Abstract
Introduction
Surveillance endoscopy detects dysplasia within Barrett's esophagus (BE) and dictates treatment. Current biopsy regimens recommend uniformly-spaced random biopsies. We assessed the distribution of dysplasia in BE to develop evidence-based biopsy regimens.
Methods
We performed analysis of the distribution of dysplasia within BE, using pre-treatment biopsy data from two randomized controlled trials (RCT) of radiofrequency ablation (RFA) for dysplastic BE: the SURF Trial and the AIM Dysplasia Trial. We used generalized linear models with generalized estimating equations to estimate prevalence differences for dysplasia depending on the standardized location of biopsies. We performed Monte Carlo simulation of biopsy regimens to estimate their yield for any dysplasia within segments.
Results
Dysplasia preferentially resides in the proximal-most half of the BE segment, which is almost twice as likely to demonstrate dysplasia as the distal-most quartile. In pooled analysis, compared to the distal-most quarter, the prevalence difference in the proximal-most quarter was 22.6%, in the second proximal-most quarter 23.1%, and in the second distal-most quarter 15.3%. The best performing biopsy regimen in simulation studies acquired 8 biopsies in the most proximal centimeter of BE, 8 biopsies in the second cm, and 2 biopsies in each cm thereafter q1cm - (8, 8, 2, 2…). A slightly simpler q2cm regimen q2cm - (12, 12, 4…) was nearly as effective.
Conclusion
Post-hoc analysis of two RCTs reveals a substantially increased prevalence of dysplasia proximally in BE segments. Our simulations suggest an altered biopsy regimen could increase sensitivity of biopsies in short-segment BE by >30%.
Introduction
Barrett's esophagus (BE) is a common, premalignant change of the normal squamous epithelium of the esophagus to a salmon-colored columnar mucosa with goblet cells.1-3 The risk of malignant transformation to esophageal adenocarcinoma varies greatly, with the most important determinant being histologic grade.4, 5 The diagnosis of dysplasia within a Barrett's segment greatly increases the rate of progression to cancer.6, 7 Because severity of dysplasia decides subsequent treatment, detection of dysplasia within a BE segment is the most important aspect of chronic care in BE, and is the purpose of surveillance endoscopy programs.8
While currently recommended biopsy regimens, such as the Seattle protocol of four quadrant biopsies every 1-2 cm,1 have the virtue of simplicity, no data regarding the distribution of dysplasia drive these recommendations. Instead, this protocol is designed to get a representative random sample of mucosa. Better characterization of the spatial distribution of dysplasia might produce biopsy regimens with decreased total numbers of biopsies and increased yield of dysplasia per biopsy. Such regimens might also decrease false negative results and, if they imply fewer overall biopsies, also decrease costs associated with histology, post-procedure pain, and any biopsy complications. The development of a regimen the sampling density of which approximates the observed distribution of dysplasia across a wide range of segment lengths would improve current practices.by drawing biopsies from the areas most likely to yield dysplasia.
Using biopsy data from two recent, methodologically rigorous randomized controlled treatment trials of BE, we sought to describe the proximal to distal distribution of dysplasia in treatment-naïve BE, and to simulate the yield of dysplasia of various hypothetical biopsy regimens for BE surveillance.
Methods
Data Sources
We performed an analysis of the distribution of dysplasia longitudinally within the BE segment, using pre-treatment biopsy data from two randomized controlled trials of radiofrequency ablation (RFA) for dysplastic Barrett's esophagus. Each trial was examined in isolation and in pooled analyses. These trials were:
1) The Surveillance vs. Radiofrequency Ablation (SURF) Trial9
The SURF Trial was conducted at nine European BE treatment centers. Eligible patients underwent endoscopy demonstrating BE with low-grade dysplasia (LGD) within 18 months prior to enrollment. An expert central pathology panel confirmed community-based diagnoses of LGD. Patients were excluded for prior endoscopic treatment for BE, history of high grade dysplasia (HGD) or adenocarcinoma, active secondary malignancy, estimated life expectancy less than 2 years (according to the enrolling physician), and age of 18 years or younger or 85 years and older.
All patients required a baseline qualifying endoscopy within six months prior to randomization to exclude visible abnormalities, HGD, or adenocarcinoma. Baseline qualifying endoscopies were performed using high-resolution endoscopy with biopsies obtained according to the modified Seattle protocol (4-quadrant biopsies/2-cm intervals) with additional biopsy and documentation of any visible abnormalities or raised lesions. Patients were subsequently randomized to either treatment or surveillance.9 Data from the baseline-qualifying exam were used in the present study.
2) The Ablation of Intestinal Metaplasia (AIM) Containing Dysplasia Trial7
The AIM Dysplasia Trial was conducted at 19 U.S. sites. Eligible patients were between 18 and 80 years of age and had endoscopically evident, non-nodular, dysplastic BE of no more than 8 cm in length. Patients with prior endoscopic mucosal resection and complete clearance of nodularity at least 8 weeks prior to enrollment were allowed. Exclusion criteria were pregnancy, active esophagitis or stricture precluding passage of the endoscope, a history of esophageal cancer, esophageal varices, uncontrolled coagulopathy, or a life expectancy of less than 2 years, as judged by the site investigator.
A central pathology lab confirmed diagnoses of dysplasia. Baseline visits were performed by either the referring gastroenterologist or at participating study sites. Endoscopic biopsies were performed with maximum-capacity or jumbo forceps in four quadrants every 1-2 cm throughout the original length of Barrett's esophagus; in addition, directed biopsies were performed at sites with any visible abnormalities.7
Statistical Methods
For the purposes of analyses in this manuscript, only pathology samples from baseline endoscopies were included. Patients with less than two biopsy levels were excluded, because such instances provided no information regarding proximal-to-distal distribution. The proximal-to-distal location of biopsy levels was standardized to the maximum distance of the BE segment from the top of gastric folds (TGF). For example, a biopsy level at TGF would be assigned a location of 0, a biopsy level 8 cm proximal to TGF within an 8 cm segment of BE would be assigned a location of 1, and a biopsy level 2 cm proximal to TGF in an 8 cm segment would be assigned a location of 0.25. In this way, spatial distributions of dysplasia within BE segments of varying lengths could be standardized to allow assessment of proximal-to-distal distribution, regardless of length of BE.
We examined the probability of any biopsy within a given biopsy level containing dysplasia using generalized estimating equations (GEE) with an auto-regressive correlation matrix, examining random effects of the two trials and controlling for repeated measures within a patient. GEE can be used to estimate correct confidence intervals and p values in the setting of multiple observations for each person. With an auto-regressive correlation matrix, this approach also allowed us to account for the possibility that areas of dysplasia are “lumped together,” i.e., that having one biopsy at a given level positive for dysplasia increased the probability that a subsequent biopsy from that or nearby levels would also be positive.
We used generalized linear models to estimate prevalence and prevalence differences for dysplasia depending on the standardized location of biopsy levels. We queried for a nonlinear relationship between the yield for dysplastic biopsies and location by fitting and statistically comparing nested models with an ordinary linear specification of the effect of location, with quadratic and cubic parameterizations, and with natural log and exponential transformations. To examine whether the prevalence difference between standardized biopsy location and the yield for dysplasia was modified by clinical or endoscopic features, we compared nested models with and without hierarchically specified interaction terms using the quasi-likelihood under the independence model criterion (QIC), a model-fit statistic to compare the fit of models estimated with GEE.10 To estimate the potential for bias due to any missing location data, we performed multiple imputation sensitivity analysis among patients with missing location data with prevalence ratios for dysplasia of 1 to 4 in the opposite direction of the observed association. In order to examine the possibility that distal biopsies in segments shorter than 4 cm may have inadvertently sampled the gastric cardia, we performed sensitivity analysis limited to patients with greater than 4 cm Prague M length. Additionally, we examined the effect of excluding the distal-most observation in each patient. To examine the possibility that prior endoscopic mucosal resection (EMR) perturbed the natural distribution of dysplasia at baseline in these trials, we also performed analysis excluding patients with prior EMR.
Simulation Methods
A simulation study was performed to examine the hypothetical yield of biopsy regimens reflecting the observed spatial distribution of dysplasia. Pooled subjects' baseline qualifying endoscopies from both trials with dysplasia in at least one biopsy fragment were included. In contrast to the model for the prior outcome, which estimated the probability of finding any dysplasia within any sample at a given level, the model for simulation predicted the likelihood of finding dysplasia in a single fragment in the entirety of the biopsy run. This best reflects current management of these patients, since the worst dysplasia finding in any single fragment dictates the patient's care. In addition to the best fitting quadratic parameterization, this model included a hierarchically specified interaction term with segment length. GEE were specified similarly to the primary analysis.
Monte Carlo sampling of simulated biopsy fragments was stratified by segment length and 1 or 2 cm increments of location, depending on the regimen being simulated. Samples for 2 cm regimens were evenly split between the top and bottom 1cm halves. Each sample was taken from a Bernoulli distribution (which is used for “yes or no” probabilities) of the absolute prevalence risk of dysplasia, informed by the location and segment length of the model for simulation. If one or more samples from a simulated patient yielded dysplasia, the sampling regimen was considered to be sensitive for detecting dysplasia. For segments of 1 cm in length, it was assumed that one half of the recommended biopsies from the q2 cm regimen would be taken. We reported the mean for sensitivity as a function of segment length, increasing the number of Monte Carlo samples until simulation standard error was negligible. In analyses of distribution of dysplasia, dysplasia found in targeted biopsies was included in addition to that found in random biopsies, while in simulations of hypothetical random biopsy protocols, only dysplasia found on random biopsy was included.
Results
Of 140 patients in the SURF study and 127 patients in the AIM Dysplasia trial, 115 and 77 respectively had fragment-level information regarding the location and histologic grade of all biopsy fragments at the baseline endoscopy complete and detailed enough to allow the analyses described above (figure 1). Of these 93 and 64, respectively, had at least two biopsy levels, yielding 157 patients for the pooled spatial analysis. Patients in both trials were predominantly male, Caucasian, and older. The mean Prague M length in the SURF study was 5.6 cm, and the mean in the AIM Dysplasia trial, which was limited to patients with no more than 8 cm of BE, was 4.4 cm (table 1). In the AIM Dysplasia trial 50 of 127 (39%) patients had incomplete data for histology locations at baseline; in the SURF study 25 of 140 (18%) patients had incomplete data; in pooled analysis 75 of 267 (28%) patients had incomplete data.
Figure 1.
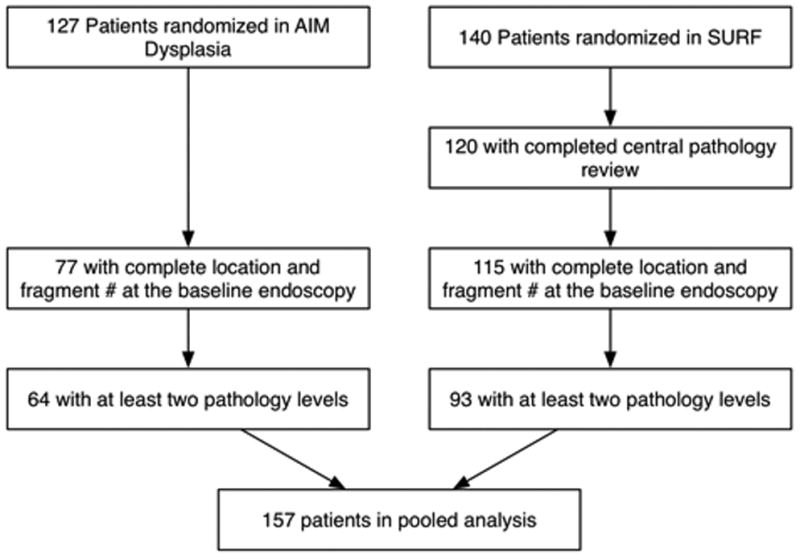
Inclusion and Exclusion of 127 AIM Dysplasia Subjects and 140 SURF Subjects Randomized in Each Trial for Post-hoc Analysis. Patients with complete biopsy location data and with at least two biopsies were included from each trial.
Table 1. Baseline Clinical and Endoscopic Characteristics of 93 Patients Included from the SURF Trial and 63 Patients included from the AIM Dysplasia Trial.
| Mean (SD) / N (%) | AIM Dysplasia | SURF | Pooled |
|---|---|---|---|
| Age | 66.5 (9.5) | 64.1 (7.9) | 65.1 (8.7) |
| Male sex | 54 (84%) | 78 (84%) | 132 (84%) |
| BMI (mg/k2) | 29.5 (5.7) | 27.3 (4.1) | 28.2 (5.0) |
| Low grade dysplasia | 35 (55%) | 93 (100%) | 128 (82%) |
| High grade dysplasia | 29 (45%) | 0 (0%) | 29 (18%) |
| Prague M Length (cm) | 4.4 (3.0) | 5.6 (3.2) | 5.0 (3.1) |
| Prague C Length (cm) | 3.1 (2.7) | 3.7 (3.8) | 3.5 (3.4) |
| Hiatal Hernia (cm) | 3.4 (1.7) | 2.8 (1.6) | 3.2 (1.7) |
| Caucasian | 61 (95%) | 90 (97%) | 151 (96%) |
| African American | 2 (3%) | 0 (0%) | 2 (1%) |
| Hispanic | 1 (2%) | 0 (0%) | 1 (1%) |
| Unknown | 0 (0%) | 3 (3%) | 3 (2%) |
Independent analysis of each trial yielded a statistically significant, positive linear association between more proximal location of biopsy level within the BE segment and the finding of dysplasia among any of the fragments at that level (table 2). Overall, biopsies from the proximal-most quarter of the BE segment were almost twice as likely to demonstrate dysplasia as those from the most distal quarter (46.6% vs. 24.0%). Considering the trials individually, in the AIM Dysplasia trial, compared to biopsy levels in the distal-most quarter segment, biopsy levels in the proximal-most quarter segment had a 38.1% (95% CL: [22.7%, 53.5%]) higher prevalence of dysplasia. The second-most proximal segment had a prevalence difference of 34.6% (95% CL: [17.6%, 50.7%]) and the second-most distal segment had a prevalence difference of 23.2% (95% CL: [7.2%, 39.3%]). In the SURF trial, compared to biopsy levels in the distal-most quarter segment, biopsy levels in the proximal-most quarter segment had an absolute 12.9% (95% CL: [1.0%, 24.8%]) higher prevalence of dysplasia. The second-most proximal segment had a prevalence difference of 16.8% (95% CL: [2.5%, 31.0%]) and the second-most distal segment had a prevalence difference of 11.6% (95% CL: [0.0%, 24.5%]). These findings were insensitive to exclusion of targeted biopsies. The absolute prevalence of dysplasia was higher in the AIM Dysplasia trial but followed a similar pattern to the SURF trial.
Table 2. Proportion of Biopsy Levels with Dysplasia in at Least One Fragment in the SURF and AIM Dysplasia Trials by Proximal-Distal Quarter Segment.
| Combined Trials | SURF Trial | AIM Dysplasia Trial | ||||
|---|---|---|---|---|---|---|
| Proportion (%) | 95% CL | Proportion (%) | 95% CL | Proportion (%) | 95% CL | |
| Proximal most fourth | 46.6 | 39.1-54.0 | 35.1 | 26.2-44.0 | 64.3 | 53.3-75.3 |
| Second most proximal fourth | 47.0 | 38.1-56.0 | 38.9 | 27.3-50.6 | 60.3 | 47.2-73.5 |
| Second most distal fourth | 39.2 | 30.3-48.1 | 33.8 | 22.9-44.7 | 49.4 | 34.8-64.1 |
| Distal most fourth | 24.0 | 16.8-31.1 | 22.2 | 13.0-31.4 | 26.2 | 15.1-37.3 |
CL, confidence limits.
In the pooled trials, compared to biopsy levels in the distal-most quarter segment, biopsy levels in the proximal-most quarter segment had an absolute 22.6% (95% CL: [12.8%, 32.5%]) higher prevalence of dysplasia. The second-most proximal segment had a prevalence difference of 23.1% (95% CL: [12.1%, 34.1%]) and the second-most distal segment had a prevalence difference of 15.3% (95% CL: [5.1%, 24.5%]).
The best-fitting nonlinear parameterization based on QIC was the quadratic fit (figure 2), which was a significantly better fit than the simple linear parameterization (quasi-likelihood ratio statistic = 5.56, p = 0.018). The prevalence of dysplasia in individual biopsy fragments, rather than biopsy levels, followed a similar distribution (figure 3). Multiple imputation analysis for missing data found that a risk ratio of greater than two in the opposite direction of that observed among patients with missing samples would be required to reverse the direction of effect. The effect remained statistically significant in 99% of random normal imputations of missing biopsy levels.
Figure 2.
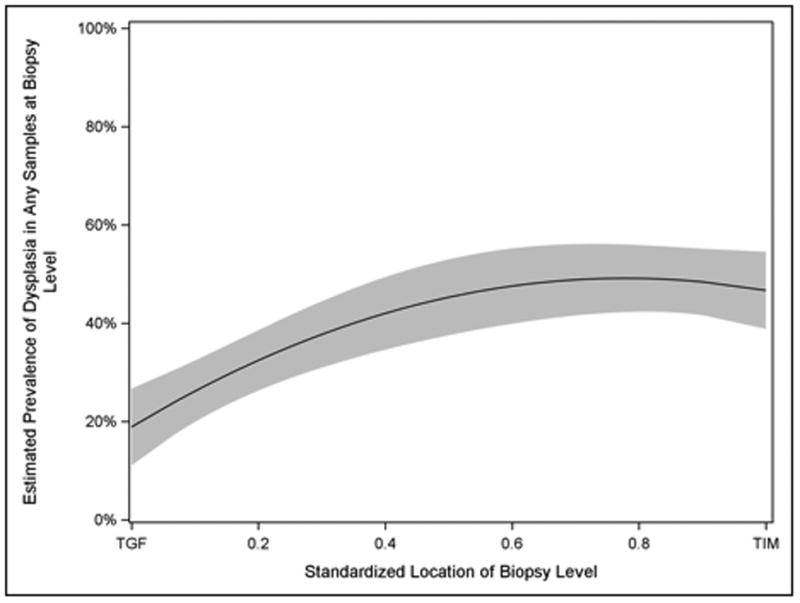
Estimated Prevalence of Dysplasia in Biopsy Levels among 157 Patients by Sampling Level in Pooled Analysis of the AIM Dysplasia and SURF Trials. Dysplasia was nearly twice as common in the top halves of Dysplastic BE segments as at TGF.
Figure 3.
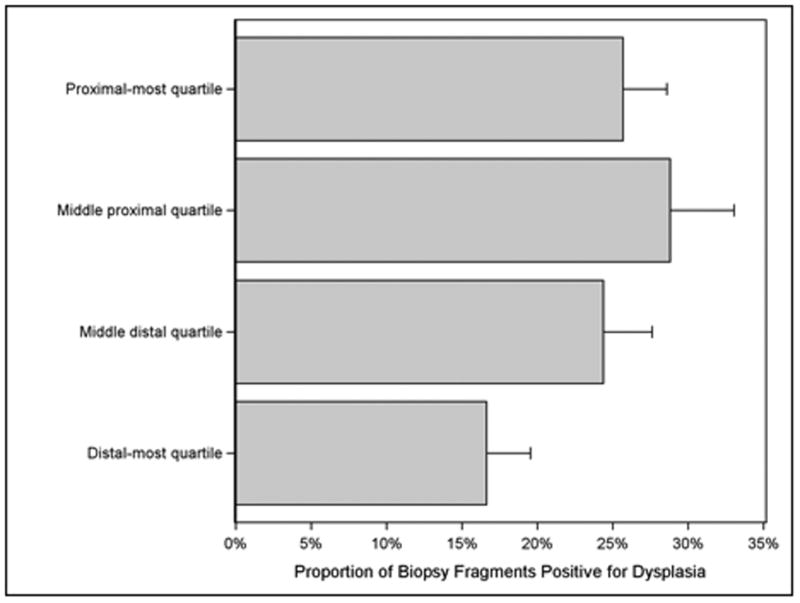
Prevalence of Dysplasia in Individual Biopsies among 157 Patients by Quartile of Sampling Level in Pooled Analysis of the AIM Dysplasia and SURF Trials. At all levels, individual biopsy fragments had a modest prevalence of positivity for dysplasia. Biopsy fragments in the proximal Barrett's segment were more likely to be positive for dysplasia.
The relationship between proximal-to-distal location and the prevalence of dysplasia was largely insensitive to baseline clinical and endoscopic characteristics. However, there was statistically significant modification of the prevalence difference at an alpha threshold of 0.05 by age, where younger patients had a greater proximal predisposition of dysplasia and older patients had a weaker predisposition. There were insignificant modifications of the prevalence difference for BMI (p = 0.098), where patients with higher BMI had a stronger proximal predisposition, and for Prague C length (p = 0.105), where patients with longer Prague C length had a weaker proximal predisposition. No other potential modifiers of the risk difference approached statistical significance.
Sensitivity analyses suggest that the spatial distribution of dysplasia was insensitive to segment length or the inclusion of patients with prior EMR. Limitation of the analysis to BE segments over four cm had no significant effect on the risk difference from TGF to TIM from 24.9% (95% CL: [14.0%, 35.8%]) to 24.3% (95% CL: [7.4%, 41.3%]). Excluding the distal-most sample from each patient attenuated the effect, but it remained statistically significant at 20.0% (95% CL: [0.6%, 40.0%]). Excluding the 11 patients with prior EMR also had no significant effect, yielding a risk difference of 26.1% (95% CL: [14.8%, 37.4%]).
Monte Carlo simulation of biopsy fragments among patients with dysplasia at the baseline endoscopy suggested that the sensitivity of biopsy regimens varied widely depending on segment length. Among patients with shorter segments, random draws of biopsy fragments often failed to identity any dysplasia (figure 4). Sensitivity decreases of >30% were observed at segment lengths ≤3 cm by random error in biopsy fragment selection. Simulations of biopsy regimens with increased proximal samples suggested that such regimens would perform better than a uniform regimen. The best overall regimen was a “q1cm - 8, 8, 2, 2…” regimen in which 8 biopsies were taken at the proximal-most 1 cm level, 8 biopsies at the next 1 cm level, and 2 biopsies in each subsequent, more distal 1 cm level. This regimen decreased the number of biopsies in longer segments of BE, but increased the number of biopsies in shorter segments. Interestingly, the worst performing regimen we modeled was 4 quadrant biopsies every 2 cm, which was projected to miss at least one in five patients with dysplasia who had a segment length of the BE of <4 cm. In contrast, a highly accurate q2 cm regimen could be constructed, but only with marked over-sampling of the proximal BE (12 biopsies over 2 cm, figure 4). Figure 5 demonstrates the total number of biopsies necessary to complete each modeled regimen as a function of BE segment length. While short segments of BE require higher numbers of biopsies in the proposed weighted regimens, longer segments of disease actually result in fewer biopsies, despite achieving sensitivities equal to or better than standard regimens.
Figure 4.
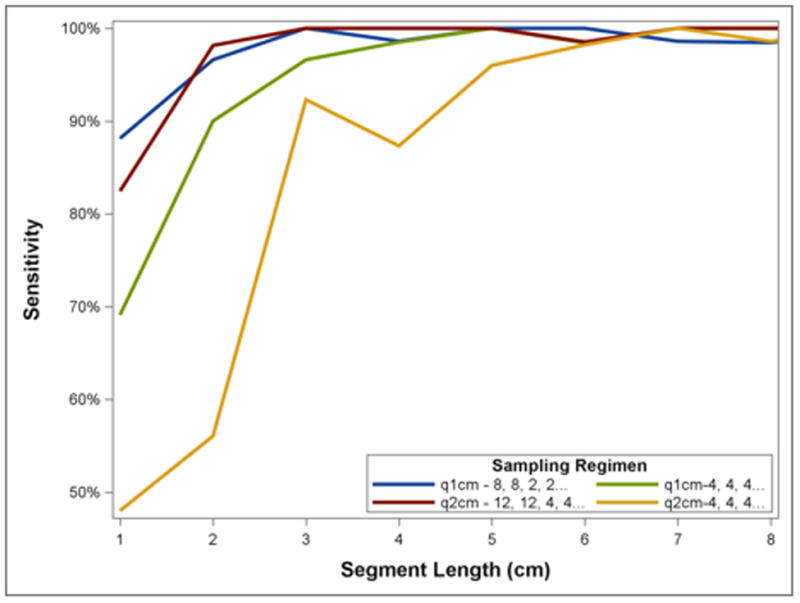
Simulated Yield for Dysplasia within Any Samples for a Given Patient under Selected Classical and Novel Biopsy Regimens by Segment Length. Biopsy regimens missed dysplasia in simulations of shorter segments because of less total biopsies introducing the possible of missing a single dysplastic biopsy fragment at random. All regimens performed well among longer segments. Note that q2cm regimens' sensitivity tended to drop slightly at even numbered segment lengths because the same number of biopsies was performed over a larger area compared to a segment one cm shorter.
Figure 5.
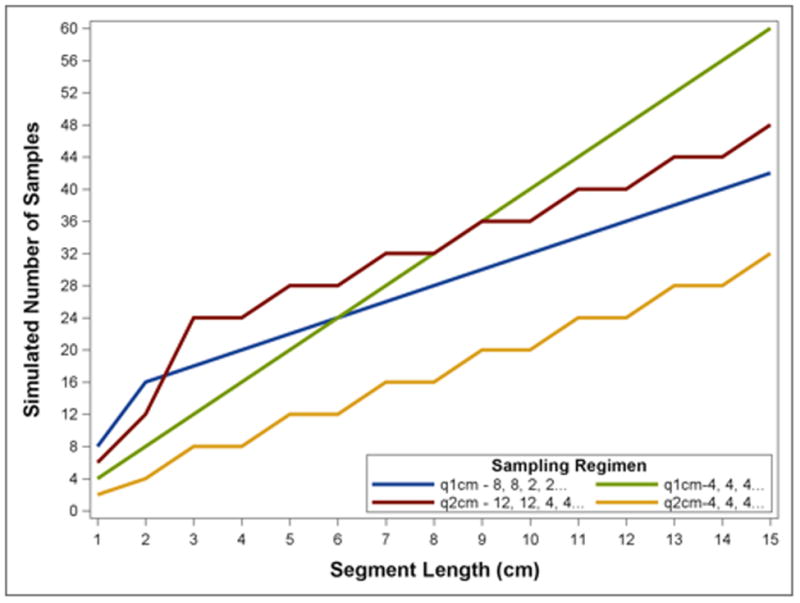
Simulated Number of Biopsies under Selected Classical and Novel Biopsy Regimens by Segment Length. In short segments the proposed biopsy regimens require more biopsies than uniform regimens, while at longer segment lengths they require more than uniform q2cm biopsies but less than q1cm.
Discussion
In a post-hoc analysis of two randomized controlled trials we found a significantly increased prevalence of dysplasia proximally within a field of BE. This relationship was stable over most baseline clinical and endoscopic characteristics, but was significantly increased among younger patients. We also present simulation studies that suggest that proximal oversampling could allow better sensitivity in those with shorter segments of BE, and for fewer biopsies in patients with longer segment BE. Simulations suggest such regimens could increase the sensitivity for dysplasia among patients with short-segment BE by >30% and decrease the number of biopsies among patients with long-segment BE of greater than 6 to 9 cm without compromising sensitivity.
BE management depends on accurate risk stratification. Surveillance endoscopy taking both random and targeted biopsies of any mucosal abnormalities throughout the BE segment is the standard of care.11-13 Most patients with BE have non-dysplastic disease.14, 15 Both the AIM Dysplasia and SURF trials demonstrated a benefit of ablative therapy in patients with low-grade dysplasia and high-grade dysplasia.7, 9 As such, the identification of dysplasia in a patient with BE is vital, as it is the deciding factor in treatment decisions for BE. Optimization of sampling techniques has the potential to benefit a large population of patients.
Given that the distribution of dysplasia at baseline endoscopies in these two trials is not linear or uniform, biopsy regimens that would perfectly reflect this distribution would be complicated. Much of the burden of surveillance of non-dysplastic BE falls to community gastroenterologists or surgeons who may not specialize in esophageal disease. As such, widespread adoption of novel biopsy regimens will be facilitated if they are memorable and simple to implement. The best performing simple regimen in simulation studies was the q1cm - “8, 8, 2, 2…” regimen, which requires 8 biopsies in the proximal-most 1 cm level and 8 biopsies at the second most proximal 1 cm level, with 2 biopsies at each more distal 1 cm level. This regimen has the advantages of ease of recall for the endoscopist, increase in the sensitivity in shorter segment BE, and decrease the number of biopsies in long segment BE. A q2cm regimen, q2cm - “12, 12, 2…” had a similar distribution of biopsies and performed almost as well.
Our findings are novel, but are generally consistent with prior studies regarding the yield of biopsies in BE surveillance. Previous work suggests that when utilizing the Seattle protocol, q1cm four quadrant biopsies detect more dysplasia than q2cm regimens, a finding replicated in our simulation.16 A subsequent study countered this finding, but had a small sample size and could only detect a very large difference in yield between the q1cm and q2cm regimens tested.17 A larger study found a very large increase in detection with systematic rather than random biopsies.18 Early studies of dysplasia in esophagectomy specimens and a study of early neoplasia did not find a spatial predisposition, but given their sample sizes, had limited power to detect effects of the magnitude we report.19-23 These studies suggested that areas of high-grade dysplasia were generally contiguous but could vary greatly in location and extent.
In distinction to our findings, a study set in Germany found a strong predisposition of esophageal and junctional adenocarcinoma (T1-T3) to the distal third of Barrett's segments, but this was after neoadjuvant chemotherapy and esophagectomy.24 These findings may reflect a substantively different population, a more advanced stage of disease, or a setting in an era before ablative therapies were the standard of care for dysplastic BE. Esophageal adenocarcinoma is generally more common in the distal third of the esophagus.25, 26 Because Barrett's carcinogenesis is a multi-step process over time, the exposures and events that cause progression from non-dysplastic to dysplastic BE may, on average, have a different spatial distribution in the esophagus than the exposures and events that cause progression of dysplasia to early cancer.
The proximal predisposition of dysplasia seems counterintuitive. Most other manifestations of reflux disease worsen with increasing acid exposure. For instance, while the degree of proximal extension of the refluxate is associated with severity of erosive disease, it is clear the distal esophagus is exposed to refluxate more often, and for longer periods of time, than more proximal areas.27-29 Therefore, it would seem most logical for the distal BE to preferentially harbor dysplasia – the exact opposite of what we actually observed. What might explain our findings? Several possibilities exist. Since the acidity of the refluxate decreases as it extends proximally, the observed pattern would make sense if weakly acidic/neutral refluxate promoted dysplasia within BE more so than strongly acidic refluxate, or if there was a protective effect of strongly acidic refluxate in BE.30 This pattern might also be consistent with a selection process that favors increased proliferation of a dysplastic clone into more proximal areas.31, 32 Perhaps the multilayered epithelium at the new squamocolumnar junction is especially prone to transformation and is a fertile area for the development of dysplasia.33, 34 While these or other mechanisms could underlie the observed distribution of dysplasia in this study, inference as to the pathogenesis explaining these findings is not possible from the present data.
This study has several limitations. Because the AIM Dysplasia protocol did not mandate sampling of the cardia, we have restricted our analyses and recommendations to the tubular esophagus. Also, a minority of patients had missing location data in each study, but multiple imputation analysis found the potential for bias to be small unless missing data were strongly biased in the opposite direction of our observed effect, which is unlikely. Next, while we examined effect measure modification, statistical power to detect modification of effects is generally much less than to detect the effect itself. It is relatively unlikely that we would detect subtle effect measure modifiers in a study of this size. Additionally, insufficient data prevented us from examining whether a differential effect existed for high-grade compared with low-grade dysplasia. Simulation studies such as those performed here are limited in that random draws of biopsies or biopsy levels may fail to approximate the actual clinical process of biopsy selection, since each additional hypothetical biopsy is presumed to have the same yield as the actual biopsies taken at that level. Our study determined only the longitudinal, not circumferential, predisposition to dysplasia. Studies suggest that, at least in the case of early adenocarcinoma or high-grade dysplasia, there may additionally be a circumferential clustering of disease.35, 36 Furthermore, this is a single analysis and, despite being performed on two, high quality, multicenter, randomized clinical trials, should be replicated in further observational or randomized studies.
These results are likely generalizable to populations that include patients with BE with dysplasia under similar conditions to the study population regardless of the underlying prevalence of dysplasia, given that the biological factors underlying dysplasia distribution would not be expected to be impacted by dysplasia prevalence. However, differences in the performance of EGD or the quality of the histological grading of dysplasia have the potential to change both the test characteristics of surveillance endoscopy, and, potentially, the findings we report here. Therefore, inference from this study is most reliable in cases of dysplasia as diagnosed by an endoscopist with expertise in managing BE in concert with a dedicated gastrointestinal pathologist. A further limitation of sampling from referral populations such as these is the possibility of selection bias. It is possible that the distribution of dysplasia in patients referred to such centers does not reflect that in general populations. However, community-based studies may also have serious threats to internal validity due to missing data for biopsy location and the possibility non-systematic sampling regimens.
The study also has several strengths. Because the same spatial predisposition of dysplasia is observed independently in these two multicenter, randomized controlled trials, our findings are likely robust to both random variation and idiosyncratic effects of individual centers. In contrast to many observational datasets, patients selected for these trials were known to be treatment-naïve, were subject to carefully recorded systematic biopsy, and had rigorous review of their histologic grade.
In summary, we report the results of pooled analysis of two multicenter, randomized controlled trials that independently support a proximal predisposition of dysplasia within Barrett's segments with dysplasia. We also propose a “q1cm - 8, 8, 2, 2…” biopsy regimen and an alternative “q2cm - 12, 12, 2…” regimen that both take advantage of the observed distribution of dysplasia and allow for a decreased number of biopsies in patients with longer segments of BE compared to a q1cm four quadrant regimen. These simulations also demonstrate that in shorter segments, even with weighted sampling regimens, dysplasia detection was very sensitive to sample number. Even small decrements in the number of samples taken were reflected in diminished sensitivity of the regimens. Given that poor adherence to rigorous sampling regimens has been described, this finding speaks to the need for novel mucosal sampling technologies.37 Such a regimen would complement, but not replace, careful examination of the Barrett's segment with targeted biopsy of mucosal abnormalities. If these results can be replicated in further studies, this change in practice has the potential to improve risk stratification of patients with BE without requiring additional time or expense. (3984/4000)
Summary Box.
What is current knowledge?
Diagnosis of dysplasia within a Barrett's esophagus (BE) segment is central to management decisions in BE.
The addition of biopsies not targeted to visible lesions improves the sensitivity of biopsy regimens over targeted biopsies alone,, and systematic biopsy regimens outperform unstructured regimens.
What is new here?
Dysplasia is more common in the proximal than distal regions of Barrett's segments. Biopsy regimens that oversample the proximal Barrett's segment perform better than uniform regimens in simulations. In short segment BE, prevalent dysplasia is frequently missed due to a small number of samples and random error.
Acknowledgments
Grant Support: NIH T32 DK 007634, NIH K24DK100548 and NIH P30 DK 034987 funded this work. The SURF and AIM-D trials were originally funded by Barrx Medical, now a subsidiary of Medtronics.
Abbreviations
- BE
Barrett's Esophagus
- RFA
radiofrequency ablation
- SURF
Surveillance vs. Radiofrequency Ablation Trial
- LGD
low grade dysplasia
- HGD
high grade dysplasia
- AIM
Ablation of Intestinal Metaplasia (AIM) Containing Dysplasia Trial
- TGF
top of gastric folds
- GEE
generalized estimating equations
- QIC
quasi-likelihood under the independence model criterion, CL, confidence limits
- q1cm
every one centimeter
- q2cm
every two centimeters
Footnotes
Author Contributions: Cary C Cotton, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis; Lucas C Duits, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis; W Asher Wolf, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis. Anne F Peery, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis; Evan S. Dellon, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis; Jacques J. Bergman, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content; Nicholas J Shaheen, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content.
Disclosures: Dr. Shaheen receives research funding from CSA Medical, Covidien Medical, NeoGenomics, Takeda Pharmaceuticals and Oncoscope. He is a consultant for Oncoscope. Dr. Bergman receives research support from Olympus Endoscopy, Cook Medical, Boston Scientific, GI Solutions Covidien, Erbe and Ninepoint Medical; receives financial support for training programs from GI Solutions Covidien; and receives honorarium-consultancy-speakers fee from Cook Medical, Boston Scientific and GI Solutions Covidien.
The other authors have no conflicts to declare.
References
- 1.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association technical review on the management of Barrett's esophagus. Gastroenterology. 2011;140:e18–52. doi: 10.1053/j.gastro.2011.01.031. quiz e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354–9. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 3.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–31. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 4.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375–83. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 5.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–57. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394–8. doi: 10.1016/j.gie.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 8.Committee ASoP. Evans JA, Early DS, et al. The role of endoscopy in Barrett's esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087–94. doi: 10.1016/j.gie.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. Jama. 2014;311:1209–17. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 10.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57:120–5. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 11.Kadri S, Lao-Sirieix P, Fitzgerald RC. Developing a nonendoscopic screening test for Barrett's esophagus. Biomark Med. 2011;5:397–404. doi: 10.2217/bmm.11.40. [DOI] [PubMed] [Google Scholar]
- 12.Leggett CL, Gorospe E, Owens VL, et al. Volumetric laser endomicroscopy detects subsquamous Barrett's adenocarcinoma. Am J Gastroenterol. 2014;109:298–9. doi: 10.1038/ajg.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sturm MB, Piraka C, Elmunzer BJ, et al. In vivo molecular imaging of Barrett's esophagus with confocal laser endomicroscopy. Gastroenterology. 2013;145:56–8. doi: 10.1053/j.gastro.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–7. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 15.Ward EM, Wolfsen HC, Achem SR, et al. Barrett's esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol. 2006;101:12–7. doi: 10.1111/j.1572-0241.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 16.Reid BJ, Blount PL, Feng Z, et al. Optimizing endoscopic biopsy detection of early cancers in Barrett's high-grade dysplasia. Am J Gastroenterol. 2000;95:3089–96. doi: 10.1111/j.1572-0241.2000.03182.x. [DOI] [PubMed] [Google Scholar]
- 17.Kariv R, Plesec TP, Goldblum JR, et al. The Seattle protocol does not more reliably predict the detection of cancer at the time of esophagectomy than a less intensive surveillance protocol. Clin Gastroenterol Hepatol. 2009;7:653–8. doi: 10.1016/j.cgh.2008.11.024. quiz 606. [DOI] [PubMed] [Google Scholar]
- 18.Abela JE, Going JJ, Mackenzie JF, et al. Systematic four-quadrant biopsy detects Barrett's dysplasia in more patients than nonsystematic biopsy. Am J Gastroenterol. 2008;103:850–5. doi: 10.1111/j.1572-0241.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 19.Nishimaki T, Hölscher AH, Schüler M, et al. Histopathologic characteristics of early adenocarcinoma in Barrett's esophagus. Cancer. 1991;68:1731–6. doi: 10.1002/1097-0142(19911015)68:8<1731::aid-cncr2820680814>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Reid BJ, Weinstein WM, Lewin KJ, et al. Endoscopic biopsy can detect high-grade dysplasia or early adenocarcinoma in Barrett's esophagus without grossly recognizable neoplastic lesions. Gastroenterology. 1988;94:81–90. doi: 10.1016/0016-5085(88)90613-0. [DOI] [PubMed] [Google Scholar]
- 21.Cameron AJ, Carpenter HA. Barrett's esophagus, high-grade dysplasia, and early adenocarcinoma: a pathological study. Am J Gastroenterol. 1997;92:586–91. [PubMed] [Google Scholar]
- 22.McArdle JE, Lewin KJ, Randall G, et al. Distribution of dysplasias and early invasive carcinoma in Barrett's esophagus. Hum Pathol. 1992;23:479–82. doi: 10.1016/0046-8177(92)90123-k. [DOI] [PubMed] [Google Scholar]
- 23.Cassani L, Sumner E, Slaughter JC, et al. Directional distribution of neoplasia in Barrett's esophagus is not influenced by distance from the gastroesophageal junction. Gastrointest Endosc. 2013;77:877–82. doi: 10.1016/j.gie.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Theisen J, Stein HJ, Feith M, et al. Preferred location for the development of esophageal adenocarcinoma within a segment of intestinal metaplasia. Surg Endosc. 2006;20:235–8. doi: 10.1007/s00464-005-0187-5. [DOI] [PubMed] [Google Scholar]
- 25.Coupland VH, Allum W, Blazeby JM, et al. Incidence and survival of oesophageal and gastric cancer in England between 1998 and 2007, a population-based study. BMC Cancer. 2012;12:11. doi: 10.1186/1471-2407-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon J, Luebeck EG, Moolgavkar SH. Age effects and temporal trends in adenocarcinoma of the esophagus and gastric cardia (United States) Cancer Causes Control. 2006;17:971–81. doi: 10.1007/s10552-006-0037-3. [DOI] [PubMed] [Google Scholar]
- 27.Bredenoord AJ, Hemmink GJ, Smout AJ. Relationship between gastro-oesophageal reflux pattern and severity of mucosal damage. Neurogastroenterol Motil. 2009;21:807–12. doi: 10.1111/j.1365-2982.2009.01306.x. [DOI] [PubMed] [Google Scholar]
- 28.Bredenoord AJ, Weusten BL, Timmer R, et al. Characteristics of gastroesophageal reflux in symptomatic patients with and without excessive esophageal acid exposure. Am J Gastroenterol. 2006;101:2470–5. doi: 10.1111/j.1572-0241.2006.00945.x. [DOI] [PubMed] [Google Scholar]
- 29.Savarino E, Tutuian R, Zentilin P, et al. Characteristics of reflux episodes and symptom association in patients with erosive esophagitis and nonerosive reflux disease: study using combined impedance-pH off therapy. Am J Gastroenterol. 2010;105:1053–61. doi: 10.1038/ajg.2009.670. [DOI] [PubMed] [Google Scholar]
- 30.Emerenziani S, Ribolsi M, Sifrim D, et al. Regional oesophageal sensitivity to acid and weakly acidic reflux in patients with non-erosive reflux disease. Neurogastroenterol Motil. 2009;21:253–8. doi: 10.1111/j.1365-2982.2008.01203.x. [DOI] [PubMed] [Google Scholar]
- 31.Geddert H, Heep HJ, Gabbert HE, et al. Expression of cyclin B1 in the metaplasia-dysplasia-carcinoma sequence of Barrett esophagus. Cancer. 2002;94:212–8. doi: 10.1002/cncr.10152. [DOI] [PubMed] [Google Scholar]
- 32.Merlo LM, Pepper JW, Reid BJ, et al. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–35. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald RC, Abdalla S, Onwuegbusi BA, et al. Inflammatory gradient in Barrett's oesophagus: implications for disease complications. Gut. 2002;51:316–22. doi: 10.1136/gut.51.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glickman JN, Chen YY, Wang HH, et al. Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of Barrett's esophagus. Am J Surg Pathol. 2001;25:569–78. doi: 10.1097/00000478-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Kariyawasam VC, Bourke MJ, Hourigan LF, et al. Circumferential location predicts the risk of high-grade dysplasia and early adenocarcinoma in short-segment Barrett's esophagus. Gastrointest Endosc. 2012;75:938–44. doi: 10.1016/j.gie.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Enestvedt BK, Lugo R, Guarner-Argente C, et al. Location, location, location: does early cancer in Barrett's esophagus have a preference? Gastrointest Endosc. 2013;78:462–7. doi: 10.1016/j.gie.2013.03.167. [DOI] [PubMed] [Google Scholar]
- 37.Peters FP, Curvers WL, Rosmolen WD, et al. Surveillance history of endoscopically treated patients with early Barrett's neoplasia: nonadherence to the Seattle biopsy protocol leads to sampling error. Dis Esophagus. 2008;21:475–9. doi: 10.1111/j.1442-2050.2008.00813.x. [DOI] [PubMed] [Google Scholar]


