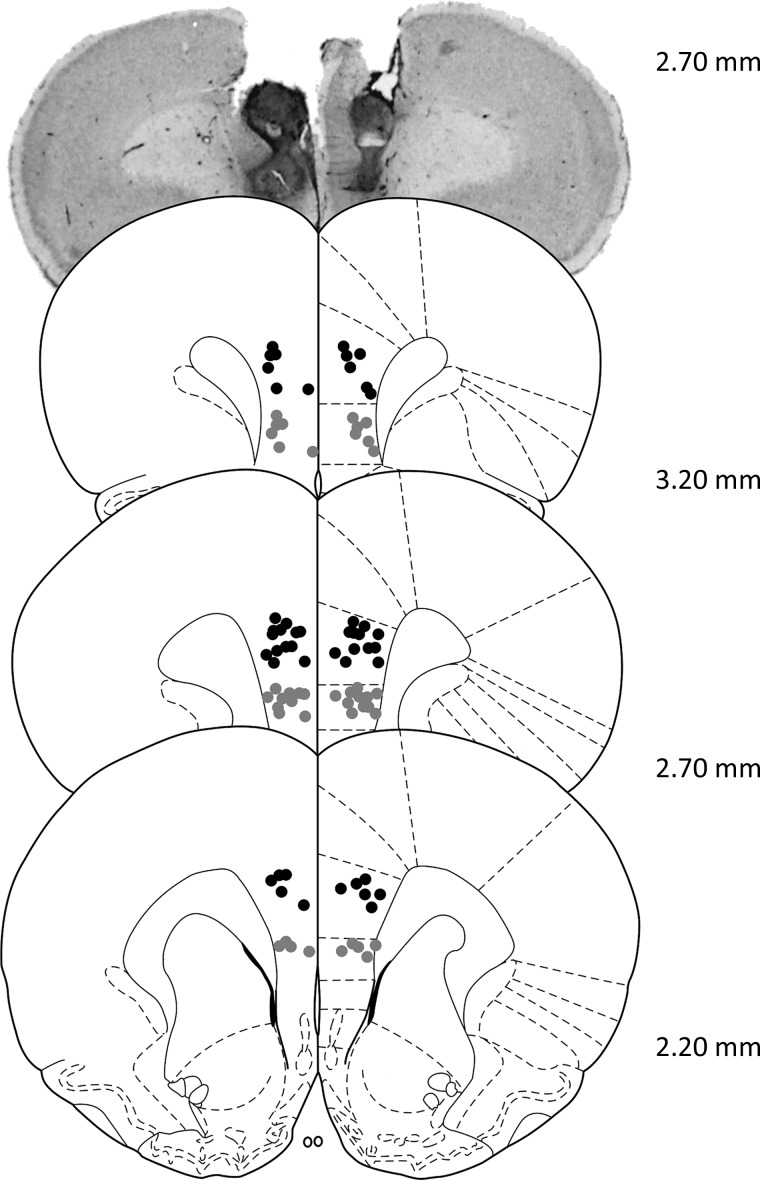
The locations of all acceptable placements, as well as a representative sample of mPFC cannulation, are depicted in Figure 2. Two animals were excluded because of inaccurate placements in one or both hemispheres, leaving a total of 24 animals for analysis (workers: n = 14; slackers: n = 10).
Figure 2.
Histological analysis of cannulae implantation. Location of all acceptable PFC infusions (black dots: PL cortex; gray dots: IL cortex), including a representative photomicrograph. Coordinates are relative to bregma. Plates modified from Paxinos and Watson (1998).
PL Cortex Inactivation
Choice Behavior, Accuracy, and Premature Responses
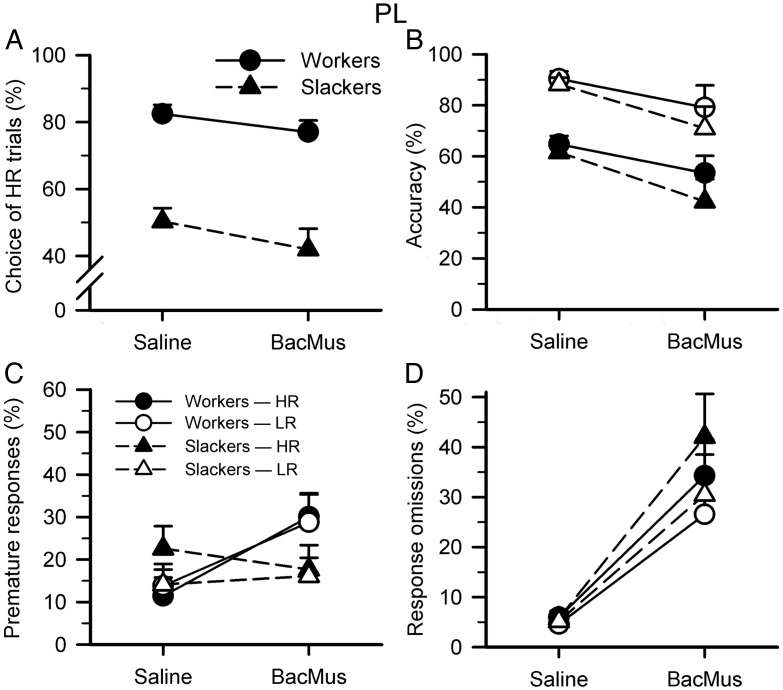
Baseline behavior has been discussed in detail elsewhere (Cocker, Hosking et al. 2012; Hosking, Cocker et al. 2014) and thus will only be briefly addressed. Animals chose high-effort/high-reward (HR) trials more than low-effort/low-reward (LR) trials when the PL was infused with saline (choice: F1,21 = 39.638, P < 0.001). Substantial individual variation in choice behavior remained, and workers continued to choose a greater proportion of HR trials than slackers (saline only—group: F1,21 = 38.390, P < 0.001). Inactivation of the PL significantly decreased all animals' choice of HR (Fig. 3A, inactivation: F1,21 = 5.236, P = 0.033; inactivation × group: F1,21 = 0.078, NS).
Figure 3.
Effects of PL cortex inactivations on the rCET. (A) Infusion of baclofen/muscimol (BacMus) into the PL significantly decreased all animals' choice of HR (inactivation: F1,21 = 5.236, P = 0.033; inactivation × group: F1,21 = 0.078, NS). (B) PL inactivation decreased all animals' accuracy for both trial types (inactivation: F1,21 = 6.385, P = 0.020; inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 0.552, NS). (C) PL inactivation had no main effect on premature responding, although there was a trend for increased premature responding in workers (inactivation/choice × inactivation/choice × inactivation × group: all Fs < 2.125, NS; inactivation × group: F1,21 = 3.979, P = 0.059; workers only—inactivation: F1,12 = 4.356, P = 0.059; inactivation × choice/slackers only—inactivation/inactivation × choice: all Fs < 2.439, NS). (D) Inactivation of the PL dramatically increased nosepoke-response omissions for all animals on both trial types (inactivation: F1,21 = 39.760, P < 0.001; inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 1.574, NS). Data shown are the mean percent for each variable (±SEM).
As previously demonstrated, animals were more accurate on LR versus HR trials (saline only—choice: F1,21 = 113.923, P < 0.001) and there were no differences in accuracy between workers and slackers, indicating that choice preferences were not solely driven by animals' ability to perform the task (saline only—choice × group/group: all Fs < 1.003, NS). PL inactivation decreased all animals' accuracy for both trial types (Fig. 3B, inactivation: F1,21 = 6.385, P = 0.020; inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 0.552, NS).
All animals showed equivalent levels of premature responding for LR and HR trials (saline only—choice/choice × group/group: all Fs < 2.026, NS), reiterating that choice preferences were not simply driven by individuals' level of motor impulsivity (Cocker, Hosking et al. 2012). PL inactivation had no main effect on premature responding, although there was a trend for increased premature responding in workers (Fig. 3C, inactivation/choice × inactivation/choice × inactivation × group: all Fs < 2.125, NS; inactivation × group: F1,21 = 3.979, P = 0.059; workers only—inactivation: F1,12 = 4.356, P = 0.059; inactivation × choice/slackers only—inactivation/inactivation × choice: all Fs < 2.439, NS).
Other Behavioral Measures
For all animals at saline conditions, nosepoke-response omissions were equivalent for LR and HR (choice/choice × group/group: all Fs < 2.491, NS). Inactivation of the PL dramatically increased these response omissions for all animals on both trial types, indicating a fundamental impairment to maintain attention on the five-hole stimulus array (Fig. 3D, inactivation: F1,21 = 39.760, P < 0.001; inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 1.574, NS). However, PL inactivation did not increase the number of lever/choice omissions (Table 1, inactivation/inactivation × group: all Fs < 1.070, NS), suggesting that the PL inactivation's effects on response omissions were not simply driven by motor impairments. Inactivation of the PL had no effect on the latency to choose between LR and HR levers (inactivation/inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 3.214, NS) but increased the latency to make a correct nosepoke-response for all animals on both trial types (inactivation: F1,21 = 24.218, P < 0.001; inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 3.609, NS). Similar to previous cohorts, there was a trend for animals to collect reward faster following HR versus LR trials (saline only—choice: F1,21 = 4.095, P = 0.056; choice × group/group: all Fs < 1.676, NS), indicating that both workers and slackers differentiated the 2 reward contingencies (i.e., slackers were not indifferent to the options, despite roughly equivalent choice of LR and HR at saline). PL inactivation increased this collection latency for all animals on both trial types (inactivation: F1,21 = 6.107, P = 0.022; inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 1.243, NS). Finally, inactivation of the PL decreased the number of completed trials for all animals (inactivation: F1,21 = 29.312, P < 0.001; inactivation × group: F1,21 = 1.040, NS).
Table 1.
Other rCET behavioral measurements during PL inactivation
| Measure | LR—saline | LR—BacMus | HR—saline | HR—BacMus |
|---|---|---|---|---|
| Choice latency | 3.04 | 2.89 | 3.39 | 2.86 |
| SEM | 0.20 | 0.21 | 0.18 | 0.26 |
| Correct latency | 0.57 | 0.93 | 0.57 | 0.75 |
| SEM | 0.04 | 0.08 | 0.03 | 0.06 |
| Collection latency | 1.76 | 3.25 | 1.62 | 4.55 |
| SEM | 0.09 | 0.65 | 0.06 | 1.35 |
| Choice omissions | (ALL) | 8.09 | 11.35 | |
| SEM | 1.61 | 2.56 | ||
| Completed trials | (ALL) | 104.13 | 62.35 | |
| SEM | 5.37 | 7.25 | ||
IL Cortex Inactivation
Choice Behavior, Accuracy, and Premature Responses
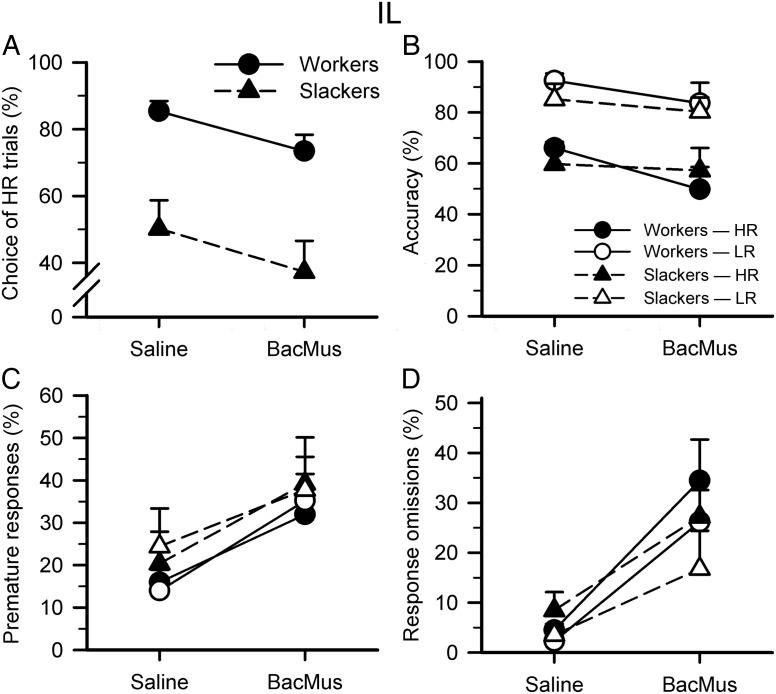
Inactivation of the IL decreased choice of HR for all animals (Fig. 4A, inactivation: F1,20 = 6.111, P = 0.023). However, IL inactivation had no significant effect on animals' accuracy (Fig. 4B, inactivation/inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 1.338, NS). IL inactivation also significantly increased premature responding for all animals across both trial types (Fig. 4C, inactivation: F1,20 = 6.766, P = 0.017; inactivation × group/choice × inactivation/choice × inactivation × group/group: all Fs < 2.570, NS).
Figure 4.
Effects of IL cortex inactivations on the rCET. (A) Baclofen/muscimol (BacMus) inactivation of the IL decreased choice of HR for all animals (inactivation: F1,20 = 6.111, P = 0.023). (B) However, IL inactivation had no significant effect on animals' accuracy (inactivation/inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 1.338, NS). (C) IL inactivation also significantly increased premature responding for all animals across both trial types (inactivation: F1,20 = 6.766, P = 0.017; inactivation × group/choice × inactivation/choice × inactivation × group/group: all Fs < 2.570, NS). (D) Inactivation of the IL increased nosepoke-response omissions for all animals across both trial types, indicating difficulties remaining engaged with the trial (inactivation: F1,20 = 19.041, P < 0.001; inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 1.380, NS). Data shown are the mean percent for each variable (±SEM).
Other Behavioral Measures
Inactivation of the IL increased the proportion of nosepoke-response omissions for all animals across both trial types, indicating difficulties remaining engaged with the trial (Fig. 4D, inactivation: F1,20 = 19.041, P < 0.001; inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 1.380, NS), whereas it had no effect on lever/choice omissions (Table 2; inactivation/inactivation × group: all Fs < 0.579, NS). IL inactivation did not affect lever/choice latency (inactivation/inactivation × group/choice × inactivation/choice × inactivation × group: all Fs < 0.684, NS) but increased the latency to correctly nosepoke for all animals (inactivation: F1,20 = 13.622, P = 0.001; inactivation × group/choice × inactivation/choice × inactivation x group: all Fs < 1.899, NS) and increased all animals' latency to collect reward, especially following successful LR trials (inactivation: F1,20 = 10.975, P = 0.003; choice × inactivation: F1,20 = 5.314, P = 0.032; inactivation × group/choice × inactivation × group: all Fs < 0.323, NS; LR only—inactivation: F1,20 = 10.743, P = 0.004; HR only—inactivation: F1,20 = 3.818, P = 0.065). Finally, inactivation of the IL decreased the number of completed trials for all animals (inactivation: F1,20 = 28.759, P < 0.001; inactivation × group: F1,20 = 1.381, NS).
Table 2.
Other rCET behavioral measurements during IL inactivation
| Measure | LR—saline | LR—BacMus | HR—saline | HR—BacMus |
|---|---|---|---|---|
| Choice latency | 3.24 | 3.07 | 3.01 | 2.93 |
| SEM | 0.29 | 0.28 | 0.20 | 0.29 |
| Correct latency | 0.62 | 0.77 | 0.54 | 0.82 |
| SEM | 0.04 | 0.04 | 0.05 | 0.07 |
| Collection latency | 2.06 | 2.70 | 1.53 | 1.97 |
| SEM | 0.35 | 0.27 | 0.07 | 0.16 |
| Choice omissions | (ALL) | 16.5 | 17.18 | |
| SEM | 2.61 | 2.29 | ||
| Completed trials | (ALL) | 81.23 | 35.68 | |
| SEM | 8.02 | 4.74 | ||
Discussion
Here, for the first time, we demonstrate PL and IL contributions to a rodent model of effort-based decision making. Temporary inactivation of either region of the medial PFC decreased all animals' willingness to expend cognitive effort. Some dissociation was also observed between the 2 regions: PL inactivations decreased animals' performance (i.e., accuracy), whereas IL inactivations increased animals' motor impulsivity (i.e., premature responding), a finding that parallels previous reports (Muir et al. 1996; Passetti et al. 2002; Chudasama et al. 2003). This consistency across studies suggests that IL, but not PL, is a critical region in the circuitry of self-regulation, and loss of function in this region leads to disinhibited motor output, that is, impulsive action. Response omissions also sharply increased for inactivations of both regions, and when considered in tandem with accuracy effects, it appears that PFC inactivations greatly impaired animals' ability to perform the task via decreasing attention. Taken together, these data imply that the PFC contributes to attentional resources, and when these resources are diminished, animals will shift their choice (via other brain regions) toward a more judicious strategy.
Alternatively, it is possible that changes in arousal, rather than decreased willingness to exert mental effort, may explain the current data. Prefrontal regions are reciprocally connected to most of the major neuromodulatory systems, and loss of IL or PL causes the activity in a large number of these interconnected regions to adjust, even during transient inactivation (Jodo et al. 1998; Amat et al. 2006; Patton et al. 2013). However, it is unlikely that animals' decreased choice of HR was primarily due to decreased arousal, as driven by perhaps the noradrenergic and/or cholinergic systems. First, in a previous study using the rCET, potentiating norepinephrine via systemic yohimbine or atomoxetine had no effect on animals' choice (Hosking, Floresco et al. 2014). Second, systemic administration of the cholinergic drugs nicotine and scopolamine affected animals' choice on the rCET but had no main effect on accuracy (Hosking, Lam et al. 2014). In sum, it appears unlikely that changes in arousal were responsible for the changes in choice behavior; a more parsimonious explanation is that the prefrontal inactivations themselves were primarily responsible for changes in accuracy and choice.
One important consideration is that the most posterior PL placements of the current study were in proximity to the ACC. With ∼1 mm diffusion for these microinfusions (Floresco et al. 2006; Marquis et al. 2007), some of the baclofen/muscimol inactivations may have spread into adjacent regions. Furthermore, the role of ACC in effort-based decision making has been demonstrated across a number of human and animal-model studies using multiple methodologies (Schweimer and Hauber 2005; Rudebeck et al. 2006; Walton et al. 2006; Croxson et al. 2009; Hauber et al. 2010). We have previously performed inactivations of the ACC on the rCET, targeting the border of Cg1 and Cg2, and found behavioral results that in many respects overlap with IL inactivations (Hosking, Cocker et al. 2014). However, it is the dorsal (i.e., PL) inactivations in the current study that are most likely to have spread to the ACC, whereas spread from IL would not be sufficient to reach the ACC. Furthermore, PL inactivations differ markedly from previous ACC inactivations in 2 of the 4 key measures here, namely accuracy and premature responding. Thus, the loss of ACC function appears minimal during PL inactivation, as the behavioral data do not correspond.
Another consideration is the rCET's effort costs as they relate to prefrontal functioning. In contrast to the current data, previous rat models of effort-based decision making found that selective IL–PL lesions had no effect on animals' willingness to exert effort (Walton et al. 2002, 2003). However, these studies utilized a T-maze task wherein animals could scale a barrier in one arm for a larger reward or enter an open arm for a smaller reward; in other words, the task's effort demands were physical rather than cognitive in nature, and this difference may underlie the divergence from the current results. A growing body of rCET research supports the notion of interrelated-yet-distinct neurobiological mechanisms for cognitive versus physical effort (Cocker, Hosking et al. 2012; Hosking, Cocker et al. 2014; Hosking, Floresco et al. 2014), and at least 1 human neuroimaging study suggests the same, noting that lateral PFC activity is increased for mental but not physical effort expenditure (Schmidt et al. 2012). Nevertheless, PFC engagement has been observed in human decision making with both mental and physical effort costs (McGuire and Botvinick 2010; Treadway, Buckholtz et al. 2012), and to the best of our knowledge, no other established physical effort decision-making task in rats has been used to examine IL–PL contributions. Furthermore, it has long been argued that high-effort conditions necessitate increased attention, regardless of whether they are mentally or physically demanding (Kahneman 1973). One possibility is therefore that the T-maze paradigm's HR option is not sufficiently demanding to recruit the attentional resources embodied by the PFC.
While the PFC undoubtedly contributes to many cognitive processes (e.g., behavioral flexibility; Grace et al. 2007), converging evidence has long implicated prefrontal activity in voluntary, or “top-down,” attentional processes (Jansen et al. 1955; Buschman and Miller 2007; Squire et al. 2013). Research with PFC-lesioned patients suggests that lateral, and to a lesser extent medial, PFC contributes to many aspects of voluntary attention, including novelty processing and anticipatory attention (Solbakk and Lovstad 2014). Such lesions negatively affect both divided and sustained attention, increasing individuals' propensity to be distracted by irrelevant stimuli (Godefroy and Rousseaux 1996); rats demonstrate a similar pattern of deficits following medial PFC lesions (Granon et al. 1998; Broersen and Uylings 1999). In addition to the direct impairments of attention in the current study (i.e., animals' accuracy), PL and IL inactivations increased rats' response omissions, that is, failures to nosepoke any hole following stimulus presentation. It is at present impossible to determine whether this reflects animals trying but failing to detect the target, being distracted before attending to the apertures, or suffering some unrelated motor slowing; however, the latter interpretation appears less likely, as these animals do not demonstrate motor impairments on similar behavioral measures, such as lever (choice) omissions or choice latency. Like the inactivations of the current study, lesions encompassing PL and IL also decreased animals' accuracy on the 5CSRTT, the precursor to the rCET (Muir et al. 1996). Altogether, inactivations of the PFC appear to have decreased animals' ability to sustain attention and increased their distractibility during the task, an interpretation that is strongly supported by the literature.
Decision making comprises a variety of constituent processes and as such also requires contributions from a number of brain regions (Dolan 2012). A substantial body of literature implicates cortico-limbic-striatal circuits in various forms of cost/benefit decision making and goal-oriented behavior (Grace et al. 2007; Floresco et al. 2008; Hosking, Cocker et al. 2014). These regions, which include much of the frontal cortex, ACC, amygdala, hippocampus, midbrain, and striatum, have been shown to subserve unique and overlapping components of decision making, with both PFC and the striatum implicated in the choice, or action selection, process (Cools et al. 2004; O'Doherty 2004, 2011; Ridderinkhof et al. 2004; Rushworth et al. 2005; Nicola 2007; Kimchi and Laubach 2009; Seo et al. 2012; Tai et al. 2012). Thus, one interpretation of the current data is that PFC inactivations impaired animals' ability to select actions based on the options' associated benefits and costs. However, this seems unlikely as a sole explanation for a number of reasons. First, inactivations sites were relatively small as compared with lesions, encompassing approximately a 1-mm spread per hemisphere and thus would have left much of the PFC intact for all conditions. Second, PFC inactivations did not drive animals' behavior toward indifference (i.e., 50% choice of HR), nor did they cause behavioral inflexibility (i.e., exacerbate existing choice preferences); rather, all animals decreased their choice of HR, regardless of their baseline choice preferences, with some animals (slackers) in fact moving “away” from 50% and toward 0%. Together, these data suggest that PFC inactivations caused a greater detriment to attentional processes than action selection and suggest that other regions within the decision-making circuit, for example the striatum, drove changes in choice behavior in response to decreased attentional reserves.
One frequently reported finding is that individuals living below the poverty line demonstrate greater risk- and delay-discounting than those more financially secure (for a review, see Haushofer and Fehr 2014). In addition to the effects of this chronic stress, poorer individuals have fewer financial resources to spend but the same biological, social, and evolutionary needs to fulfill, and thus they opt instead for smaller, sooner, sure gains. Such behavior may be identified as “irrational” from an economics perspective, but cognitive biases such as risk aversion appear relatively well conserved across mammalian species (Cocker, Dinelle et al. 2012; Rogers et al. 2013; Yamada et al. 2013; Cocker and Winstanley 2014; Tremblay et al. 2014) and thus may indeed positively contribute to an organism's fitness. A similar case can be made for mental resources. All other task contingencies being equal, individuals will avoid options with higher mental effort demands (Kool et al. 2010). Greater subjective sensitivity to mental effort predicts greater avoidance of those high-effort options (McGuire and Botvinick 2010; Kool et al. 2013), and common motivational nodes such as the striatum appear to subtract mental effort costs from their associated benefits in order to arrive at a net value for action selection (Botvinick et al. 2009). When brain regions responsible for effort expenditure (in this case, the PFC) are compromised, along with the faculties they provide, the striatum may therefore adjust behavior according to a new net value. This model is supported both by the current data and aberrant effortful decision making observed in individuals with putative PFC dysfunction (Treadway, Bossaller et al. 2012; Gold et al. 2013; but see Gold et al. 2014).
One obvious hypothesis that arises from this research is that improvements to an individual's cognitive resources should concomitantly increase their willingness to expend said mental effort. As such, therapeutic approaches that aim to boost the resources that decision making requires, irrespective of the decision-making process per se, should be effective at ultimately improving choice. One option may be to exploit drugs that benefit attention, such as those that increase acetylcholine function (Wilens and Decker 2007; Klinkenberg et al. 2011; Bracco et al. 2014). However, in all the experiments, we have performed to date using the rCET, choice of the HR option and ability to perform the HR trials are not predictive of each other in healthy animals. Put another way, cognitively lazy animals (i.e., slackers) are just as accurate on HR trials as workers; ability is not synonymous with endeavor. We recently observed a particularly powerful example of this disconnect in that nicotine administration decreased animals' choice of HR despite increasing their accuracy on the rCET (Hosking, Lam et al. 2014). As such, increasing attentional abilities by any means may not increase willingness to exert cognitive effort.
However, bearing all these data in mind, perhaps the most important aspect of the current results is that they indicate quite clearly that ability to work and willingness to work are tightly coupled at the level of the PFC; when input is lost from this region, accuracy decreases and/or impulsivity rises and the strategy switches to one that requires less effort. By extension, restoring prefrontal activity in individuals with PFC dysfunction, or boosting the connections between this region and action-selection areas such as the striatum, should enhance ability and willingness to work in tandem. Identifying the mechanisms by which ability and effort are regulated cohesively as well as those that lead to independent modulation of these processes may improve our understanding of how engagement in cognitively effortful processes can be encouraged in both the healthy and diseased brain.
The ability to rebalance cost/benefit decision making would be of benefit not only to those diagnosed with mental illness but may also have an impact on those whose quality of life, and subsequently their decision making, is altered due to sociocultural factors (e.g., poverty and chronic stress). Delineating the circumstances under which neural pathways are activated to reallocate resources toward strategies, high or low in cognitive effort may therefore be of significant value to neuroscientists and economists alike.
Funding
This work was supported by a discovery grant awarded to C.A.W. from the Canadian Natural Sciences and Engineering Council. C.A.W. also receives salary support through the Michael Smith Foundation for Health Research and the Canadian Institutes of Health Research (CIHR) New Investigator Award program. J.G.H. was supported by a CIHR Doctoral Research Award.
Notes
Conflict of Interest: The authors declare that over the past 3 years, C.A.W. has sat on an Advisory Board for Shire and received due compensation. No authors have any other conflicts of interest or financial disclosures to report.
References
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. 2006. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 26:13264–13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. 2011. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. IntJ Develop Neurosci. 29:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N. 2014. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts a motivation and functional impairment. J Abnorm Psychol. 123:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM, Roesch MR. 2013. Neural structures underlying set-shifting: roles of medial prefrontal cortex and anterior cingulate cortex. Behav Brain Res. 250:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. 2009. Effort discounting in human nucleus accumbens. Cogn Affect Behav Neurosci. 9:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracco L, Bessi V, Padiglioni S, Marini S, Pepeu G. 2014. Do cholinesterase inhibitors act primarily on attention deficit? A naturalistic study in Alzheimer's disease patients. J Alzheimer's Dis. 40:737–742. [DOI] [PubMed] [Google Scholar]
- Brase GL. 2014. The nature of thinking, shallow and deep. Front Psychol. 5:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broersen LM, Uylings HB. 1999. Visual attention task performance in Wistar and Lister hooded rats: response inhibition deficits after medial prefrontal cortex lesions. Neuroscience. 94:47–57. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Bravo-Rivera H, Quirk GJ. 2013. Prelimbic and infralimbic neurons signal distinct aspects of appetitive instrumental behavior. PLoS One. 8:e57575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. 2007. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 315:1860–1862. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. 2003. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 146:105–119. [DOI] [PubMed] [Google Scholar]
- Cocker PJ, Dinelle K, Kornelson R, Sossi V, Winstanley CA. 2012. Irrational choice under uncertainty correlates with lower striatal D(2/3) receptor binding in rats. J Neurosci. 32:15450–15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocker PJ, Hosking JG, Benoit J, Winstanley CA. 2012. Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision-making task. Neuropsychopharmacology. 37:1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocker PJ, Winstanley CA. 2014. Irrational beliefs, biases and gambling: Exploring the role of animal models in elucidating vulnerabilities for the development of pathological gambling. Behav Brain Res. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW. 2004. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 24:1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O'Reilly JX, Behrens TE, Rushworth MF. 2009. Effort-based cost-benefit valuation and the human brain. J Neurosci. 29:4531–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. 2001. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci. 21:4908–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ. 2012. Neuroscience of Preference and Choice: Cognitive and Neural Mechanisms. London, Waltham, MA: Academic Press/Elsevier. [Google Scholar]
- Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. 2006. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 26:2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. 2008. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 8:375–389. [DOI] [PubMed] [Google Scholar]
- Gigerenzer G, Selten R. 2001. Bounded Rationality: the Adaptive Toolbox. Cambridge, Mass: MIT Press. [Google Scholar]
- Gleichgerrcht E, Ibanez A, Roca M, Torralva T, Manes F. 2010. Decision-making cognition in neurodegenerative diseases. Nat Rev Neurol. 6:611–623. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Rousseaux M. 1996. Divided and focused attention in patients with lesion of the prefrontal cortex. Brain Cogn. 30:155–174. [DOI] [PubMed] [Google Scholar]
- Gold JM, Kool W, Botvinick MM, Hubzin L, August S, Waltz JA. 2014. Cognitive effort avoidance and detection in people with schizophrenia. Cogn Affect Behav Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. 2013. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 74:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goschke T. 2014. Dysfunctions of decision-making and cognitive control as transdiagnostic mechanisms of mental disorders: advances, gaps, and needs in current research. Int J Methods Psychiatr Re. 23(Suppl 1):41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. 2007. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 30:220–227. [DOI] [PubMed] [Google Scholar]
- Granon S, Hardouin J, Courtier A, Poucet B. 1998. Evidence for the involvement of the rat prefrontal cortex in sustained attention. Q J Exp Psychol B. 51:219–233. [DOI] [PubMed] [Google Scholar]
- Hauber W, Endepols H, Sommer S, Backes H, Wiedermann D, Graf R. 2010. Effort-based decision making in the rat: an [(18)F] fluorodeoxyglucose micro positron emission tomography study. J Neurosci. 30:9708–9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haushofer J, Fehr E. 2014. On the psychology of poverty. Science. 344:862–867. [DOI] [PubMed] [Google Scholar]
- Hong LE, Schroeder M, Ross TJ, Buchholz B, Salmeron BJ, Wonodi I, Thaker GK, Stein EA. 2011. Nicotine enhances but does not normalize visual sustained attention and the associated brain network in schizophrenia. Schizophr Bull. 37:416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking JG, Cocker PJ, Winstanley CA. 2014. Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacology. 39:1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking JG, Floresco SB, Winstanley CA. 2014. Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking JG, Lam FC, Winstanley CA. 2014. Nicotine increases impulsivity and decreases willingness to exert cognitive effort despite improving attention in "Slacker" rats: insights into cholinergic regulation of cost/benefit decision making. PLoS One. 9:e111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J, Jr, Andersen P, Kaada BR. 1955. Subcortical mechanisms in the searching or attention response elicited by prefrontal cortical stimulation in unanesthetized cats. Yale J Biol Med. 28:331–341. [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G. 1998. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 83:63–79. [DOI] [PubMed] [Google Scholar]
- Kahneman D. 1973. Attention and Effort. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Kimchi EY, Laubach M. 2009. Dynamic encoding of action selection by the medial striatum. J Neurosci. 29:3148–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I, Sambeth A, Blokland A. 2011. Acetylcholine and attention. Behav Brain Res. 221:430–442. [DOI] [PubMed] [Google Scholar]
- Kool W, McGuire JT, Rosen ZB, Botvinick MM. 2010. Decision making and the avoidance of cognitive demand. J Exp Psychol Gen. 139:665–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool W, McGuire JT, Wang GJ, Botvinick MM. 2013. Neural and behavioral evidence for an intrinsic cost of self-control. PLoS One. 8:e72626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzban R, Duckworth A, Kable JW, Myers J. 2013. An opportunity cost model of subjective effort and task performance. Behav Brain Sci. 36:661–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis JP, Killcross S, Haddon JE. 2007. Inactivation of the prelimbic, but not infralimbic, prefrontal cortex impairs the contextual control of response conflict in rats. Eur J Neurosci. 25:559–566. [DOI] [PubMed] [Google Scholar]
- McGuire JT, Botvinick MM. 2010. Prefrontal cortex, cognitive control, and the registration of decision costs. Proc Natl Acad Sci USA. 107:7922–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JT, Kable JW. 2013. Rational temporal predictions can underlie apparent failures to delay gratification. Psychol Rev. 120:395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Ayduk O, Berman MG, Casey BJ, Gotlib IH, Jonides J, Kross E, Teslovich T, Wilson NL, Zayas V, et al. 2011. ‘Willpower’ over the life span: decomposing self-regulation. Soc Cogn Affect Neurosci. 6:252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. 1989. Delay of gratification in children. Science. 244:933–938. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. 1996. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex. 6:470–481. [DOI] [PubMed] [Google Scholar]
- Nicola SM. 2007. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl). 191:521–550. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. 2011. Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Ann N Y Acad Sci. 1239:118–129. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. 2004. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 14:769–776. [DOI] [PubMed] [Google Scholar]
- Paelecke-Habermann Y, Pohl J, Leplow B. 2005. Attention and executive functions in remitted major depression patients. J Affect Disord. 89:125–135. [DOI] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. 2002. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex. 12:1254–1268. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, O'Connell MT, Everitt BJ, Robbins TW. 2000. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci. 12:3051–3058. [DOI] [PubMed] [Google Scholar]
- Patton MH, Bizup BT, Grace AA. 2013. The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J Neurosci. 33:16865–16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1998. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press. [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. 2004. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 56:129–140. [DOI] [PubMed] [Google Scholar]
- Robbins TW. 2002. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl). 163:362–380. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Fukuda M, Kato N. 2004. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res. 50:1–11. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Wong A, McKinnon C, Winstanley CA. 2013. Systemic administration of 8-OH-DPAT and eticlopride, but not SCH23390, alters loss-chasing behavior in the rat. Neuropsychopharmacology. 38:1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS. 2006. Separate neural pathways process different decision costs. Nature Neuroscience. 9:1161–1168. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Gough PM, Alexander IH, Kyriazis D, McDonald KR, Passingham RE. 2005. Attentional selection and action selection in the ventral and orbital prefrontal cortex. J Neurosci. 25:11628–11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam TR, Wilson NL, Shoda Y, Mischel W, Ayduk O. 2013. Preschoolers’ delay of gratification predicts their body mass 30 years later. J Pediatr. 162:90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L, Lebreton M, Clery-Melin ML, Daunizeau J, Pessiglione M. 2012. Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biol. 10:e1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweimer J, Hauber W. 2005. Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn Mem. 12:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. 1995. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 109:1063–1073. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. 2008. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 14:249–262. [DOI] [PubMed] [Google Scholar]
- Seo M, Lee E, Averbeck BB. 2012. Action selection and action value in frontal-striatal circuits. Neuron. 74:947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiei N, Gray M, Viau V, Floresco SB. 2012. Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology. 37:2194–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbakk AK, Lovstad M. 2014. Effects of focal prefrontal cortex lesions on electrophysiological indices of executive attention and action control. Scand J Psychol. 55:233–243. [DOI] [PubMed] [Google Scholar]
- Squire RF, Noudoost B, Schafer RJ, Moore T. 2013. Prefrontal contributions to visual selective attention. Annu Rev Neurosci. 36:451–466. [DOI] [PubMed] [Google Scholar]
- Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. 2012. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci. 15:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. 2012. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 121:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. 2012. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 32:6170–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M, Cocker PJ, Hosking JG, Zeeb FD, Rogers RD, Winstanley CA. 2014. Dissociable effects of basolateral amygdala lesions on decision making biases in rats when loss or gain is emphasized. Cogn Affect Behav Neurosci. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. 1981. The framing of decisions and the psychology of choice. Science. 211:453–458. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. 2003. Do rats have a prefrontal cortex? Behav Brain Res. 146:3–17. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Hyde J, Gilchrist ID, Tytherleigh M, Plummer S. 2000. Acute stress, memory, attention and cortisol. Psychoneuroendocrino. 25:535–549. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MFS. 2003. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 23:6475–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MFS. 2002. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 22:10996–11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PEM, Rushworth MFS. 2006. Weighing up the benefits of work: Behavioral and neural analyses of effort-related decision making. Neural Netw. 19:1302–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Decker MW. 2007. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition. Biochem Pharmacol. 74:1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. 2003. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl). 167:304–314. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Zeeb FD, Bedard A, Fu K, Lai B, Steele C, Wong AC. 2010. Dopaminergic modulation of the orbitofrontal cortex affects attention, motivation and impulsive responding in rats performing the five-choice serial reaction time task. Behav Brain Res. 210:263–272. [DOI] [PubMed] [Google Scholar]
- Yamada H, Tymula A, Louie K, Glimcher PW. 2013. Thirst-dependent risk preferences in monkeys identify a primitive form of wealth. Proc Natl Acad Sci USA. 110:15788–15793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. 2009. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 34:2329–2343. [DOI] [PubMed] [Google Scholar]