Abstract
Cortical development extensively depends on sensory experience. Effects of congenital monaural and binaural deafness on cortical aural dominance and representation of binaural cues were investigated in the present study. We used an animal model that precisely mimics the clinical scenario of unilateral cochlear implantation in an individual with single-sided congenital deafness. Multiunit responses in cortical field A1 to cochlear implant stimulation were studied in normal-hearing cats, bilaterally congenitally deaf cats (CDCs), and unilaterally deaf cats (uCDCs). Binaural deafness reduced cortical responsiveness and decreased response thresholds and dynamic range. In contrast to CDCs, in uCDCs, cortical responsiveness was not reduced, but hemispheric-specific reorganization of aural dominance and binaural interactions were observed. Deafness led to a substantial drop in binaural facilitation in CDCs and uCDCs, demonstrating the inevitable role of experience for a binaural benefit. Sensitivity to interaural time differences was more reduced in uCDCs than in CDCs, particularly at the hemisphere ipsilateral to the hearing ear. Compared with binaural deafness, unilateral hearing prevented nonspecific reduction in cortical responsiveness, but extensively reorganized aural dominance and binaural responses. The deaf ear remained coupled with the cortex in uCDCs, demonstrating a significant difference to deprivation amblyopia in the visual system.
Keywords: asymmetric hearing, auditory development, binaural cochlear implants, interaural time difference, sensory deprivation
Introduction
Development of the auditory cortex extensively depends on hearing experience (Kral and Sharma 2012). In contrast to monocular deprivation in the visual system, unilateral hearing is traditionally considered sufficient to provide the cues required for auditory development. In childhood, the incidence of monaural deafness is high, estimated to be approximately 2 per 1000 children (Tharpe and Sladen 2008; Shargorodsky et al. 2010). It remains a matter of discussion how to clinically approach this condition, since unilateral hearing allows the development of spoken language. Nonetheless, more detailed studies provided evidence that unilateral deafness negatively affected the development of language and therefore requires therapy (Lieu et al. 2010; Wie et al. 2010; Sarant et al. 2014). Recent studies showed that the untreated deaf ear is put into a disadvantage in access of cortical neurons if an early sensitive period for the treatment of congenital unilateral deafness is missed (Kral, Heid, et al. 2013; Kral, Hubka, et al. 2013), thus likely affecting binaural hearing.
Binaural hearing is required for spatial localization of the sound source. It improves speech comprehension in a complex multisource environment. Binaural cues are extracted in the olivary complex and transmitted up to the auditory cortex (Grothe et al. 2010). Different cortical fields contribute differentially to localization performance (Casseday and Neff 1973; Neff and Casseday 1977; Malhotra and Lomber 2007; Lee and Middlebrooks 2013). The primary auditory cortex (field A1) is essential in this function; deactivation of or damage to field A1 leads to extensive deficits in localization (Casseday and Neff 1973; Neff and Casseday 1977; Malhotra and Lomber 2007; Lee and Middlebrooks 2013). Field A1 represents the contralateral acoustic space using a population code (Kitzes et al. 1980; Middlebrooks et al. 1994; Irvine et al. 1996; Lee and Middlebrooks 2013). The strongest excitatory drive for A1 neurons comes from the contralateral (“crossed”) ear, leading to a contralateral aural dominance (Phillips and Irvine 1983; Kral et al. 2009). Nonetheless, very few A1 neurons are purely monaural (Zhang et al. 2004; Middlebrooks 2015). The ipsilateral (“uncrossed”) ear can have excitatory or inhibitory influences (Zhang et al. 2004). Sensitivity to both interaural time and level differences is high in field A1 (Kitzes et al. 1980; Irvine et al. 1996) and periods of unilateral hearing loss are known to affect aural dominance (Moore and Irvine 1981; Irvine et al. 1996; Vale et al. 2004; Polley et al. 2013). Binaural sensitivity is believed to be less developmentally affected by asymmetry in hearing; it is more the behavioral importance of binaural (interaural time and level differences) versus monaural (spectral pinna) cues that are plastic in mammals (King et al. 2011). However, this hypothesis is based only on outcomes of moderate conductive hearing loss and may not apply for profound unilateral deafness.
While effects of monocular deprivation in the visual system were studied extensively, the effects of congenital unilateral deafness on binaural computations are unknown. Pioneering studies using cochlear ablation demonstrated that the hearing ear becomes overrepresented in the midbrain and the cortex in unilateral hearing (Kitzes and Semple 1985; Reale et al. 1987; McAlpine et al. 1997; Hsieh and Cramer 2006). Cochlear ablation, however, damaged the auditory nerve severely and by that precluded the testing of responses to the ablated ear.
Using a natural animal deafness model (Kral and Lomber 2015), here we compared responses of cortical neurons with monaural and binaural stimulation in congenital unilateral deafness. Unilaterally deaf cats were selected out of the colony of deaf white cats by a hearing-screening procedure (Heid et al. 1998). They were very rare (<1%) (Geigy et al. 2007), but mimic the clinical condition of inborn single-sided deafness (SSD). Consequently, these animals allow exceptional insights into the brain processes underlying binaural sensitivity in this extreme condition.
By probing the functional properties of the cortex using binaural cochlear implants, we demonstrate shifts in monaural responsiveness and reductions in facilitatory binaural interactions in binaural congenital deafness. Unilateral deafness caused additional effects on the primary auditory cortex. While responsiveness to the congenitally deaf ear was never completely lost in unilateral deafness, there was a further reduction in interaural time difference (ITD) sensitivity. Degradation of sensitivity for ITDs showed a hemispheric specificity in unilateral deafness.
Materials and Methods
The present study was carried out on 12 cats. In part, the methods are given in more detail elsewhere (Tillein et al. 2010) and will be only briefly recapitulated here. Five congenitally deaf cats (CDCs) were selected from a colony of deaf white cats using early hearing screening with brainstem evoked responses and stimulation above 120 dB SPL (Heid et al. 1998). The animals' hearing status was additionally confirmed at the beginning of the acute experiments, using the same procedure. Five animals had normal hearing (lowest hearing threshold <40 dB SPL, detected using brainstem evoked responses). To prevent electrophonic hearing (electrical stimulation of hair cells) in hearing controls, these animals were deafened at the beginning of the experiment by intrascalar application of neomycin (Hartmann et al. 1984). In what follows, the previously hearing adult animals with destroyed hair cells at the beginning of the acute experiment will be referred to as hearing controls (HCs). The adjective “hearing” does not only refer to the functional state of the cochlea during the experiment but also to the developmental and functional state of the central auditory system. The 2 unilaterally CDCs (uCDCs) were exceptionally rare animals: In the early hearing-screening procedure, they were diagnosed as deaf in one ear only, with excellent hearing in the other ear (lowest sensitivity <40 dB SPL, detected using brainstem evoked responses). This condition corresponds to the clinical situation of congenital SSD. Also in these animals, the hearing ear was treated with neomycin as in hearing controls.
The experiments were approved by the local state authorities and were performed in compliance with the Guidelines of the European Community for the care and use of laboratory animals (EU VD 86/609/EEC) and the German Animal Welfare Act (TierSchG).
Cochlear Implant
The cochlear implant consisted of a medical-grade silicone tube with 5 intrascalar contacts: A small golden sphere at the tip (diameter 0.8 mm) and 4 golden rings, with a distance of 1 mm between all electrodes (Behrendt 1999). The intrascalar part of the implant was tapered in the apical direction from a diameter of 1.6–0.8 mm. The extracochlear silicone tube had a diameter of 1.6 mm. The gold contacts were connected to a seven-strand Teflon-coated stainless-steel braided wire. The stimulation mode was wide bipolar (most apical vs. the fourth intracochlear electrode in the basal direction; distance between active electrodes was thus 3 mm).
Experimental Procedures
All animals were premedicated with 0.25 mg atropine i.p. and initially anesthetized with ketamine hydrochloride (24.5 mg/kg, Ketavet, Parker-Davis, Germany) and propionyl promazine phosphate (2.1 mg/kg, Combelen, Bayer, Germany). The animals were then tracheotomized and artificially ventilated with 50% O2 and 50% N2O, with the addition of 0.2–1.5% concentration of isoflurane (Lilly, Germany) to maintain a controlled depth of anesthesia (Kral et al. 1999). End-tidal CO2 was monitored and maintained below 4%, and core temperature was kept above 37.5 °C using a homeothermic blanket. The animal's status was further monitored by blood gas concentration measurements, pH, bicarbonate concentration and base excess, glycemia, and oxygen saturation determined from capillary blood. A modified Ringer's solution containing bicarbonate and plasma expander was infused i.v. with additional bicarbonate depending on the acid–base status. Monitoring and correction of the acid–base balance was performed every 12 h.
The animal's head was fixed in a stereotactic holder (Horsley-Clarke). Both bullae and ear canals were exposed. To record evoked auditory brainstem responses, a small trephination was drilled at the vertex and a silver-ball electrode (diameter 1 mm) was attached epidurally. The indifferent electrode used for the recordings was inserted medially into the neck muscles.
Hearing status was verified using brainstem evoked responses with condensation clicks applied through a modified DT 48 speaker (Bayer Dynamics, Germany). The speaker membrane was positioned approximately 1 cm from the tympanic membrane within a custom-made acoustically calibrated sound-delivery device (closed system) inserted into the remaining part of the external auditory meatus after the pinna was removed. Brainstem evoked signals were recorded using an epidural vertex electrode against a reference at the midline of the neck, were preamplified (60 dB, Otoconsult V2 low-impedance amplifier), amplified at a second stage (40 dB, Otoconsult Amplifier-Filter F1, filters 0.010–10 kHz), and recorded using National Instruments MIO cards. The signals were averaged (200 sweeps, repetition rate 33 Hz, Audiology Laboratory, Otoconsult, Frankfurt am Main, Germany). The absence of acoustically evoked brainstem responses (including wave I, generated within the auditory nerve) to clicks above 120 dB SPL verified complete deafness. In HCs, the thresholds were <40 dB SPL before the animals were deafened by slow instillation of 300 µL of neomycin sulfate into the scala tympani (within 5 min). The neomycin was left in place for a further 5 min and subsequently washed out by slow instillation of Ringer's solution. Total absence of brainstem evoked responses verified that the deafening procedure was successful. The same procedure was used to remove hair cells in the hearing ear of uCDCs.
For electrical stimulation, the animals were bilaterally implanted with a cochlear implant (CI) electrode array in each cochlea (Fig. 1) inserted via the round window. To ensure insertion depth was comparable between the 2 ears, the electrode array was inserted until the most basal electrode of the implant was just observed behind the rim of the round window (insertion depth ∼6 mm). The bulla was sealed with bone wax. Charge-balanced pulses (200 µs/phase, repetition rate 2 Hz) were applied to the CI (wide bipolar stimulation: most apical vs. fourth electrode, stimulation interelectrode distance ∼3 mm). Stimulation was performed with optically isolated current sources (CS1, Otoconsult, Germany). To verify that the position of the cochlear implants was comparable, electrically evoked auditory brainstem response (EABR) with single biphasic pulses was recorded and lowest current levels evoking a brainstem response (EABR threshold currents) were determined.
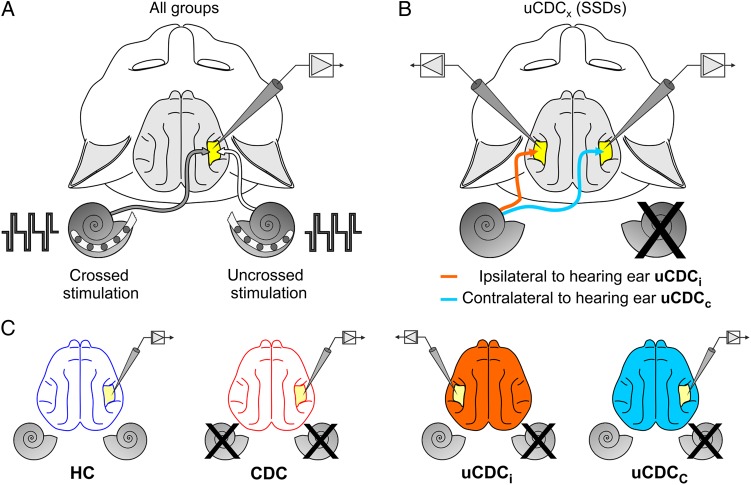
Figure 1.
(A) Schematic illustration of stimulation with bilateral cochlear implants. Stimulus: 3 biphasic charge-balanced pulses delivered to each ear at 6 dB above eABR threshold, 200 µs/phase, 500 Hz repetition rate. Recording in the primary auditory cortex, field A1 (yellow area). The terms “crossed” and “uncrossed” indicate the side of stimulated cochlea with respect to the recorded cortical hemisphere: crossed = stimulated cochlea contralateral to the recording side (dark arrow), uncrossed = stimulated cochlea and recorded cortex at the same side (light arrow). (B) Scheme illustrating the recording conditions in animals with unilateral congenital deafness (uCDCx), corresponding to the condition of SSD. Responses collected from the hemisphere of the same side as the hearing ear (ipsilateral) are designated uCDCi (red arrow); those contralateral to the hearing ear are designated uCDCc (blue arrow). (C) Animals investigated in the present study: HC: acutely deafened hearing controls; CDC: bilaterally congenitally deaf cats; uCDCi, uCDCc: unilaterally congenitally deaf cats recorded ipsilateral or contralateral to the hearing ear.
Trephination was performed above the auditory cortex and the dura was removed. The cortex was photographed to document the recording positions. Using an x-y-z micromotor (1 µm precision in all directions), a silver-ball macroelectrode (diameter 1 mm) was positioned at 9 cortical positions on the primary auditory cortex (field A1). The dorsal end of the posterior ectosylvian sulcus was used as a reference point. Signals (local field potentials, LFPs) recorded in response to an electric biphasic pulse applied through a cochlear implant were preamplified (60 dB, Otoconsult V2 low-impedance amplifier), amplified at a second stage (20 dB, Otoconsult Amplifier-Filter F1, filters 0.010–10 kHz), recorded using National Instruments MIO cards, and averaged (100 sweeps, repetition rate 1.97 Hz). The signals were stored and threshold current levels were evaluated at all recording positions with a precision of ±1 dB.
To determine the extent of the cortical activated region, a Ringer-filled glass microelectrode (impedance <6 MΩ) was used. LFPs on the cortical surface were recorded at 75–150 cortical positions during stimulation with the cochlear implant, using single biphasic pulses (200 µs/phase, wide bipolar stimulation at both the ipsilateral and contralateral ear, stimulation current 10 dB above the lowest cortical threshold determined with the macroelectrode). Fifty responses were averaged to obtain evoked LFPs. Amplitudes of these middle-latency responses (peak to baseline) were used to construct cortical activation maps (Kral et al. 2009) using custom-made software programmed in MATLAB© (MathWorks, Inc.). Convergence of bilateral activity in the same locations of the primary auditory cortex was verified in all investigated animals (cf. Kral et al. 2009).
After the functional activation map in the cortex was determined, the cortical tissue was penetrated at the cortical spots with the largest LFPs (“hot spots”) using a single-shank multielectrode array (NeuroNexus, USA, single shank, 16 contacts, spacing 150 µm, 177 µm2 contact area, electrode array length 2.4 mm, impedance ∼1–2 MΩ) such that the last electrode was just at the level of the cortical surface. The movement of the cortex was stabilized using a modified Davies chamber filled with agar and sealed with melted bone wax.
The signals were amplified by a 64-channel Cheetah amplifier (Neuralynx, Tucson, AZ, USA; 5000× amplified, filters 1 Hz–9 kHz), fed to the input of a National Instruments MIO card, and stored on a computer using custom-made MATLAB routines. The signals were offline high-pass filtered (elliptic IIR filter, second-order, edge frequency of 400 Hz), and stimulus artifacts were blanked. Zero-phase digital filtering was performed to avoid induced latency shifts. Unit activity was detected by an automatic thresholding procedure. Thresholds were computed according to the formula:
| (1) |
where x is the high-pass filtered recorded signal, abs(x) denotes the absolute value of x and the constants are taken from a previous study on automated spike detection (Quiroga et al. 2004). Resulting multiunit spike trains were further analyzed.
Binaural Stimulation
Binaural stimulation was performed using a train of 3 biphasic charge-balanced pulses (200 µs/phase) at a repetition rate of 500 pps. Thus, the total stimulation duration was <5 ms in all binaural conditions.
Responses to stimulation of each ear were recorded separately with increasing intensity (1–2 dB precision, interstimulus interval of 1537 ms). Afterwards, with reference to the individual EABR thresholds, intensities at both ears co-varied from at least 1 dB below threshold to at least 9 dB above threshold. EABR threshold was used as a reference so as to compensate for the possible differences in exact location of the cochlear implants within the scala tympani. ITDs were pseudorandomly varied across a range from 0 to 600 µs (0, 100, 200, 300, 400, 500, and 600 µs), with stimuli leading at either the ipsilateral or contralateral ear. ITD sensitivity was tested for different binaural levels. Each stimulus condition was repeated 30 times, so that robust response properties could be determined.
Throughout the experiment, stimulation was verified by visual inspection of current monitor signals for both current sources (at the contralateral and ipsilateral ears) using an oscilloscope. At the end of the experiment, current monitor signals were additionally recorded on the computer.
Data Processing
The data processing involved has been described in detail before (Tillein et al. 2010). Units were considered to be responding to the stimuli if the spike pattern in analyzed poststimulus time windows could not be explained by a Poisson process using the spontaneous rate observed in the prestimulus time window (Chase and Young 2007). Probabilities were computed for all spikes in the analyzed time window. The unit was deemed to be responding to the stimulus if the probability that the spike train in the analyzed window was produced by a spontaneous Poisson process was <0.001 [for details, see Supplementary Material in Tillein et al. (2010)].
Dynamic range (DR) of the units was assessed by an automatic fitting procedure using a sigmoidal function template in the following equation:
| (2) |
where L represents data from the rate-level function, with DRcenter and DRwidth corresponding to the level at the half width of DR. A and B represent scaling and shifting parameters of sigmoid function along the level axis, respectively. Threshold and saturation point of the rate-level functions were then defined by subtraction (DRcenter − DRwidth) and summation (DRcenter + DRwidth) of the sigmoid function parameters. DR is then determined directly from the DRwidth parameter (DR = 2 × DRwidth) that corresponds to the difference between the level at the saturation point and at the threshold of a rate-level function.
Aural dominance index (ADI) was computed in order to survey general distribution of the proportion of crossed and uncrossed responses according to following formula:
| (3) |
FRcontra and FRipsi represent the summed firing rates from the DR of individual multiunit activity (MUA) evoked by crossed and uncrossed monoaural stimulation, respectively. ADI ranges from −1 to 1 corresponding to purely uncrossed to purely crossed preference, which corresponds to classes from 7 to 1 in ocular dominance histograms used in visual studies (Hubel and Wiesel 1963). Balanced crossed and uncrossed responses give rise to zero ADI.
Binaural Interactions
Binaural interactions were assessed using the scheme proposed by Zhang et al. (2004): first, the “driving influence” of each ear (crossed and uncrossed, Fig. 1) was determined (Fig. 2A). In cases when both the crossed and the uncrossed ears were able to evoke the cortical response, the unit was classified as excitatory–excitatory (EE). In cases when the uncrossed ear did not drive the units at the recorded position, but the crossed ear did, the units were classified as E0. Conversely, 0E denoted units that responded to stimulation of the uncrossed ear alone. Those cells that did not respond when individual ears were stimulated, but responded to stimulation of both ears, were classified as preferentially binaural (PB).
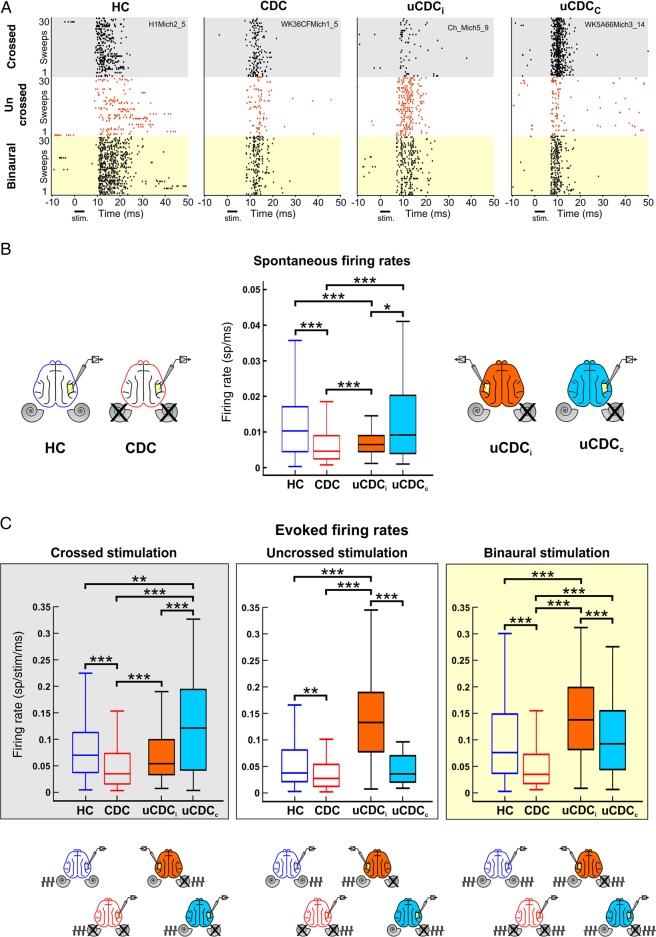
Figure 2.
Spontaneous and evoked firing rate analysis. (A) Raster plots of cortical responses to 30 repetitions of crossed (gray boxes), uncrossed (white boxes), and bilateral stimulation (light yellow boxes). Each dot represents an action potential. Responses were stronger for crossed than uncrossed stimulation in all conditions except uCDCi (unilateral animals, hemisphere ipsilateral to the hearing ear) where a stronger uncrossed response was observed. (B) Spontaneous firing rate computed from all recording sites in binaurally normal-hearing and deaf animals (empty boxes) and unilateral deaf animals (filled boxes). The boxes cover the second and third quartiles of the distribution; the intersection line inside the box shows the median and the whiskers the range of the data. The drawings adjacent to the box plots illustrate the relation between recording hemisphere and the cochlea in deaf animals (crossed). Spontaneous firing rate was reduced in binaurally deaf cats and in the ipsilateral cortex of unilateral animals (uCDCi). No difference between hearing control and contralateral cortex of unilateral animal was observed (uCDCc). (C) Box plots of evoked firing rates for all groups and stimulus conditions. The stimulus, recording sides, and groups are depicted below the box plots. Firing rates were analyzed within a response window of 50 ms and calculated for 1 ms. Evoked firing rates for all stimulus conditions were lowest in CDCs, while highest rates were found in the contralateral cortex of the unilateral animal (uCDCc) with crossed stimulation. In the ipsilateral cortex of the unilateral animal (uCDCi), both uncrossed and binaural stimulation evoked the highest firing rates of all groups. Two-tailed Wilcoxon–Mann–Whitney test, *P < 0.05; **P < 0.01; ***P < 0.001.
Units were additionally classified according to the relation between binaural and both monaural responses. When binaural stimulation resulted in a firing rate of <80% of the preferred responses (a larger response in unilateral stimulation), the unit was classified as suppressive. If the response was between 80% of the preferred responses and 80% of the summed crossed and uncrossed responses, the interaction was classified as occlusion. Interactions between 80% and 120% of the sum of monaural responses were considered neutral, and those exceeding 120% of the summed monaural response were deemed to be facilitation. To guarantee the robustness of the findings, the summed firing rates from the DR of individual MUA responses (i.e., intensities between a threshold and an initial point of saturation; Fig. 3, top) were used.
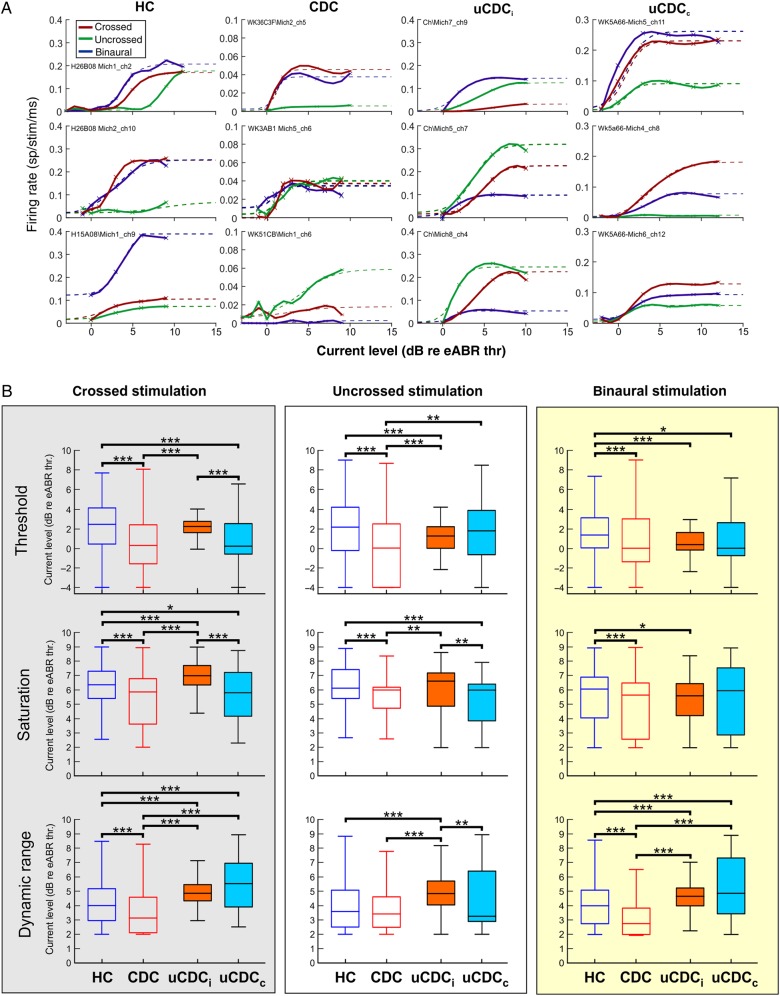
Figure 3.
Basic unit response properties. (A) Rate-level functions for all groups and stimulus conditions (red = crossed, green = uncrossed, and blue = binaural stimulation). Broken lines indicate the sigmoid function fitted to the response curve. For each group, 3 examples of rate-level functions are shown to demonstrate the variability of responses. (B) Quantitative analysis of threshold (first row), saturation (second row), and DR (lower row) calculated from fitted rate-level functions for all groups and stimulus conditions (same scheme as in Fig. 2C). CDCs show the lowest stimulation thresholds and smallest DRs among all groups and for all stimulus conditions (two-tailed Wilcoxon–Mann–Whitney test *P < 0.05, **P < 0.01, ***P < 0.001).
ITD Processing
The ITD data-processing procedure has been described in detail elsewhere (Tillein et al. 2010). For each ITD, responsiveness was determined separately as described above from 30 stimulus repetitions. For an ITD responsiveness, however, a significant response to one ITD was not considered enough: It had to occur for at least 3 “successive” ITDs (Tillein et al. 2010). From such ITD responsive sites, ITD functions from the first response (poststimulus time 0–15 ms) were automatically classified at intensities of 3, 6, 9, and 12 dB above EABR threshold using the custom-made software in MATLAB. Templates for classification were similar to those in previous studies (Smith and Delgutte 2007; Tillein et al. 2010) and resulted in classification into peak, trough, sigmoid, and biphasic shapes. Specifically, peak and trough ITD functions were fitted by a Gaussian function. Monotonic ITDs were similarly fitted using a sigmoid function. ITD responses with maxima and minima were modeled by a biphasic function (i.e., the difference between 2 Gaussian functions). Responsive ITD functions with nonsignificant fit all templates [determined by the correlation between data and fitted function; significance level for 18 degrees of freedom at r < 0.56, ≤1%; details in Tillein et al. (2010)] were considered nonclassified ITD responses. If the criterion for ITD responsiveness, as defined above, was not achieved, the unit was deemed ITD nonresponsive.
ITDBest was the parameter defining the ITD with the strongest (peak, biphasic, and sigmoid function) or weakest (trough function) response. ITDcenter was defined as the point at the center of the rising phase of a fitting function (inflection point, see Fig. 6C inlay).
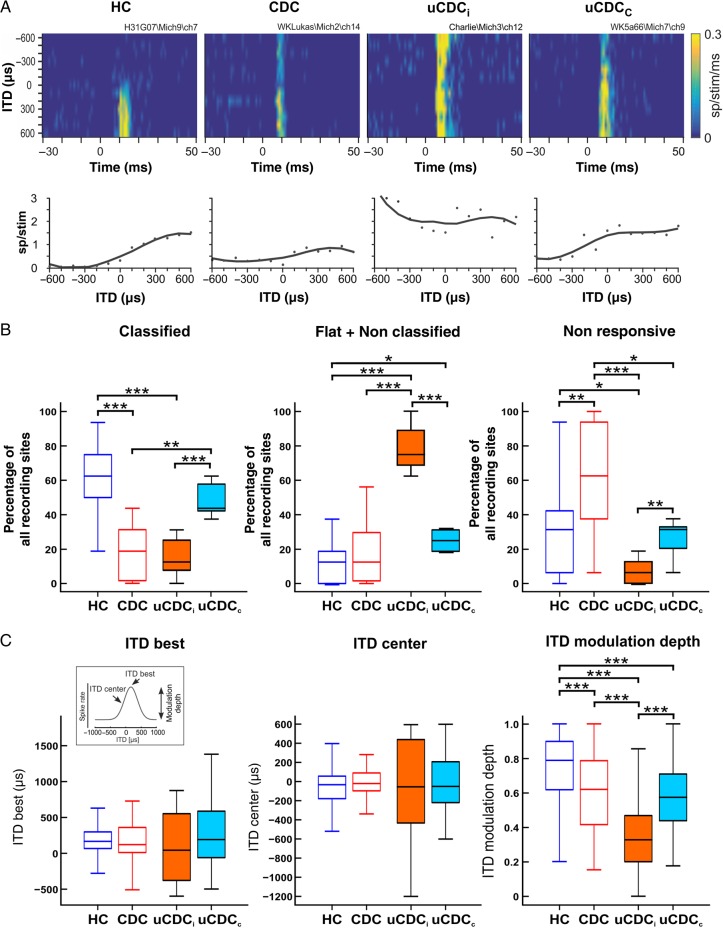
Figure 6.
ITD sensitivity (stimuli presented at interaural level difference of 0 dB). (A) Firing rate (color) as a function of peristimulus time (abscissa) and ITD (ordinate) of 4 typical units showing sensitivity to ITDs (one example per animal group). The lower panels show the number of spikes per stimulus as a function of ITD evaluated from the corresponding color plot. The line fitted along the original data points represents the ITD function showing changes in responsiveness with respect to ITD. (B) Statistical evaluation of ITD sensitivity, presented for all animal groups. Classified units (left) represent those units that systematically changed the firing rate with ITD, thus representing the true ITD-sensitive units (cf. Tillein et al. 2010). The majority of such units were observed in normal-hearing animals (HCs) and in the contralateral hemisphere of the unilateral animals (uCDCc), with only a few in the hemisphere ipsilateral to the hearing ear (uCDCi), and in the binaurally deaf animals (CDC). Correspondingly, nonclassified units and flat units (middle, responding with similar firing rates to different ITDs) were more frequent in the hemisphere ipsilateral to the hearing ear (uCDCi). Units nonresponsive to stimulation (right) were most frequently found in binaurally deaf cats (CDCs), while they were very rare in the ipsilateral cortex of unilateral animals (uCDCi). (C) Comparison of the properties of classified units in all animals and conditions. Analyzed ITD parameters are indicated in the inset. Both ITDbest and ITDcenter showed larger spread over the tested ITD range in uCDCs, but no significant differences were found between groups and conditions (left and middle). MDs of ITD function (right), quantifying ITD sensitivity in the classified units, were lowest in the ipsilateral cortex of the unilateral animals. Two-tailed Wilcoxon–Mann–Whitney test, *P < 0.05, **P < 0.01, ***P < 0.001.
Modulation depth (MD) of the responses was defined as the ratio of the difference between maximal and minimal response firing rate and maximal firing rate. ITD functions with MD <50% were classified as flat. All parameters obtained from the significantly fitted templates (flat class excluded) were compared among studied groups at 6 dB above the individual EABR threshold.
Statistical Evaluation
Results were statistically compared using the two-tailed Wilcoxon–Mann–Whitney test in MATLAB. For distribution testing, a two-sample Kolmogorov–Smirnov test was performed. An alpha-level of 5% was considered significant.
Results
First, surface LFP activation maps were determined using microelectrode recordings of LFPs. Using this approach, the cortical representation of the stimulated region of the auditory nerve was determined. Most responsive cortical regions as defined from the LFP maps were identified and defined as hot spots (e.g., Kral et al. 2009; Tillein et al. 2010).
The mapping procedure established functionally corresponding recording locations in all 3 groups of animals (cf. Kral et al. 2009; Kral, Heid, et al. 2013). Within the spot with the largest LFPs, unit responses to monaural and binaural electrical stimulation were recorded using a single-shank Neuronexus probe. Recordings covered the central part of the hot spots through all layers, characterizing the functional state of this cortical region in each animal very well. In total, 336 multiunit recordings were taken from hearing controls, 320 units from CDCs, and 256 multiunit recordings were collected in unilateral animals. Responding recording sites were selected based on the statistical procedure described above.
Terminology
In the present study, the term “crossed” refers to the situation in which stimulation and recordings sites are located on opposite sides of the brain, whereas “uncrossed” indicates that stimulation and recording are located on the same side (Fig. 1A).
The terms “contralateral” and “ipsilateral” are always used with reference to the side of the hearing ear in unilateral animals (Fig. 1B). The ipsilateral cortex is that on the same side of the brain as the hearing ear. In uCDCs, this redefinition allows the hemispheres to be differentiated (relative to the hearing ear) from the stimulation/recording configuration. Figure 1C shows the investigated groups of animals, including hearing controls, CDCs, and uCDCs, as well as the designation of the recorded hemisphere (and the color code) relative to the hearing ear in unilateral (single-sided) hearing animals.
Spontaneous Firing Rates
When spontaneous activity was compared between HCs, CDCs, and uCDCs, a significant reduction was observed in CDCs compared with HCs (Fig. 2B, two-tailed Wilcoxon–Mann–Whitney test, P = 2.7 × 10−15). The uCDCs exhibited a significant difference between the hemispheres (P = 0.014): Whereas the ipsilateral hemisphere showed a lower firing rate than in HCs (P = 1.3 × 10−5), the contralateral hemisphere was not significantly different from that in HCs [P = 0.86; for corresponding data on cochlear ablation, see McAlpine et al. (1997)]. In uCDCs, spontaneous firing rates in the ipsilateral hemispheres were in between those of HCs and CDCs, whereas in the contralateral hemisphere they were more comparable with HCs than with CDCs.
Maximum Evoked Firing Rates
Maximum evoked firing rates were processed at a current level of 6 dB above brainstem response threshold, which is about the middle of the population's DR (Tillein et al. 2010; see below). In this study—as in a previous investigation by Tillein et al. (2010)—CDCs showed significantly lower firing rates than HCs in all configurations (Fig. 2A). In uCDCs, the 2 hemispheres were again significantly different in all stimulation configurations, demonstrating a hemispheric-specific effect of the reorganization.
Remarkably, the deaf ear of uCDCs was not disconnected from the cortex. The firing rate evoked by the deaf ear was not different from that in HCs in either condition (crossed: P = 0.13 and uncrossed P = 0.38). However, when directly comparing responses to the deaf and the hearing ear in uCDCs, the hearing ear evoked higher firing rates in all configurations (Fig. 2C, orange vs. light blue, crossed: P = 0.0003; uncrossed: P = 1.2 × 10−12). Consequently, the representation of the hearing ear was stronger than that of the deaf ear in uCDCs [for LFP data, see Kral, Heid, et al. (2013) and Kral, Hubka, et al. (2013)]. The firing rates for the hearing ear were even higher than those in HCs (Fig. 2C, crossed: P = 0.0022; uncrossed: P = 4.6 × 10−21). Finally, there was a genuine hemispheric difference in uCDCs, as the same stimulation (of both ears) resulted in significantly higher firing rates at the ipsilateral cortex than at the contralateral cortex (Fig. 2C, P = 0.0006). Again, this supports the conclusions of a previous LFP study on hemispheric differences after unilateral deafness or unilateral cochlear implantation (Kral, Heid, et al. 2013).
Rate-Level Functions
Rate-level functions were subsequently investigated in detail. The rate-level combinations were fitted with template functions for crossed, uncrossed, and binaural conditions (Fig. 3A). The response thresholds (Fig. 3B, top) were consistently lower in CDCs than in HCs (crossed: P = 4.4 × 10−14; uncrossed: P = 3.6 × 10−13; binaural: P = 1.7 × 10−6). Furthermore, the DR was—except in the uncrossed condition—smaller in CDCs than in HCs (Fig. 3B, bottom, crossed: P = 4.8 × 10−5; uncrossed: P = 0.42; binaural: P = 5.7 × 10−10). Lower thresholds and smaller DR are consistent with “hypersensitivity” to auditory inputs. However, the decrease in spontaneous firing rate in CDCs (Fig. 2B) indicates an additional factor, possibly the fact that fewer neurons are connected to the deprived ear.
In uCDCs, there were no hemispheric differences in threshold, saturation, and DR for binaural stimulation (Fig. 3B). The results for the individual ears were mixed. Threshold and saturation levels were significantly lower for crossed stimulation in the contralateral hemisphere, and thus for stimulation of the hearing ear. In the DR, however, both hemispheres in the uCDCs showed an increase compared with HCs and CDCs.
In total, the present data thus indicate that in some (but not all) comparisons, in uCDCs, the response to the hearing ear is more similar to that in HCs, the response to the deaf ear is similar to that in CDCs, and the binaural response is similar to that in the HCs (Fig. 3C). However, in general, greater variability of the responses in unilateral animals compared with the other groups was noted in these response properties.
Classes of Monaural Responsiveness
Previous studies have investigated aural interaction in cortical units based on the type of monaural and binaural responsiveness (Zhang et al. 2004). In the present study, the same classification was used (Fig. 4); however, stimulation was electrical in all cases.
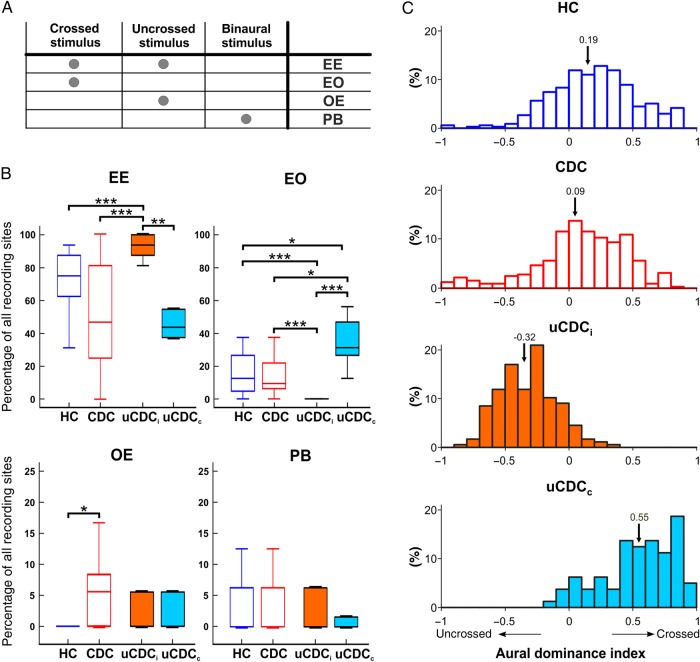
Figure 4.
Monaural response classification. (A) Classification of monaural responses [based on Zhang et al. (2004)]. Depending on the response to crossed and uncrossed stimulation units, these are classified into EE (responding to the stimulation of crossed and uncrossed ear), E0 (responding only to the stimulation of the crossed ear), and 0E (responding only to the stimulation of the uncrossed ear). Units responding solely to binaural stimulation are designated PB. Testing with binaural stimuli was performed at ITD = 0 µs and ILD = 0 dB. (B) Statistical analysis of the distribution within the response classes. Most differences were found for EE and E0 responses. In the ipsilateral cortex of unilateral animals (uCDCi), the highest number of EE responses was found, while the same group showed the lowest number of E0 responses. Two-tailed Wilcoxon–Mann–Whitney test, *P < 0.05; **P < 0.01; ***P < 0.001. (C) Histograms of ADI of multiunit responses in all studied groups 6 dB above EABR thresholds. Means of the population are marked by an arrow, and mean values are given above arrows. In hearing controls (blue color), the index was predominantly positive, demonstrating stronger responses in crossed ear stimulation. In CDCs (red color), the distribution is similar but with smaller mean. In uCDCi (orange color, filled), a prominent shift in favor of the uncrossed ear (negative values) is observed. Again, a hemispheric specificity shows up, with crossed ear preference in uCDCc (light blue color, filled). ADI = −1 corresponds to the ocular dominance score 7 in the visual system and ADI = 1 to the ocular dominance score of 1. For details on statistics, see text.
In all animal groups, the predominant type of monaural response was the EE type, whereas no significant difference was found between HCs and CDCs (Fig. 4B, EE panel, P = 0.062). A tendency toward lower EE counts in CDCs was observed, with very high variability in CDCs. However, considerably more 0E units were found in CDCs (P = 0.012). This indicates that some of the units responsive to both ears became units responsive solely to the uncrossed ear after binaural deafness, a class exceptionally rare in HCs. In consequence, the normal bias toward the crossed ear in HCs (more E0 than 0E) was reduced in CDCs, consistent with the reduced contralateral dominance in binaural deafness observed previously (Kral et al. 2009; Tillein et al. 2010).
Pronounced hemispheric differences were found in uCDCs (Fig. 4B). At the hemisphere ipsilateral to the hearing ear, more EE interactions were found, this being potentially due to increase in responsiveness to the ipsilateral (i.e., hearing) ear. There were nearly no E0 interactions at this (ipsilateral) hemisphere, further supporting the notion that the units normally responsive to the crossed (deaf) ear became responsive to both ears in uCDCs. The contralateral hemisphere in uCDCs had similar counts of EE units to those in CDCs; again, however, the number was not significantly different from that in the HCs (CDCs: P = 1.00; HCs: P = 0.07). There were few 0E and PB interactions in uCDCs. In contrast to the visual system, binaural units were not lost; in fact, EE units were the predominant type in the present sample. Rather, the counts in the different classes indicate that monaural units responsive to the deaf ear only (in uCDCis corresponding to E0; uCDCi = hemisphere ipsilateral to the hearing ear) were most likely transformed into binaural units (responsive to both the deaf and the hearing ear, EE).
An ADI (Fig. 4C) was computed from the difference in firing rate evoked by the crossed ear and the uncrossed one, divided by the sum of stimulation of each ear (see Materials and Methods). In hearing controls, the responses favored crossed responses (0.19 ± 0.33) and shifted closer to balanced input in bilaterally deaf CDCs (0.09 ± 0.36, two-tailed Wilcoxon–Mann–Whitney test, P = 0.003). In uCDCs, the situation differed in the 2 hemispheres. At the ipsilateral cortex, the index was reversed to favor of uncrossed stimulation (−0.32 ± 0.22; compared with HCs: P = 1.8 × 10−51; CDCs: P = 1.8 × 10−37). Finally, the contralateral hemisphere in uCDCs showed an extensive preference for uncrossed stimulation (0.55 ± 0.29, compared with HCs: P = 4.2 × 10−16, compared with CDCs: P = 2.6 × 10−21). This finding convincingly demonstrates an extensive use-related plasticity and shift in aural dominance (preference) toward the hearing ear. The histograms of this index allow direct comparison with outcomes of monocular deprivation studies in the visual system, where ocular dominance score 7 corresponds to ADI = −1 and ocular dominance score 1 to ADI = 1.
Binaural Interactions
To quantify binaural interactions, all firing rates between threshold and saturation level of the function were summed for crossed, uncrossed, and binaural responses. Binaural interactions were then assessed based on the relation of the sum of monaural responses (stimulation of crossed and uncrossed ear separately) to the binaural response (stimulation at both ears simultaneously; see Fig. 5A). Binaural responses were recorded at 0 dB interaural level difference and at ITD = 0 µs. Interactions were classified as facilitation, neutral interaction, occlusion, and suppression (Zhang et al. 2004).
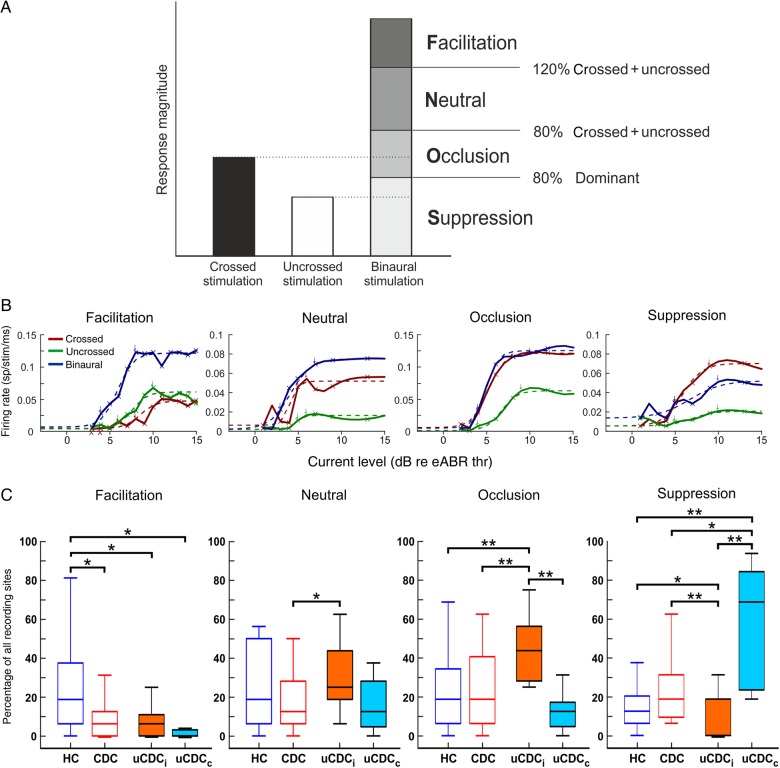
Figure 5.
Binaural response classification. (A) Classification of responses based on the relation between monaural and binaural responses [F = facilitation; N = neutral interaction; O = occlusion; S = suppression, after Zhang et al. (2004)]. Testing was performed at ITD = 0 µs and ILD = 0 dB. (B) Examples of rate-level functions for facilitatory, neutral occlusive, and suppression interactions (for description of rate-level functions, see Fig. 3A). (C) Distribution and statistics of binaural interaction classes for all animal groups. A significant reduction of facilitatory interactions was found for CDCs and both unilateral groups compared with HCs. The highest number of occlusions was found in the ipsilateral cortex of uCDCs, while the contralateral cortex showed the highest number of suppressions. Two-tailed Wilcoxon–Mann–Whitney test,*P < 0.05; **P < 0.01.
In HCs, facilitation, neutral interaction, occlusion, and suppression were evenly distributed. CDCs showed a similar pattern with the exception of facilitation, which was less frequent in CDCs (P = 0.011). Facilitation was most prominent in HCs. As facilitation shows boosted responses (supralinear summation) in the binaural condition, it can be concluded that auditory experience is required for true binaural benefit at the cortical single-neuron level.
In uCDCs, facilitation was in both hemispheres as rare in CDCs, despite the considerable increase in EE units at the ipsilateral hemisphere (Fig. 4). Binaural responsiveness was strongly (but differentially) affected in the 2 hemispheres. The ipsilateral A1 showed more neutral and occlusion interactions (∼77% of all sites). The contralateral cortex showed a bias toward suppression (57.5 ± 33.8% of all recorded sites), indicating a strong suppressive influence of the deaf ear on the prevalent excitatory activation of the hearing ear (Fig. 5C).
ITD Sensitivity
ITD sensitivity in binaurally deaf cats has been investigated before (Tillein et al. 2010, 2011). The present findings are consistent with the previous report. Therefore, the results from the HCs and CDCs in the present study are discussed only in comparison with uCDCs. The automatic classification procedure of ITD functions introduced in the previous study (Tillein et al. 2010) was used here to compare ITD sensitivity in uCDCs relative to HCs and CDCs. The classification used 4 classes of ITD responses: peak, sigmoid, biphasic, and trough. Here, for reasons of simplification, these classes were pooled and only the relative numbers of classified and nonclassified units are shown.
As in the previous study (Tillein et al. 2010), the number of classified units was significantly smaller in CDCs than in HCs (Fig. 6B, P = 1.4 × 10−5). In uCDCs, the hemispheres were different with respect to ITD sensitivity: The contralateral hemisphere had more classified units than the ipsilateral hemisphere (P = 0.0004), whereas in the hemisphere contralateral to the hearing ear this number was higher than in CDCs (P = 0.004), with no difference to the HCs (P = 0.17). At the ipsilateral hemisphere, most units were nonclassified or flat. This finding suggests that the frequency of the classified responses was dominated by the character of the crossed ear: If it was hearing, the proportion was similar to that in HCs. If it was deaf, it was similar to CDCs. The number of responsive but nonclassified (or flat) ITD functions was much higher in unilateral animals, whereas these too showed hemispheric specificity: The contralateral hemisphere was similar to that in deaf cats, but the ipsilateral hemisphere had more nonclassified responses than in both hearing and deaf cats. This indicates a specific loss of ITD sensitivity that is more extensive in the ipsilateral hemisphere. A sensitivity analysis demonstrated that the effect size in all significant differences was large (Cohen's d >2), with power close to 100%.
One of the functional deficits observed in CDCs was reduced responsiveness [see Tillein et al. (2010)]. This deficit, not specific to ITD, disappeared in unilateral animals: The number of nonresponsive units was similar to that in HCs in the contralateral hemisphere (P = 0.93) and smaller than in HCs in the ipsilateral hemisphere (P = 0.013). In both hemispheres of uCDCs, the nonresponsive units were less frequent than in CDCs (ipsilateral hemisphere: P = 3.54 × 10−5; contralateral hemisphere: P = 0.02). With respect to general responsiveness, unilateral animals thus compared better with hearing animals than with bilaterally deaf animals. However, binaural interactions were significantly less specific for ITDs, as suggested by an increase in flat and nonclassified responses.
To further quantify the ITD specificity of the classified responses, the parameters of the templates in the fitted ITD functions were compared in the classified groups (Hancock et al. 2010; Tillein et al. 2010). ITDbest did not show significant differences between the groups (Fig. 6C). Thus, ITD sensitivity was not compensating for the loss of hearing in one ear by means of cue remapping.
ITDcenter in uCDCs at the ipsilateral hemisphere was more variable than both in hearing cats and in CDCs (HC: Kolmogorov–Smirnov test, P = 0.03; CDCs: Kolmogorov–Smirnov test, P = 0.04). Except for higher variability, no systematic effect was observed in ITDcenter among uCDCs. Finally, MD, a measure of ITD sensitivity of the classified responses, was reduced in uCDCs compared with HCs in both hemispheres (Fig. 6C; ipsilateral: P = 5.8 × 10−40; contralateral: P = 1.3 × 10−9). Here, again, the ipsilateral hemisphere demonstrated a more pronounced deficit (P = 4.9 × 10−11, cf. Tillein et al. 2010). The ipsilateral hemisphere in uCDCs had lower MD than in CDCs (P = 5 × 10−15), whereas the contralateral hemisphere was similar to that in CDCs (P = 0.27). Consequently, compared with binaurally deafness, unilateral deafness leads to a further decrease in sensitivity to ITDs, particularly at the ipsilateral hemisphere. It would appear that the ipsilateral hemisphere traded binaural sensitivity, including ITD sensitivity, for responsiveness to the only hearing ear.
Discussion
The present study is the first to compare cortical binaural responsiveness in congenital monaural and binaural deafness. It demonstrates extensive aural dominance plasticity induced by hearing asymmetry. Binaural facilitation, the true binaural advantage, was more abundant in animals with binaural hearing experience (Fig. 5C). Thus, although the presence of rudimental sensitivity to binaural cues in congenitally deaf animals demonstrates their inborn nature, the extensive modifications in unilateral animals show that they are maintained by experience and can be modified by abnormal hearing, specifically in each hemisphere.
The evoked firing rate in uCDCs was higher for stimulation at the hearing ear, and in other words there was a preference for the hearing ear on both hemispheres [Fig. 2C, comp. also Kral, Heid, et al. (2013) and Kral, Hubka, et al. (2013)]. The present study further showed that the uncrossed projections reorganize more than the crossed ones. The uncrossed stimulation caused suppression in one hemisphere (uCDCc) but excitation in the other hemisphere (uCDCi). Compared with the uncrossed stimulations, the crossed stimulations resulted in much more similar properties in both hemispheres in the single-sided deaf group.
A hemispheric-specific effect was observed for several binaural properties in unilateral animals, with an extensive loss in ITD sensitivity at the hemisphere ipsilateral to the hearing ear (Fig. 6). Thus, compared with complete deafness, unilateral hearing had a beneficial consequence, preventing the loss of responsiveness, but also a deleterious consequence, reducing the sensitivity to binaural cues.
Methodological Discussion
The present study used electrical stimuli to investigate binaural sensitivity. Electrical stimulation differs from acoustic stimulation by higher synchrony, less stochasticity, and a reduced DR. A previous study directly compared ITD sensitivity under acoustic and electric stimulation in hearing cats (Smith and Delgutte 2007) and found only minor differences, including a slight decrease in ITDbest in electric stimulation. Even though we did not use acoustic stimulation, the similarity of the electrical responses in the present study and the previous study (Smith and Delgutte 2007) demonstrates that the present approach is valid in investigation of ITD sensitivity.
Loss of spiral ganglion cells in deaf animals could have affected the outcomes of the present study. However, in contrast to pharmacologically deafened animals, the preservation of spiral ganglion cells is high in the cochlear base of CDCs (where the cochlear implant is placed), with no significant loss up to 2 years of age and with slowly decreasing counts within the following years (Heid et al. 1998). In contrast to pharmacologically deafened animals, response thresholds of brainstem responses evoked by cochlear implants were not significantly different in HCs and CDCs and also brainstem response amplitudes were not different from HCs (Tillein et al. 2012). Furthermore, the hemispheric-specific effects of the present and a previous study (Kral, Heid, et al. 2013) cannot be caused by spiral ganglion degeneration, since response to the same ear was compared. The analysis of response onset timing in uCDCs excludes a strong influence of spiral ganglion degeneration on the outcomes (Kral, Hubka, et al. 2013). In total, while a biasing influence of the spiral ganglion state cannot be excluded, it does not seem to have dominated the present outcomes. Finally, the investigated animals (including spiral ganglion changes) precisely mimic the condition of single-sided deafness in children, and thus is of high clinical relevance.
The main limitation of the present study is due to the exceptionally rare nature of unilateral congenital deafness combined with unilateral normal hearing. However, these rare animals are scientifically and clinically valuable, as they allow insights into neuronal consequences of this most extreme hearing asymmetry. Owing to the use of multielectrode arrays, the obtained neuronal sample in the unilateral deaf animals was high. The present results were clearly related to the hearing status and were consistent with mechanisms associated with asymmetric hearing. A significant reduction of suppressive interactions, more on the ipsilateral hemisphere, was also found after unilateral ear plugging (Silverman and Clopton 1977; Moore and Irvine 1981; Vale et al. 2004). Thus, where comparable (see below), the present outcomes are consistent with previous studies.
Discussion of Results
The focus of the present study was a particular aspect of binaural hearing, namely the developmental consequences of unilateral and bilateral deafness and their hemispheric specificity. To consider the behavioral ability to localize sound, one obviously has to include other cues into consideration (interaural level difference sensitivity and spectral monaural cues). Additionally, further cortical areas contributing to localization behavior have to be taken into account (Malhotra and Lomber 2007). As a starting point, here we concentrate on the primary auditory cortex, monaural and binaural interactions, and the dominant binaural cue, the ITDs.
The present outcomes extend previous findings with cochlear ablation, demonstrating reorganization of responsiveness toward the nonablated ear (Kitzes and Semple 1985; Moore and Kitzes 1985; Reale et al. 1987; Kitzes et al. 1995; Moore et al. 1995; McAlpine et al. 1997). However, in the above studies, responses to the ablated ear (or binaural stimuli) could not be tested. A shift of the auditory system toward the hearing ear has been demonstrated following unilateral ear plugging in behavioral experiments (King et al. 2001, 2011) as well as in electrophysiological studies (Silverman and Clopton 1977; Moore and Irvine 1981; Brugge et al. 1985; Vale et al. 2004; Popescu and Polley 2010). In hearing ferrets, ear plugging in adulthood in the absence of localization training degrades spatial localization ability, whereas in young animals plastic adaptation to the altered cues is observed in the space maps [King et al. 2011; for similar data in the visual system, see Sillito et al. (1981)]. The compensating effect of the ear plug is based on a greater emphasis on monaural cues and low-frequency binaural cues (Kumpik et al. 2010; Keating et al. 2013; Keating and King 2013). However, this experimental approach is different from the present one: in the above studies, unilateral hearing loss was induced in a previously normal-hearing ear. Thus, before intervention, the animals had normal-hearing experience. The present investigations represent more extensive manipulation in which the brain has never been patterned by auditory input from both ears.
The present outcomes indicate that uCDCs traded binaural cues (which are not available in unilateral deafness) for increased representation of the hearing ear, potentially putting more emphasis on monaural cues (Keating and King 2013). In contrast to the barn owl (Knudsen 2002), mammals do not show extensive shifts in ITD tuning after early asymmetric conductive hearing loss (Brugge et al. 1985; King et al. 2011). Here, we revealed a profound reduction in ITD sensitivity and an increase in variance of ITDcenter in residually ITD-sensitive units in uCDCs. The methodological difference to the previous study of Brugge et al. (1985) is the complete absence of hearing on one side in the present study. In the present extreme condition (uCDCs), a near loss of ITD representation was observed in one hemisphere. This is supported by morphological rearrangements in the olivary complex following unilateral deprivation (Feng and Rogowski 1980) and indicates that the amount of residual hearing affects the outcome significantly.
Interestingly, spatial localization is compromised after binaural implantations in unilaterally deaf children as well (Firszt et al. 2008, 2012; Litovsky et al. 2009, 2010; Gordon et al. 2013). Therefore, it appears plausible that reorganization similar to that reported here takes place in the brain of unilaterally deaf humans (Gordon et al. 2015). Another related observation is the poor performance of the second-implanted ear in pediatric sequential implantations (Graham et al. 2009) and the surprisingly slow learning of speech understanding using this ear (Illg et al. 2013). The present results provide evidence of the physiological substrate underlying these observations and are consistent with previous LFP data showing an aural preference for the hearing ear (Kral, Hubka, et al. 2013).
As the cortex does not directly receive aural-specific input, the binaural interactions described in the present study must be inherited from subcortical structures, with contribution of additional cortical effects due to its role in learning and translation of sensory input into behavior. Subcortical deficits have been described in deafness (Moore and Irvine 1981; Shepherd et al. 1999; Vale et al. 2004; Baker et al. 2010; Hancock et al. 2010; O'Neil et al. 2010). For example, a reduced inhibitory drive from the hearing ear was observed in the inferior colliculus following unilateral deprivation (Silverman and Clopton 1977; Moore and Irvine 1981; Vale et al. 2004). This is consistent with our findings on differential activation patterns in the 2 hemispheres.
A variety of additional possible cellular mechanisms can be involved in the present findings. The basic response properties indicate hypersensitivity in the sense of reduced DR and lower thresholds (Kral et al. 2005; Kotak et al. 2008; Fallon et al. 2009). However, a simple excitatory gain control increase (as a consequence of homeostatic plasticity) in deaf cats does not explain the present data, namely the reduced DR and threshold (Fig. 3B), combined with reduced spontaneous firing rate and reduced evoked firing rate (Fig. 2B,C). Inhibitory changes (Kotak et al. 2008) are additionally involved in both CDCs and uCDCs (Tillein et al. 2010; Kral, Hubka, et al. 2013), as demonstrated here by reorganization in suppressive binaural interactions (Fig. 5). Inhibition plays also a role in ITD extraction in the olivary complex (Brand et al. 2002; Grothe 2003).
Hyperpolarization-activated channels are known to be involved in fast response properties in the brainstem, also in binaural hearing as investigated here. Cochlear ablations before hearing onset affect hyperpolarization-activated ionic channels in the brainstem neurons (Hassfurth et al. 2009). However, these findings cannot be directly extrapolated to the present condition, since loss of hair cells (or cochlear ablation) before hearing onset differs from congenital deafness by more extensive degenerations in the brainstem (including neuronal loss; Tong et al. 2015).
A rearrangement of the binaural projection patterns within the auditory pathway may have smeared interaural timing and likely contribute to the present findings. Large-scale shifts in the ipsilateral and contralateral projection patterns may occur in unilateral deprivation (Nordeen et al. 1983; Russell and Moore 1995; Hsieh and Cramer 2006), but have been demonstrated following cochlear ablations before hearing onset. In CDCs, some more subtle rearrangement of subcortical (Heid et al. 1997) as well as thalamocortical (Barone et al. 2013) projection patterns were observed. Reduced cochleotopic gradients have been demonstrated throughout the auditory pathway as a consequence of early deafness (Leake et al. 2002; Fallon et al. 2009; Barone et al. 2013). This, particularly in unilateral deafness, might lead to less-precise inputs from the ears (Clause et al. 2014), affecting both spectral processing and sound localization. Furthermore, congenital deafness rearranges the endbulb of Helds both morphologically as well as functionally (Ryugo et al. 2005; Baker et al. 2010). That again may contribute to the deficits in response timing and have consequences for binaural processing. Taken together, the present findings are likely to be caused by a combination of several cellular mechanisms related to auditory deprivation.
Comparison with Visual Deprivation
Correspondingly to the ocular dominance shifts in the visual system, also the present study observed shifts in auditory dominance (Fig. 4C). In unilateral congenital deafness the cortex remained responsive to the deaf ear. This contrasts the condition of monocular deprivation (Hubel and Wiesel 1970; Shatz and Stryker 1978; Sillito et al. 1981), where responsiveness to the blind eye is extensively reduced. In congenital unilateral deafness units monaurally responsive to the deaf ear were rare, but units that are binaurally responsive (EE) became unusually frequent, indicating that units solely responsive to the deaf ear were overtaken by the hearing ear, but continued to respond to the deaf ear, even though weaker than to the hearing ear (Fig. 4B).
The fundamental difference between the 2 sensory systems is in the integration of inputs from the 2 sensory organs. While the primary visual cortex receives monocular input (the inputs from each eye to the layer IV are segregated and binocular integration is observed in supragranular layers), in the auditory system binaural integration is observed already in the olivary complex. The primary auditory cortex, in contrast, does receive binaural and not monaural inputs. Furthermore, the auditory system has 2 binaural integration routes, the interaural time and the level difference route. This has no correspondence in the visual system—particularly there is nothing similar to the ITD cue. Developmental manipulations in the 2 sensory systems consequently must lead to different outcomes.
Therefore, the outcome of congenital unilateral deafness is in part distinct from consequences of monocular deprivation (deprivation amblyopia).
Clinical Implications
Effects of partial or total deprivation on the auditory pathway show sensitive periods in rodents (Clopton and Silverman 1977; Mowery et al. 2015; Polley et al. 2013), ferrets (McAlpine et al. 1997), cats (Kral et al. 2006; Kral, Heid, et al. 2013; Kral, Hubka, et al. 2013), and humans (Sharma et al. 2005; Kral and Sharma 2012). Although binaural properties show up early in the development of the cat (Blatchley and Brugge 1990), the present data demonstrate that ear-balanced auditory experience during development is required to preserve and refine binaural hearing.
The present study implies that both binaural and unilateral deafness impair binaural extraction of sound-source location information. Consequently, hearing should be restored symmetrically by means of binaural cochlear implantations as soon as possible to prevent deleterious effects on the representation of binaural cues. In the case of sequential implantations, the interimplant intervals should be kept as short as possible in children (Firszt et al. 2012; Sarant et al. 2014; Gordon et al. 2015; Zheng et al. 2015). The present data further substantiate findings of an aural preference for the hearing ear and a residual responsiveness to the deaf ear (Kral, Hubka, et al. 2013). The input from the deaf ear is not lost. However, it is put at a disadvantage compared with the hearing ear and results in compromised binaural computations. Appropriate training procedures might help to compensate for this condition after binaural hearing has been restored in individuals with congenital unilateral deafness.
Funding
This work was supported by the German Research Foundation (DFG Kr 3370/1-3 and Cluster of Excellence Hearing4All). Funding to pay the Open Access publication charges for this article was provided by the authors.
Notes
We are grateful to Dr J.J. Eggermont for comments on the earlier version of this manuscript. Conflict of Interest: J.T. is employed by MED-EL Starnberg Germany. As the manuscript focuses on basic research and is not related to any product, no conflict of interest exists.
References
- Baker CA, Montey KL, Pongstaporn T, Ryugo DK. 2010. Postnatal development of the endbulb of held in congenitally deaf cats. Front Neuroanat. 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P, Lacassagne L, Kral A. 2013. Reorganization of the connectivity of cortical field DZ in congenitally deaf cat. PLoS ONE. 8:e60093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt M. 1999. Entwicklung und Herstellung eines Cochlea-Implantates zur chronischen Stimulation von gehörlosen weißen Katzen. MD Thesis J.W. Goethe University, Frankfurt am Main, Germany. [Google Scholar]
- Blatchley BJ, Brugge JF. 1990. Sensitivity to binaural intensity and phase difference cues in kitten inferior colliculus. J Neurophysiol. 64:582–597. [DOI] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. 2002. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 417:543–547. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Orman SS, Coleman JR, Chan JC, Phillips DP. 1985. Binaural interactions in cortical area AI of cats reared with unilateral atresia of the external ear canal. Hear Res. 20:275–287. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Neff WD. 1973. Localization of pure tones. J Acoust Soc Am. 54:365. [DOI] [PubMed] [Google Scholar]
- Chase SM, Young ED. 2007. First-spike latency information in single neurons increases when referenced to population onset. Proc Natl Acad Sci USA. 104:5175–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clause A, Kim G, Sonntag M, Weisz CJ, Vetter DE, Rübsamen R, Kandler K. 2014. The precise temporal pattern of prehearing spontaneous activity is necessary for tonotopic map refinement. Neuron. 82:822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopton BM, Silverman MS. 1977. Plasticity of binaural interaction. II. Critical period and changes in midline response. J Neurophysiol. 40:1275–1280. [DOI] [PubMed] [Google Scholar]
- Fallon JB, Irvine DR, Shepherd RK. 2009. Cochlear implant use following neonatal deafness influences the cochleotopic organization of the primary auditory cortex in cats. J Comp Neurol. 512:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng AS, Rogowski BA. 1980. Effects of monaural and binaural occlusion on the morphology of neurons in the medial superior olivary nucleus of the rat. Brain Res. 189:530–534. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Holden LK, Reeder RM, Cowdrey L, King S. 2012. Cochlear implantation in adults with asymmetric hearing loss. Ear Hear. 33:521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firszt JB, Reeder RM, Skinner MW. 2008. Restoring hearing symmetry with two cochlear implants or one cochlear implant and a contralateral hearing aid. J Rehabil Res Dev. 45:749–768. [DOI] [PubMed] [Google Scholar]
- Geigy CA, Heid S, Steffen F, Danielson K, Jaggy A, Gaillard C. 2007. Does a pleiotropic gene explain deafness and blue irises in white cats? Vet J. 173:548–553. [DOI] [PubMed] [Google Scholar]
- Gordon K, Henkin Y, Kral A. 2015. Asymmetric hearing during development: the aural preference syndrome and treatment options. Pediatrics. 136:141–153. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Wong DD, Papsin BC. 2013. Bilateral input protects the cortex from unilaterally-driven reorganization in children who are deaf. Brain. 136:1609–1625. [DOI] [PubMed] [Google Scholar]
- Graham J, Vickers D, Eyles J, Brinton J, Al Malky G, Aleksy W, Martin J, Henderson L, Mawman D, Robinson P et al. 2009. Bilateral sequential cochlear implantation in the congenitally deaf child: evidence to support the concept of a ‘critical age’ after which the second ear is less likely to provide an adequate level of speech perception on its own. Cochlear Implants Int. 10:119–141. [DOI] [PubMed] [Google Scholar]
- Grothe B. 2003. New roles for synaptic inhibition in sound localization. Nat Rev Neurosci. 4:540–550. [DOI] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. 2010. Mechanisms of sound localization in mammals. Physiol Rev. 90:983–1012. [DOI] [PubMed] [Google Scholar]
- Hancock KE, Noel V, Ryugo DK, Delgutte B. 2010. Neural coding of interaural time differences with bilateral cochlear implants: effects of congenital deafness. J Neurosci. 30:14068–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R, Topp G, Klinke R. 1984. Discharge patterns of cat primary auditory fibers with electrical stimulation of the cochlea. Hear Res. 13:47–62. [DOI] [PubMed] [Google Scholar]
- Hassfurth B, Magnusson AK, Grothe B, Koch U. 2009. Sensory deprivation regulates the development of the hyperpolarization-activated current in auditory brainstem neurons. Eur J Neurosci. 30:1227–1238. [DOI] [PubMed] [Google Scholar]
- Heid S, Hartmann R, Klinke R. 1998. A model for prelingual deafness, the congenitally deaf white cat—population statistics and degenerative changes. Hear Res. 115:101–112. [DOI] [PubMed] [Google Scholar]
- Heid S, Jähn-Siebert TK, Klinke R, Hartmann R, Langner G. 1997. Afferent projection patterns in the auditory brainstem in normal and congenitally deaf white cats. Hear Res. 110:191–199. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Cramer KS. 2006. Deafferentation induces novel axonal projections in the auditory brainstem after hearing onset. J Comp Neurol. 497:589–599. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1970. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 206:419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1963. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. J Neurophys. 26:994–1002. [DOI] [PubMed] [Google Scholar]
- Illg A, Giourgas A, Kral A, Büchner A, Lesinski-Schiedat A, Lenarz T. 2013. Speech comprehension in children and adolescents after sequential bilateral cochlear implantation with long interimplant interval. Otol Neurotol. 34:682–689. [DOI] [PubMed] [Google Scholar]
- Irvine DR, Rajan R, Aitkin LM. 1996. Sensitivity to interaural intensity differences of neurons in primary auditory cortex of the cat. I. Types of sensitivity and effects of variations in sound pressure level. J Neurophysiol. 75:75–96. [DOI] [PubMed] [Google Scholar]
- Keating P, Dahmen JC, King AJ. 2013. Context-specific reweighting of auditory spatial cues following altered experience during development. Curr Biol. 23:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating P, King AJ. 2013. Developmental plasticity of spatial hearing following asymmetric hearing loss: context-dependent cue integration and its clinical implications. Front Syst Neurosci. 7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Dahmen JC, Keating P, Leach ND, Nodal FR, Bajo VM. 2011. Neural circuits underlying adaptation and learning in the perception of auditory space. Neurosci Biobehav Rev. 35:2129–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Kacelnik O, Mrsic-Flogel TD, Schnupp JW, Parsons CH, Moore DR. 2001. How plastic is spatial hearing? Audiol Neurootol. 6:182–186. [DOI] [PubMed] [Google Scholar]
- Kitzes LM, Kageyama GH, Semple MN, Kil J. 1995. Development of ectopic projections from the ventral cochlear nucleus to the superior olivary complex induced by neonatal ablation of the contralateral cochlea. J Comp Neurol. 353:341–363. [DOI] [PubMed] [Google Scholar]
- Kitzes LM, Semple MN. 1985. Single-unit responses in the inferior colliculus: effects of neonatal unilateral cochlear ablation. J Neurophysiol. 53:1483–1500. [DOI] [PubMed] [Google Scholar]
- Kitzes LM, Wrege KS, Cassady JM. 1980. Patterns of responses of cortical cells to binaural stimulation. J Comp Neurol. 192:455–472. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. 2002. Instructed learning in the auditory localization pathway of the barn owl. Nature. 417:322–328. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Takesian AE, Sanes DH. 2008. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb Cortex. 18:2098–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Heid S, Hubka P, Tillein J. 2013. Unilateral hearing during development: hemispheric specificity in plastic reorganizations. Front Syst Neurosci. 7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Hubka P, Heid S, Tillein J. 2013. Single-sided deafness leads to unilateral aural preference within an early sensitive period. Brain. 136:180–193. [DOI] [PubMed] [Google Scholar]
- Kral A, Lomber SG. 2015. Deaf white cats. Curr Biol. 25:R351–R353. [DOI] [PubMed] [Google Scholar]
- Kral A, Sharma A. 2012. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 35:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Tillein J, Hartmann R, Klinke R. 1999. Monitoring of anaesthesia in neurophysiological experiments. Neuroreport. 10:781–787. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Heid S, Hartmann R, Klinke R. 2005. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 15:552–562. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Heid S, Klinke R, Hartmann R. 2006. Cochlear implants: cortical plasticity in congenital deprivation. Prog Brain Res. 157:283–313. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Hubka P, Schiemann D, Heid S, Hartmann R, Engel AK. 2009. Spatiotemporal patterns of cortical activity with bilateral cochlear implants in congenital deafness. J Neurosci. 29:811–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpik DP, Kacelnik O, King AJ. 2010. Adaptive reweighting of auditory localization cues in response to chronic unilateral earplugging in humans. J Neurosci. 30:4883–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT. 2002. Postnatal refinement of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 448:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Middlebrooks JC. 2013. Specialization for sound localization in fields A1, DZ, and PAF of cat auditory cortex. J Assoc Res Otolaryngol. 14:61–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu JE, Tye-Murray N, Karzon RK, Piccirillo JF. 2010. Unilateral hearing loss is associated with worse speech-language scores in children. Pediatrics. 125:e1348–e1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Jones GL, Vanhoesel R. 2010. Effect of auditory deprivation on binaural sensitivity in bilateral cochlear implant users. J Acoust Soc Am. 127:1812. [Google Scholar]
- Litovsky RY, Parkinson A, Arcaroli J. 2009. Spatial hearing and speech intelligibility in bilateral cochlear implant users. Ear Hear. 30:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S, Lomber SG. 2007. Sound localization during homotopic and heterotopic bilateral cooling deactivation of primary and nonprimary auditory cortical areas in the cat. J Neurophysiol. 97:26–43. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Martin RL, Mossop JE, Moore DR. 1997. Response properties of neurons in the inferior colliculus of the monaurally deafened ferret to acoustic stimulation of the intact ear. J Neurophysiol. 78:767–779. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. 2015. Sound localization. Handb Clin Neurol. 129:99–116. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Clock AE, Xu L, Green DM. 1994. A panoramic code for sound location by cortical neurons. Science. 264:842–844. [DOI] [PubMed] [Google Scholar]
- Moore DR, Irvine DR. 1981. Plasticity of binaural interaction in the cat inferior colliculus. Brain Res. 208:198–202. [DOI] [PubMed] [Google Scholar]
- Moore DR, Kitzes LM. 1985. Projections from the cochlear nucleus to the inferior colliculus in normal and neonatally cochlea-ablated gerbils. J Comp Neurol. 240:180–195. [DOI] [PubMed] [Google Scholar]
- Moore DR, Russell FA, Cathcart NC. 1995. Lateral superior olive projections to the inferior colliculus in normal and unilaterally deafened ferrets. J Comp Neurol. 357:204–216. [DOI] [PubMed] [Google Scholar]
- Mowery TM, Kotak VC, Sanes DH. 2015. Transient hearing loss within a critical period causes persistent changes to cellular properties in adult auditory cortex. Cereb Cortex. 25:2083–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff WD, Casseday JH. 1977. Effects of unilateral ablation of auditory cortex on monaural cat's ability to localize sound. J Neurophysiol. 40:44–52. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Killackey HP, Kitzes LM. 1983. Ascending projections to the inferior colliculus following unilateral cochlear ablation in the neonatal gerbil, Meriones unguiculatus. J Comp Neurol. 214:144–153. [DOI] [PubMed] [Google Scholar]
- O'Neil JN, Limb CJ, Baker CA, Ryugo DK. 2010. Bilateral effects of unilateral cochlear implantation in congenitally deaf cats. J Comp Neurol. 518:2382–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DP, Irvine DR. 1983. Some features of binaural input to single neurons in physiologically defined area AI of cat cerebral cortex. J Neurophysiol. 49:383–395. [DOI] [PubMed] [Google Scholar]
- Polley DB, Thompson JH, Guo W. 2013. Brief hearing loss disrupts binaural integration during two early critical periods of auditory cortex development. Nat Commun. 4:2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. 2010. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 65:718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga RQ, Nadasdy Z, Ben-Shaul Y. 2004. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 16:1661–1687. [DOI] [PubMed] [Google Scholar]
- Reale RA, Brugge JF, Chan JC. 1987. Maps of auditory cortex in cats reared after unilateral cochlear ablation in the neonatal period. Brain Res. 431:281–290. [DOI] [PubMed] [Google Scholar]
- Russell FA, Moore DR. 1995. Afferent reorganisation within the superior olivary complex of the gerbil: development and induction by neonatal, unilateral cochlear removal. J Comp Neurol. 352:607–625. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Kretzmer EA, Niparko JK. 2005. Restoration of auditory nerve synapses in cats by cochlear implants. Science. 310:1490–1492. [DOI] [PubMed] [Google Scholar]
- Sarant J, Harris D, Bennet L, Bant S. 2014. Bilateral versus unilateral cochlear implants in children: a study of spoken language outcomes. Ear Hear. 35:396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan SG, Curhan GC, Eavey R. 2010. Change in prevalence of hearing loss in US adolescents. JAMA. 304:772–778. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Kral A. 2005. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 203:134–143. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. 1978. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol. 281:267–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Baxi JH, Hardie NA. 1999. Response of inferior colliculus neurons to electrical stimulation of the auditory nerve in neonatally deafened cats. J Neurophysiol. 82:1363–1380. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA, Blakemore C. 1981. The role of GABAergic inhibition in the cortical effects of monocular deprivation. Nature. 291(5813):318–320. [DOI] [PubMed] [Google Scholar]
- Silverman MS, Clopton BM. 1977. Plasticity of binaural interaction. I. Effect of early auditory deprivation. J Neurophysiol. 40:1266–1274. [DOI] [PubMed] [Google Scholar]
- Smith ZM, Delgutte B. 2007. Sensitivity to interaural time differences in the inferior colliculus with bilateral cochlear implants. J Neurosci. 27:6740–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharpe AM, Sladen DP. 2008. Causation of permanent unilateral and mild bilateral hearing loss in children. Trends Amplif. 12:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillein J, Heid S, Lang E, Hartmann R, Kral A. 2012. Development of brainstem-evoked responses in congenital auditory deprivation. Neural Plast. 2012:182767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillein J, Hubka P, Kral A. 2011. Sensitivity to interaural time differences with binaural implants: is it in the brain? Cochlear Implants Int. 12(Suppl 1):S44–S50. [DOI] [PubMed] [Google Scholar]
- Tillein J, Hubka P, Syed E, Hartmann R, Engel AK, Kral A. 2010. Cortical representation of interaural time difference in congenital deafness. Cereb Cortex. 20:492–506. [DOI] [PubMed] [Google Scholar]
- Tong L, Strong MK, Kaur T, Juiz JM, Oesterle EC, Hume C, Warchol ME, Palmiter RD, Rubel EW. 2015. Selective deletion of cochlear hair cells causes rapid age-dependent changes in spiral ganglion and cochlear nucleus neurons. J Neurosci. 35:7878–7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Juiz JM, Moore DR, Sanes DH. 2004. Unilateral cochlear ablation produces greater loss of inhibition in the contralateral inferior colliculus. Eur J Neurosci. 20:2133–2140. [DOI] [PubMed] [Google Scholar]
- Wie OB, Pripp AH, Tvete O. 2010. Unilateral deafness in adults: effects on communication and social interaction. Ann Otol Rhinol Laryngol. 119:772–781. [PubMed] [Google Scholar]
- Zhang J, Nakamoto KT, Kitzes LM. 2004. Binaural interaction revisited in the cat primary auditory cortex. J Neurophysiol. 91:101–117. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Godar SP, Litovsky RY. 2015. Development of sound localization strategies in children with bilateral cochlear implants. PLoS ONE. 10:e0135790. [DOI] [PMC free article] [PubMed] [Google Scholar]