Abstract
Noradrenaline (NA) is a key neuromodulator for the regulation of behavioral state and cognition. It supports learning by increasing arousal and vigilance, whereby new experiences are “earmarked” for encoding. Within the hippocampus, experience-dependent information storage occurs by means of synaptic plasticity. Furthermore, novel spatial, contextual, or associative learning drives changes in synaptic strength, reflected by the strengthening of long-term potentiation (LTP) or long-term depression (LTD). NA acting on β-adrenergic receptors (β-AR) is a key determinant as to whether new experiences result in persistent hippocampal synaptic plasticity. This can even dictate the direction of change of synaptic strength.
The different hippocampal subfields play different roles in encoding components of a spatial representation through LTP and LTD. Strikingly, the sensitivity of synaptic plasticity in these subfields to β-adrenergic control is very distinct (dentate gyrus > CA3 > CA1). Moreover, NA released from the locus coeruleus that acts on β-AR leads to hippocampal LTD and an enhancement of LTD-related memory processing. We propose that NA acting on hippocampal β-AR, that is graded according to the novelty or saliency of the experience, determines the content and persistency of synaptic information storage in the hippocampal subfields and therefore of spatial memories.
Keywords: β-adrenergic receptors, hippocampus, memory, noradrenaline, synaptic plasticity
Introduction
Noradrenaline (NA) belongs to a group of neurotransmitters able to modulate synaptic functions that are referred to as neuromodulators. In the central nervous system, the primary source of NA is the locus coeruleus (Sara et al. 1994; Kitchigina et al. 1997), a structure located in the brain stem that sends noradrenergic projections to many brain regions (Jones et al. 1977). The activation of the noradrenergic system strongly depends on an animal's behavioral state, with novel or emotive stimuli resulting in burst firing, the intensity and duration of which is determined by the nature and saliency of the experience (Crow 1968; Kety 1970, 1972; Aston-Jones and Bloom 1981; Sara and Segal 1991; Sara 2009). It was suggested almost 4 decades ago that the noradrenergic system might play a powerful role in supporting learning and memory processes (Kety 1972). Subsequent experiments highlighted the role of NA as an enhancer of neuronal responses toward discrete stimuli and thereby of signal-to-noise ratios that support information detection and encoding (Woodward et al. 1979; Sara 1985; Servan-Schreiber et al. 1990).
NA in the Hippocampus
NA plays an extremely important role in the regulation of hippocampal function. Elevations of NA levels increase neuronal excitability in the dentate gyrus (Lacaille and Harley 1985; Stanton and Sarvey 1985; Harley 1991) and in the CA subfields (Mueller et al. 1981; Heginbotham and Dunwiddie 1991; Dunwiddie et al. 1992; Jurgens et al. 2005), via activation of β-adrenergic receptors (β-AR) (Kitchigina et al. 1997). Furthermore, the enhanced responsiveness of hippocampal neurons to NA release from the locus coeruleus can persist for 24 h (Walling and Harley 2004), suggesting that NA also engages in metaplastic regulation (Abraham and Tate 1997; Maity et al. 2015) of hippocampal information encoding. In doing so, it will inevitably exert control over the readiness of the hippocampus to store new memories or retrieve old ones. The NA receptors are subdivided into α-adrenergic receptors (α-AR) and β-AR (Ahlquist 1948). Although α-AR can influence hippocampal function, largely through regulating neuronal excitability (Segal et al. 1991), the β-AR exert very specific effects on synaptic information encoding, whereby they strongly regulate synaptic plasticity, even in the absence of ostensible effects on neuronal excitability (Kemp and Manahan-Vaughan 2008a).
Projections of the LC to the hippocampus are differentiated, with inputs to the dentate gyrus being particularly dense (Ungerstedt 1971; Jones and Moore 1977; Loy et al. 1980). Afferents of the LC also terminate in the Stratum lucidum of area CA3 (Loy et al. 1980) where mossy fibers originating from the dentate gyrus form synapses. The LC exhibits firing activity that fluctuates across the circadian cycle (Bouret and Sara 2004), with activity being lower during sleep and increasing during the transition from sleep to wakefulness (Berridge 2008). Tonic firing activity correlates with behavioral state, with low tonus being associated with low vigilance states (Aston-Jones and Bloom 1981; Aston-Jones and Cohen 2005) and phasic burst firing being triggered by phenomena such as exploration of novelty, or arousing stimuli (Aston-Jones and Bloom 1981; Grant et al. 1988; Sara et al. 1994; Hervé-Minvielle and Sara 1995; Vankov et al. 1995), decision-making, or during the transition from a lower to a higher vigilance state (Aston-Jones and Bloom 1981; Rajkowski et al. 2004). In effect, the LC serves as a change detector.
The locus coeruleus is part of the ascending reticular activating system (ARAS) (Jelinger 2009), and its tonic activity is a key component of the waking cycle (Berridge 2008). In this function, it is reasonable to expect that it plays a key role in modulating thresholds for incoming cortical information (Devilbiss et al. 2006). Under conditions where an arousal change is triggered (by a novel event), phasic activity of the locus coeruleus changes excitability levels in the hippocampus (Lacaille and Harley 1985; Stanton and Sarvey 1985; Harley 1991), and indeed in other cortical areas (see Marzo et al. 2014), such that salient information is retained and/or encoded (Lemon et al. 2009; Lemon and Manahan-Vaughan 2012). This property of change detection enables selective information encoding that may be rendered more specific by activity-related local NA release networks that enable localized and highly selective information processing in discrete synaptic populations (Mather et al. 2015), thus conferring an even greater precision to the modulation by NA of synaptic responses and encoding. We propose that β-AR are a critical transducer of LC-mediated change detection, particularly pertaining to the promotion of encoding of salient experiences. This possibility is corroborated by observations that activation of the LC potently modulates β-AR-dependent and input-specific synaptic strength in the hippocampus, that is associated with hippocampus-dependent learning and memory (Kemp and Manahan-Vaughan 2007, 2011; Hansen and Manahan-Vaughan 2015a).
β-Adrenoreceptor Expression in the Hippocampus
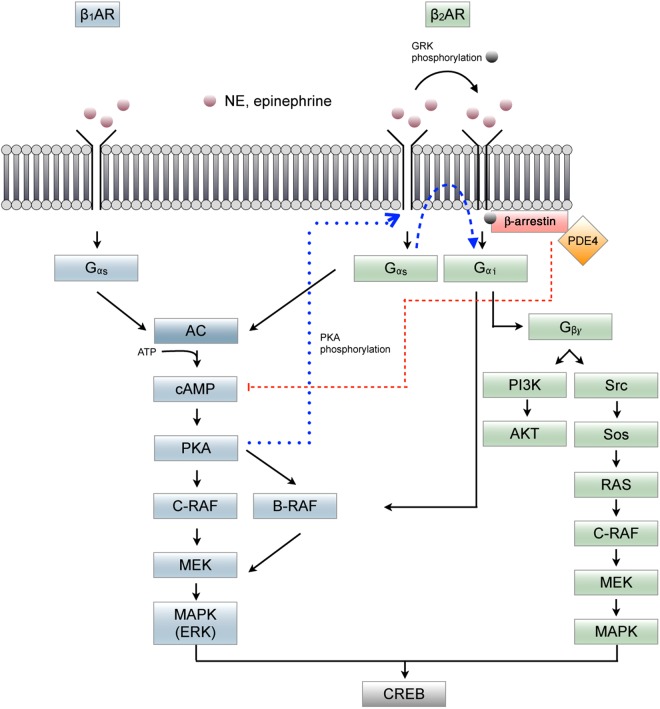
Different subtypes of β-AR are expressed in the hippocampus. They are classified into β1-, β2-, and β3-subtypes on the basis of their affinity toward adrenaline and NA (Lands et al. 1967) and are G protein-coupled receptors (GPCRs) that are positively coupled to adenylyl cyclase via Gs. However, depending on which receptor (β1- or β2-AR) is activated by ligands, different effects are observed based on the activation of downstream cascades (Fig. 1). Ligand binding to β1- or β2-AR leads to activation of guanine nucleotide-binding regulatory Gs-proteins. This, in turn, activates adenylate cyclase (AC), followed by an increase of intracellular cyclic adenosine monophosphate (cAMP) and subsequent activation of protein kinase A (PKA) (Seeds and Gilman 1971; Maguire et al. 1977; Strulovici et al. 1984; Hausdorff et al. 1989). In addition to the activation of Gs-proteins, ligand binding to β2-adrenergic receptors can also activate extracellular signal-regulated kinases (ERK), MAPKs, Akt, and tyrosine kinase transactivation, a pathway mediated by Gi/Go proteins (Abramson et al. 1988; Xiao et al. 1995; Daaka et al. 1997; Maudsley et al. 2000; Zhu et al. 2001).
Figure 1.
Summary of the different signaling pathways that respond to activation of β1AR (blue) and β2AR (green). PKA phosphorylation mediates the switch from Gα to Gβ activation (dotted blue line). An inhibitory effect resulting from β-arrestin and PDE4 action is shown by the red dotted line. AC, adenylate cyclase; PI3K, phosphatidylinositol 3-kinase; ATP, adenosine triphosphate; B-Raf, proto-oncogene B-Raf; cAMP, 3′-5′-cyclic adenosine monophosphate; C-Raf, RAF proto-oncogene serine/threonine kinase; CREB, cAMP-responsive element-binding protein; ERK, extracellular signal-regulated kinase; Gαs, Gαi, Gβɣ, G-proteins; GRK, G protein-coupled receptor kinase; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase; NE, norepinephrine; PDE4, phosphodiesterase-4; PKA, protein kinase; RAS, membrane-associated guanine nucleotide-binding protein; Src, proto-oncogene tyrosine-protein kinase; SOS, Son of Sevenless, genes encoding guanine nucleotide exchange factors (Winder et al. 1999; Schmitt and Stork 2000; Xiao 2001; Lefkowitz et al. 2002; Baillie et al. 2003; Shenoy et al. 2006; Lemon et al. 2009; Meitzen et al. 2011).
Of the different subtypes, the β1- and β2-AR subtypes are of particular interest due to their role in hippocampal synaptic plasticity (Yang et al. 2002; Gelinas et al. 2008; Kemp and Manahan-Vaughan 2008a): activation of these subtypes results in stimulation of extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) that is a key step in the activation of the cAMP response element-binding protein (CREB) that mediates protein transcription (Fig. 1) and thus strongly supports persistent synaptic plasticity (Braunewell and Manahan-Vaughan 2001; Ahmed and Frey 2005) and long-term memory (Kandel 2012). β3-AR are exclusively expressed in a subset of precursor cells in the subgranular zone of the DG (Jhaveri et al. 2010).
The expression of β1 and β2-AR in the hippocampus is not homogenous. Both, receptor subtypes are expressed in pyramidal cells in area CA1, CA3 and the dentate gyrus (DG) (Booze et al. 1993; Milner et al. 2000; Guo and Li 2007; Cox et al. 2008), whereas relatively low levels of both receptor subtypes have been reported in CA3 (Booze et al. 1993). A relatively wide distribution of β1-AR has been reported for the CA3 region, however, whereby expression is prominent on perikarya and to some extent on proximal dendrites (Jurgens et al. 2005). In the CA1 and CA3 regions, β1- and β2-AR are preferentially expressed in neurons as poopsed to astrocytes. β2-AR have been reported in neuronal membranes and cytoplasm, as well as in the nucleus of neurons in the CA1 and CA3 regions, whereas β1-AR are expressed only in membranes and cytoplasm (Guo and Li 2007). In the DG, β-AR are distributed within granule cells at postsynaptic sites (Milner et al. 2000). Furthermore, β1- and β2-AR are found on interneurons with a similar distribution across all 3 hippocampal regions for β2-AR (Cox et al. 2008). Compared with the CA1 region, the DG contains the highest NA content and the highest fiber density of noradrenergic innervation relative to the other hippocampal subregions (Loy et al. 1980).
In addition to postsynaptic expression of β-AR, it has been reported that β-AR also occur on presynaptic sites (Heider et al. 1997; Nejtek and Dahl 1997). Presynaptic activation of β-AR results in action potential initiation in the Schaffer collateral-CA1, but not in the perforant path-CA1 pathway. These differences may derive from pathway-specific modulation of the Schaffer collaterals mediated by β-AR (Nejtek and Dahl 1997) and suggest that activation of pre- or postsynaptic β-AR may result in different functional outcomes; but as yet, little is known about this possibility.
β-Adrenergic Regulation of Hippocampal Synaptic Plasticity
Evidence is increasing that synaptic plasticity enables the encoding of memory (Manahan-Vaughan and Braunewell 1999; Straube et al. 2003a; Kemp and Manahan-Vaughan 2004; Whitlock et al. 2006; Nabavi et al. 2014). Moreover, LTP and LTD may contribute to different aspects of the encoding of an experience. LTP is tightly related to context-dependent fear memory (Whitlock et al. 2006; Nabavi et al. 2014) and to the encoding of novel space, particularly in the concept of a novel scene or scene change, whereby learning about general properties of space or substantial changes in a known environment strengthens hippocampal LTP (Straube et al. 2003b; Kemp and Manahan-Vaughan 2004). Moreover, this is an encoding property exhibited by all hippocampal subfields (Kemp and Manahan-Vaughan 2004, 2008b; Hagena and Manahan-Vaughan 2012).
LTD, on the other hand, appears to encode the content and the specific features of space: whereby the precise characteristics of the spatial content determines which hippocampal subfield responds to this experience with the expression of LTD (Kemp and Manahan-Vaughan 2007). Thus, learning about the navigational or orientational (large and distal) content of space strengthens LTD in the dentate gyrus, or at mossy fiber-CA3 synapses, whereas learning about discrete (small and proximal) environmental features, or more subtle details of spatial content, enables LTD in the CA1 region (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2011; Goh and Manahan-Vaughan 2013a) and in associational commissural (AC)-CA3 synapses (Hagena and Manahan-Vaughan 2012). A picture is emerging, which strongly suggests that LTP and LTD work together to encode the components and elements of a spatial representation (Kemp and Manahan-Vaughan 2007), whereby we propose that LTP encodes the initial scaffold, and perhaps context, of the spatial experience, and LTD contributes details and content to this representation. This is supported by our observations that hippocampal synaptic potentiation appears instantaneously upon exposure of a rodent to novel space that includes novel content (Manahan-Vaughan and Braunewell 1999). Within several minutes, this potentiation response is superceded by persistent synaptic depression. This suggests that LTP may select the synaptic network in which the new experience is to be encoded, whereas LTD refines and optimizes this network into the final robust and unique representation.
What is particularly compelling about β-AR is that these receptors play a central role in this process, by driving the direction of change of synaptic strength (Lemon et al. 2009; Hansen and Manahan-Vaughan 2015b) and in grading the persistency of synaptic plasticity in the different hippocampal subfields (Kemp and Manahan-Vaughan 2008a; Hagena and Manahan-Vaughan 2012; Hansen and Manahan-Vaughan 2015b). Bearing in mind the significance of LTP and LTD for long-term spatial memory, and given the postulated roles of the different hippocampal subfields in the encoding of memory aspects such as working memory (Nakao et al. 2002; Kesner 2007; Bikbaev et al. 2008; Kesner and Warthen 2010), pattern completion, pattern separation (Kesner et al. 2004; Leutgeb and Leutgeb 2007; Leutgeb et al. 2007; Bakker et al. 2008; Kesner and Warthen 2010; Hagena and Manahan-Vaughan 2011; Neunuebel and Knierim 2014), mismatch detection (Lisman and Otmakhova 2001; Lee et al. 2005; Kumaran and Maguire 2007; Duncan et al. 2012), and the holistic completion of spatial representation (Kemp and Manahan-Vaughan 2007), this places the β-AR in a unique position as a determinant of the content and persistency of synaptic information storage and memory.
LTP
Influence of β-Adrenergic Receptors on LTP in the Dentate Gyrus
Almost 30 years ago, Stanton and Sarvey (1985) demonstrated that destroying noradrenergic fibers with selective dorsal bundle injections of 6-OHDA and thereby depleting NA, reduced the occurrence and magnitude of LTP in the DG. In the DG, application of a β-AR agonist causes lasting potentiation of the population spike (Lethbridge et al. 2014) and application of NA itself to the DG causes LTP of the population spike in slice preparations (Bramham et al. 1997; Swanson-Park 1999), as well as in anesthetized rats (Neuman and Harley 1983; Winson and Dahl 1985; Chaulk and Harley 1998). LTP of both the population spike and dendritic excitatory postsynaptic potential resulting from NA application to the DG has also been described in vitro (Stanton and Sejnowski 1989), suggesting that NA modulates both somatic and dendritic excitability in this hippocampal subfield. In addition, in the DG in vivo, LTP is reinforced by β-AR agonism or NA application (Almaguer-Melian et al. 2005; Hansen and Manahan-Vaughan 2015b). Evidence also exists that the increased extracellular NA release that occurs in response to high-frequency stimulation (HFS) of the perforant path is an important component in inducing LTP in the DG (Bronzino et al. 2001). This process may relate to glutamate release-mediated localized NA release, as reported by Mather et al. (2015).
This prominent influence of β-AR in the DG can be attributed to the fact that noradrenergic innervation originating from the LC is very dense in the DG, and the NA content in this hippocampal subfield may be higher compared with the CA1 and CA3 regions (Loy et al. 1980) (Fig. 2). In addition, the highest expression of β1- and β2-AR is found in the DG compared with the CA1 and CA3 region (Booze et al. 1993; Milner et al. 2000), suggesting that the DG may be more sensitive to noradrenergic control (via β-AR) than the other hippocampal subfields.
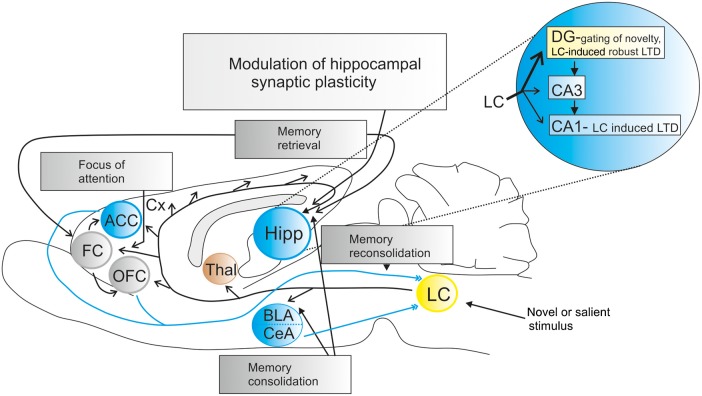
Figure 2.
Noradrenergic projections of the locus coeruleus involved in the modulation of persistent hippocampal synaptic plasticity in the rat brain. The locus coeruleus (LC) sends projections to the basolateral amygdala (BLA), thalamus (Thal), orbitofrontal cortex (OFC), frontal cortex (FC), anterior cingulate cortex (ACC), Cortex (Cx), and hippocampus (Hipp). The projection to the hippocampus is responsible for modulating hippocampal synaptic plasticity (Harley 2007; Lemon et al. 2009; Sara 2009; Lemon and Manahan-Vaughan 2012). The DG receives the strongest noradrenergic projection from the LC (indicated by the big black arrow in the magnification of the hippocampus on the upper right side) compared with CA1 and CA3 (smaller black arrows indicate weaker LC projections) (Loy et al. 1980; Fallon and Loughlin 1982) underpinning its role in novelty gating within hippocampal information processing. Electrical activation of the LC induces β-AR-dependent long-term depression in SC-CA1 and DG synapses (Lemon et al. 2009; Hansen and Manahan-Vaughan 2015b) indicating the important role of the LC in encoding novel information. In addition, the different stages of memory are dependent on a complex interaction within this noradrenaline (NA)–LC network. Memory consolidation is promoted by the Hipp and BLA (Eichenbaum 2000; Roozendaal et al. 2008), whereas memory retrieval is enabled by an interaction between the Hipp and FC (Corbetta and Shulman 2002; Sara 2010). The projection from the FC and ACC to the LC is believed to contribute to the reconsolidation of memory processes (Sara 2000, 2009, 2010). The FC regulates the control of attention to a novel or salient stimulus (Corbetta et al. 2008; Robbins and Arnsten 2009). According to the integrative theory of NA-LC function, the OFC and ACC send projections to the LC, driving transitions between the LC modes and phasic LC responses to adapt synaptic gain (Aston-Jones and Cohen 2005).
Strikingly, β-AR activation in the DG may serve to determine the direction of change in synaptic strength in medial and lateral perforant path-DG synapses: combining β-AR agonism with stimulation of the medial perforant path induces LTP (in the form of NELLP: “norepinephrine” long-lasting potentiation), whereas stimulating the lateral perforant path induces LTD (in the form of NELLD: “norepinephrine” long-lasting depression) (Dahl and Sarvey 1989; Pelletier et al. 1994). These differential effects of NA may be important for selective hippocampal information processing between these anatomically and biochemically distinct pathways (Dahl and Sarvey 1989).
Influence of β-Adrenergic Receptors on LTP in the CA3 Region
In area CA3, two important pathways converge onto CA3 pyramidal cells: the mossy fibers (mf) that originate from dentate gyrus granule cells, and the associational commissural (AC-CA3) fibers that are, in effect, recurrent afferents that arise from ipsilateral CA3 pyramidal cells, or commissural projections from contralateral CA3 pyramidal cells. Both synapses display different properties in terms of receptor involvement in long-term changes of synaptic plasticity, whereby, for example, the mossy fiber (mf)–CA3 synapses express LTP that does not require N-methyl-d-aspartate receptor (NMDAR) activation and integrate presynaptic processes (Harris and Cotman 1986; Nicoll and Schmitz 2005). Furthermore, synaptic plasticity at mf-CA3 and AC-CA3 synapses are differently regulated by the metabotropic glutamate receptor, mGlu5 (Hagena and Manahan-Vaughan 2015). The molecular distinctions of synaptic plasticity at mf-CA3 and AC-CA3 synapses may reflect distinct functional roles in terms of hippocampal information encoding and memory (Hagena and Manahan-Vaughan 2011).
Area CA3 receives massive projections from the LC, which terminate in the Stratum lucidum (Moore and Bloom 1979; Loy et al. 1980), the termination site of mf projections from the DG. As in area CA1 and the DG, LTP in the CA3 region can be distinguished into different phases, called early (E)-LTP that lasts for up to 2 h in vivo, and late (L)-LTP, that lasts for several hours or days. E-LTP requires activation of mGlu receptors (Bashir et al. 1993) and protein kinases (Malenka et al. 1989) (and to some degree phosphatases), L-LTP requires the expression of immediate early genes (Jones et al. 2001), as well as protein translation (Connor et al. 2011). Interestingly, in vitro studies have shown that differences exist between the different inputs to CA3 at the level of β-AR. Activation of these receptors induced early- and late-LTP at mf-CA3 synapses but not at AC-CA3 synapses in vitro, and antagonism of β-AR blocks late-LTP, suggesting that β-AR play a crucial role in modulating information processing mf-CA3 synapses (Hopkins and Johnston 1988; Huang and Kandel 1996). In line with this, other studies, conducted on rat brain slices, reported that β1-AR activation has enhancing effects on LTP at mf–CA3 synapses (Hopkins and Johnston 1984, 1988).
Very few studies have explored synaptic plasticity in CA3 synapses of the intact rodent. Recently, we have shown a differentiated involvement of β-AR in persistent plasticity at mf-CA3 synapses in freely behaving rats. Interestingly, LTP or LTD elicited with electrical stimulation, and which lasts for over 24 h, is not altered by propranolol, a β-AR antagonist (Hagena and Manahan-Vaughan 2012). However, β-AR activation results in strengthening of weak synaptic potentiation at mf-CA3 and AC-CA3 synapses in vivo, although weak depression remains unaffected (Hagena and Manahan-Vaughan 2012).
Influence of β-Adrenergic Receptors on LTP in the CA1 Region
The role of β-AR in the CA1 region has been extensively investigated both in vitro and in vivo, whereby in vitro studies of synaptic plasticity are typically limited to the first 60 min of the plasticity event. It has been shown, for example, that LTP induced by prolonged theta-tetanus in vitro (PTT; one train of 900 stimuli at 5 Hz) depends on β-AR in mice and rats (Thomas et al. 1996; Katsuki et al. 1997; Gelinas and Nguyen 2005; Hu et al. 2007; Qian et al. 2012). Other in vitro studies showed that LTP induction in area CA1 depends on recruiting noradrenergic innervation (Yang et al. 2002). These processes may rely on joint activation of other neuromodulatory systems: co-activation of β-adrenergic and cholinergic receptors enhances LTP induction in area CA1 in vitro (Watabe et al. 2000), and dopamine D1 receptors mediate LC effects on hippocampal synaptic plasticity, which also recruit β-AR (Lemon et al. 2009).
LTP can be induced by activation of β-AR within a variety of stimulation frequencies. LTP induced with 5 Hz afferent stimulation depends on β-AR, both in mice (Thomas et al. 1996) and in rats (Lin et al. 2003), and more recently, a new role has emerged for β2-AR in LTP induced by theta stimulation (Qian et al. 2012). LTP induced by different afferent stimulation patterns and using varying frequencies (5, 10, or 100 Hz) is also enhanced by applying a β-AR agonist before or during stimulation (Thomas et al. 1996; Katsuki et al. 1997; Cohen et al. 1999; Gelinas et al. 2008; Li et al. 2013). Surprisingly, low-frequency stimulation (LFS) paired with β-AR activation causes LTP in area CA1 in vitro (Gelinas and Nguyen 2005). This β-AR-dependent LFS-induced LTP may result from decreased A-type potassium channel activity in pyramidal cell dendrites that lead to increased dendritic cell excitability (O'Dell et al. 2010). We have observed in vivo that LC stimulation results in LTD in the hippocampus (Lemon et al. 2009; Lemon and Manahan-Vaughan 2012; Hansen and Manahan-Vaughan 2015b).
Controversies in the Presumed Role of β-AR in Regulating LTP
It should be mentioned, however, that some in vitro studies have contradicted the abovementioned findings and report that application of β-AR antagonists or agonists does not alter LTP, particularly when it is induced by a strong afferent stimulation pattern (Dunwiddie et al. 1982; Schimanski et al. 2007). One possible explanation for this discrepancy may be the time frame during which LTP was monitored. Dunwiddie and colleagues monitored LTP responses for ca. 20 min. Thus, it may be that β-AR effects on the subsequent phases of LTP were missed. In the study by Schimanski et al. (2007), genetically modified animals were used that exhibit different levels of endogenous NA. We have observed that LC stimulation that occurs immediately prior to high-frequency afferent stimulation prevents hippocampal LTP that would normally endure for <4 h, but does not prevent LTP that would normally persist for over 24 h (Hansen and Manahan-Vaughan 2015a). These observations align with the abovementioned studies and suggest that under circumstances where the novel experience is extremely potent in its emotive force, encoding of this information is prioritized by the hippocampus and overrides regulatory control by NA released from the LC that acts on β-AR. Here, associative shock or fearful experiences spring to mind. Many studies have shown that fear conditioning that uses foot shock or other forms of acute and potent stress leads to robust LTP in the hippocampus (Korz and Frey 2003, 2005; Ahmed et al. 2006; Whitlock et al. 2006).
LTD
Regulation by β-Adrenergic Receptors of LTD in the Dentate Gyrus
Intrahippocampal application of a β-AR agonist induces LTD in the DG in vivo (Lethbridge et al. 2014). At higher agonist concentrations, LTP occurs (Lethbridge et al. 2014), suggesting (as mentioned already above) that the degree of β-AR activation may be decisive for the type of synaptic plasticity induced. Strikingly, however, brief direct activation of the locus coeruleus in vivo, which leads to NA release in the hippocampus (Lemon et al. 2009), promotes the expression of hippocampal LTD in CA1 (Lemon et al. 2009; Lemon and Manahan-Vaughan 2012) and in the dentate gyrus (Hansen and Manahan-Vaughan 2015b). Agonist activation of β-AR also facilitates weak LTD into persistent (>24 h) LTD in vivo (Hansen and Manahan-Vaughan 2015b). This suggests that the bias of β-AR regulation of DG plasticity may be toward LTD.
In line with this, in vitro studies showed that in the absence of patterned afferent stimulation, NA induces LTD in the DG (Dahl and Sarvey 1990), and agonist activation of β-AR also elicits LTD at lateral perforant path-dentate gyrus synapses (Dahl and Sarvey 1990). Moreover, as mentioned earlier, the combination of β-AR antagonism with stimulation of the medial perforant path induces LTD in the DG (in the form of NELLD) (Dahl and Sarvey 1989; Pelletier et al. 1994). In freely behaving rats, persistent LTD that lasts for over 24 h at medial perforant path-dentate gyrus synapses critically depends on β-AR activation (Hansen and Manahan-Vaughan 2015b).
Regulation by β-Adrenergic Receptors of LTD in the CA3 Region
Despite the importance of the CA3 region in the acquisition and processing of spatial information, only one study has addressed the role of β-AR in LTD in area CA3. Here, it was shown that at mf-CA3 synapses, antagonism of β-AR had no effect on LTD (>24 h) in freely behaving rats that was induced solely by patterned afferent stimulation (1 Hz, 900 pulses) (Hagena and Manahan-Vaughan 2012). Effects were not dose dependent: increasing the antagonist concentration failed to alter the resistance of LTD to regulation by β-AR. This is in striking contrast to the DG, where β-AR antagonism prevents persistent LTD. This may relate to the putative role of the CA3 in specific forms of information processing, such as pattern completion (Kesner 2007), or to the very unique molecular mechanisms underlying synaptic plasticity, and persistent LTD, at mf-CA3 synapses (Klausnitzer and Manahan-Vaughan 2008; Hagena and Manahan-Vaughan 2010).
Regulation by β-Adrenergic Receptors of LTD in the CA1 Region
In vitro studies as to the role of β-AR receptors in LTD in area CA1 are few and contradictory: CA1-LTD induced by LFS is unaffected by β-AR modulation in vitro (Yang et al. 2002), whereas other in vitro studies reported an involvement of β-AR activation in preventing CA1-LTD (Katsuki et al. 1997). We believe these discrepancies derive from differences in experimental preparations across laboratories, such as methodological differences in the preparation of hippocampal slices, slice treatment in the in vitro chamber, and the afferent stimulation protocols used, but they also derive from the fact that most in vitro studies examine plasticity effects for at most 60 min after patterned afferent stimulation (see e.g.: Bliss and Collingridge 1993; Bear and Malenka 1994; Malenka and Bear 2004 for reviews of the hippocampal plasticity field). Although this would be considered a heretic statement among in vitro plasticity researchers, plasticity responses that persist for 60 min are not truly long-term effects. Here, rather one is examining E-LTP or E-LTD in in vitro studies of this kind. And strikingly, where LTD was followed for hours or days in vivo, a critical involvement of β-AR was described: different labs have reported that short-term depression (STD) that is induced by weak LFS is prolonged into late LTD in Schaffer collateral-CA1 synapses in freely behaving rats and mice following activation of β-AR (Straube and Frey 2003; Kemp and Manahan-Vaughan 2008a).
It is striking that LTP and LTD that are elicited by strong stimulation of mf-CA3 synapses are resistant to regulation by β-AR. This suggests that under conditions where the LC is strongly activated, information processing in the CA1 and DG (but not CA3) will be subject to modulation by NA. The CA3 region has been proposed to be responsible for pattern completion (Kesner 2007), whereby encountering a fragment of a previously learned experience results in the retrieval of the entire stored representation. The DG and CA1 may be more important for pattern separation, whereby novel experience is distinguished from a similar previously learned experience and encoded as a new “engram” (Kesner et al. 2004). Thus, given the fact that NA is released from the LC based on the novelty, or saliency, of an experience, it is plausible that it supports new information encoding (at the level of the DG or CA1) more than processes that might involve retrieval or updating of established engrams (in the CA3 region).
Cellular Mechanisms Underlying β-Adrenergic Receptor Regulation of Hippocampal Synaptic Plasticity
Bath application of a β-AR agonist (to a hippocampal slice) may mimic a tonic elevation in NA (and thereby tonic activation of β-AR), whereas brief stimulation of the LC may emulate phasic NA release (onto β-AR). This suggests that the pattern and duration of β-AR activation that occurs in coincidence with, for example, entorhinal cortex informational input into the hippocampus, may be decisive in determining the direction of change in synaptic strength. In line with this, we have observed that brief activation of the LC in vivo prevents weaker forms of hippocampal LTP (Hansen and Manahan-Vaughan 2015a), whereas an elevation in “tonic” activation by means of intracerebral agonist treatment can strengthen weak potentiation into persistent LTP (Hansen and Manahan-Vaughan 2015a) and under specific state-dependent circumstances, phasic activation of the LC triggers NA-LTP (Walling et al. 2011). Conversely, an emulation of “tonic” LC activation (by brief electrical stimulation of the LC) that occurs in conjunction with test-pulse stimulation of afferent fibers to the hippocampus results in very robust LTD at CA1 (Lemon et al. 2009) and DG synapses (Hansen and Manahan-Vaughan 2015b).
β-AR do not only influence the direction of change in synaptic strength; they also modulate the persistency of synaptic plasticity. Early (E-LTP) that normally lasts for just 1–2 h is reinforced by β-AR activation (Hsu et al. 2002) and β-AR antagonism attenuates E-LTP in vitro (Connor et al. 2011). β-AR activation also prolongs L-LTP in the CA1 region of rat hippocampal slices (Gelinas and Nguyen 2005). Moreover, β-AR contribute to the maintenance of LTP in area CA1 in different mouse strains (Schimanski et al. 2007). Agonist activation of β-AR prolongs E-LTD into L-LTD in the DG (Hansen and Manahan-Vaughan 2015b), but not in the CA1 region (Kemp and Manahan-Vaughan 2008a) in putative alignment with the density of LC projections in these hippocampal subfields (Loy et al. 1980).
Evidence suggests that β-AR-dependent L-LTP is based on dendritic translation, but not transcription involving an upregulation of the eukaryotic initiation factor (eIF) 4F complex in CA1 pyramidal cell dendrites during β-AR-dependent LTP (O'Dell et al. 2010). Formation of the eIF4F complex plays a key role during initiation of translation (O'Dell et al. 2010). β-AR regulate postsynaptic initiation of protein translation through the ERK and mTOR pathways (Winder et al. 1999; Gelinas et al. 2007). The translation of plasticity-related proteins can, in turn, facilitate hippocampal synaptic plasticity (O'Dell et al. 2010). β-AR activation can also enhance short-term plasticity through modulation of intrinsic excitability of neurons. This involves the attenuation of Ca2+-dependent potassium currents in the DG, and in CA1 pyramidal neurons (Haas and Konnerth 1983; Haas and Rose 1987), along with potentiated activity of voltage-dependent Ca2+-channels in the DG (Gray and Johnston 1987) and at mf-CA3 synapses (Fisher and Johnston 1990).
The abovementioned examples highlight that β-AR not only influence the direction of change of synaptic strength, but they also regulate the duration of hippocampal synaptic plasticity and therefore may also contribute to the precision of synaptic encoding in the hippocampus. Thus, β-AR activation also augments LTP in CA1 synapses at an input other than the stimulated input (i.e., they modulate heterosynaptic LTP) (Connor et al. 2011).
Taken together, these observations suggest that β-AR mediate multifarious and diverse regulation of both early and late components of LTP and LTD, suggesting that their role in enabling this form of synaptic plasticity is quite pivotal. This in turn may relate to the abovementioned roles of tonically (Bouret and Sara 2004), phasically (Aston-Jones and Bloom 1981; Grant et al. 1988; Sara et al. 1994; Hervé-Minvielle and Sara 1995; Vankov et al. 1995), and locally (Mather et al. 2015) released NA in modulating very distinct kinds of information processing and storage.
Molecular Mechanisms Underlying β-Adrenergic Receptor Regulation of Hippocampal Synaptic Plasticity
Several mechanisms have been proposed to explain how LTP is modulated by β-AR on a molecular level. NA, or activation of β-AR, induces phosphorylation of the GluA1 subunit of the AMPA receptor, at Ser845 and Ser831 sites (Vanhoose and Winder 2003; Joiner et al. 2010; Zhou et al. 2012) that in turn facilitates the synaptic delivery of GluA1 containing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) that are subject to NA modulation (Hu et al. 2007; O'Dell et al. 2010). Thus, the threshold for use-dependent alterations in hippocampal synaptic plasticity can be lowered, for instance to induce LTP. Furthermore, an elevated NA level in the hippocampus that is induced by emotional arousal causes GluA1 phosphorylation (Hu et al. 2007). The increase of extrasynaptic GluA1 mediated by NA-induced phosphorylation may be achieved by mechanisms that link GluA1 with molecules that support its delivery to the synaptic membrane (Leonard et al. 1998; Chen et al. 2000; Esteban et al. 2003; Rouach et al. 2005).
A further aspect of β-AR regulation of glutamate receptors pertains to NMDAR that comprise a pivotal component of many forms of hippocampal LTP (Bliss and Collingridge 1993). Here, a modulation by β-AR of NMDAR-mediated processes has been reported: deficits in LTP in GluN2A knockout mice can be reversed by activating β-AR, such that the ERK pathway is stimulated, and an increase in GluA1 phosphorylation occurs (Moody et al. 2011). These are important properties of β-AR, as phosphorylation of AMPAR GluA1 is considered to be a key step in the bidirectional modification of hippocampal synaptic plasticity (Lee et al. 2000): when GluA1 remains phosphorylated, memory storage can be facilitated (Hu et al. 2007).
β-AR also regulate PKA and AC (Zhang et al. 2013), two further critical components of hippocampal synaptic plasticity signaling cascades (Muller et al. 1991). Moreover, PKA plays an important role in β-AR-dependent LTP (Gelinas et al. 2007). PKA can itself promote the surface extrasynaptic pool of GluA1 (Oh et al. 2006) that in turn is regulated by NA. LTP can also be facilitated through direct phosphorylation effects of PKA on NMDAR (Chen and Sara 2007; O'Dell et al. 2010). β-AR activation can also inhibit protein phosphatases 1 and 2A to facilitate LTP induction in CA1 mouse slices (Thomas et al. 1996). Furthermore, protein synthesis-dependent L-LTP requires the activation of β-AR (Straube and Frey 2003; Straube et al. 2003a). In addition to β-AR modulation of long-term synaptic plasticity through the regulation of NMDAR and AMPAR, β-AR activation also modulates the processes of synaptic tagging and capture of LTP (see review from O'Dell et al. 2015).
Special Role of the Locus Coeruleus in the Regulation of Hippocampal Synaptic Plasticity
LC activation, in response to arousal or novelty, induces NA release in different hippocampal subfields (Yavich et al. 2005; Lemon et al. 2009). The exploration of a novel object in a holeboard causes a tonic increase in the population spike lasting 50–75 s in the DG (Kitchigina et al. 1997). The highest content of endogenous NA compared with other hippocampal subfields occurs in the DG, which receives the strongest noradrenergic projections from the LC (Loy et al. 1980). Thus, to date, most studies have focused on LC regulation of this hippocampal subfield.
LC activation via glutamate with the goal of triggering NA release in the hippocampus, and most specifically in the DG, has been implemented in diverse in vitro (Harley and Milway 1986) and in vivo studies (Klukowski and Harley 1994; Walling and Harley 2004), and also in anesthetized rats (Reid and Harley 2010; Edison and Harley 2012). This glutamate-induced LC activation led to different results in the perforant path-DG synapse: an immediate short-term potentiation of the population spike in vitro (Harley 1987) and in vivo (Klukowski and Harley 1994), an intermediate population spike potentiation lasting 3 h (Edison and Harley 2012) and a potentiation of the slope of the excitatory postsynaptic potential occurring 24 h after LC activation (Walling and Harley 2004). Both the LTP of the field excitatory postsynaptic potential, and the short-term potentiation of the population spike in perforant path-DG synapses, were dependent on β-AR activation (Harley and Milway 1986; Walling and Harley 2004). Moreover, β-AR activation was required for LC-induced, protein synthesis-dependent LTP in the DG (Walling and Harley 2004). Another study revealed that pairing LC stimulation with afferent perforant path stimulation induced a potentiation of both excitatory postsynaptic potential and the population spike in anesthetized rats (Reid and Harley 2010).
Interestingly, the medial and lateral perforant path inputs to the DG can be selectively potentiated depending on the interstimulus interval between pairs of utilized path stimulation (Edison and Harley 2012). A further study showed that the repeated pairing of electrical LC stimulation with perforant path stimulation enabled an LTP of the population spike in vivo (Harley et al. 1989). What is interesting here is that β-AR antagonism with propranolol did not block the potentiation, but it did suppress the effect seen by glutamate activation (Harley et al. 1989). Thus, as seen in β-AR-dependent LTP (Straube et al. 2003a), the dependency on β-AR can be overcome with strong repeated afferent stimulation protocols. This may relate to the fact that strong LC activation is likely to induce co-release of dopamine with NA from the LC (Smith and Greene 2012), whereas brief LC stimulation triggers a small DA release in the CA1 region (Lemon et al. 2009), which also influences synaptic plasticity that is enabled by LC activation (Lemon and Manahan-Vaughan 2012).
The LC may play a very particular role in the regulation of hippocampal information encoding through LTD: electrical stimulation of the LC, coupled with test-pulse stimulation of either the Schaffer collaterals or the medial perforant path, results in β-AR-dependent LTD in the CA1 region and DG, respectively. The resultant LTD lasts for <24 h in the CA1 region, and for longer than 24 h in the DG in vivo, reflecting perhaps the direct innervation of the DG by the LC and the higher density of β-AR in this subfield (Loy et al. 1980; Booze et al. 1993; Milner et al. 2000).
β-Adrenergic Receptors and Learning-Facilitated Plasticity
Learning-facilitated plasticity describes the ability of hippocampal synapses to respond with persistent LTP, or LTD, when a learning event is coupled with afferent stimulation that is subthreshold for the induction of lasting plasticity, or coupled with test-pulse stimulation of hippocampal afferents (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004, 2007, 2008b; Goh and Manahan-Vaughan 2013b). The disadvantage of examining robust forms of plasticity that are simply induced by strong afferent activation by means of electrical stimulation is that we do not know how physiological these forms of plasticity are, nor do we know how closely they relate to the putative role of synaptic plasticity in long-term memory. Studies of learning-facilitated plasticity circumvent this dilemma: the types of plasticity induced by afferent stimulation are very weak, or non-existent and the robust forms of LTD or LTD that emerge when these protocols are coupled with learning events, give valuable insight into the role of LTP and LTD in long-term information encoding of spatial experience (Kemp and Manahan-Vaughan 2007). Here, we have observed that LTP is tightly associated with the acquisition of knowledge about new space or spatial change (Kemp and Manahan-Vaughan 2004, 2007), whereas LTD is tightly associated with learning about the specific content details of space. A hierarchy with regard to the involvement of hippocampal subfields in this process has become evident: LTD in the dentate gyrus and in mf-CA3 synapses is enabled by novel learning about large navigational content (e.g., distal or large items), whereas LTD at AC-CA3 and Schaffer collateral-CA1 synapses is enabled by novel learning about small, subtle content details (such as hidden items or items that can only be detected when the animal is immediately proximal to them) (Manahan-Vaughan and Braunewell 1999; Kemp and Manahan-Vaughan 2004, 2007, 2008b; Goh and Manahan-Vaughan 2012; Hagena and Manahan-Vaughan 2012).
What is striking is that very consistent results have emerged as to the role of β-AR in learning-facilitated plasticity. LTP (>24 h) that is enabled by the learning about novel space, or spatial change, is prevented by antagonism of β-AR. Effects are consistent for learning-facilitated LTP evoked in the CA1, CA3, or DG subfields (Kemp and Manahan-Vaughan 2008a; Hagena and Manahan-Vaughan 2012; Goh and Manahan-Vaughan 2013b). The reinforcement of LTP in the DG by appetitive or aversive stimuli is also mediated by β-AR (Seidenbecher et al. 1997).
Furthermore, LTD that is facilitated by learning about novel spatial content, in the CA3 region and DG, is also tightly dependent upon β-AR and has been reported both in rats and in mice in vivo (Kemp and Manahan-Vaughan 2008a; Hagena and Manahan-Vaughan 2012; Goh and Manahan-Vaughan 2013b). The only exception to this is the CA1 region where, in rats, β-AR antagonism does not block learning-facilitated LTD (Kemp and Manahan-Vaughan 2008a), whereas in mice, it does (Goh and Manahan-Vaughan 2013b).
Taken together, these findings indicate that β-AR are also very important for the regulation of physiologically relevant forms of synaptic plasticity. We propose that NA acting on β-AR plays a decisive role in determining not only whether a novel experience should be encoded, but also in determining how this information is encoded, at the level of LTP and LTD. We believe this relates in turn to the behavioral saliency of the novel experience: very emotive experiences may be encoded by robust LTP (Korz and Frey 2003; Ahmed et al. 2006; Whitlock et al. 2006) that bypass the need for β-AR modulation. Spatial experiences that relate for example, the encoding of new space or of spatial change, may be encoded by LTP (Kemp and Manahan-Vaughan 2004, 2007). Strong phasic activity of the LC, such as during periods of strong wakefulness or anticipation (Bouret and Sara 2005), may predispose hippocampal synapses to encoding of this kind that is mediated by β-AR. During periods of tonic LC activity, that relate to increased vigilance related to a novel experience, a higher degree of encoding of content details of this experience will be enabled by hippocampal LTD (Kemp and Manahan-Vaughan 2007). This is strongly supported by NA acting on β-AR (Kemp and Manahan-Vaughan 2008a; Lemon et al. 2009; Hansen and Manahan-Vaughan 2015b), whereby the degree and specificity of this form of content encoding (“distal” content encoding in the DG, “proximal” content encoding in, e.g., the CA1 region) are enabled by LTD within the different hippocampal subfields (Kemp and Manahan-Vaughan 2007; Hagena and Manahan-Vaughan 2012) that is in turn modulated by β-AR (Kemp and Manahan-Vaughan 2008a; Hagena and Manahan-Vaughan 2012; Hansen and Manahan-Vaughan 2015b).
Role of β-Adrenergic Receptor Activity in Hippocampus-Dependent Learning
Given its significance for learning-facilitated LTD, and the plasticity-related forms of learning detailed in the earlier sections, it is not surprising that substantial evidence exists to support a role for other forms or aspects of β-AR hippocampus-dependent memory. For example, β-AR-blockade leads to deficits in spatial reference memory in the water maze task (Ji et al. 2003b) and in contextual fear memory (Ji et al. 2003a). Furthermore, LC activation leads to a β-AR-dependent enhancement of episodic-like memory in rats that is coupled to enhancements of LTD (Lemon et al. 2009). Differentiated roles for β1-AR in short-term memory and β2-AR in long-term memory and memory consolidation have also been proposed (Gibbs and Summers 2002). Other hippocampus-dependent tasks, such as extinction learning in a spatial context (André et al. 2015), memory retrieval (Thomas 2015), or reinstatement of memories may also be mediated by activation of β-AR: exposure to a novel environment within 1 h prior to or after extinction learning results in reduced fear reinstatement in rodents. This effect is prevented by a β-AR antagonist (Liu et al. 2015), whereas β-AR activation by NA induces reinstatement of previously extinguished fear memories (Morris et al. 2005).
The formation and maintenance of memory are strongly influenced by emotional experience. It is frequently the case that memories, obtained during strong emotional events, are more salient and persistent. Furthermore, short-term memory, acquired in the hippocampus, is vulnerable to interference. But consolidation, over time, strengthens this memory, making it persistent and thus less vulnerable (McGaugh 2000). Activation of emotional and arousal systems can affect memory encoding, leading to the strengthening of acquired information (Cahill et al. 1994). The noradrenergic system is particularly influential in this regard (Harley 1991; Bouret and Sara 2005). It is activated by novel stimuli or reinforcement, accomplished by bursting responses of LC cells (Sara et al. 1994). Furthermore, emotional states such as pain, fear, or arousal can have enhancing effects on memory by activating the noradrenergic system (Rochford and Dawes 1993; Debiec and Ledoux 2004).
A memory can be divided into different phases. Initially, a new memory has to be learned (acquisition). Subsequently consolidation occurs, in which a memory is strengthened and retained, if salient enough. Lastly, a stored memory must be retrievable. During all these processes, neuromodulators such as NA play a crucial role (Sara 2000, 2009, 2010). In vitro studies have shown that NA is an important component in hippocampus-dependent contextual (Murchison et al. 2011) and emotional memory retrieval (Schutsky et al. 2011). β-AR are particularly important for these processes (Table 1). Stimulation of the LC facilitates memory retrieval via a β-AR-dependent mechanism (Devauges and Sara 1991) and enhances episodic-like memory in rats (Lemon et al. 2009). Furthermore, NA-deficient dopamine β-hydroxylase null mutant (Dbh−/−) mice that display impaired contextual and spatial memory retrieval show recovery of these deficits following β-AR agonist treatment (Murchison et al. 2004). Interestingly, other studies have shown that β-AR antagonism disrupts the consolidation of episodic memory when the antagonist is applied during a narrow time window before memory acquisition (Cahill et al. 1994; McGaugh 2000) and that it impairs consolidation and reconsolidation during an inhibitory avoidance task in rats (Liang et al. 1986; Przybyslawski et al. 1999). Thus, not only the timing and intensity of LC activation, but also the nature of the behavioral experience may determine to what extent β-AR contribute to memory processes.
Table 1.
Effects of β-AR antagonists and agonists on synaptic plasticity in vitro
| Drug/knockout | Subtype | Subregion | Effect on plasticity | References |
|---|---|---|---|---|
| Isoproterenol | β1, β2 | CA1 | Induction of LTP (>40 min) | Thomas et al. (1996) |
| Blocks depotentiation | Thomas et al. (1996) | |||
| STP strengthened |
Winder et al. (1999); Gelinas et al. (2008) Gelinas et al. (2008) |
|||
| No effect on LTP (>2 h) | Hsu et al. (2002) | |||
| Enhances E-LTP and L-LTP | Giovannini et al. (2001) | |||
| Induction of TPS-LTP (1 h) | Brown et al. (2000) | |||
| Induction of TPS-LTP (30 min) no effect on induction (1 train, 5 Hz) |
Watabe et al. (2000) | |||
| Enhances induction of LTP | Cohen et al. (1999); Swanson-Park (1999) | |||
| LTP enhanced | Tenorio et al. (2010) | |||
| LTP after low-frequency stimulation | Reis et al. (2005) | |||
| LTP induction enhanced | Moody et al. (1998) | |||
| LTP enhanced (1 h) | Katsuki et al. (1997); Winder et al. (1999) | |||
| LTD blocked | Katsuki et al. (1997) | |||
| No effect on NA-induced LTP | Izumi and Zorumski (1999) | |||
| LTP enhanced (1 h) | Li et al. (2013) | |||
| CA3 | STP strengthened | Huang and Kandel (1996) | ||
| No effect on LTP | Moody et al. (1998) | |||
| Propranolol | β1, β2 | DG | Blocked LTP in LPP and MPP LTP blocked |
Bramham et al. (1997) Swanson-Park (1999) |
| CA1 | No effect on LTP (>3 h) | Swanson-Park (1999) | ||
| Dose-dependently blocks LTP | Li et al. (2013) | |||
| LTP blocked No effect on PS of LTP |
Connor et al. (2011) Kobayashi et al. (1997) |
|||
| CA3 | Blocks E-LTP and L-LTP | Huang and Kandel (1996) | ||
| LTP (3 h) blocked | Huang and Kandel (1996) | |||
| R(AB) + Isoproterenol | CA1 | LTP enhanced | Gelinas et al. (2008) | |
| β1, β2 | β1, β2 | CA1 | LTP impaired | Winder et al. (1999) |
β-Adrenergic Receptors and Arousal
It has been suggested that phasic activation of LC neurons that occurs in conjunction with cognitive (attentional) shifts supports dynamic reorganization of target neural networks, permitting rapid behavioral adaptation to changing environmental information. This would be reflected in rapid network plasticity, and a network reset, that serves to support the creation of new functional networks (Bouret and Sara 2005). Empirical evidence for this derives from in vivo observations that LC activation, resulting in NA release that acts on β-AR, causes a change in network oscillations in the form of a reduction of theta power that accompanies the triggering of hippocampal LTD and the enhancement of episodic-like memory in rats (Lemon et al. 2009). We postulate that this modulation of hippocampal network oscillations may reflect a means by which salient information is distinguished by the hippocampus for subsequent synaptic processing (Lemon et al. 2009). This assumption is also in line with Kety's (1970) interpretation that NA release in the brain changes signal-to-noise ratios “suppressing most, but permitting or even accentuating activity in those [synapses] that are transmitting novel or significant stimuli.” We furthermore postulate that it is the graded release of NA acting on β-AR, based on the degree of arousal that determines the direction of change of synaptic strength in the hippocampus and the relative sensitivity of synaptic plasticity to β-AR in the hippocampal subfields.
This assumption is based on observations that the sensitivity of the hippocampal subfields to regulation of persistent forms of synaptic plasticity by β-AR in vivo is in the order of DG > CA3 > CA1 (Kemp and Manahan-Vaughan 2008a; Lemon et al. 2009; Hagena and Manahan-Vaughan 2012; Goh and Manahan-Vaughan 2013b; Hansen and Manahan-Vaughan 2015b) (Table 2). Thus, for example, LC stimulation that releases NA onto β-AR, triggers LTD in the DG that endures for longer periods than LTD triggered under the same conditions in the CA1 region (Lemon et al. 2009; Hansen and Manahan-Vaughan 2015b). This observation, coupled with the fact that the LC innervates the DG strongly, suggests that moderate arousal states that provoke NA release in the hippocampus may influence DG information processing but not CA3 or CA1 information processing. This is particularly interesting in the context of learning-facilitated LTD. In the dentate gyrus, large (distal) landmark cues facilitate LTD, whereas this type of novel spatial experience does not promote LTD in the CA1 region (Kemp and Manahan-Vaughan 2008b). In contrast, small proximally located spatial features promote LTD in the CA1 region but have no impact on LTD in the DG (Kemp and Manahan-Vaughan 2008b). Intuitively, one would expect that when one first explores a novel environment, it is the distal, navigational, and orientational information that will be learned first. More subtle aspects of the environment, such as subtle local details, will be learned subsequently and will depend on the degree of attention paid to the environment. This may be mediated by a differentiated encoding by LTD in the DG, CA1 (and CA3 region: [Hagena and Manahan-Vaughan 2012]). NA release acting on β-AR in hippocampal subfields may drive this differentiation in encoding by LTD, based on a graded relative sensitivity to NA that arises from differences in receptor distribution and LC innervation. The locus coeruleus is ideally placed to support this kind of graded encoding (Sara and Bouret 2012), based on evidence that the duration and intensity of NA release from the LC is determined by the saliency of the novel experience (Aston-Jones and Bloom 1981; Grant et al. 1988; Sara et al. 1994; Hervé-Minvielle and Sara 1995; Vankov et al. 1995).
Table 2.
Effects of β-AR antagonists and agonists on synaptic plasticity in vivo
Conclusions: β-Adrenergic Receptors Determine the Content and Persistency of Synaptic Information Storage
Based on studies that describe the role of β-AR in hippocampal synaptic plasticity, it is becoming clear that activation of β-AR promotes long-term modifications of synaptic efficacy in hippocampal subfields, and that these changes may underlie the impact of this receptor on learning and memory processes.
We propose three levels of β-AR-mediated control of synaptic information storage following NA release from the LC:
During strong tonic release of NA from the LC (Aston-Jones and Bloom 1981), excitability thresholds in the hippocampus become elevated (Lacaille and Harley 1985; Stanton and Sarvey 1985; Harley 1991), this predisposes the hippocampus toward the expression of synaptic plasticity, and under these circumstances, information encoding by means of LTP is particularly promoted by NA acting on β-AR (Hansen and Manahan-Vaughan 2015a; Lethbridge et al. 2014). This condition could be anticipated to occur during periods of strong wakefulness and sustained vigilance (Bouret and Sara 2004).
During tonic LC firing, that is triggered by discrete episodes of novel experience (Grant et al. 1988; Sara et al. 1994; Hervé-Minvielle and Sara 1995; Vankov et al. 1995), NA acting on β-AR selectively facilitates synaptic information encoding by means of LTD (Lemon et al. 2009; Lemon and Manahan-Vaughan 2012; Hansen and Manahan-Vaughan 2015b), to the disadvantage of encoding via LTP (Hansen and Manahan-Vaughan 2015a). We believe that this comprises a cellular mechanism through which attention to and retention of the precise details of a novel experience are encoded.
NA released from the LC can continue to modulate neural activity for hours after the tonic activity has ceased (Walling and Harley 2004). Recently, it has been proposed that local “hotspots” of NA release in discrete synaptic networks can occur (Mather et al. 2015). We propose that this may comprise a mechanism whereby NA acting on β-AR can serve to support consolidation (Gibbs and Summers 2002) of a recently encoded experience.
In summary, we postulate that β-AR comprise a key molecular mechanism for the determination of the nature, content, and persistency of synaptic information content. This synaptic “choreography” is enabled by the state-dependent patterns of NA release that are mediated by the LC, that act in turn on hippocampal β-AR.
Funding
This work is supported by a grant from the German research foundation (Deutsche Forschungsgemeinschaft, www.dfg.de) to D.M.-V. (Ma1843/6-2). Funding to pay the Open Access publication charges for this article was provided by the German Research Foundation.
Notes
Conflict of Interest: None declared.
References
- Abraham WC, Tate WP. 1997. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 52:303–323. [DOI] [PubMed] [Google Scholar]
- Abramson SN, Martin MW, Hughes AR, Harden TK, Neve KA, Barrett DA, Molinoff PB. 1988. Interaction of beta-adrenergic receptors with the inhibitory guanine nucleotide-binding protein of adenylate cyclase in membranes prepared from cyc-S49 lymphoma cells. Biochem Pharmacol. 37:4289–4297. [DOI] [PubMed] [Google Scholar]
- Ahlquist RP. 1948. A study of the adrenotropic receptors. Am J Physiol. 153:586–600. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Frey JU. 2005. Plasticity-specific phosphorylation of CaMKII, MAP-kinases and CREB during late-LTP in rat hippocampal slices in vitro. Neuropharmacology. 49:477–492. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Frey JU, Korz V. 2006. Long-term effects of brief acute stress on cellular signaling and hippocampal LTP. J Neurosci. 26:3951–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaguer-Melian W, Rojas-Reyes Y, Alvare A, Rosillo JC, Frey JU, Bergado JA. 2005. Long-term potentiation in the dentate gyrus in freely moving rats is reinforced by intraventricular application of norepinephrine, but not oxotremorine. Neurobiol Learn Mem. 83:72–78. [DOI] [PubMed] [Google Scholar]
- André MA, Wolf OT, Manahan-Vaughan D. 2015. Beta-adrenergic receptors support attention to extinction learning that occurs in the absence, but not the presence, of a context change. Front Behav Neurosci. 9:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. 1981. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1:887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. 2005. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 28:403–450. [DOI] [PubMed] [Google Scholar]
- Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. 2003. Beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci USA. 100:940–945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bakker A, Kirwan CB, Miller M, Stark CEL. 2008. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 319:1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. 1993. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 363:347–350. [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. 1994. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 4:389–399. [DOI] [PubMed] [Google Scholar]
- Berridge CW. 2008. Noradrenergic modulation of arousal. Brain Res Rev. 58:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikbaev A, Neyman S, Ngomba RT, Conn PJ, Conn J, Nicoletti F, Manahan-Vaughan D. 2008. MGluR5 mediates the interaction between late-LTP, network activity, and learning. PLoS ONE. 3:e2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 361:31–39. [DOI] [PubMed] [Google Scholar]
- Booze RM, Crisostomo EA, Davis JN. 1993. Beta-adrenergic receptors in the hippocampal and retrohippocampal regions of rats and guinea pigs: autoradiographic and immunohistochemical studies. Synapse. 13:206–214. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. 2005. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28:574–582. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. 2004. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur J Neurosci. 20:791–802. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Bacher-Svendsen K, Sarvey JM. 1997. LTP in the lateral perforant path is beta-adrenergic receptor-dependent. Neuroreport. 8:719–724. [DOI] [PubMed] [Google Scholar]
- Braunewell K-H, Manahan-Vaughan D. 2001. Long-term depression: a cellular basis for learning? Rev Neurosci. 12:121–140. [DOI] [PubMed] [Google Scholar]
- Bronzino JD, Kehoe P, Mallinson K, Fortin DA. 2001. Increased extracellular release of hippocampal NE is associated with tetanization of the medial perforant pathway in the freely moving adult male rat. Hippocampus. 11:423–429. [DOI] [PubMed] [Google Scholar]
- Brown GP, Blitzer RD, Connor JH, Wong T, Shenolikar S, Iyengar R, Landau EM. 2000. Long-term potentiation induced by theta frequency stimulation is regulated by a protein phosphatase-1-operated gate. J Neurosci. 20:7880–7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. 1994. Beta-adrenergic activation and memory for emotional events. Nature. 371:702–704. [DOI] [PubMed] [Google Scholar]
- Chaulk PC, Harley CW. 1998. Intracerebroventricular norepinephrine potentiation of the perforant path-evoked potential in dentate gyrus of anesthetized and awake rats: a role for both alpha- and beta-adrenoceptor activation. Brain Res. 787:59–70. [DOI] [PubMed] [Google Scholar]
- Chen F-J, Sara SJ. 2007. Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neuroscience. 144:472–481. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. 2000. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 408:936–943. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Coussens CM, Raymond CR, Abraham WC. 1999. Long-lasting increase in cellular excitability associated with the priming of LTP induction in rat hippocampus. J Neurophysiol. 82:3139–3148. [DOI] [PubMed] [Google Scholar]
- Connor SA, Wang YT, Nguyen P. 2011. Activation of {beta}-adrenergic receptors facilitates heterosynaptic translation-dependent long-term potentiation. J Physiol. 589:4321–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron. 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 3:201–215. [DOI] [PubMed] [Google Scholar]
- Cox DJ, Racca C, LeBeau FEN. 2008. Beta-adrenergic receptors are differentially expressed in distinct interneuron subtypes in the rat hippocampus. J Comp Neurol. 509:551–565. [DOI] [PubMed] [Google Scholar]
- Crow TJ. 1968. Cortical synapses and reinforcement: a hypothesis. Nature. 219:736–737. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. 1997. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 390:88–91. [DOI] [PubMed] [Google Scholar]
- Dahl D, Sarvey JM. 1990. Beta-adrenergic agonist-induced long-lasting synaptic modifications in hippocampal dentate gyrus require activation of NMDA receptors, but not electrical activation of afferents. Brain Res. 526:347–350. [DOI] [PubMed] [Google Scholar]
- Dahl D, Sarvey JM. 1989. Norepinephrine induces pathway-specific long-lasting potentiation and depression in the hippocampal dentate gyrus. Proc Natl Acad Sci USA. 86:4776–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE. 2004. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 129:267–272. [DOI] [PubMed] [Google Scholar]
- Devauges V, Sara SJ. 1991. Memory retrieval enhancement by locus coeruleus stimulation: evidence for mediation by beta-receptors. Behav Brain Res. 43:93–97. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Page ME, Waterhouse BD. 2006. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J Neurosci. 26:9860–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Ketz N, Inati SJ, Davachi L. 2012. Evidence for area CA1 as a match/mismatch detector: a high-resolution fMRI study of the human hippocampus. Hippocampus. 22:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Roberson NL, Worth T. 1982. Modulation of long-term potentiation: effects of adrenergic and neuroleptic drugs. Pharmacol Biochem Behav. 17:1257–1264. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Taylor M, Heginbotham LR, Proctor WR. 1992. Long-term increases in excitability in the CA1 region of rat hippocampus induced by beta-adrenergic stimulation: possible mediation by cAMP. J Neurosci. 12:506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison HT, Harley CW. 2012. Medial and lateral perforant path evoked potentials are selectively modulated by pairing with glutamatergic activation of locus coeruleus in the dentate gyrus of the anesthetized rat. Hippocampus. 22:501–509. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi S-H, Wilson C, Nuriya M, Huganir RL, Malinow R. 2003. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 6:136–143. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. 2000. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 1:41–50. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Loughlin SE. 1982. Monoamine innervation of the forebrain: collateralization. Brain Res Bull. 9:295–307. [DOI] [PubMed] [Google Scholar]
- Fisher R, Johnston D. 1990. Differential modulation of single voltage-gated calcium channels by cholinergic and adrenergic agonists in adult hippocampal neurons. J Neurophysiol. 64:1291–1302. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen P. 2007. ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem. 282:27527–27535. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen P. 2005. Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci. 25:3294–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Tenorio G, Lemon N, Abel T, Nguyen P. 2008. Beta-adrenergic receptor activation during distinct patterns of stimulation critically modulates the PKA-dependence of LTP in the mouse hippocampus. Learn Mem. 15:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME, Summers RJ. 2002. Role of adrenoceptor subtypes in memory consolidation. Prog Neurobiol. 67:345–391. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Blitzer RD, Wong T, Asoma K, Tsokas P, Morrison JH, Iyengar R, Landau EM. 2001. Mitogen-activated protein kinase regulates early phosphorylation and delayed expression of Ca2+/calmodulin-dependent protein kinase II in long-term potentiation. J Neurosci. 21:7053–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JJ, Manahan-Vaughan D. 2013b. Hippocampal long-term depression in freely behaving mice requires the activation of beta-adrenergic receptors. Hippocampus. 23:1299–1308. [DOI] [PubMed] [Google Scholar]
- Goh JJ, Manahan-Vaughan D. 2012. Spatial object recognition enables endogenous LTD that curtails LTP in the mouse hippocampus. Cereb Cortex. 23:1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JJ, Manahan-Vaughan D. 2013a. Synaptic depression in the CA1 region of freely behaving mice is highly dependent on afferent stimulation parameters. Front Integr Neurosci. 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SJ, Aston-Jones G, Redmond DE. 1988. Responses of primate locus coeruleus neurons to simple and complex sensory stimuli. Brain Res Bull. 21:401–410. [DOI] [PubMed] [Google Scholar]
- Gray R, Johnston D. 1987. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 327:620–622. [DOI] [PubMed] [Google Scholar]
- Guo N-N, Li B-M. 2007. Cellular and subcellular distributions of beta1- and beta2-adrenoceptors in the CA1 and CA3 regions of the rat hippocampus. Neuroscience. 146:298–305. [DOI] [PubMed] [Google Scholar]
- Haas HL, Konnerth A. 1983. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. Nature. 302:432–434. [DOI] [PubMed] [Google Scholar]
- Haas HL, Rose GM. 1987. Noradrenaline blocks potassium conductance in rat dentate granule cells in vitro. Neurosci Lett. 78:171–174. [DOI] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. 2010. Frequency facilitation at mossy fiber- CA3 synapses of freely behaving rats contributes to the induction of persistent LTD via an adenosine A1 receptor-regulated mechanism. Cereb Cortex. 20:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. 2012. Learning-facilitated long-term depression and long-term potentiation at mossy fiber-CA3 synapses requires activation of β-adrenergic receptors. Front Integr Neurosci. 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. 2011. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb Cortex. 21:2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. 2015. mGlu5 acts as a switch for opposing forms of synaptic plasticity at mossy fiber-CA3 and commissural associational-CA3 synapses. J Neurosci. 35:4999–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen N, Manahan-Vaughan D. 2015a. Hippocampal long-term potentiation that is elicited by perforant path stimulation or that occurs in conjunction with spatial learning is tightly controlled by beta-adrenoreceptors and the locus coeruleus. Hippocampus. doi:10.1002/hipo.22436. [DOI] [PMC free article] [PubMed]
- Hansen N, Manahan-Vaughan D. 2015b. Locus coeruleus stimulation facilitates long-term depression in the dentate gyrus that requires activation of β-adrenergic receptors. Cereb Cortex. 25:1889–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW. 2007. Norepinephrine and the dentate gyrus. Prog Brain Res. 163:299–318. [DOI] [PubMed] [Google Scholar]
- Harley C. 1991. Noradrenergic and locus coeruleus modulation of the perforant path-evoked potential in rat dentate gyrus supports a role for the locus coeruleus in attentional and memorial processes. Prog Brain Res. 88:307–321. [DOI] [PubMed] [Google Scholar]
- Harley C, Milway JS, Lacaille JC. 1989. Locus coeruleus potentiation of dentate gyrus responses: evidence for two systems. Brain Res Bull. 22:643–650. [DOI] [PubMed] [Google Scholar]
- Harley CW. 1987. A role for norepinephrine in arousal, emotion and learning?: limbic modulation by norepinephrine and the Kety hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 11:419–458. [DOI] [PubMed] [Google Scholar]
- Harley CW, Milway JS. 1986. Glutamate ejection in the locus coeruleus enhances the perforant path-evoked population spike in the dentate gyrus. Exp Brain Res Experimentelle Hirnforschung Expérimentation Cérébrale. 63:143–150. [DOI] [PubMed] [Google Scholar]
- Harris EW, Cotman CW. 1986. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett. 70:132–137. [DOI] [PubMed] [Google Scholar]
- Hausdorff WP, Bouvier M, O'Dowd BF, Irons GP, Caron MG, Lefkowitz RJ. 1989. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J Biol Chem. 264:12657–12665. [PubMed] [Google Scholar]
- Heginbotham LR, Dunwiddie TV. 1991. Long-term increases in the evoked population spike in the CA1 region of rat hippocampus induced by beta-adrenergic receptor activation. J Neurosci. 11:2519–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider M, Schliebs R, Rossner S, Bigl V. 1997. Basal forebrain cholinergic immunolesion by 192IgG-saporin: evidence for a presynaptic location of subpopulations of alpha 2- and beta-adrenergic as well as 5-HT2A receptors on cortical cholinergic terminals. Neurochem Res. 22:957–966. [DOI] [PubMed] [Google Scholar]
- Hervé-Minvielle A, Sara SJ. 1995. Rapid habituation of auditory responses of locus coeruleus cells in anaesthetized and awake rats. Neuroreport. 6:1363–1368. [DOI] [PubMed] [Google Scholar]
- Hopkins W, Johnston D. 1984. Frequency-dependent noradrenergic modulation of long-term potentiation in the hippocampus. Science. 226:350–352. [DOI] [PubMed] [Google Scholar]
- Hopkins WF, Johnston D. 1988. Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J Ioiol. 59:667–687. [DOI] [PubMed] [Google Scholar]
- Hsu K-S, Huang C-C, Liang Y-C, Wu H-M, Chen Y-L, Lo S-W, Ho W-C. 2002. Alterations in the balance of protein kinase and phosphatase activities and age-related impairments of synaptic transmission and long-term potentiation. Hippocampus. 12:787–802. [DOI] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang M-G, Ledoux J, Huganir RL, Malinow R. 2007. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 131:160–173. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. 1996. Modulation of both the early and the late phase of mossy fiber LTP by the activation of beta-adrenergic receptors. Neuron. 16:611–617. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. 1999. Norepinephrine promotes long-term potentiation in the adult rat hippocampus in vitro. Synapse. 31:196–202. [DOI] [PubMed] [Google Scholar]
- Jelinger KA. 2009. Functional pathology of consciousness. Neuropsychiatr. 23:115–133. [PubMed] [Google Scholar]
- Jhaveri DJ, Mackay EW, Hamlin AS, Marathe SV, Nandem LS, Vaidya VA, Bartlett PF. 2010. Norepinephrine directly activates adult hippocampal precursors via beta3-adrenergic receptors. J Neurosci. 30:2795–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Zhang X, Li B. 2003a. Beta-adrenergic modulation of in vivo long-term potentiation in area CA1 and its role in spatial learning in rats. Sci China C Life Sci. 46:605–614. [DOI] [PubMed] [Google Scholar]
- Ji JZ, Zhang XH, Li B-M. 2003b. Deficient spatial memory induced by blockade of beta-adrenoceptors in the hippocampal CA1 region. Behav Neurosci. 117:1378–1384. [DOI] [PubMed] [Google Scholar]
- Joiner M-LA, Lisé M-F, Yuen EY, Kam AYF, Zhang M, Hall DD, Malik ZA, Qian H, Chen Y, Ulrich JD et al. 2010. Assembly of a beta2-adrenergic receptor—GluR1 signalling complex for localized cAMP signalling. EMBO J. 29:482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, Halaris AE, McIlhany M, Moore RY. 1977. Ascending projections of the locus coeruleus in the rat. I. Axonal transport in central noradrenaline neurons. Brain Res. 127:1–21. [DOI] [PubMed] [Google Scholar]
- Jones BE, Moore RY. 1977. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 127:25–53. [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. 2001. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 4:289–296. [DOI] [PubMed] [Google Scholar]
- Jurgens CWD, Rau KE, Knudson CA, King JD, Carr PA, Porter JE, Doze VA. 2005. Beta1 adrenergic receptor-mediated enhancement of hippocampal CA3 network activity. J Pharmacol Exp Ther. 314:552–560. [DOI] [PubMed] [Google Scholar]
- Kandel ER. 2012. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H, Izumi Y, Zorumski CF. 1997. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol. 77:3013–3020. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. 2008a. Beta-adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb Cortex. 18:1326–1334. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. 2008b. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 18:968–977. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. 2004. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci USA. 101:8192–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. 2007. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 30:111–118. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. 2011. Passive spatial perception facilitates the expression of persistent hippocampal long-term depression. Cereb Cortex. 22:1614–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. 2007. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 14:771–781. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. 2004. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 15:333–351. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Warthen DK. 2010. Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus. 20:550–557. [DOI] [PubMed] [Google Scholar]
- Kety SS. 1970. Genetic: environmental interactions in schizophrenia. Trans Stud Coll Physicians Phila. 38:124–134. [PubMed] [Google Scholar]
- Kety SS. 1972. The possible role of the adrenergic systems of the cortex in learning. Res Publ Assoc Res Nerv Ment Dis. 50:376–389. [PubMed] [Google Scholar]
- Kitchigina V, Vankov A, Harley C, Sara SJ. 1997. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 9:41–47. [DOI] [PubMed] [Google Scholar]
- Klausnitzer J, Manahan-Vaughan D. 2008. Frequency facilitation at mossy fiber- CA3 synapses of freely behaving rats is regulated by adenosine A1 receptors. J Neurosci. 28:4836–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klukowski G, Harley CW. 1994. Locus coeruleus activation induces perforant path-evoked population spike potentiation in the dentate gyrus of awake rat. Exp Brain Res Experimentelle Hirnforschung Expérimentation Cérébrale. 102:165–170. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ohno M, Shibata S, Yamamoto T, Watanabe S. 1997. Concurrent blockade of beta-adrenergic and muscarinic receptors suppresses synergistically long-term potentiation of population spikes in the rat hippocampal CA1 region. Brain Res. 777:242–246. [DOI] [PubMed] [Google Scholar]
- Korz V, Frey JU. 2005. Bidirectional modulation of hippocampal long-term potentiation under stress and no-stress conditions in basolateral amygdala-lesioned and intact rats. J Neurosci. 25:7393–7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korz V, Frey JU. 2003. Stress-related modulation of hippocampal long-term potentiation in rats: Involvement of adrenal steroid receptors. J Neurosci. 23:7281–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. 2007. Match mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci. 27:8517–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC, Harley CW. 1985. The action of norepinephrine in the dentate gyrus: beta-mediated facilitation of evoked potentials in vitro. Brain Res. 358:210–220. [DOI] [PubMed] [Google Scholar]
- Lands AM, Arnold A, McAuliff JP, Luduena FP, Brown TG. 1967. Differentiation of receptor systems activated by sympathomimetic amines. Nature. 214:597–598. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. 2000. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 405:955–959. [DOI] [PubMed] [Google Scholar]
- Lee I, Hunsaker MR, Kesner RP. 2005. The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci. 119:145–153. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Pierce KL, Luttrell LM. 2002. Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol Pharmacol. 62:971–974. [DOI] [PubMed] [Google Scholar]
- Lemon N, Aydin-Abidin S, Funke K, Manahan-Vaughan D. 2009. Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on beta-adrenergic receptor activation. Cereb Cortex. 19:2827–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. 2012. Dopamine D1/D5 receptors contribute to de novo hippocampal LTD mediated by novel spatial exploration or locus coeruleus activity. Cereb Cortex. 22:2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. 1998. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 273:19518–19524. [DOI] [PubMed] [Google Scholar]
- Lethbridge RL, Walling SG, Harley CW. 2014. Modulation of the perforant path-evoked potential in dentate gyrus as a function of intrahippocampal β-adrenoceptor agonist concentration in urethane-anesthetized rat. Brain Behav. 4:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser M-B, Moser EI. 2007. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 315:961–966. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. 2007. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 14:745–757. [DOI] [PubMed] [Google Scholar]
- Li S, Jin M, Zhang D, Yang T, Koeglsperger T, Fu H, Selkoe DJ. 2013. Environmental novelty activates β2-adrenergic signaling to prevent the impairment of hippocampal LTP by Aβ oligomers. Neuron. 77:929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. 1986. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 368:125–133. [DOI] [PubMed] [Google Scholar]
- Lin Y-W, Min M-Y, Chiu T-H, Yang H-W. 2003. Enhancement of associative long-term potentiation by activation of beta-adrenergic receptors at CA1 synapses in rat hippocampal slices. J Neurosci. 23:4173–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. 2001. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 11:551–568. [DOI] [PubMed] [Google Scholar]
- Liu J-F, Yang C, Deng J-H, Yan W, Wang H-M, Luo Y-X, Shi H-S, Meng S-Q, Chai B-S, Fang Q et al. 2015. Role of hippocampal β-adrenergic and glucocorticoid receptors in the novelty-induced enhancement of fear extinction. J Neurosci. 35:8308–8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy R, Koziell DA, Lindsey JD, Moore RY. 1980. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol. 189:699–710. [DOI] [PubMed] [Google Scholar]
- Maguire ME, Ross EM, Gilman AG. 1977. Beta-adrenergic receptor: ligand binding properties and the interaction with adenylyl cyclase. Adv Cyclic Nucleotide Res. 8:1–83. [PubMed] [Google Scholar]
- Maity S, Rah S, Sonenberg N, Gkogkas CG, Nguyen P. 2015. Norepinephrine triggers metaplasticity of LTP by increasing translation of specific mRNAs. Learn Mem. 22:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. 2004. LTP and LTD: an embarrassment of riches. Neuron. 44:5–21. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN. 1989. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 340:554–557. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell K-H. 1999. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci USA. 96:8739–8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo A, Totah NK, Neves RM, Logothetis NK, Eschenko O. 2014. Unilateral electrical stimulation of rat locus coeruleus elicits bilateral response of norepinephrine neurons and sustained activation of medial prefrontal cortex. J Neurophysiol. 111:2570–2588. [DOI] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley CW. 2015. Norepinephrine ignites local hot spots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav Brain Sci. 1:1–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley S, Pierce KL, Zamah AM, Miller WE, Ahn S, Daaka Y, Lefkowitz RJ, Luttrell LM. 2000. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem. 275:9572–9580. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. 2000. Memory—a century of consolidation. Science. 287:248–251. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Stern CM, Mermelstein PG. 2011. β1-Adrenergic receptors activate two distinct signaling pathways in striatal neurons. J Neurochem. 116:984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Shah P, Pierce JP. 2000. β-Adrenergic receptors primarily are located on the dendrites of granule cells and interneurons but also are found on astrocytes and a few presynaptic profiles in the rat dentate gyrus. Synapse. 36:178–193. [DOI] [PubMed] [Google Scholar]
- Moody TD, Thomas MJ, Makhinson M, O'Dell TJ. 1998. 5-Hz stimulation of CA3 pyramidal cell axons induces a beta-adrenergic modulated potentiation at synapses on CA1, but not CA3, pyramidal cells. Brain Res. 794:75–79. [DOI] [PubMed] [Google Scholar]
- Moody TD, Watabe AM, Indersmitten T, Komiyama NH, Grant SGN, O'Dell TJ. 2011. Beta-adrenergic receptor activation rescues theta frequency stimulation-induced LTP deficits in mice expressing C-terminally truncated NMDA receptor GluN2A subunits. Learn Mem. 18:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. 1979. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 2:113–168. [DOI] [PubMed] [Google Scholar]
- Morris RW, Westbrook RF, Killcross AS. 2005. Reinstatement of extinguished fear by beta-adrenergic arousal elicited by a conditioned context. Behav Neurosci. 119:1662–1671. [DOI] [PubMed] [Google Scholar]
- Mueller AL, Hoffer BJ, Dunwiddie TV. 1981. Noradrenergic responses in rat hippocampus: evidence for medication by alpha and beta receptors in the in vitro slice. Brain Res. 214:113–126. [DOI] [PubMed] [Google Scholar]
- Muller D, Buchs PA, Stoppini L, Boddeke H. 1991. Long-term potentiation, protein kinase C, and glutamate receptors. Mol Neurobiol. 5:277–288. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Schutsky K, Jin S-H, Thomas SA. 2011. Norepinephrine and ß1-adrenergic signaling facilitate activation of hippocampal CA1 pyramidal neurons during contextual memory retrieval. Neuroscience. 181:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison CF, Zhang X-Y, Zhang W-P, Ouyang M, Lee A, Thomas SA. 2004. A distinct role for norepinephrine in memory retrieval. Cell. 117:131–143. [DOI] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. 2014. Engineering a memory with LTD and LTP. Nature. 511:348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K, Ikegaya Y, Yamada MK, Nishiyama N, Matsuki N. 2002. Hippocampal long-term depression as an index of spatial working memory. Eur J Neurosci. 16:970–974. [DOI] [PubMed] [Google Scholar]
- Nejtek V, Dahl D. 1997. Pathway specificity of l-isoproterenol indicates beta-adrenergic modulation of the Schaffer collateral pathway in field CA1 in the rat hippocampal slice. Neuroreport. 8:745–749. [DOI] [PubMed] [Google Scholar]
- Neuman RS, Harley CW. 1983. Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Res. 273:162–165. [DOI] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ. 2014. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron. 81:416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. 2005. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 6:863–876. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Connor SA, Gelinas JN, Nguyen P. 2010. Viagra for your synapses: Enhancement of hippocampal long-term potentiation by activation of beta-adrenergic receptors. Cell Signal. 22:728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell TJ, Connor SA, Guglietta R, Nguyen P. 2015. β-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn Mem. 22:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. 2006. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 281:752–758. [DOI] [PubMed] [Google Scholar]
- Pelletier MR, Kirkby RD, Jones SJ, Corcoran ME. 1994. Pathway specificity of noradrenergic plasticity in the dentate gyrus. Hippocampus. 4:181–188. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. 1999. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 19:6623–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Matt L, Zhang M, Nguyen M, Patriarchi T, Koval OM, Anderson ME, He K, Lee H-K, Hell JW. 2012. The β2 adrenergic receptor supports prolonged theta tetanus - induced LTP. J Neurophysiol. 107:2703–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J, Majczynski H, Clayton E, Aston-Jones G. 2004. Activation of monkey locus coeruleus neurons varies with difficulty and performance in a target detection task. J Neurophysiol. 92:361–371. [DOI] [PubMed] [Google Scholar]
- Reid AT, Harley CW. 2010. An associativity requirement for locus coeruleus-induced long-term potentiation in the dentate gyrus of the urethane-anesthetized rat. Exp Brain Res. 200:151–159. [DOI] [PubMed] [Google Scholar]
- Reis GF, Lee MB, Huang AS, Parfitt KD. 2005. Adenylate cyclase-mediated forms of neuronal plasticity in hippocampal area CA1 are reduced with aging. J Neurophysiol. 93:3381–3389. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT. 2009. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 32:267–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford J, Dawes P. 1993. Effect of naloxone on the habituation of novelty-induced hypoalgesia: the collateral inhibition hypothesis revisited. Pharmacol Biochem Behav. 46:117–123. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. 2008. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 90:576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Byrd K, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. 2005. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 8:1525–1533. [DOI] [PubMed] [Google Scholar]
- Sara SJ. 1985. The locus coeruleus and cognitive function: attempts to relate noradrenergic enhancement of signal/noise in the brain to behavior. Physiol Psychol. 13:151–162. [Google Scholar]
- Sara SJ. 2009. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 10:211–223. [DOI] [PubMed] [Google Scholar]
- Sara SJ. 2010. Reactivation, retrieval, replay and reconsolidation in and out of sleep: connecting the dots. Front Behav Neurosci. 4:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. 2000. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 7:73–84. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S. 2012. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 76:130–141. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Segal M. 1991. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog Brain Res. 88:571–585. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Hervé A. 1994. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 35:457–465. [DOI] [PubMed] [Google Scholar]
- Schimanski LA, Ali DW, Baker GB, Nguyen P. 2007. Impaired hippocampal LTP in inbred mouse strains can be rescued by beta-adrenergic receptor activation. Eur J Neurosci. 25:1589–1598. [DOI] [PubMed] [Google Scholar]
- Schmitt JM, Stork PJ. 2000. Beta 2-adrenergic receptor activates extracellular signal-regulated kinases (ERKs) via the small G protein rap1 and the serine/threonine kinase B-Raf. J Biol Chem. 275:25342–25350. [DOI] [PubMed] [Google Scholar]
- Schutsky K, Ouyang M, Thomas SA. 2011. Xamoterol impairs hippocampus-dependent emotional memory retrieval via Gi/o-coupled β2-adrenergic signaling. Learn Mem. 18:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds NW, Gilman AG. 1971. Norepinephrine stinulated increase of cyclic AMP levels in developing mouse brain cell cultures. Science. 174:292. [DOI] [PubMed] [Google Scholar]
- Segal M, Markram H, Richter-Levin G. 1991. Actions of norepinephrine in the rat hippocampus. Prog Brain Res. 88:323–330. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Reymann KG, Balschun D. 1997. A post-tetanic time window for the reinforcement of long-term potentiation by appetitive and aversive stimuli. Proc Natl Acad Sci USA. 94:1494–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servan-Schreiber D, Printz H, Cohen JD. 1990. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science. 249:892–895. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. 2006. Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 281:1261–1273. [DOI] [PubMed] [Google Scholar]
- Smith CC, Greene RW. 2012. CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci. 32:6072–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. 1985. Depletion of norepinephrine, but not serotonin, reduces long-term potentiation in the dentate gyrus of rat hippocampal slices. J Neurosci. 5:2169–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sejnowski TJ. 1989. Associative long-term depression in the hippocampus induced by hebbian covariance. Nature. 339:215–218. [DOI] [PubMed] [Google Scholar]
- Straube T, Frey JU. 2003. Involvement of beta-adrenergic receptors in protein synthesis-dependent late long-term potentiation (LTP) in the dentate gyrus of freely moving rats: the critical role of the LTP induction strength. Neuroscience. 119:473–479. [DOI] [PubMed] [Google Scholar]
- Straube T, Korz V, Balschun D, Frey JU. 2003a. Requirement of beta-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J Physiol. 552:953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Korz V, Frey JU. 2003b. Bidirectional modulation of long-term potentiation by novelty-exploration in rat dentate gyrus. Neurosci Lett. 344:5–8. [DOI] [PubMed] [Google Scholar]
- Strulovici B, Cerione RA, Kilpatrick BF, Caron MG, Lefkowitz RJ. 1984. Direct demonstration of impaired functionality of a purified desensitized beta-adrenergic receptor in a reconstituted system. Science. 225:837–840. [DOI] [PubMed] [Google Scholar]
- Swanson-Park J. 1999. A double dissociation within the hippocampus of dopamine D1/D5 receptor and β-adrenergic receptor contributions to the persistence of long-term potentiation. Neuroscience. 92:485–497. [DOI] [PubMed] [Google Scholar]
- Tenorio G, Connor SA, Guévremont D, Guévremont D, Abraham WC, Williams J, O'Dell TJ, Nguyen P. 2010. “Silent” priming of translation-dependent LTP by ß-adrenergic receptors involves phosphorylation and recruitment of AMPA receptors. Learn Mem. 17:627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA. 2015. Neuromodulatory signaling in hippocampus-dependent memory retrieval. Hippocampus. 25:415–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O'Dell TJ. 1996. Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron. 17:475–482. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 1971. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 367:1–48. [DOI] [PubMed] [Google Scholar]
- Vanhoose AM, Winder DG. 2003. NMDA and beta1-adrenergic receptors differentially signal phosphorylation of glutamate receptor type 1 in area CA1 of hippocampus. J Neurosci. 23:5827–5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankov A, Hervé-Minvielle A, Sara SJ. 1995. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 7:1180–1187. [DOI] [PubMed] [Google Scholar]
- Walling SG, Brown RA, Milway JS, Earle AG, Harley CW. 2011. Selective tuning of hippocampal oscillations by phasic locus coeruleus activation in awake male rats. Hippocampus. 21:1250–1262. [DOI] [PubMed] [Google Scholar]
- Walling SG, Harley CW. 2004. Locus ceruleus activation initiates delayed synaptic potentiation of perforant path input to the dentate gyrus in awake rats: a novel beta-adrenergic- and protein synthesis-dependent mammalian plasticity mechanism. J Neurosci. 24:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe AM, Zaki PA, O'Dell TJ. 2000. Coactivation of beta-adrenergic and cholinergic receptors enhances the induction of long-term potentiation and synergistically activates mitogen-activated protein kinase in the hippocampal CA1 region. J Neurosci. 20:5924–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. 2006. Learning induces long-term potentiation in the hippocampus. Science. 313:1093–1097. [DOI] [PubMed] [Google Scholar]
- Winder DG, Martin KC, Muzzio IA, Rohrer D, Chruscinski A, Kobilka B, Kandel ER. 1999. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors. Neuron. 24:715–726. [DOI] [PubMed] [Google Scholar]
- Winson J, Dahl D. 1985. Action of norepinephrine in the dentate gyrus. II. Iontophoretic studies. Exp Brain Res Experimentelle Hirnforschung Expérimentation Cérébrale. 59:497–506. [DOI] [PubMed] [Google Scholar]
- Woodward DJ, Moises HC, Waterhouse BD, Hoffer BJ, Freedman R. 1979. Modulatory actions of norepinephrine in the central nervous system. Fed Proc. 38:2109–2116. [PubMed] [Google Scholar]
- Xiao RP. 2001. Beta-adrenergic signaling in the heart: dual coupling of the beta2-adrenergic receptor to G(s) and G(i) proteins. Sci STKE. 2001:re15–re15. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Ji X, Lakatta EG. 1995. Functional coupling of the beta 2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol. 47:322–329. [PubMed] [Google Scholar]
- Yang H-W, Lin Y-W, Yen C-D, Min M-Y. 2002. Change in bi-directional plasticity at CA1 synapses in hippocampal slices taken from 6-hydroxydopamine-treated rats: the role of endogenous norepinephrine. Eur J Neurosci. 16:1117–1128. [DOI] [PubMed] [Google Scholar]
- Yavich L, Jäkälä P, Tanila H. 2005. Noradrenaline overflow in mouse dentate gyrus following locus coeruleus and natural stimulation: real-time monitoring by in vivo voltammetry. J Neurochem. 95:641–650. [DOI] [PubMed] [Google Scholar]
- Zhang M, Patriarchi T, Stein IS, Qian H, Matt L, Nguyen M, Xiang YK, Hell JW. 2013. Adenylyl cyclase anchoring by a kinase anchor protein AKAP5 (AKAP79/150) is important for postsynaptic β-adrenergic signaling. J Biol Chem. 288:17918–17931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Hoogenraad CC, Joëls M, Krugers HJ. 2012. Combined β-adrenergic and corticosteroid receptor activation regulates AMPA receptor function in hippocampal neurons. J Psychopharmacol (Oxford). 26:516–524. [DOI] [PubMed] [Google Scholar]
- Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. 2001. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA. 98:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]