Abstract
Nogo receptor 1 (NgR1) is expressed in forebrain neurons and mediates nerve growth inhibition in response to Nogo and other ligands. Neuronal activity downregulates NgR1 and the inability to downregulate NgR1 impairs long-term memory. We investigated behavior in a serial behavioral paradigm in mice that overexpress or lack NgR1, finding impaired locomotor behavior and recognition memory in mice lacking NgR1 and impaired sequential spatial learning in NgR1 overexpressing mice. We also investigated a role for NgR1 in drug-mediated sensitization and found that repeated cocaine exposure caused stronger locomotor responses but limited development of stereotypies in NgR1 overexpressing mice. This suggests that NgR1-regulated synaptic plasticity is needed to develop stereotypies. Ex vivo magnetic resonance imaging and diffusion tensor imaging analyses of NgR1 overexpressing brains did not reveal any major alterations. NgR1 overexpression resulted in significantly reduced density of mature spines and dendritic complexity. NgR1 overexpression also altered cocaine-induced effects on spine plasticity. Our results show that NgR1 is a negative regulator of both structural synaptic plasticity and dendritic complexity in a brain region-specific manner, and highlight anterior cingulate cortex as a key area for memory-related plasticity.
Keywords: cocaine sensitization, dendritic structure, NgR1, spatial memory, spine plasticity
Introduction
The ability of the brain to learn and to store memories is essential for our very existence, and alterations of synaptic structure are regarded as a key element in the formation of long-term memories (Holtmaat and Svoboda 2009; Xu et al. 2009; Yang et al. 2009). For new memories to form and consolidate, neuronal compartments, therefore, need to become temporarily plastic to allow alterations of synaptic and dendritic configurations. In contrast, the default condition in both gray and white matter appears to be one of nerve growth inhibition to maintain status quo. This inhibition is in part provided by the Nogo-signaling system (Schwab 2010; Mironova and Giger 2013). The Nogo-signaling machinery encompasses an increasing number of proteins. This system, and Nogo receptor 1 (NgR1) in particular, are strongly expressed in forebrain neurons endowed with marked plasticity also in adulthood in mice, rats, and humans alike (Fournier et al. 2001; Josephson et al. 2002; Laurén et al. 2003).
The forces that inhibit neuronal outgrowth have to be overcome for new connections to sprout. One mechanism to accomplish this appears to be activity-induced down-regulation of NgR1, as shown to occur in a variety of experimental conditions such as treatment with kainic acid or amphetamine, electroconvulsive stimulation, spinal cord injury, traumatic brain injury, running, and exposure to a novel environment (Josephson et al. 2003; Endo et al. 2009; Wills et al. 2012; Guo et al. 2013; Nordgren et al. 2013). A strong stimulus (kainic acid) also leads to upregulation of the NgR1 antagonist Lotus, possibly to further neutralize NgR1-mediated signaling (Karlsson, Koczy et al. 2013). Moreover, the neuronal activity induced by kainic acid upregulates NgR2 and NgR3 messenger RNA (Karlsson, Koczy et al. 2013), which should increase sensitivity to the white matter-specific inhibitor myelin associated glycoprotein (Venkatesh et al. 2005), and to chondroitin sulfate proteoglycans (Dickendesher et al. 2012), respectively. This suggests ways in which synaptic plasticity of individual synapses becomes restricted to very local territories (Karlsson, Koczy et al. 2013).
When NgR1 is absent, the ocular dominance shift phenomenon remains into adulthood in mice, showing that lack of NgR1 is associated with an increased degree of synaptic plasticity (McGee et al. 2005). Conversely, and consistent with such a role for NgR1, we found that overexpression of the NgR1 gene in forebrain neurons impairs the formation of lasting memories, suggesting impaired plasticity when NgR1 cannot be downregulated as needed (Karlén et al. 2009). Moreover, both Nogo-A and OMgp can attenuate long term potention (LTP) induction through NgR1 (Raiker et al. 2010; Zemmar et al. 2014), while blocking the Nogo-A-Δ20—S1PR2 signaling pathway markedly enhances LTP (Kempf et al. 2014), showing that this effect of Nogo can be mediated by both the Nogo-A-Δ20 and the Nogo 66 domains. It has also been shown that NgR1 limits the formation of excitatory synapses in neurons during development (Wills et al. 2012) and NgR1 has been implicated as a regulator of spine dynamics and maturation (Lee et al. 2008; Akbik et al. 2013).
In terms of behavioral consequences, the role of NgR1 has not been evaluated in a more demanding setting. Typically, mice are exposed to only one or two behavior tests, quite distinct from the complex and demanding learning tasks that take place in the wild. Here, we directly compare the performance of control mice with that of mice that either overexpress or lack NgR1 in a more demanding setting with an initial motor test, followed by a novel object recognition test and 2 different spatial memory tests, to test several different aspects of memory formation. The test battery engages partly different brain areas (motor control: cortical premotor/motor areas, striatum, cerebellum; object memory: cerebral cortex, hippocampus; spatial orientation: hippocampus, entorhinal cortex). In addition, in a separate experiment, we assessed the role of NgR1 overexpression in drug-induced plasticity. We show that overexpression of NgR1 can significantly impair spatial learning in this more natural sequential learning paradigm, while lack of NgR1 impairs novel object recognition and locomotor function (rescuable with training).
While a relationship between NgR1 availability and dendritic spine structure has been established, there is also lack of knowledge about the role of NgR1 for the formation of mature spines during development and in response to perturbations in adulthood, not the least with respect to differences between different brain areas. Likewise, there is a lack of information about the role of NgR1 for dendritic architecture.
Our hypothesis was that overexpression of NgR1 (causing an inability to down-regulate NgR1 when needed (Karlén et al. 2009) in forebrain neurons would inhibit structural synaptic plasticity, and that this in turn would impair learning. We address these issues by comparing control mice with constitutively NgR1 overexpressing mice and also by subjecting these 2 types of mice to a cocaine sensitization protocol, known to induce structural changes (Russo et al. 2010). We demonstrate that overexpression of NgR1 significantly alters both spine structure and density. Consistent with our hypothesis, the density of mature mushroom spines is decreased in all 3 brain areas analyzed. Spine alterations are paralleled by alterations of several structural parameters of the dendritic arbor itself in a brain area-specific manner. We also hypothesized that the plasticity blocking effect of NgR1 overexpression might be overcome by a sufficiently strong stimulus such as cocaine. While behavioral responses to cocaine, and effects of cocaine on spine and dendrite morphology were also found to be regulated by NgR1, we noted that cocaine seemed able to increase spine densities in NgR1 overexpressing mice, supporting our hypothesis. Notably, we find that NgR1 overexpressing mice develop less stereotypies in response to a cocaine sensitization protocol. Together, these findings show that NgR1 is a negative regulator of structural synaptic plasticity as well as dendritic complexity, and that not only dendritic spines but also the dendritic tree that carries them can become markedly rearranged by drugs in a short period of time in adult animals.
Experimental Procedures
Mice
Mice were housed with siblings in cages with a small house and tissues for nesting. Food and water were available ad libitum. Lights were set to a 12-h on/off alternating cycle. The NgR1 knockout mice (Nogo Receptor MTL B2) (Zheng et al. 2005) were kindly provided by Dr Marc Tessier-Lavigne. For the knockout studies, we chose to study NgR1+/− mice versus mice with complete lack of NgR1. We have carried out western blots to verify that the NgR1 protein levels in NgR1+/− mice are about half those found in NgR1+/+ mice (data not shown). The NgR1 knockout construct we obtained also carries LacZ. By comparing NgR1−/− and NgR1+/− animals, any possible influence of LacZ is minimized. This strategy to control for the insertion of exogenous genetic material was deemed “the most conservative approach” to study effects of altered NgR1 expression levels. Indeed, we found significant differences between the 2 studied genotypes in 3 of the 4 behavior tests carried out. Had we not found differences between the homo- and heterozygous mice in our tests, control mice with 2 intact NgR1 genes would have been needed, but since robust differences were found, we were able to conclude that the presence of NgR1 matters. A similar approach, comparing homozygous and heterozygous NgR1 knockout mice, has also been used by Strittmatter's group (Akbik et al. 2013).
To generate mice that overexpress NgR1 in the forebrain, we obtained mice that express the tetracycline transactivator under the control of the CamKII promoter (Jackson laboratories). These mice were crossed with mice that express a tetracycline-responsive element driving the expression of an NgR1 transgene, as previously described (Karlén et al. 2009). For the NgR1 overexpressing mice, the control mice consisted of monotransgenic mice carrying either the CamkII-tTA gene or the tetracycline-responsive element-NgR1 gene. We have previously compared these 2 types of controls to each other and to wild-type mice with respect to behavior parameters, and not found any differences. For both studies, the rational was to have control mice that had undergone genetic manipulations similar to those of the corresponding experimental groups. This strategy to control for the insertion of exogenous genetic material was deemed the most conservative approach to study the effects of altered NgR1 expression levels. When mixed genders were used, a possible gender effect was first examined and none was found for the current experimental conditions. A gender stratification of data analysis was therefore not used. The series of behavior tests was composed to challenge different types of learning related to different brain domains and functions, such as motor control areas (cortical motor areas, striatum, cerebellum), object memory (cerebral cortex, hippocampus), and spatial orientation (hippocampus, entorhinal cortex). All animal studies were approved by the Northern Stockholm Animal Ethical Committee.
Rotarod Performance
Mice were trained on the accelerating Rotarod for 5 days with 4 trials per day and allowed to rest 40 min between trials. The rod accelerated from 4 RPM to a maximum of 80 RPM in 7.5 min and the RPM at which the mouse fell off was recorded. If mice hung on to the rod, they were lightly touched and if they did not start running, the RPM at the start of the hanging behavior was recorded. Eighteen NgR1 overexpressing (10 males, 8 females) and 17 control (9 males, 8 females) mice were used. For the NgR1 knockout study, 22 NgR1−/− (12 males, 10 females) and 21 NgR1+/− (11 males, 10 females) mice were used.
Novel Object Recognition
The mice were first put into the arena (42 × 42 cm) for 10 min of habituation after which they were returned to their home cages. After 1 h, they were put back into the arena for familiarization with 2 identical objects for 10 min. They were again returned to their home cage for 1 h and then returned for the novel object phase, during which one of the two objects was an identical copy of the object used during the familiarization phase, and one object was new to the mouse. Mouse behavior was digitally recorded and the time mice spent investigating each object was obtained from visual observation of the movies by an individual blinded to the genotype and novelty of the object, and the preference for the objects was scored during 4 min (as determined by a pilot experiment). The inclusion criterion for the novel object test was that the total time spent to investigate both objects was at least 4 s. A total of 16 NgR1 overexpressing mice (8 males, 8 females) and 14 controls (7 males, 7 females) were used to study the effects of NgR1 overexpression. For the NgR1 knockout study, 12 knockouts (7 males, 5 females) and 11 controls (6 males and 5 females) were used.
Barnes Maze
Mice were placed at the center of a circular board (1.25 m in diameter) placed in a brightly lit room (Barnes 1979). The board has 36 holes along the outer perimeter and one of these holes allowed the mice to escape into a small compartment. The mice were trained during 5 days with 4 trials (180 s) per day. On the sixth day, the mice were subjected to a probe trial where the escape compartment had been removed, and the proportion of pokes into the previous escape hole and the 2 closest neighboring holes was used as a measure of escape hole memory. Mice that spent prolonged periods of time without significant locomotion were excluded from the test. For the NgR1 overexpression study, 12 NgR1 overexpressing mice (6 males, 6 females) and 17 controls (9 males, 8 females) met the inclusion criteria. For the NgR1 knockout study, 16 NgR1 null mice (12 males, 4 females) and 18 controls (11 males, 7 females) met the criteria.
Morris Water Maze
For the Morris water maze test (Morris 1984), mice were placed in a pool (1.8 m in diameter) at semi-random starting positions (south, east, north, and west; once each per day) and trained with 4 trials per day to find the location of a submerged escape platform (15 cm in diameter). On the first 2 days, the mice underwent a visual platform test with a curtain around the pool and with a flag on the platform. Only 1 mouse did not manage to complete this stage (1 Ngr1 knockout mouse that was removed from the rest of the study). At day 3, the mice were trained with the hidden version of the test without a visual landmark on the platform. The curtain was now removed and distal cues were placed around the pool to allow visual navigation. Mice were trained for 7 days for the hidden version of the test. Mice that did not actively swim (“floaters”) were removed from the study. Eleven NgR1 overexpressing mice (5 males, 6 females) and 16 controls (9 males, 7 females) met the criteria. Sixteen NgR1 knockout mice (10 males and 6 females) and 21 controls (12 males, 9 females, all heterozygous for the NgR1 null allele) used in the study of lack of NgR1 met the criteria.
Locomotor Sensitization to a Drug Challenge
Male NgR1 overexpressing mice and littermate controls were equally divided into cocaine (Sigma Aldrich, St Louis, MO, USA) and saline groups, and subjected to a locomotor sensitization paradigm initiated by 2 days of saline injections to investigate baseline locomotor activity in response to injections and a new environment. This was followed by 10 days of saline (10 mL/kg) or cocaine (15 mg/kg in 10 mL/kg saline) injections. The cocaine dose was chosen as it has been commonly used by others (Robinson and Kolb 2004). After each injection (day 1–12), locomotor activity (distance in cm) and stereotypic-like behavior (measured as repeated crossings of the same light beam as occurring during grooming, head bobbing, etc.) were registered in activity boxes (42 × 42 cm, Accuscan Instruments, OH, USA) during 1.5 h. The experimenter was blinded with regard to genotype until the final data were analyzed. For each of the 4 groups (control mice saline/cocaine and NgR1 overexpressing mice saline/cocaine), 24 mice were used. Four NgR1 overexpressing mice were removed from the analysis for being highly overactive in response to the first cocaine injections, and because the standard deviation of the 4 excluded mice was 4.3, 5.8, 6.5, and 4.0, respectively, above the mean of the included mice.
Spine and Dendrite Analyses
Mice were sacrificed 24 h after the last saline or cocaine injection (day 13) and brains were processed for Golgi staining (FD rapid GolgiStain Kit, FD NeuroTechnologies, Ellicott City, MD, USA) according to the manufacturer's manual. Tissues were cryosectioned at −25°C (Microm HM500M; Microm HM560; Thermo Scientific) into 160-µm-thick coronal sections.
Selection of Brain Areas
We chose 3 brain areas: frontal association cortex, due to its importance for decision-making and executive functions, the anterior cingulate cortex for its important role in the limbic system, and emotional integration, and the nucleus accumbens for its role in reward-associated learning. The choice of areas also reflects the cocaine sensitization arm of the experiment since the frontal association cortex and the nucleus accumbens are both strongly involved in the addictive response (Robinson and Kolb 2004) and since the cingulate cortex is strongly linked to cocaine use in both humans and rodents (Aron and Paulus 2007). We hypothesized that the plasticity blocking effect of NgR1 overexpression might be overcome by a sufficiently strong stimulus such as cocaine.
Selection of Neurons
Areas of interest were selected using dedicated software for dendrite and spine analysis (Neurolucida MBF Bioscience, VT, USA). Neurons suitable for analysis were randomly selected if fulfilling our inclusion criteria: Cortical pyramidal neurons should have their soma in layer 3, their apical dendrite should be contained in the section, and a substantial portion of the distal parts should not overlay other dendrites to allow accurate spine counting and avoid ambiguity in dendritic reconstruction. In nucleus accumbens, we targeted medium spiny projection neurons, the dominant class of neurons in this region (Robison and Nestler 2011). Within the selected region of interest, we randomly selected neurons with dendritic trees contained in the section and without the disturbance of other Golgi-stained cells.
Spine Analysis
Dendritic spines on distal dendrite branches, (≥fourth order dendrites) in nucleus accumbens, the cingulate cortex, and the frontal association cortex were analyzed by light microscopy (Zeiss Axio Imager M2 light microscope, Carl Zeiss Microscopy, Germany) and dedicated software (Neurolucida). Spines were quantified and morphologically categorized as filopodia (if clearly more than 2 times as long as wide), thin (if approximately twice as long as wide), or mushroom (showing a head at least 2× wider when compared with the neck) types. The filopodia-type spines were found to be too few to allow sufficient power for analysis and were therefore not included. Approximately 8 neurons were counted per brain and area. The analysis was blinded to genotype and drug treatment. A total of 77 452 spines from 1115 neurons were counted and classified (frontal association cortex: 368 neurons, cingulate cortex: 363 neurons, nucleus accumbens: 384 neurons, used to analyze 4 groups with 12 animals in each group). All counting and classification of spines was carried out by one trained individual.
Dendritic Tree Analyses
The Sholl analysis principle (Sholl 1953) was applied to the same populations of neurons as used for dendritic spine analysis using the same dedicated software as used for spine analysis (Neurolucida). In addition to 1) the Sholl analysis plot of dendritic complexity at different distances from the neuron soma, we also calculated 2) total dendrite length, 3) number of dendrite endings, and 4) the distribution of different dendrite lengths.
Ex Vivo Magnetic Resonance Imaging (MRI)
Formalin fixed brains were positioned in syringes filled with Fomblin (Solvay Solexis, Italy) to avoid image artifacts due to susceptibility mismatch, while providing a dark background. A horizontal 9.4 Tesla magnetic resonance scanner (Agilent, Yarnton, UK) equipped with a birdcage coil (16 mm inner diameter, Rapid Biomed, Rimpar, Germany) was used for data acquisition. A diffusion-weighted spin echo with diffusion-weighted gradients applied in 30 different directions, as well as a reference image with the diffusion encoding gradients set to zero, was used (TR = 2.1 s, TE = 20.65 ms, NEX = 4, matrix = 192 × 128, field of view = 19.2 × 19.2 mm2, 50 contiguous 0.3-mm-thick slices). The data were zero-filled to 256 × 256 points before Fourier transformation. The diffusion-weighted images were interpreted using the diffusion tensor model, and fractional anisotropy (FA) and mean diffusivity (MD) were analyzed (ImageJ, NIH) by a person blinded to the genotype. For the diffusion tensor imaging (DTI) and MR images shown, the levels were modified and nonbrain areas removed to achieve a clearer picture. The exact same settings were used for control and overexpressing brain images and these changes were not applied to the pictures used for analysis.
Statistical Analysis
Data were analyzed by t-tests when only 2 groups were compared (probe trials). Generalized linear models were used for all tests with multiple groups and generalized estimated equations were used for all longitudinal data. When appropriate, post hoc tests were performed with Bonferroni correction for multiple testing. All analyses were performed using the same analysis program (SPSS 22, IBM, USA).
Results
Within the brain, several different systems have the ability to form their own specific types of memories (Squire 2004). Mice are often tested with respect to only one of these different memory domains. While the Nogo system has been seen to affect learning in some of these different modalities (Mironova and Giger 2013), a thorough examination of the different aspects of memory formation has not yet been performed. We choose to subject mice with excess NgR1 and mice lacking NgR1 to several behavior tasks intended to test these different aspects of memory. The mice were first exposed to the Rotarod, a task that requires the basal ganglia, areas of the premotor, and motor cortex as well as cerebellum. This was followed by the novel object test that puts a high demand on frontal and parietal regions of the cerebral cortex as well as hippocampus. We then challenged the mice in 2 consecutive spatial tests that both require the hippocampus. We reasoned that repetitive spatial learning would put a higher demand on the hippocampal circuitry.
Rotarod Performance Is Impaired in NgR Knockout Mice
The behavioral testing began by the examination of motor learning of NgR1 overexpressing and knockout (NgR1−/−) mice using the accelerating Rotarod (Jones and Roberts 1968). Both control and overexpressing mice improved performance with training (Fig. 1A) without any significant effect of NgR1 overexpression (genotype P = 0.3, day P < 0.001 and genotype × day P = 0.5). As the mice were subjected to 4 trials per day, we thought that motivation might differ between the groups and performance on some trials might lower the average. Therefore, the maximal speed achieved during the entire training paradigm was also compared between the groups. This parameter supported our initial finding of no significant difference between genotypes (Fig. 1B, P = 0.22).
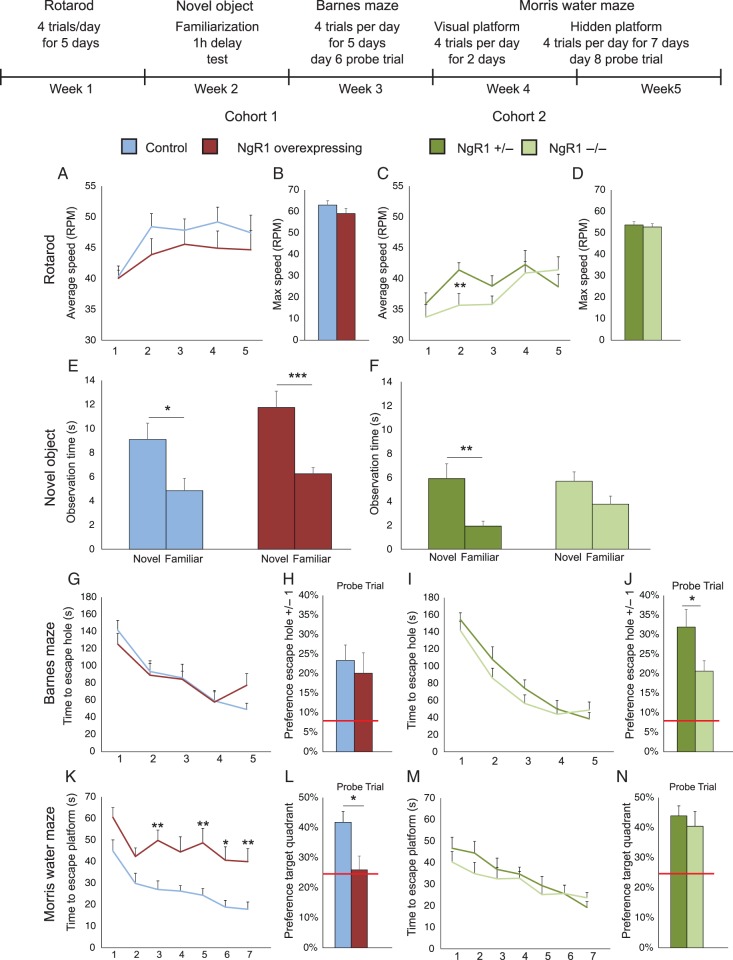
Figure 1.
Overexpression of NgR1 impairs sequential spatial learning, lack of NgR1 impairs locomotion and novel object recognition. This compound figure presents 2 separate studies, one in which NgR1 overexpressing mice were compared with litter mate controls, and a second study in which NgR1−/− mice were compared with littermate NgR+/− mice. Statistical comparisons are only made within, not between groups. At the top is shown a time line of the tests and the experimental steps of each test (A,C) Performance during 5 days (4 trials per day) of Rotarod training. (B,D) Average of the maximal speeds achieved by mice in the corresponding groups. (E,F) Novel object recognition, shown as time spent looking at a novel, compared with a familiar object. (G,I) Learning performance during 5 days of training in Barnes maze with 4 trials per day. (H,J) Probe trials performed 1 day after the training period. (K,M) Performance during 7 days of training in Morris water maze. (L,N) Probe trials of the same groups in Morris water maze carried out 1 day after the last training session. Red bars in (H,J,L,N) indicate chance levels of performance. *P < 0.05, **P < 0.01, ***P ≤ 0.001.
While NgR1 overexpression did not significantly affect performance, mice lacking NgR1 performed significantly worse during the initial phase of Rotarod training (Fig. 1C, genotype P = 0.34, day P < 0.001 and genotype × day P = 0.003) compared with control mice, heterozygous for the NgR1 null mutation (NgR1+/−). Toward the end of the training session, the performance of NgR1−/− mice improved to a similar level when compared with that of NgR1+/− mice. The initial deficit in Rotarod performance was thus rescued by training, and the maximum speed achieved was not significantly different between NgR1−/− and NgR1+/− mice (Fig. 1D, P = 0.66).
Novel Object Recognition Intact in NgR1 Overexpressing Mice, Impaired in NgR1 Knockout Mice
We next performed a novel object recognition test to assess if overexpression or lack of NgR1 would affect short-term (1 h) recognition memory. Both control and NgR1 overexpressing mice demonstrated a significant preference for the novel object (Fig. 1E, P = 0.034 and P < 0.001, respectively). The same was true for NgR1+/− mice (Fig. 1F, P = 0.003). Interestingly, NgR1−/− mice did not show a significant preference for the novel object (P = 0.467). This lack of significant novel object recognition in NgR1−/− mice was not due to a difference in the time spent looking at the novel object, instead NgR1−/− mice spent more time looking at the familiar object. To verify that the impairment seen in NgR1−/− mice was not caused by less exploration of the objects during the preceding familiarization phase, we compared the time the mice spent investigating each object during this first session, and found no significant difference (Supplementary Fig. 1, P = 0.16); if anything NgR1−/− mice spent more time than NgR1+/− mice investigating the objects. This suggests that the inability to downregulate NgR1 does not impair short-term (1 h) object memory, while complete lack of NgR1 does.
Sequential Spatial Learning Is Severely Impaired in NgR1 Overexpressing Mice
To assess spatial memory, we subjected the mice to 2 different behavioral tests; the Barnes maze followed by the Morris water maze. In the initial Barnes maze test, NgR1 overexpressing mice learned the task as well as controls (Fig. 1G, genotype P = 0.94, day P < 0.001 and genotype × day P = 0.22). To verify that the mice had learned the location of the escape hole, we performed a probe trial 1 day after the end of the learning period without the escape hole (day 6, Fig. 1H), and found no significant difference between the 2 groups (P = 0.46). NgR1−/− mice also learned the maze as well as their controls (Fig. 1I, genotype P = 0.34, day P < 0.001 and genotype × day P = 0.3). In the following probe trial, both groups showed strong memory for the former escape location. The NgR1+/− mice had a significantly stronger preference for the former escape location than NgR1−/− mice (Fig. 1J, P = 0.03). This difference was not caused by low preference in NgR1−/− mice, but instead due to very strong preference in the NgR1+/− group. This suggests that day-to-day learning is intact in both NgR1 over- and underexpressing mice.
To further investigate spatial memory abilities, we finally subjected the same cohorts of mice to the Morris water maze one week after the Barnes test. All groups started with 2 days of training with a visual platform and no difference was seen in how fast either group found the platform or in their swim speed (Supplementary Figure 2). After the visual platform session, the mice were trained for another 7 days in the Morris water maze with a hidden platform. In this second spatial memory test, the NgR1 overexpressing mice were significantly impaired (Fig. 1K, genotype P < 0.001, day P < 0.001, genotype × day P = 0.72). A probe trial performed the day after the training phase confirmed this deficit by showing that NgR1 overexpressing mice performed significantly worse than controls and (Fig. 1L, P = 0.013) no better than at chance level.
NgR1−/− mice learned the Morris water maze equally well as NgR1+/− mice (Fig. 1M, genotype P = 0.47, day P < 0.001, genotype × day P = 0.29). In the following probe trial, both groups demonstrated strong memory for the platform location (Fig. 1N) without any significant difference between the groups (P = 0.56).
Ex Vivo MRI Suggests Intact Gross Anatomy and White Matter Tracts in NgR1 Overexpressing Mice
While the consequences of a lack of NgR1 have been well studied, less is known about how overexpression of NgR1 in forebrain neurons may affect brain development and structure. To assess this, we performed T2-weighted ex vivo 9.4 T MRI scans of brains from NgR1 overexpressing and control mice. We first analyzed the gross anatomy of the brain and found no significant differences in the volume of the brainstem, cerebellum, cerebrum, or hippocampal formation (Fig. 2D). We also analyzed the thickness of the cerebral cortex at several different locations and found no significant effects related to overexpression of NgR1 (Fig. 2E).
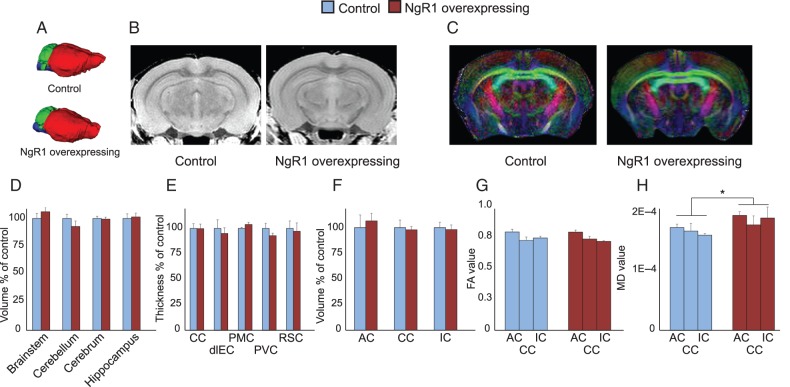
Figure 2.
Gross brain neuroanatomy is not affected by NgR1 overexpression. Ex vivo MRI analysis of brain parameters. (A) 3D representation of brain structures of control and NgR1 overexpressing mice. Forebrain (red), cerebellum (green), and brain stem (blue). (B) Representative T2 images used for measurement of cortical thickness. (C) Representative DTI images, used for white matter analysis. (D) Volumes of 4 major brain regions in NgR1 overexpressing mice compared with controls. (E) Thickness of 5 cortical areas in NgR1 overexpressing mice compared with controls. CC, cingulate cortex; dlEC, dorsolateral entorhinal cortex; PMC, premotor cortex; PVC, primary visual cortex; RSC, retrosplenial cortex (F) Volumes of 3 white matter tracts in NgR1 overexpressing mice compared with controls; AC, anterior commissure; CC, corpus callosum; IC, internal capsule. (G) Fractional anisotropy and (H) mean diffusivity of the same white matter areas as shown in (F). *P < 0.05.
The Nogo system is important for both myelin-based nerve growth inhibition and myelination per se (Chong et al. 2012). We therefore analyzed the size of some of the major axonal pathways in the brain to see if NgR1 overexpression had affected their structure. The volumes of corpus callosum, the anterior commissure, and the internal capsule were measured and found to be similar to those of control mice (Fig. 2F). To further investigate if NgR1 overexpression affects white matter structure, we measured FA, a measurement of uniformity of axonal tracts. No significant differences were detected in the 3 white matter regions investigated (Fig. 2G), suggesting that white matter uniformity had not been altered by the NgR1 transgene. We also measured MD, a measurement of how much diffusion of water is inhibited, and found a small, but significant increase in NgR1 overexpressing mice (Fig. 2H, genotype P = 0.02, region P = 0.546 genotype × region P = 0.675). Together, these findings indicate that overexpression of NgR1 from birth have very limited effects on gross neuroanatomy.
NgR1 Overexpression Enhances Locomotor Sensitization While Inhibiting the Development of Stereotypic Behavior
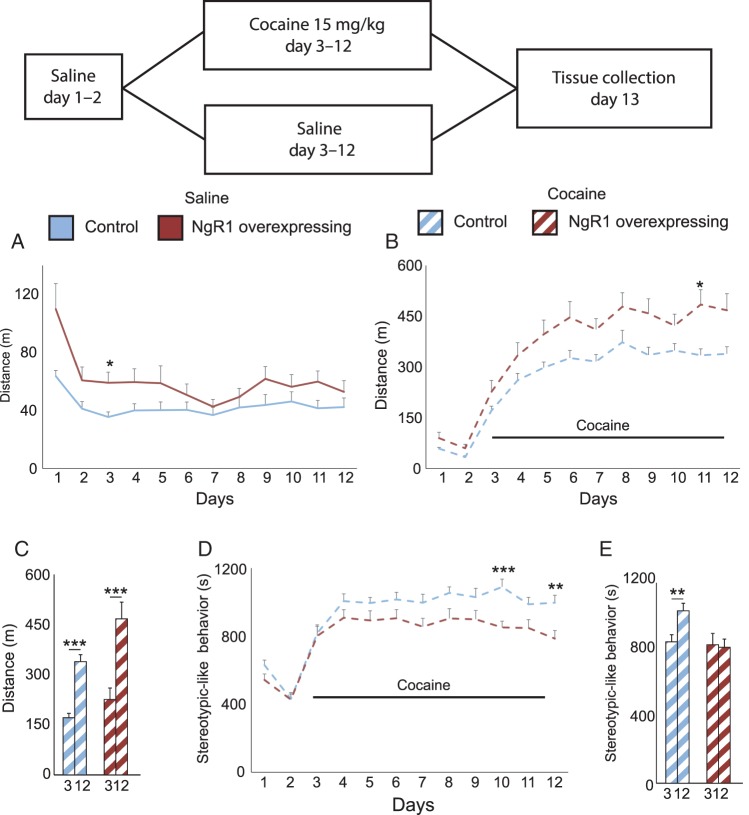
We subjected a new cohort of NgR1 overexpressing and control mice to a cocaine sensitization protocol in order to assess how NgR1 would affect the development of locomotor sensitization. The mice received either saline injections for 12 days or saline for the first 2 days, followed by cocaine for the next 10 days. NgR1 overexpressing mice displayed a stronger initial locomotion activity during the first days of saline treatment (Fig. 3A, genotype P = 0.035 day P < 0.001 and genotype × day P = 0.039). This normalized with time such that there was no significant difference in locomotion following saline injections at the end of the experiment. For the groups destined to be challenged with cocaine, we detected the same increased baseline locomotion of the NgR1 overexpressing mice during the initial 2 days of saline treatment (Fig. 3B, genotype P = 0.041, day P < 0.001 and genotype × day P = 0.641). When first injected with cocaine at day 3, both NgR1 overexpressing and control mice showed a characteristic increase in locomotion (Fig. 3B, genotype P = 0.005, day < 0.001 and genotype × day P = 0.487) and over the time course of the experiment NgR1 overexpressing mice developed and maintained a stronger locomotor response to cocaine than controls. To more clearly visualize the amount of sensitization, we compared the locomotion on day 3 with the locomotion on day 12 and could see that both groups sensitized in a strongly significant manner (Fig. 3C control P < 0.001 and NgR1 overexpressing P < 0.001).
Figure 3.
Excess NgR1 potentiates cocaine sensitivity while limiting the development of stereotypies. At the top is shown a time line for the test and information about treatments of the 2 groups. Locomotion of control and NgR1 overexpressing mice was monitored during (A) 12 days of saline injections or (B) 2 days of saline injections, followed by 10 days of cocaine injections. (C) Locomotion responses of control and NgR1 overexpressing mice to the first (day 3) and the last day (day 12) of cocaine. (D) The amount of time spent performing stereotypy-like behavior. (E) The amount of stereotypy-like behavior after the first and the last cocaine dose. *P < 0.05, **P < 0.01, ***P < 0.001.
We next asked if a difference in the amount of stereotypy-like movements might be the cause of the difference seen in locomotion between the groups. We did find that control mice significantly increased the time spent performing stereotypy-like movements from day 3 to day 12 (Fig. 3, genotype P = 0.007, day P < 0.001 and genotype × day P = 0.051) such that there was a clear difference between day 3 and 12 in controls but not in NgR1 overexpressing mice (Fig. 3E controls P = 0.0043; NgR1 overexpressing mice P = 0.85). This shows that the inability to downregulate NgR1 counteracts the drug-induced shift of behavior from locomotion to stereotypy-like behavior seen in control mice.
Forebrain NgR1 Levels Regulate Dendritic Spine Densities and Spine Responses to Cocaine
The day after the last injection, the saline- and cocaine-treated control and NgR1 overexpressing mice were sacrificed and brain sections from all 4 groups were Golgi-stained (Supplementary Fig. 3). The frontal association cortex, the cingulate cortex, and nucleus accumbens were selected (Supplementary Fig. 4) for spine and dendrite analysis.
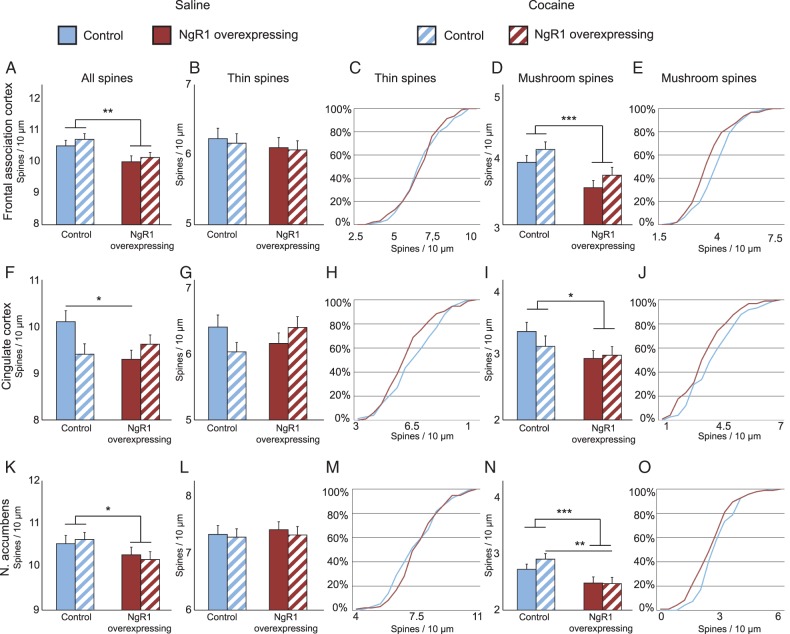
In the frontal association cortex, we found that the apical dendrites of pyramidal neurons had significantly lower spine densities in NgR1 overexpressing mice compared with control mice (Fig. 4A, genotype P = 0.003). To further analyze the cause of this reduction, we stratified spines into thin and mushroom types. There was no significant difference between controls and NgR1 overexpressing mice with regard to the density of thin spines (Fig. 4B). We plotted the frequency distribution of thin spine densities and could not see any effect of NgR1 overexpression on the density frequency distribution (Fig. 4C). In contrast to thin spine density, mushroom spine density was significantly reduced in NgR1 overexpressing mice (Fig. 4D, genotype P < 0.001). This caused a left shift of the density frequency distribution, showing that the reduction of mushroom spines had affected neurons with different mushroom spine densities evenly (Fig. 4E). Furthermore, there was a tendency for cocaine to increase the density of mushroom spines in both control and NgR1 overexpressing mice (treatment P = 0.088).
Figure 4.
NgR1 limits the number of mature mushroom spines in vivo; cocaine affects control and NgR1 overexpressing mice differently. Density of all spines (A,F,K), thin spines (B,G,L), and mushroom spines (D,I,N) in 3 brain areas from control and NgR1 overexpressing mice 24 h after last treatment with saline or cocaine. Frequency distribution charts for thin (C,H,M) and mushroom spines (E,J,O) (n > 60 neurons per group from 12 mice per group). Significances between groups with Bonferroni correction: *P < 0.05, **P < 0.01, ***P < 0.001.
In the cingulate cortex, we observed a lower spine density on the apical dendrites of pyramidal neurons in NgR1 overexpressing mice compared with controls (Fig. 4F). Interestingly, there was a significant interaction between genotype and cocaine in this area, such that while control mice subjected to cocaine showed a reduction in spine density compared with control mice treated with saline, the opposite was seen in NgR1 overexpressing mice (Fig. 4F, genotype × treatment P = 0.016). Unlike the case in frontal association cortex, the change of thin spine density in the cingulate cortex mirrored that seen for total spine density, by also showing a different response to cocaine depending on genotype (Fig. 4G, genotype × treatment P = 0.025) and density frequency distribution (Fig. 4H). We found a significantly lower density of mushroom spines in NgR1 overexpressing mice, similar to that seen in the frontal association cortex (Fig. 4I; genotype P = 0.046) but no interaction between genotype and treatment was found for mushroom spines. The spine density distribution chart for mushroom spines in saline-treated mice (Fig. 4J) showed a similar left shift for NgR1 overexpressing mice as that seen in the frontal association cortex.
In nucleus accumbens, the density of spines on medium spiny neuron dendrites was reduced in mice overexpressing NgR1 (Fig. 4K, genotype P = 0.045), but not significantly affected by cocaine treatment. The lower spine density of NgR1 overexpressing mice was not due to a reduction of thin spines as these were comparable between control and overexpressing mice (Fig. 4L,M). The difference in spine density was instead due to a significant decrease in the density of mushroom spines (Fig. 4N, genotype P < 0.001). The spine density distribution was again shifted to the left for NgR1 overexpressing mice (Fig. 4O). However, in accumbens, the shift was more pronounced among the group of neurons with the lowest spine densities. Notably, after cocaine sensitization, control mice showed a significantly higher mushroom spine density in nucleus accumbens than NgR1 overexpressing mice.
NgR1 Affects Dendritic Structure in the Cerebral Cortex but Not in Accumbens
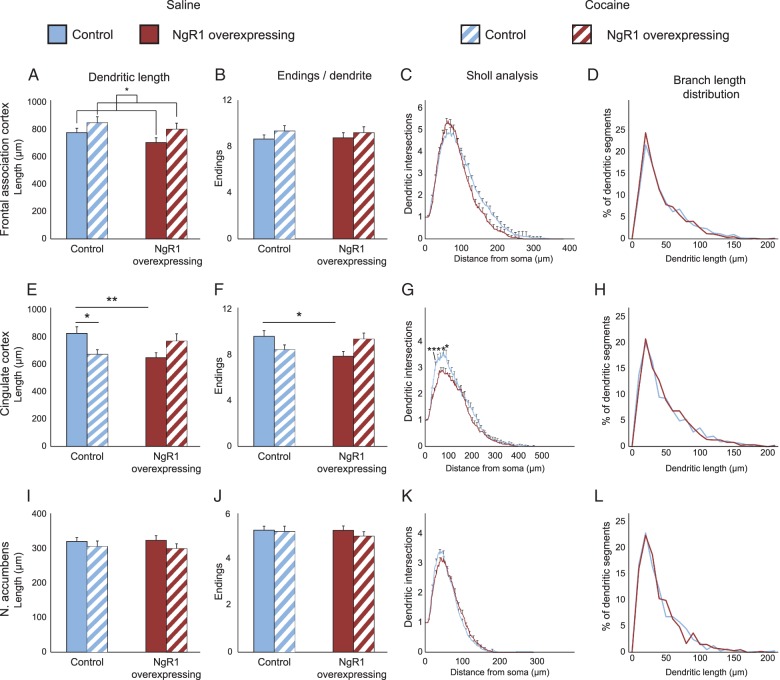
An important determinant of the afferent information to a neuron is the size and complexity of its dendritic arbor (Fig. 5). We, therefore, examined the total length of the dendritic tree, the distribution of lengths of the individual branches, the number of endings and as well as the complexity using Sholl analysis (representative examples of neurons in Supplementary Fig. 5).
Figure 5.
Dendritic length, number of branch points, and complexity are limited by NgR1. Same groups as in Figure 4. (A,E,I) Total length of dendrites of neurons used to analyze dendritic spines in 3 brain areas of control and NgR1 overexpressing mice treated with saline or saline followed by cocaine. (B,F,J) Number of dendritic endings per analyzed dendrite in the same 4 groups. (C,G,K) Sholl analysis of dendrite complexity with respect to distance from cell body. (D,H,L) Frequency distribution of different dendritic lengths. Significance between groups with Bonferroni correction: *P < 0.05, **P < 0.01.
In the frontal association cortex, the length of the dendritic tree tended to be shorter in NgR1 overexpressing mice than in controls (Fig. 5A, genotype P = 0.1). After mice had been exposed to cocaine, for 10 days, their dendritic trees increased significantly in length (P = 0.02). The number of endings per dendrite was also analyzed as a measure of dendritic complexity and did not change significantly in frontal association cortex (Fig. 5B). Sholl analysis of dendritic complexity showed that, for saline-treated animals, there was no difference between NgR1 overexpressing mice and controls (Fig. 5C, for the remaining groups see Supplementary Fig. 6). To see if NgR1 affected the growth of a subset of dendritic branches, we plotted a frequency distribution of the length of individual branches and found them to be very similar (Fig. 5D and remaining groups Supplementary Fig. 7).
In the cingulate cortex, control mice had significantly larger dendritic trees than NgR1 overexpressing mice after saline treatment (P = 0.008). For control mice, the exposure to cocaine resulted in a significant decrease in the length of the dendritic arbor (P = 0.037), while there was a nonsignificant increase in dendritic length in NgR1 overexpressing mice (Fig. 5E). The cocaine-induced decrease of dendritic length in control mice and the nonsignificant increase noted in NgR1 overexpressing mice together constituted a significant interaction between genotype and cocaine (genotype × treatment P = 0.001) in the cingulate cortex. This pattern was closely mirrored by the numbers of endings (Fig. 5F) that also exhibited a strong interaction between genotype and treatment (genotype × treatment P = 0.003). The significant decrease in dendritic length seen in saline-treated NgR1 overexpressing mice appear to be due to less branching as they had a significantly reduced number of dendritic endings (P = 0.02). There was a tendency for cocaine to reduce the endings in control mice (P = 0.07) while a small nonsignificant increase was noted in NgR1 overexpressing mice. Sholl analysis confirmed the reduction in dendritic complexity in NgR1 overexpressing mice (Fig. 5G, genotype P < 0.001). The dendritic branch length distribution analysis (Fig. 5H and Supplementary Fig. 7) showed no difference, indicating that NgR1 mostly affects formation of new branches and not the growth of existing ones.
In the nucleus accumbens, we found the dendrites to be much more stable and thus affected neither by overexpression of NgR1 nor by the exposure to cocaine. Dendrite length, number of dendrite endings, Sholl analysis, and dendrite branch length frequency analysis were all similar between the groups (Fig. 5I–L).
Together, the analysis of dendritic architecture shows that the 3 analyzed brain areas differ markedly with respect to NgR1 and cocaine effects on dendritic structure and identifies cingulate cortex as an area with a profound role of NgR1 signaling for cocaine-induced plasticity of dendrite architecture. Our data reveal profound effects of NgR1 levels on dendritic architecture and show that cocaine can effectively alter dendritic architecture in adult animals in an NgR1-dependent manner.
Discussion
Research during the last decade has provided strong support for a role for the Nogo-signaling system, and particularly NgR1, in plasticity underlying learning (Mironova and Giger 2013; Schwab and Strittmatter 2014). NgR1 has been shown to be temporarily downregulated in a number of neuron-activating situations, including physiological conditions and following central nervous system (CNS) lesions, drug exposure, or electroconvulsive stimuli (Josephson et al. 2003; Endo et al. 2009; Wills et al. 2012; Guo et al. 2013; Nordgren et al. 2013). Since NgR1 downregulation appears to be closely coupled to many forms of neuronal activation, we previously generated mice with an additional NgR1 gene, driven by the Cam kinase II promoter. While the endogenous NgR1 gene is downregulated by activity also in these mice, the transgene is not. We found that transgenic NgR1 overexpression, presumably by counteracting downregulation of the endogenous NgR1 gene, impaired the formation of long-term memory (Karlén et al. 2009). This supports previous as well as later findings that loss of NgR1 instead enhances cortical plasticity (McGee et al. 2005; Wills et al. 2012; Akbik et al. 2013; Zemmar et al. 2014) and learning (Akbik et al. 2013; Zemmar et al. 2014). Another study has reported learning impairments in NgR1−/− mice (van Gaalen et al. 2012), which is conceivable as a result of too much plasticity, which might impair consolidation and long-term maintenance of newly formed circuitries. In support of a role for Nogo signaling in learning, there is also evidence that rats with age-related cognitive impairment have an increased expression of proteins from the Nogo family (VanGuilder et al. 2011).
While a role for NgR1 in plasticity appears well established in principle, there are also partially contradictory results. Information has also been limited with regard to the relative role of NgR1 downregulation for synaptic plasticity in different brain areas, and to which degree not only spines but also the dendritic tree itself is affected. To address these issues, we took advantage of mice overexpressing NgR1 in forebrain neurons to obtain a better understanding of when and where NgR1 levels matter for behavior and gray matter plasticity. We subjected control mice and mice with overexpression or underexpression of NgR1 to a variety of different memory tests in sequence, starting with a motor skill test, followed by a recognition memory test and ending with 2 spatial memory tests, thereby testing several aspects of memory function in the same mice. The entire test battery took approximately 1 month, considerably more time than most test batteries of behavior. We subjected a different group of control and NgR1 overexpressing mice to either a saline or a cocaine sensitization protocol and studied cocaine-induced behaviors as well as spine and dendrite structure in all 4 groups. Taken together, our studies demonstrate key roles for NgR1 in forebrain neurons for the ability to manage hippocampus-dependent tests under demanding conditions and for reactions to drugs of abuse. Importantly, we demonstrate brain area-specific dendritic and spine density alterations caused by NgR1 overexpression and by cocaine.
NgR1 Overexpression Does Not Disturb Gross Brain Anatomy or Animal Vitality
To verify that NgR1 overexpression does not impair overall brain development, we performed high-resolution ex vivo MRI analyses of the brains of adult NgR1 overexpressing and control mice and found no significant differences in the size of selected gray and white matter brain regions. This shows that the presence of the NgR1 transgene does not cause any major disturbances of brain development. The only significant finding was a slightly higher MD value in white matter, but as the FA value was not affected, our data suggest normal or near-normal overall condition of both gray and white matter. These observations and the generally healthy appearance and behavior of NgR1 overexpressing mice show that such overexpression during development and in adulthood does not severely impair vital qualities of life.
Lacking NgR1 Modestly Impairs Motor Learning, Training Rescues It
In the initial Rotarod test, we saw no significant difference in performance between NgR1 overexpressing mice and controls. Mice lacking NgR1 had a modest, but significant impairment of early motor performance as measured by the Rotarod. These findings are consistent with previous studies (Kim et al. 2004; Akbik et al. 2013; Park et al. 2014). However, we found that intense training allowed NgR1−/− mice to reach a level of performance equal to that of NgR1+/− controls, but no tendencies of faster learning. Thus, forebrain overexpression of NgR1 does not impair motor learning, and training rescues the NgR1−/− motor endophenotype.
Lacking NgR1 Impairs Short-Term Object Memory
Similar to their performance in the Rotarod test, NgR1 overexpressing mice were not impaired in a novel object test, suggesting that the presence of the NgR1 transgene does not impair recognition memory. Surprisingly, NgR1−/− mice did not show a significant preference for the novel object, while NgR1+/− mice did. This was not due to a lack of exploratory behavior as the NgR1−/− mice spent more time investing the objects during the familiarization phase. Furthermore, the lack of preference was not due to fewer visits to the novel object than made by the NgR1+/− control mice, but to a higher interest also in the “familiar” object. A likely reason is that the objects were not memorized well enough by NgR1−/− mice during the familiarization phase. Hence, while NgR1−/− mice continued to view the familiar object as interesting, NgR1+/− did not. These findings broaden the understanding of memory disturbances caused by lack of NgR1, and, when combined with the indication of impaired working memory in NgR1−/− mice (Budel et al. 2008), suggest that lack of NgR1 causes disturbances of short-term memory.
NgR1 Overexpression Limits Spatial Memory Capacity
We have previously shown that NgR1 overexpression does not impair learning in the Morris water maze but impairs the formation of lasting memories (Karlén et al. 2009; Karlsson, Karlen et al. 2013). When we now increased difficulty by subjecting the mice to 4 consecutive tests, the third being Barnes maze, directly followed by Morris water maze, the performance of NgR1−/− mice was as good as that of NgR1+/− mice; both groups showed efficient learning in both mazes. NgR1 overexpressing mice navigated the Barnes maze as well as control mice, supporting our previous finding that day-to-day development of spatial memory is not impaired. However, while NgR1 overexpressing mice had no problem to learn the visual version of Morris water maze, they did not manage to learn how to navigate the Morris water maze when the platform was hidden. The finding that NgR1 overexpressing mice are not impaired in Barnes maze strengthens our previous findings that NgR1 overexpression does not impair learning of a spatial memory. There are several possible reasons that overexpressing mice are impaired in Morris water maze when subjected to this test directly after having undergone Barnes maze test but not when Morris test is the only test. For instance, overexpressing mice might not be capable of learning a new escape strategy. We find this to be unlikely, however, as NgR1 overexpressing mice had no problem learning to swim to the visual platform. Alternatively, the NgR1 overexpressing mice could have a reversal problem when using similar ques for a different test. However, NgR1 overexpressing mice are not impaired in reversal learning when tested only in Morris water maze (Karlén et al. 2009). Another possibility is that the decreased number of mushroom spines caused by NgR1 overexpression (see Discussion) may become insufficient as memory tasks accumulate. This is supported by the fact that learning of a spatial task correlates well with the number of mature mushroom spines (Mahmmoud et al. 2015).
To summarize findings from the series of behavior tests, lack of NgR1 appears to result in a slight impairment in motor skill that can be overcome by training, and an impairment in recognition memory, while spatial learning is not affected. In contrast, overexpression of NgR1 does not affect motor learning or recognition memory, but diminishes the ability to learn multiple spatial tests.
NgR1 Overexpression Alters Responses to Cocaine Sensitization
We next asked if NgR1 overexpression would modulate the behavioral response to a strong and addictive psychostimulant and subjected control and overexpressing mice to a cocaine sensitization paradigm. The first dose of cocaine caused proportionally similar increases of locomotion behavior in control and NgR1 overexpressing mice. However, during the next 3 days of cocaine treatments, overexpressing mice increased their cocaine responses more than controls although both controls and NgR1 overexpressing mice showed strongly significant sensitization. For control mice, the increased locomotion was paralleled with an increase in stereotypic-like behavior. This was not the case for NgR1 overexpressing mice that kept their stereotypic behavior at a constant level, leaving more time for the overexepressing mice to perform locomotive behavior. This increased sensitization of NgR1 overexpressing mice suggests that a persistent high level of NgR1 impairs the ability to adapt to repeated cocaine treatments.
Increased Versus Decreased Nogo-A—NgR1 Signaling: Opposite Effects on Spines and Dendritic Architecture?
Recent work from several laboratories suggest that the Nogo-A—NgR1 signaling pathway plays a pivotal role in maintaining synaptic circuitry and regulating experience-dependent structural plasticity as needed in CNS gray matter. Using Golgi staining and in vivo two-photon imaging, alterations of dendritic structure, and density and turnover of dendritic spines have been described in response to removal/neutralization of Nogo-A or NgR1. The overall finding of decreasing Nogo-A signaling is increased size of dendritic arbors, and in certain models increased spine density or a shift toward more immature spine types (Papadopoulos et al. 2006; Zagrebelsky et al. 2010; Petrinovic et al. 2013). Conversely, overexpression of Nogo-A has been shown to lead to smaller dendritic trees in the cerebellum and while we investigated a different area, it appears that overexpression of NgR1 phenocopies the effects on Nogo-A overexpression, strengthening the support for Nogo-A to NgR1 signaling as an important regulator for dendritic structure (Petrinovic et al. 2013).
As for NgR1, structural studies at the dendrite and spine level have previously only been carried out following receptor deletion, and the picture is less clear. Studies have reported no effect on total spine counts, but a shift toward a decreased density of thin and mushroom spines and an increase of stubby spines (Lee et al. 2008), increased experience-dependent spine turnover and enhanced learning without marked effects on spine density (Akbik et al. 2013), or no effect on spine turnover or spine stability, despite altered memory functions (Park et al. 2014). Still others have reported learning impairments in NgR1−/− mice (van Gaalen et al. 2012), which is conceivable as a result of too much plasticity, impairing consolidation and long-term maintenance of newly formed circuitries. In support of a role for Nogo signaling in learning, there is also evidence that rats with age-related cognitive impairment have an increased expression of Nogo family proteins (VanGuilder et al. 2011).
Here, we provide a missing link for the understanding of the structural effects of altered Nogo-A—NgR1 signaling at the spine and dendrite level, by examining the effects of increased NgR1 levels. This approach is particularly relevant for NgR1, because its dynamic involvement in the regulation of synaptic plasticity appears to be temporary downregulation, rather than upregulation episodes, in response to increased neuronal activity (Josephson et al. 2003). Further support for the rationale to study structural sequelae of NgR1 overexpression is our finding that inability to downregulate NgR1 impairs formation of lasting memories (Karlén et al. 2009).
General Reductions of Mature Spine Densities in Brain Areas of NgR1 Overexpressing Mice
To explain the behavioral effects of NgR1 overexpression in forebrain neurons, we hypothesized that the increased NgR1 levels would lead to decreased spine densities and possibly smaller dendritic trees in affected brain areas. To investigate this, we studied adult control and NgR1 overexpressing mice. We added structural analysis of treatment of adult mice with cocaine, a drug known to induce synaptic plasticity (Robinson and Kolb 2004). Three areas were chosen due to their strong involvement in decision-making (frontal association cortex), emotional regulation, and “top-down control of memory retrieval” (cingulate cortex), as well as rewarding behavior (nucleus accumbens) (Robinson and Kolb 2004; Russo et al. 2010; Rajasethupathy et al. 2015). Our detailed analysis of spines and dendritic trees of neurons in these 3 brain regions showed that the presence of an NgR1 transgene that is not downregulated by neuronal activity, and thus presumably counteracts plastic events (Karlén et al. 2009), leads to reduced density of mushroom-type (mature) spines, consistent across the frontal association cortex, the cingulate cortex, and nucleus accumbens. This resulted in an overall lower spine density in overexpressing mice when compared with controls. We conclude that NgR1 is a robust limiter of the number of mature dendritic spines in vivo with clear functional consequences.
The analyses of secondary and tertiary dendrites in dorsal CA1 in NgR1−/− mice have shown increased density of stubby spines at the expense of thin and mushroom spines (Lee et al. 2008), while there was no effect on total spine density. In the barrel cortex, neither spine density nor distribution of spine types was found to be affected by loss of NgR1 (Akbik et al. 2013). While the loss of NgR1 therefore does not appear to affect total spine density and to have different effects on spine types in different regions, we can now show that NgR1 overexpression in forebrain neurons results in robustly reduced total spine density and a reduction in mature mushroom spines in 3 out of 3 investigated brain areas.
Area-Specific Alterations of Dendritic Structure in NgR1 Overexpressing Mice
Our observation that dendritic length in saline-treated NgR1 overexpressing mice was decreased in the cingulate cortex, and that there was a similar tendency in the frontal association cortex, but not in the subcortical nucleus accumbens shows that the effects of NgR1 vary between brain regions and/or types of neurons. The normal expression of NgR1 in accumbens is very low (Barrette et al. 2007). One possibility for the difference seen in accumbens between spines (decreased density) and dendrite lengths (not affected) could thus be that presynaptic NgR1 in afferent projections regulates the formation of mushroom spines on the dendrites of accumbal neurons, while effects on dendrite structure may require increased NgR1 on the dendrites themselves (as is the case in the 2 cortical areas studied).
In the most NgR1 responsive area in our study, the cingulate cortex, dendritic branching was decreased by NgR1 overexpression, and Sholl analysis showed decreased dendritic complexity. Thus, NgR1 overexpression decreased all 3 investigated dendritic parameters, length, branching, and complexity in this area. The difference in dendritic length did not seem to be due to the growth of individual dendritic branches, but instead appeared to most closely correlate with the number of endings, indicating a role for NgR1 in the initiation of branching but not in the prolongation of the segments. This is in agreement with previous studies, indicating that NgR1 is important for the initial steps in outgrowth, but not neurite growth per se (Chivatakarn et al. 2007).
NgR1 Overexpression Alters Structural Reponses to Cocaine in the Cingulate Cortex
The cocaine sensitization protocol increased dendritic length in frontal association cortex. Thus adult pyramidal neurons can undergo such alterations in response to a 10-day drug treatment. The tendencies for cocaine to increase mushroom spines in frontal association cortex and nucleus accumbens are in line with the findings by others (Russo et al. 2010). However, the most striking structural effects of cocaine sensitization were observed in the cingulate cortex, in which cocaine decreased spine densities in control mice, but increased spine densities in NgR1 overexpressing mice. A similar pattern was observed with respect to dendrite lengths and dendrite endings. The significantly shorter dendrite lengths and dendrite endings seen in saline-treated NgR1 mice compared with saline-treated control mice were no longer significant in the corresponding cocaine-treated groups. This suggests that a strong stimulus, such as cocaine, can overcome the inhibitory effect of NgR1 overexpression and partially normalize the cell although spine densities in NgR1 overexpressing mice do not exceed densities found in nontreated control animals. The fact that cocaine decreases spine densities in the cingulate cortex of control mice might reflect the ability of control mice to dampen the sensitization effect of repeated cocaine injections more effectively than NgR1 overexpressing mice.
Conclusions
Taken together, we show that constitutive overexpression of NgR1 significantly limits learning and the density of mature spines in forebrain neurons in all areas examined (frontal association cortex, cingulate cortex, and nucleus accumbens) as well as limits dendritic branching. We also show that NgR1 overexpression blocks the emergence of stereotypic-like behavior induced by cocaine and alters structural responses to this drug. A role for NgR1 emerges as a master regulator of synaptic plasticity and memory, also in the adult animal, and identifies the cingulate cortex as a key brain area for drug-induced NgR1-regulated dendritic and spine plasticity.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This study is supported by an ERC Advanced Investigator grant (322744; L.O.), the Swedish Research Council (K2012-62X-03185-42-4; L.O.), Swedish Brain Power (L.O., T.K.), StratNeuro, Wings for Life, Karolinska Institutet Research Foundations (L.O.), the Swedish Brain Foundation (A.J.), NIH grant 5R21DA030067 (S.B.), and the Karolinska DPA (L.O.). The MRI scanning was performed at the Department of Comparative Medicine/Karolinska Experimental Research and Imaging Centre at Karolinska University Hospital, Solna, Sweden. Funding to pay the Open Access publication charges for this article was provided by the Swedish Research Council.
Supplementary Material
Notes
We thank Peter Damberg and Sahar Nikkhou Aski for performing the ex vivo MRI and DTI scans, and Karin Pernold, Karin Lundströmer, Eva Lindqvist, and Margareta Widing for technical support. Conflict of Interest: None declared.
References
- Akbik F, Bhagat S, Patel P, Cafferty W, Strittmatter S. 2013. Anatomical plasticity of adult brain is titrated by Nogo Receptor 1. Neuron. 77:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron JL, Paulus MP. 2007. Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 102(Suppl 1):33–43. [DOI] [PubMed] [Google Scholar]
- Barnes CA. 1979. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 93:74–104. [DOI] [PubMed] [Google Scholar]
- Barrette B, Vallieres N, Dube M, Lacroix S. 2007. Expression profile of receptors for myelin-associated inhibitors of axonal regeneration in the intact and injured mouse central nervous system. Mol Cell Neurosci. 34:519–538. [DOI] [PubMed] [Google Scholar]
- Budel S, Padukkavidana T, Liu B, Feng Z, Hu F, Johnson S, Lauren J, Park J, McGee A, Liao J et al. 2008. Genetic variants of Nogo-66 receptor with possible association to schizophrenia block myelin inhibition of axon growth. J Neurosci. 28:13161–13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivatakarn O, Kaneko S, He Z, Tessier-Lavigne M, Giger RJ. 2007. The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J Neurosci. 27:7117–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SY, Rosenberg SS, Fancy SP, Zhao C, Shen YA, Hahn AT, McGee AW, Xu X, Zheng B, Zhang LI et al. 2012. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci USA. 109:1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickendesher T, Baldwin K, Mironova Y, Koriyama Y, Raiker S, Askew K, Wood A, Geoffroy C, Zheng B, Liepmann C et al. 2012. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 15:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Tominaga T, Olson L. 2009. Cortical changes following spinal cord injury with emphasis on the Nogo signaling system. Neuroscientist. 15:291–299. [DOI] [PubMed] [Google Scholar]
- Fournier A, GrandPre T, Strittmatter S. 2001. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 409:341–346. [DOI] [PubMed] [Google Scholar]
- Guo M, Xue B, Jin D, Mao L, Wang J. 2013. Dynamic downregulation of Nogo receptor expression in the rat forebrain by amphetamine. Neurochem Int. 633:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. 2009. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 10:647–658. [DOI] [PubMed] [Google Scholar]
- Jones BJ, Roberts DJ. 1968. The quantitative measurement of motor inco-ordination in naive mice using an accelerating rotarod. J Pharm Pharmacol. 20:302–304. [DOI] [PubMed] [Google Scholar]
- Josephson A, Trifunovski A, Scheele C, Widenfalk J, Wahlestedt C, Brene S, Olson L, Spenger C. 2003. Activity-induced and developmental downregulation of the Nogo receptor. Cell Tissue Res. 311:333–342. [DOI] [PubMed] [Google Scholar]
- Josephson A, Trifunovski A, Widmer H, Widenfalk J, Olson L, Spenger C. 2002. Nogo-receptor gene activity: cellular localization and developmental regulation of mRNA in mice and humans. J Comp Neurol. 453:292–304. [DOI] [PubMed] [Google Scholar]
- Karlén A, Karlsson T, Mattsson A, Lundstromer K, Codeluppi S, Pham T, Backman C, Ogren S, Aberg E, Hoffman A et al. 2009. Nogo receptor 1 regulates formation of lasting memories. Proc Natl Acad Sci USA. 106:20476–20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson TE, Karlen A, Olson L, Josephson A. 2013. Neuronal overexpression of Nogo receptor 1 in APPswe/PSEN1(DeltaE9) mice impairs spatial cognition tasks without influencing plaque formation. J Alzheimers Dis. 33:145–155. [DOI] [PubMed] [Google Scholar]
- Karlsson TE, Koczy J, Brene S, Olson L, Josephson A. 2013. Differential conserted activity induced regulation of Nogo receptors (1-3), LOTUS and Nogo mRNA in mouse brain. PLoS One. 8:e60892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf A, Tews B, Arzt ME, Weinmann O, Obermair FJ, Pernet V, Zagrebelsky M, Delekate A, Iobbi C, Zemmar A et al. 2014. The sphingolipid receptor S1PR2 is a receptor for Nogo-a repressing synaptic plasticity. PLoS Biol. 12:e1001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Liu B, Park J, Strittmatter S. 2004. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 44:439–451. [DOI] [PubMed] [Google Scholar]
- Laurén J, Airaksinen M, Saarma M, Timmusk T. 2003. Two novel mammalian Nogo receptor homologs differentially expressed in the central and peripheral nervous systems. Mol Cell Neurosci. 24:581–594. [DOI] [PubMed] [Google Scholar]
- Lee H, Raiker SJ, Venkatesh K, Geary R, Robak LA, Zhang Y, Yeh HH, Shrager P, Giger RJ. 2008. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 28:2753–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmmoud RR, Sase S, Aher YD, Sase A, Groger M, Mokhtar M, Hoger H, Lubec G. 2015. Spatial and working memory is linked to spine density and mushroom spines. PLoS One. 10:e0139739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee A, Yang Y, Fischer Q, Daw N, Strittmatter S. 2005. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 309:2222–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironova YA, Giger RJ. 2013. Where no synapses go: gatekeepers of circuit remodeling and synaptic strength. Trends Neurosci. 36:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 11:47–60. [DOI] [PubMed] [Google Scholar]
- Nordgren M, Karlsson T, Svensson M, Koczy J, Josephson A, Olson L, Tingstrom A, Brene S. 2013. Orchestrated regulation of Nogo receptors, LOTUS, AMPA receptors and BDNF in an ECT model suggests opening and closure of a window of synaptic plasticity. PLoS One. 8:e78778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos C, Tsai S, Cheatwood J, Bollnow M, Kolb B, Schwab M, Kartje G. 2006. Dendritic plasticity in the adult rat following middle cerebral artery occlusion and Nogo-a neutralization. Cereb Cortex. 16:529–536. [DOI] [PubMed] [Google Scholar]
- Park J, Frantz M, Kast R, Chapman K, Dorton H, Stephany C-É, Arnett M, Herman D, McGee A. 2014. Nogo receptor 1 limits tactile task performance independent of basal anatomical plasticity. PLoS One. 9:e112678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrinovic MM, Hourez R, Aloy EM, Dewarrat G, Gall D, Weinmann O, Gaudias J, Bachmann LC, Schiffmann SN, Vogt KE et al. 2013. Neuronal Nogo-A negatively regulates dendritic morphology and synaptic transmission in the cerebellum. Proc Natl Acad Sci USA. 110:1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiker S, Lee H, Baldwin K, Duan Y, Shrager P, Giger R. 2010. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 30:12432–12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P, Sankaran S, Marshel JH, Kim CK, Ferenczi E, Lee SY, Berndt A, Ramakrishnan C, Jaffe A, Lo M et al. 2015. Projections from neocortex mediate top-down control of memory retrieval. Nature. 526:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T, Kolb B. 2004. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 47(Suppl 1):33–46. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. 2011. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 12:623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S, Dietz D, Dumitriu D, Morrison J, Malenka R, Nestler E. 2010. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 33:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M. 2010. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 11:799–811. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Strittmatter SM. 2014. Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol. 27:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. 1953. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Squire LR. 2004. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 82:171–177. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Relo AL, Mueller BK, Gross G, Mezler M. 2012. NOGO-66 receptor deficient mice show slow acquisition of spatial memory task performance. Neurosci Lett. 510:58–61. [DOI] [PubMed] [Google Scholar]
- VanGuilder H, Farley J, Yan H, Kirk C, Mitschelen M, Sonntag W, Freeman W. 2011. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 43:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Lee H, Joshi P, Kantor D, Newman B, Mage R, Rader C, Giger R. 2005. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 25:808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills Z, Mandel-Brehm C, Mardinly A, McCord A, Giger R, Greenberg M. 2012. The nogo receptor family restricts synapse number in the developing hippocampus. Neuron. 73:466–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. 2009. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 462:915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan W. 2009. Stably maintained dendritic spines are associated with lifelong memories. Nature. 462:920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagrebelsky M, Schweigreiter R, Bandtlow C, Schwab M, Korte M. 2010. Nogo-A stabilizes the architecture of hippocampal neurons. J Neurosci. 30:13220–13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemmar A, Weinmann O, Kellner Y, Yu X, Vicente R, Gullo M, Kasper H, Lussi K, Ristic Z, Luft A et al. 2014. Neutralization of nogo-a enhances synaptic plasticity in the rodent motor cortex and improves motor learning in vivo. J Neurosci. 34:8685–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Atwal J, Ho C, Case L, He X, Garcia K, Steward O, Tessier-Lavigne M. 2005. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci USA. 102:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.