Abstract
Introduction
Patients with systemic lupus erythematosus (SLE) with B-lymphocyte stimulator (BLyS) levels ≥ 2 ng/mL are at increased risk of flare. A regression analysis was undertaken to identify routine clinical measures that correlate with BLyS ≥ 2 ng/mL. Efficacy and safety of belimumab 10 mg/kg were examined in patients with BLyS ≥ 2 ng/mL and < 2 ng/mL.
Methods
Data from BLISS-52 and -76 (N = 1684) were pooled post hoc. A univariate logistic regression was employed to identify factors predictive of baseline BLyS ≥ 2 ng/mL. Factors significant at the 0.05 level then entered a stepwise logistic regression as covariates. Efficacy endpoints included SLE responder index (SRI), ≥ 4-point reduction in Safety of Estrogens in Lupus National Assessment–Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) and risk of severe flare over 52 weeks. Adverse events (AEs) were analyzed for each treatment arm and BLyS subgroup.
Results
Baseline predictors of BLyS ≥ 2 ng/mL included positive anti-Smith (≥ 15 U/mL), low complement (C) 3 (< 900 mg/L), anti-double-stranded DNA (anti-dsDNA) 80–200 and ≥ 200 IU/mL, immunosuppressant usage, proteinuria, elevated C-reactive protein (CRP), and low total lymphocyte count for all patients. Belimumab 10 mg/kg led to significantly greater SRI responses over 52 weeks versus placebo in both BLyS subgroups, though treatment differences were numerically greater at Week 52 in the BLyS ≥ 2 ng/mL group (24.1%, p < 0.0001) compared with BLyS < 2 ng/mL (8.2%, p = 0.0158). Results were similar for ≥ 4-point reduction in SELENA-SLEDAI. Risk of severe flare over 52 weeks was significantly reduced with belimumab 10 mg/kg versus placebo in the BLyS ≥ 2 ng/mL group (p = 0.0002). AEs were similar across treatment arms and BLyS subgroups.
Conclusions
Positive anti-Smith, low C3, anti-dsDNA ≥ 80 IU/mL, immunosuppressant usage, proteinuria, elevated CRP, and low total lymphocyte count were predictors of BLyS ≥ 2 ng/mL. Monitoring these factors could identify patients with BLyS ≥ 2 ng/mL who are at risk of flare.
Keywords: BLyS, belimumab, systemic lupus erythematosus, BLISS trials, regression analysis
Introduction
The course and presentation of systemic lupus erythematosus (SLE) is unpredictable and variable, and is characterized by periods of disease flare and remission.1 Treatment of SLE aims to minimize symptoms, which can be serious and life-threatening, and to minimize the risk of flares.2 Early detection of disease flares allows prompt, appropriate therapy to be initiated, and can reduce their impact.3–5 However, over time, flare can lead to organ damage, further increasing disease burden.6
BLISS-52 and BLISS-76 were randomized, double-blind, placebo-controlled multicenter trials with similar designs conducted in patients with SLE; trial design and results have been described previously.7,8 Post hoc analyses from the BLISS trials have identified baseline disease activity characteristics that were predictors of moderate-to-severe SLE flare over one year; predictors included renal, neurological, or vasculitic involvement, elevated anti-double-stranded DNA (anti-dsDNA) levels, low complement (C) 3, and elevated B-lymphocyte stimulator (BLyS) levels.9 Specifically, patients with baseline BLyS levels within the top quartile (≥ 2 ng/mL) had an increased risk of a clinically-meaningful flare over one year, when three indices of flare were applied (modified SLE Flare Index (SSF),10 one new British Isles Lupus Assessment Group (BILAG) A or two new B scores, and any BILAG A score) at Week 24 and Week 52.9
Belimumab is a monoclonal antibody with proven efficacy in the treatment of SLE.7,8 Belimumab specifically inhibits soluble BLyS and may confer additional clinical benefits in patients with high BLyS levels.11
Determining BLyS levels in patients with SLE may be informative for physicians, yet these tests are not routinely collected in clinical practice. Therefore, we examined routine clinical measures to identify those that correlate with BLyS levels ≥ 2 ng/mL, to help physicians identify patients with SLE at risk of flare.9 We also examined how patients at two BLyS levels responded over 52 weeks of belimumab treatment (BLyS levels ≥ 2 ng/mL and BLyS levels < 2 ng/mL).
Materials and methods
Study design and population
The methods and results for the BLISS trials (ClinicalTrials.gov NCT00424476 and NCT00410384) have been described previously.7,8 To identify factors that are predictive of BLyS levels ≥ 2 ng/mL, a regression analysis was performed on pooled BLISS data post hoc (Study 200619). Patients with available BLyS data from all three treatment arms (placebo, belimumab 1 mg/kg, and belimumab 10 mg/kg) were included (regression analysis population); only baseline data were examined, therefore treatment assignment was not a factor.
The effects of belimumab 10 mg/kg plus standard SLE care versus placebo plus standard SLE care (efficacy population) were examined by baseline BLyS groupings (≥ 2 ng/mL and < 2 ng/mL groups) over 52 weeks. A population comprising only patients who received belimumab 10 mg/kg was selected as it is the licensed dose of belimumab.
The regression analysis was also conducted in a subgroup of patients who met a serological definition of high disease activity12 (anti-dsDNA positive (>30 U/mL) and low C3/C4 at baseline), and who had available BLyS data (serologically active regression analysis population). This serological definition of high disease activity is associated with more severe disease, and so this population is of particular interest.1,13,14 Efficacy analyses were also conducted in patients from this population who received belimumab 10 mg/kg or placebo (serologically active efficacy population).
Study endpoints
The primary endpoint assessed baseline factors predictive of baseline BLyS levels ≥ 2 ng/mL (regression analysis). Factors such as study protocol (BLISS-52 or BLISS-76), patient demographics, concomitant SLE medications, disease activity and biomarkers, for example, anti-dsDNA, anti-Smith, C3/C4 levels, proteinuria, and lymphocyte count, were included in the regression analysis. The final regression analysis excluded Safety of Estrogens in Lupus National Assessment–Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI), BILAG, and Systemic Lupus International Collaborating Clinics (SLICC) scores, as these are not routinely collected in clinical practice.
Secondary endpoints (efficacy analysis) included SLE Responder Index (SRI) response15 over 52 weeks, SELENA-SLEDAI16 score over 52 weeks, and rate of flare and severe flare among patients with baseline BLyS levels ≥ 2 ng/mL and among patients with baseline BLyS levels < 2 ng/mL for the belimumab 10 mg/kg and placebo arms in the efficacy populations.
An SRI responder was defined as having a reduction of ≥ 4 points in SELENA-SLEDAI score, no new BILAG A organ domain score, no more than one new BILAG B organ domain score, and no worsening in physician global assessment (PGA) score from baseline (worsening defined as an increase of ≥ 0.3 points).15 Flare rate was measured using the modified SFI.10 Severe flare was defined by the SFI, modified to exclude the single criterion of an increase in the SELENA-SLEDAI score to > 12.10
In exploratory analyses, changes in prednisone dose from a baseline level of > 7.5 mg/day or ≤ 7.5 mg/day, and proportion of patients with no worsening in PGA score (defined as an increase of ≥ 0.3 points) were summarized for the efficacy populations over 52 weeks.
Safety was assessed using the incidence of adverse events (AEs) for the efficacy populations.
Statistical analysis
A univariate logistic regression was employed to identify a subset of baseline factors predictive of baseline BLyS levels ≥ 2 ng/mL. Factors significant at the p = 0.05 level entered the stepwise logistic regression as covariates. Efficacy endpoints were summarized by treatment for patients by BLyS subgroup. Odds ratios, 95% confidence intervals (OR (95% CI)) and p-values were calculated by logistic regression for belimumab 10 mg/kg versus placebo. A Cox proportional hazards model examined the time to first flare and severe flare. No corrections for multiplicity were applied.
Results
Patient population
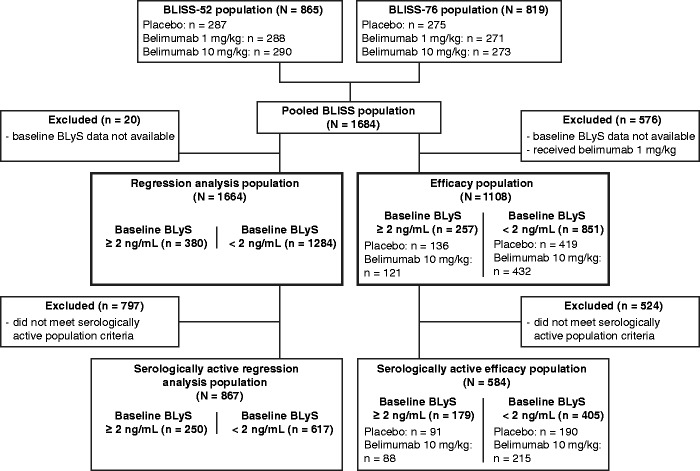
Of the 1684 patients who received any study treatment in the two BLISS trials, 1664 had available baseline BLyS data (Figure 1). Of these, 380 (22.8%) had BLyS levels ≥ 2 ng/mL. The majority of patients with BLyS ≥ 2 ng/mL were female (90.5%), mean age was 36.3 years, and the mean SELENA-SLEDAI score was 10.8 (Table 1). 867/1664 patients showed serologically active disease (Figure 1). In total, 1108 patients received belimumab 10 mg/kg or placebo and had data available for the post hoc efficacy analysis; 584 of these patients were included in the serologically active efficacy analysis, 179 (30.7%) of whom had baseline BLyS levels ≥ 2 ng/mL.
Figure 1.
Patient disposition.
Table 1.
Baseline characteristics, BLISS-52/76 pooled data
| Regression analysis population (N = 1664)a |
||
|---|---|---|
| BLyS ≥ 2 ng/mL (n = 380) | BLyS < 2 ng/mL (n = 1284) | |
| Female, n (%) | 344 (90.5) | 1221 (95.1) |
| Mean (SD) age, years | 36.3 (11.0) | 38.2 (11.7) |
| Mean (SD) SELENA-SLEDAI score | 10.8 (4.3) | 9.4 (3.5) |
| Mean proteinuria (SD), g/24 hour | 0.8 (1.2) | 0.4 (0.8) |
| Proteinuria (≥ 2 g/24 hour), n (%) | 39 (10.3) | 60 (4.7) |
| CRP (>3 mg/L), n (%) | 217 (57.1) | 442 (34.4) |
| Immunosuppressant use, n (%) | 241 (63.4) | 568 (44.2) |
| Anti-Smith+, n (%) | 175 (46.1) | 341 (26.6) |
| Anti-dsDNA (80–200 IU/mL), n (%) | 85 (22.4) | 267 (20.8) |
| Anti-dsDNA ≥ 200 IU/mL, n (%) | 190 (50.0) | 346 (26.9) |
| Anti-dsDNA (≥ 30 IU/mL), n (%) | 312 (82.1) | 843 (65.7) |
| Low C3 (< 90 mg/dl), n (%) | 225 (59.2) | 524 (40.8) |
| Low C4 (< 16 mg/dl), n (%) | 252 (66.3) | 681 (53.0) |
| Mean (SD) lymphocyte count, 105/mL, | 18.7 (9.9)b | 23.4 (11.2)c |
BLyS: B-lymphocyte stimulator; C: complement; CRP: C-reactive protein; dsDNA: double-stranded DNA; SD: standard deviation; SELENA-SLEDAI: Safety of Estrogens in Lupus National Assessment–Systemic Lupus Erythematosus Disease Activity Index.
Includes all patients with available BLyS data (patients may be missing some covariate data).
Data missing for one patient.
Data missing for four patients.
Predictors of baseline BLyS levels ≥ 2 ng/mL
Baseline parameters routinely collected in clinical practice predictive of baseline BLyS levels ≥ 2 ng/mL included positive anti-Smith (≥ 15 U/mL), low C3 (< 900 mg/L), anti-dsDNA 80–200 IU/mL and ≥ 200 IU/mL, immunosuppressant usage, proteinuria, elevated C-reactive protein (CRP; >3 mg/L), and low total lymphocyte count (Table 2).
Table 2.
Baseline predictors of baseline BLyS levels ≥ 2 ng/mL in the stepwise logistic regression analysis (regression analysis population)
| Odds ratio | 95% Wald confidence limits | χ2 | p-value | |
|---|---|---|---|---|
| Positive anti-Smith (≥ 15 U/mL) | 1.72 | 1.32, 2.25 | 15.68 | < 0.01 |
| Low C3 (< 900 mg/L) | 1.33 | 1.00, 1.77 | 3.96 | < 0.05 |
| Anti-dsDNA (> 200 IU/mL) | 1.96 | 1.43, 2.69 | 17.35 | < 0.01 |
| Anti-dsDNA (80–200 IU/mL) | 1.52 | 1.07, 2.16 | 5.51 | < 0.05 |
| Use of immunosuppressant medication | 1.85 | 1.43, 2.39 | 21.69 | < 0.01 |
| Proteinuria (≥ 0.5 g/24 hour) | 1.82 | 1.37, 2.43 | 16.76 | < 0.01 |
| Elevated CRP (> 3 mg/L) | 2.41 | 1.86, 3.13 | 44.19 | < 0.01 |
| Total lymphocyte count | 0.97 | 0.96, 0.98 | 19.46 | < 0.01 |
BLyS: B-lymphocyte stimulator; C: complement; CRP: C-reactive protein; dsDNA: double-stranded DNA.
In the serologically active patient regression analysis the baseline parameters routinely collected in clinical practice predictive of baseline BLyS levels ≥ 2 ng/mL were the same as those identified for the overall population (data not shown), with the exception of anti-dsDNA 80–200 IU/mL (p = 0.0606).
Efficacy analysis
SRI response
SRI responses over time are shown in Figure 2. SRI response was significantly higher with belimumab 10 mg/kg versus placebo among patients with BLyS ≥ 2 ng/mL at Week 16 and Weeks 28–52, with a treatment difference at Week 52 of 24.1% (p < 0.0001). When treated with belimumab 10 mg/kg, 52.1% of patients with BLyS ≥ 2 ng/mL and 50.2% of patients with BLyS < 2 ng/mL were SRI responders at Week 52. However, the treatment difference versus placebo was numerically lower among patients with BLyS < 2 ng/mL compared with BLyS ≥ 2 ng/mL (treatment difference of 8.2% at Week 52).
Figure 2.
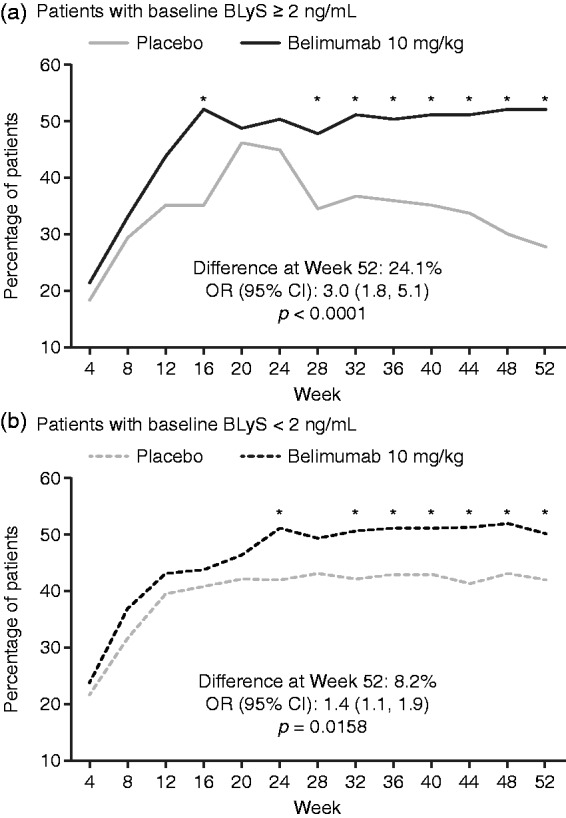
Percentage of patients with SRI response over time in those with (a) baseline BLyS ≥ 2 ng/mL and those with (b) baseline BLyS < 2 ng/mL (efficacy population). *p ≤ 0.05. p-values calculated from logistic regression for belimumab 10 mg/kg versus placebo; covariates include baseline SELENA-SLEDAI (≤ 9 versus ≥ 10), baseline proteinuria level (< 2 g/24-hour versus ≥ 2 g/24-hour equivalent), race (African descent or indigenous-American descent versus other), and study (BLISS-52 versus BLISS-76). BLyS: B-lymphocyte stimulator; CI: confidence interval; OR: odds ratio; SELENA-SLEDAI: Safety of Estrogen in Lupus Erythematosus National Assessment–Systemic Lupus Erythematosus Disease Activity Index; SRI: SLE responder index.
In the serologically active efficacy population the findings were consistent with those in the overall population.
Improvement in SELENA-SLEDAI score
Improvements in SELENA-SLEDAI (≥ 4-points) followed a similar pattern to SRI response. For both BLyS groups, significantly more patients achieved a ≥ 4-point reduction from baseline in SELENA-SLEDAI score with belimumab 10 mg/kg versus placebo from Week 32 to 52 (Figure 3). The percentage of patients achieving this reduction was significantly higher in the BLyS ≥ 2 ng/mL group at Week 16, and in the BLyS < 2 ng/mL group at Week 24.
Figure 3.
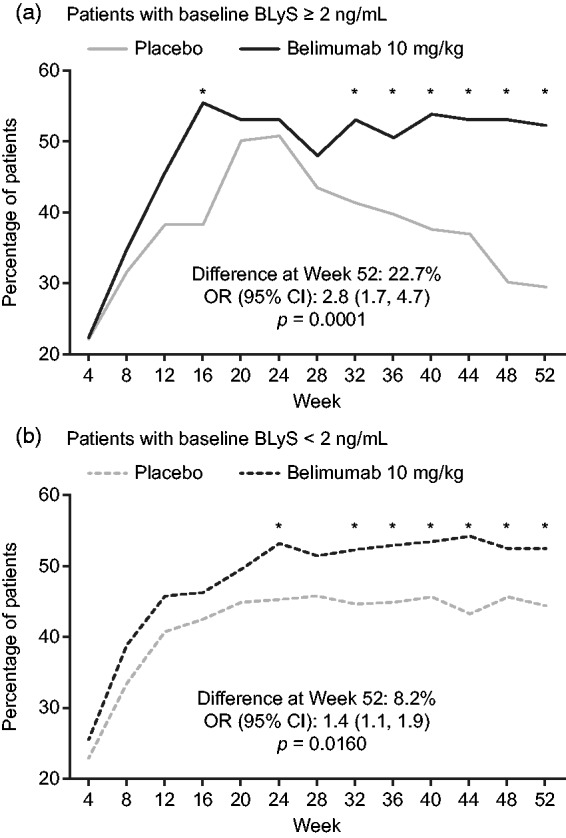
Percentage of patients with SELENA-SLEDAI reduction of at least four points over time in those with (a) baseline BLyS ≥ 2 ng/mL and those with (b) baseline BLyS < 2 ng/mL (efficacy population). *p ≤ 0.05. p-values calculated from logistic regression for belimumab. 10 mg/kg versus placebo; covariates include baseline SELENA-SLEDAI (≤ 9 versus ≥ 10), baseline proteinuria level (< 2 g/24-hour versus ≥ 2 g/24-hour equivalent), race (African descent or indigenous-American descent versus other), and study (BLISS-52 versus BLISS-76). BLyS: B-lymphocyte stimulator; CI: confidence interval; OR: odds ratio; SELENA-SLEDAI: Safety of Estrogen in Lupus Erythematosus National Assessment–Systemic Lupus Erythematosus Disease Activity Index.
In the serologically active efficacy population the findings were consistent with those in the overall population.
Risk of flare and severe flare over 52 weeks
The risk of SLE flare over 52 weeks was not significantly reduced with belimumab 10 mg/kg versus placebo in either BLyS subgroup, though there was a trend towards significance for the BLyS < 2 ng/mL group (Table 3). The risk of severe SLE flare over 52 weeks with belimumab 10 mg/kg versus placebo was significantly reduced for patients with BLyS ≥ 2 ng/mL (hazard ratio (HR) versus placebo (95% CI): 0.37 (0.22, 0.63); p = 0.0002), but was not significantly reduced for those with BLyS < 2 ng/mL (Table 3).
Table 3.
Secondary efficacy endpoints: risk of flare and severe flare (efficacy population)
| BLyS ≥ 2 ng/mL at baseline |
BLyS < 2 ng/mL at baseline |
|||
|---|---|---|---|---|
| Placebo (N = 136) | Belimumab 10 mg/kg (N = 121) | Placebo (N = 419) | Belimumab 10 mg/kg (N = 432) | |
| SLE flare (%)a | 119 (87.5) | 97 (80.2) | 332 (79.2) | 316 (73.2) |
| Median time to SLE flare (range), days | 60 (1–336) | 79 (5–329) | 85 (1–387) | 112 (1–367) |
| HR versus placebo (95% CI)b | 0.83 (0.63, 1.09) | 0.86 (0.74, 1.00) | ||
| p-valueb | 0.1707 | 0.0532 | ||
| Severe SLE flare, n (%) | 53 (39.0) | 21 (17.4) | 78 (18.6) | 66 (15.3) |
| Median time to severe SLE flare (range), days | NA (1–360)c | NA (10–323)c | NA (5–371)c | NA (1–366)c |
| HR versus placebo (95% CI)b | 0.37 (0.22, 0.63) | 0.81 (0.58, 1.12) | ||
| p-valueb | 0.0002 | 0.2071 | ||
BLyS: B-lymphocyte stimulator; CI: confidence interval; OR: odds ratio; HR: hazard ratio; NA: not available; SD: standard deviation; SELENA-SLEDAI: Safety of Estrogens in Lupus National Assessment–Systemic Lupus Erythematosus Disease Activity Index; SRI: SLE responder index; SLE: systemic lupus erythematosus.
Any increase of ≥ 3 points in SLEDAI score resulted in a mild/moderate flare.
Cox proportional hazards model adjusted for baseline SELENA-SLEDAI score (≤ 9 versus ≥ 10), baseline proteinuria level (< 2 g/24-hour versus ≥ 2 g/24-hour equivalent), race (African descent or indigenous-American descent versus other), and study (BLISS-52 versus BLISS-76).
Median days missing if the estimated probability of a flare is < 50%.
The results for the serologically active population differ slightly from those observed for the overall efficacy population in terms of SLE flare, as the risk was reduced significantly with belimumab 10 mg/kg versus placebo for those with BLyS ≥ 2 ng/mL and BLyS < 2 ng/mL (HR versus placebo (95% CI): 0.70 (0.51, 0.96) for BLyS ≥ 2 ng/mL and 0.77 (0.61, 0.96) for BLyS < 2 ng/mL; both p < 0.05). For severe SLE flare, results were consistent with the overall population.
Changes in prednisone dose
Among those with BLyS ≥ 2 ng/mL and prednisone dose > 7.5 mg/day at baseline, a higher percentage of patients who received belimumab 10 mg/kg versus placebo had a reduction in prednisone dose to ≤ 7.5 mg/day by Week 52 (Table 4). For those with BLyS < 2 ng/mL, significantly more patients who were treated with belimumab 10 mg/kg versus placebo had a reduction in prednisone dose to ≤ 7.5 mg/day.
Table 4.
Exploratory efficacy endpoints (efficacy population)
| BLyS ≥ 2 ng/mL at baseline |
BLyS < 2 ng/mL at baseline |
|||
|---|---|---|---|---|
| Placebo (N = 136) | Belimumab 10 mg/kg (N = 121) | Placebo (N = 419) | Belimumab 10 mg/kg (N = 432) | |
| Prednisone reduced to ≤ 7.5 mg/day by Week 52 a, n (%) | 9/76 (11.8) | 16/79 (20.3) | 37/240 (15.4) | 55/240 (22.9) |
| Difference versus placebo | 8.41 | 7.50 | ||
| OR (95% CI); p-value versus placebo | 2.05 (0.83, 5.09) | 1.64 (1.03, 2.61) | ||
| p-value | 0.1198 | 0.0368 | ||
| Prednisone increased to > 7.5 mg/day by Week 52 b, n (%) | 25/60 (41.7) | 13/42 (31.0) | 54/179 (30.2) | 51/192 (26.6) |
| Difference versus placebo | –10.7 | –3.61 | ||
| OR (95% CI); p-value versus placebo | 0.70 (0.29, 1.74) | 0.83 (0.52, 1.31) | ||
| p-value | 0.4472 | 0.4147 | ||
| No worsening in PGA score at Week 52 c, n (%) | 77 (56.6) | 87 (71.9) | 289 (69.0) | 326 (75.5) |
| Difference versus placebo | 15.3 | 6.5 | ||
| OR (95% CI); p-value versus placebo | 2.1 (1.2, 3.7) | 1.4 (1.0, 1.9) | ||
| p-value | 0.0066 | 0.0249 | ||
BLyS: B-lymphocyte stimulator; OR: odds ratio; PGA: Physicians Global Assessment.
Out of patients with baseline prednisone dose > 7.5 mg/day.
Out of patients with baseline prednisone dose ≤ 7.5 mg/day.
Worsening defined as an increase of ≥ 0.3 points since baseline.
Increases in prednisone dose > 7.5 mg/day (in patients who received ≤ 7.5 mg/day at baseline) occurred in a lower percentage of patients who received belimumab 10 mg/kg versus placebo, for both BLyS subgroups, though this was not statistically significant (Table 4).
Worsening in PGA score
Significantly more patients who were treated with belimumab 10 mg/kg versus placebo had no worsening in PGA score at 52 weeks compared with baseline, for both BLyS subgroups (Table 4).
AEs
Incidence of any AEs was similar across treatment arms and BLyS subgroups, and consistent with the overall population (92.1%). However, the incidence of some individual AEs, such as nausea, diarrhea, and anemia, appeared to differ slightly (Table 5). Overall, serious AEs (SAEs) were reported by 190 (17.1%) patients; rates appeared slightly higher in the BLyS ≥ 2 ng/mL group compared with BLyS < 2 ng/mL, but were comparable between treatment arms in the BLyS ≥ 2 ng/mL group. Treatment-related AEs were reported by 438 (39.5%) patients; the most common in the BLyS ≥ 2 ng/mL group were upper respiratory tract infection, reported by 6.2% of patients (8.3%, belimumab 10 mg/kg; 4.4%, placebo), and nausea and headache, both reported by 3.1% of patients overall. Upper respiratory tract infection was the most common treatment-related AE in the BLyS < 2 ng/mL group also (4.7% of patients: 3.5%, belimumab 10 mg/kg; 6.0%, placebo), and cases of headache and urinary tract infection were reported by 4.5% and 3.1% of patients overall, respectively. The incidences of AEs and SAEs leading to study discontinuation were higher for the BLyS ≥ 2 ng/mL groups compared with BLyS < 2 ng/mL; within the BLyS ≥ 2 ng/mL group, 22 (16.2%) patients who received placebo had AEs leading to discontinuation, compared with 10 (8.3%) patients who received belimumab 10 mg/kg. Incidences of AEs for the serologically active efficacy population were consistent with the overall population.
Table 5.
Summary of adverse events (efficacy population)
| BLyS ≥ 2 ng/mL at baseline |
BLyS < 2 ng/mL at baseline |
|||
|---|---|---|---|---|
| Placebo (N = 136) | Belimumab 10 mg/kg (N = 121) | Placebo (N = 419) | Belimumab 10 mg/kg (N = 432) | |
| Any AE, n (%)a | 126 (92.6) | 115 (95.0) | 384 (91.6) | 395 (91.4) |
| Headache | 24 (17.6) | 24 (19.8) | 89 (21.2) | 83 (19.2) |
| Upper respiratory tract infection | 22 (16.2) | 19 (15.7) | 81 (19.3) | 71 (16.4) |
| Nausea | 12 (8.8) | 24 (19.8) | 46 (11.0) | 42 (9.7) |
| Urinary tract infection | 14 (10.3) | 18 (14.9) | 53 (12.6) | 51 (11.8) |
| Arthralgia | 15 (11.0) | 15 (12.4) | 62 (14.8) | 56 (13.0) |
| Diarrhea | 7 (5.1) | 15 (12.4) | 39 (9.3) | 47 (10.9) |
| Nasopharyngitis | 10 (7.4) | 14 (11.6) | 36 (8.6) | 49 (11.3) |
| Anemia | 11 (8.1) | 13 (10.7) | 20 (4.8) | 15 (3.5) |
| Cough | 14 (10.3) | 12 (9.9) | 26 (6.2) | 30 (6.9) |
| Pyrexia | 12 (8.8) | 13 (10.7) | 26 (6.2) | 36 (8.3) |
| Edema peripheral | 10 (7.4) | 13 (10.7) | 32 (7.6) | 29 (6.7) |
| Hypertension | 14 (10.3) | 7 (5.8) | 35 (8.4) | 25 (5.8) |
| Any SAE, n (%) | 32 (23.5) | 25 (20.7) | 57 (13.6) | 76 (17.6) |
| Any treatment-related AE, n (%) | 58 (42.6) | 48 (39.7) | 174 (41.5) | 158 (36.6) |
| AEs resulting in study discontinuation, n (%) | 22 (16.2) | 10 (8.3) | 20 (4.8) | 27 (6.3) |
| SAEs resulting in study discontinuation, n (%) | 13 (9.6) | 9 (7.4) | 13 (3.1) | 17 (3.9) |
AE: adverse event; BLyS: B-lymphocyte stimulator; SAE: serious adverse event.
Only AEs occurring in ≥ 10% of patients in any treatment arm are listed.
Discussion
BLyS levels ≥ 2 ng/mL have been found to be predictive of SLE flare over 52 weeks;9 however, BLyS levels are not routinely or easily measured in clinical practice. This post hoc regression analysis of baseline data from two Phase III clinical trials (BLISS-52 and -76) identified routinely-collected clinical measures that could be used as surrogate markers of serum BLyS levels ≥ 2 ng/mL: positive anti-Smith (≥ 15 U/mL), low C3 (< 900 mg/L), anti-dsDNA 80–200 IU/mL and ≥ 200 IU/mL, the use of immunosuppressant medication, proteinuria, elevated CRP, and low total lymphocyte count. Taking these parameters into consideration may help healthcare professionals identify patients with SLE who are at risk of flare and manage their treatment plan accordingly.9
The BLISS trials demonstrated the efficacy of belimumab (10 mg/kg).7,8 This post hoc analysis of those trials compared belimumab 10 mg/kg (plus standard SLE care) versus placebo (plus standard SLE care) in patients with BLyS levels ≥ 2 ng/mL and BLyS levels < 2 ng/mL. Approximately half of patients who received belimumab achieved an SRI response at Week 52 in the overall population, and this was consistent across both BLyS subgroups. The treatment differences versus placebo in SRI responses were numerically greater in patients with BLyS levels ≥ 2 ng/mL (difference versus placebo of 24.1%) compared with BLyS levels < 2 ng/mL (difference versus placebo of 8.2%) and the 12% difference measured in the overall BLISS population.7,8 The differences in treatment response in the present study were brought about by the poorer response in the placebo arm in the BLyS ≥ 2 ng/mL group compared with the BLyS < 2 ng/mL group. This suggests that patients with BLyS levels ≥ 2 ng/mL, who are at risk of flare,9 had a lesser response to standard treatment than those with BLyS levels < 2 ng/mL, but benefited from belimumab, which specifically inhibits BLyS.11 There may be additional factors, other than the level of circulating BLyS, which play a role in this response.
The overall percentage of patients who received belimumab and experienced a severe flare appeared to be similar between the two BLyS groups. However, in the placebo arms the BLyS ≥ 2 ng/mL group experienced a numerically greater incidence of severe flare compared with the BLyS < 2 ng/mL group. This indicates that inhibition of BLyS lowered the risk of severe flare in the BLyS ≥ 2 ng/mL group to a level similar to that in the BLyS < 2 ng/mL group. Indeed, the reduction in risk of severe SLE flare with belimumab 10 mg/kg plus standard care versus placebo was numerically greater in patients with BLyS levels ≥ 2 ng/mL (63%) compared with BLyS levels < 2 ng/mL (19%). That BLyS levels ≥ 2 ng/mL are associated with SLE flare suggests a pathophysiological mechanism by which disease activity and exacerbations may emerge in SLE,9 and therapies that reduce BLyS may be especially beneficial in patients with levels ≥ 2 ng/mL. This theory is supported by our results, which demonstrate greater benefits of belimumab 10 mg/kg over placebo in preventing severe flare in patients with BLyS ≥ 2 ng/mL compared with patients with BLyS < 2 ng/mL.
Results of a previous post hoc analysis suggested that belimumab had a greater therapeutic benefit in patients with high disease activity, anti-dsDNA positivity, low complement, or corticosteroid treatment at baseline.12 The current study suggests that baseline BLyS levels are at least as indicative as C3 levels or dsDNA-antibody levels to predict the benefit of belimumab treatment.12 Measuring baseline BLyS levels in the clinic would add useful information to the conventional parameters already measured, further assisting physicians in their treatment choices. However, the data presented in the current study provide a guide to identify patients who are likely to have high BLyS levels, should an assay not be available.
BLyS levels did not appear to be predictive of changes in steroid doses in the present study, though a steroid-sparing effect of belimumab was evident. Reductions in steroid dose to ≤ 7.5 mg/day were observed for a higher proportion of patients receiving belimumab versus placebo in both BLyS groups. However, this was only statistically significant for patients with BLyS levels < 2 ng/mL. Similar steroid dose reductions of ≥ 25% to ≤ 7.5 mg/day were reported in the BLISS trials, though these were not statistically significant for the belimumab 10 mg/kg group versus placebo.7,8 Steroid dose analysis was exploratory in the BLISS studies, as they were not designed to examine such changes, and the protocol restricted changes in steroid dose;7,8 thus the potential steroid-sparing effects of belimumab in these studies could be masked.
There are a number of limitations associated with these post hoc analyses. Although patients with BLyS levels ≥ 2 ng/mL at baseline showed a numerically greater clinical response to belimumab than patients with BLyS levels < 2 ng/mL, no statistical analyses were performed to compare the efficacy of belimumab between these groups and no corrections for multiplicity were applied. Since these trials were not prospectively designed to examine corticosteroid changes, it is not possible to draw any firm conclusions regarding the steroid-sparing effect of belimumab. Finally, post hoc analyses are exploratory; prospective studies to confirm the relationship between patient baseline characteristics and treatment response may be warranted.
In conclusion, we identified a number of routine clinical measures that are predictive of BLyS levels ≥ 2 ng/mL in patients with SLE. These may help identify patients at risk of SLE flare who may derive benefit from treatment adjustments to prevent flares. In efficacy analyses, approximately half of patients in both BLyS subgroups achieved an SRI response with belimumab 10 mg/kg by Week 52. Patients with higher BLyS levels (≥ 2 ng/mL), who are most at risk of SLE flare, had generally poorer responses to standard SLE care alone, and derived a greater benefit from belimumab treatment; this was demonstrated by the numerically greater treatment differences for belimumab versus placebo for these patients, compared with the BLyS < 2 ng/mL group, though no statistical analyses compared the efficacy of belimumab in these two patient categories. In safety analyses, the overall incidence of AEs was similar across treatment arms and BLyS subgroups.
Acknowledgements
Medical writing assistance was provided by Louisa Pettinger of Fishawack Indicia Ltd, and was funded by GlaxoSmithKline.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: David A. Roth, April Thompson, Anne E. Hammer and David Gordon are employees of, and hold stock in, GlaxoSmithKline. Charles T. Molta and Yongqiang Tang, were employees of GlaxoSmithKline at the time of the study and hold stock in GlaxoSmithKline.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study (study 200619) was funded and conducted by GlaxoSmithKline.
References
- 1.Bertsias G, Ioannidis JP, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis 2008; 67: 195–205. [DOI] [PubMed] [Google Scholar]
- 2.Manson J, Rahman A. Systemic lupus erythematosus. Orphanet J Rare Dis 2006; 1: 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidelines for referral and management of systemic lupus erythematosus in adults. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Arthritis Rheum 1999; 42: 1785–1796. [DOI] [PubMed] [Google Scholar]
- 4.Bootsma H, Spronk P, Derksen R, et al. Prevention of relapses in systemic lupus erythematosus. Lancet 1995; 345: 1595–1599. [DOI] [PubMed] [Google Scholar]
- 5.D'Cruz DP. Systemic lupus erythematosus. BMJ 2006; 332: 890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzi S. Epidemiology of systemic lupus erythematosus. Am J Manag Care 2001; 7: S474–479. [PubMed] [Google Scholar]
- 7.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011; 63: 3918–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377: 721–731. [DOI] [PubMed] [Google Scholar]
- 9.Petri MA, van Vollenhoven RF, Buyon J, et al. Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III belimumab trials. Arthritis Rheum 2013; 65: 2143–2153. [DOI] [PubMed] [Google Scholar]
- 10.Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus 1999; 8: 685–691. [DOI] [PubMed] [Google Scholar]
- 11.Baker KP, Edwards BM, Main SH, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum 2003; 48: 3253–3265. [DOI] [PubMed] [Google Scholar]
- 12.van Vollenhoven RF, Petri MA, Cervera R, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis 2012; 71: 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biesen R, Dahnrich C, Rosemann A, et al. Anti-dsDNA-NcX ELISA: dsDNA-loaded nucleosomes improve diagnosis and monitoring of disease activity in systemic lupus erythematosus. Arthritis Res Ther 2011; 13: R26–R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasiri S, Karimifar M, Bonakdar Z, Salesi M. Correlation of ESR, C3, C4, anti-DNA and lupus activity based on British Isles Lupus Assessment Group Index in patients of rheumatology clinic. Rheumatol Int 2010; 30: 1605–1609. [DOI] [PubMed] [Google Scholar]
- 15.Furie RA, Petri MA, Wallace DJ, et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum 2009; 61: 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petri M, Kim MY, Kalunian KC, et al. Combined oral contraceptives in women with systemic lupus erythematosus. New Engl J Med 2005; 353: 2550–2558. [DOI] [PubMed] [Google Scholar]