Abstract
Ghrelin plays a key role in the regulation of growth hormone secretion and energy homeostasis. Adiponectin is exclusively secreted by adipose tissue and is abundantly present in the circulation, with important effects on metabolism. We studied five lean and five obese young men [ages: 24.2 ± 1.0 (lean) and 21.8 ± 1.6 (obese) years (difference not significant); body mass indexes: 35.0 ± 1.3 and 23.0 ± 0.3 kg/m2 (P = 0.01)], sampled blood every 7 min over 24 h, and measured ghrelin, adiponectin, and leptin in 2,070 samples for a total of 6,210 data points. Circulating 24-h ghrelin showed significant ultradian fluctuations and an orderly pattern of release in lean and obese subjects with similar pulsatility characteristics. Plasma adiponectin concentrations were significantly lower in the obese group, with lower pulse height. In contrast to leptin, which is secreted in an orderly manner, the 24-h patterns of adiponectin were not significantly different from random in both the lean and obese groups. We show here that adipocytes can simultaneously secrete certain hormones, such as leptin, in patterns that are orderly, whereas other hormones, such as adiponectin, are secreted in patterns that appear to be random. The cross-approximate entropy statistic revealed pattern synchrony among ghrelin–leptin, ghrelin–adiponectin, and leptin–adiponectin hormone time series in the lean and obese subjects. Plasma ghrelin concentrations showed a nocturnal rise that exceeded the meal-associated increases in lean subjects, and this newly identified nocturnal rise was blunted in the obese. We suggest that the blunting of the nocturnal rise of ghrelin is a biological feature of human obesity.
Ghrelin is an endogenous ligand of the growth hormone secretagogue receptor and a potent stimulator of growth hormone release in humans (1–3). Ghrelin is synthesized primarily by the stomach, and in substantially lower amounts by the bowel, pituitary, kidney, placenta, and hypothalamus (4, 5). Accumulating evidence suggests that ghrelin contributes to the short- and long-term regulation of body weight as a key element of a complex central signaling network that regulates food intake and energy expenditure (6–9). Ghrelin is the only known circulating orexigenic hormone (9); its levels increase preprandially and decrease after meals, suggesting a role in meal initiation (6, 10, 11). Intravenous administration of ghrelin stimulates food intake in humans (6). Ghrelin induces a positive energy balance and induces adiposity in rodents by decreasing fat utilization (12). Ghrelin levels are reported to be decreased in obesity (13) and increase after diet-induced weight loss (14). Preliminary data in rodents suggest that ghrelin can also affect neuroendocrine and behavioral responses to stress (15).
Circulating plasma levels of ghrelin are influenced principally by changes in energy balance. It has been suggested that insulin may play an important role in the decrease of ghrelin levels after meals (16, 17). Rodent studies indicate that the satiety-inducing effects of leptin might include the suppression of ghrelin secretion, thus identifying leptin as a possible determinant of ghrelin concentrations (18). Indeed, ghrelin levels are decreased in human obesity, whereas leptin levels are increased, and the effects of ghrelin on energy homeostasis are opposite to those of leptin (8).
Adiponectin is the most abundant serum adipokine, and it is secreted exclusively by the adipose tissue (19). Circulating basal adiponectin concentrations are decreased in obesity, type 2 diabetes, and insulin resistance (20). The degree of hypoadiponectinemia is more closely related to the degree of insulin resistance and hyperinsulinemia than to the degree of adiposity (20). Low baseline adiponectin levels predict the development of subsequent diabetes in humans (21), and this reduction is suggested to play a role in the pathogenesis of cardiovascular disease associated with obesity (22, 23). It is suggested that adiponectin is a potent insulin enhancer linking adipose tissue and whole-body glucose metabolism (24). The administration of adiponectin improves insulin sensitivity in animal models of obesity and insulin resistance (25). Daily treatment with a protease-generated fragment of the recombinant adiponectin molecule prevents diet-induced obesity in mice without affecting food intake (26).
Adiponectin has been implicated in regulating energy homeostasis, functioning in combination with leptin (25). Insulin resistance in murine models lacking adipose tissue was shown to be completely reversed by a combination of physiological doses of adiponectin and leptin but only partially by either adiponectin or leptin alone (25). In diabetic and nondiabetic subjects, weight loss resulted in decreased plasma adiponectin concentrations and increased leptin levels (27). Serum adiponectin and leptin showed out-of-phase 24-h profiles in normal-weight men (28).
In this study, we sought to characterize in detail the dynamics of ghrelin and adiponectin in human obesity. To better understand the regulation of these two hormones and their possible interrelationship with leptin, in humans, we also evaluated the dynamics of leptin, which has an established role in the long-term regulation of body weight and energy homeostasis.
Subjects and Methods
Subjects. We conducted a study of five obese and five lean Mexican-American men, collecting a total of 2,070 plasma samples that yielded 6,210 data points of ghrelin, adiponectin, and leptin over a 24-h period. Subjects were recruited specifically for this study between March and October 2003 through local advertisement at the University of California, Los Angeles. We defined Mexican-American as having at least three grandparents born in Mexico. Lean was defined as a body mass index (BMI) of 20–25 kg/m2; obese was defined as a BMI >30 kg/m2. We screened all subjects for any personal history of mental illness, medical illness, obesity, smoking, or substance abuse. None of the subjects had night-shift work, transmeridian travel, or recent weight loss or gain. All subjects had normal physical examinations, normal electrocardiograms, and normal values on a screening laboratory panel. During the 30-day period preceding each study as well as during the study, no subject took prescribed or over-the-counter medications, hormones, or dietary supplements.
Clinical Research Protocol. Using an Institutional Review Board–approved protocol, after signed informed consent was obtained, we conducted a rapid-sampling study over 24 h to obtain 207 samples per subject. We studied 10 male subjects at the University of California, Los Angeles, General Clinical Research Center under standard conditions, which are reported in ref. 29. Briefly, the subjects were acclimated for 2 days in the same research room where blood collection occurred, and sampling was performed every 7 min for 24 h, starting at 0800 hours. The subjects were exposed to light during 0700–2300 hours and were studied in bed in the dark during 2300–0700 hours, during which time they slept. Information about the subjects' usual food intake was obtained to assess their caloric intake before admission, and they consumed a standard isocaloric diet designed to maintain their admission body weight (20% protein, 25% fat, and 55% carbohydrates). The subjects ate four meals a day (breakfast, 20% of calories, 0830 hours; lunch, 35% of calories, 1230 hours; dinner, 35% of calories, 1730 hours; and evening snack, 10% of calories, 2100 hours). During the daytime of the blood sampling, the subjects were allowed to walk in the research room and participate in nonstrenuous activities.
Body composition was measured by using dual-energy x-ray absorptiometry (QDR-4500, Hologic, Waltham, MA).
Assays. Hormonal assays were performed in plasma by using the following commercially available kits (Linco Research, St. Charles, MO): ghrelin (measures both octanoylated and des-octanoylated ghrelin), limit of detection 100 pg/ml, interassay coefficient of variation (CV) 8.1%, and intraassay CV 3.9%; adiponectin, limit of detection 1 ng/ml, interassay CV 5.2%, and intraassay CV 4.8%; and leptin, limit of detection 0.5 ng/ml, interassay CV 9.7%, and intraassay CV 4.2%.
Data Analysis
Pulse Analysis. We used cluster (provided by M. Johnson, University of Virginia, Charlottesville), a well validated computerized pulse-analysis algorithm, to identify statistically significant pulses in relation to measurement error in each individual hormone time series (29–31).
Relative Increment. Because of the intensity of sample collection, it was possible to look at the relative increment (expressed in percent terms), which is the amount of relative variability within a 7-min window, as described in ref. 29.
Diurnal Variability Analysis. To test for the presence of diurnal variation, we used a semiparametric linear mixed-effects approach (32). Within each subject, we modeled the observed hormone time series by using a simple harmonic function, similar to standard cosinor analysis (33). We extended the basic model to also include an orthogonal unknown smoothing spline function. This model allows for asymmetry in the periodic hormone behavior and controls for possible meal effects that may lead to inappropriate inferences regarding diurnal variation. We pooled the data from multiple subjects within the same group using a mixed-effects framework to model B, the group-level baseline concentration, and (α, β), the group-level regression coefficients for the linearized harmonic function. We fitted our model by using restricted maximum likelihood (34) and identified diurnal variation within a group by rejecting α = β = 0 by using an approximate F test (35). Given the three different hormones measured on two different groups of subjects, we adjusted for the six multiple comparisons by using a Bonferonni correction by setting P < 0.05/6 = 0.0083 as our nominal cutoff to identify diurnal variation. In the hormone series with significant diurnal variation, we additionally reported the group-level amplitude 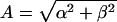 and peak time φ = tan–1 (β/α)/2π converted to clock hours.
and peak time φ = tan–1 (β/α)/2π converted to clock hours.
Cross-Correlation Analysis. Cross-correlation was calculated after lagging the concentration time series of one hormone relative to the concentration time series of another hormone, as described in ref. 31.
Approximate Entropy (ApEn) and Cross-ApEn. ApEn provides a network-dependent analysis of hormone secretion. ApEn is a scale- and translation-invariant, model-independent regularity statistic that was developed to quantify the orderliness of sequential measures (36), such as hormone time series (37). The basic derivation and calculation of ApEn are described in refs. 36 and 38–40. For this study, ApEn values were calculated for each hormone profile with window length m = 1 and tolerance parameter r = 20% of the average SD of the individual subject's hormone time series.
The cross-ApEn was used to evaluate the relative regularity or pattern synchronicity of coupled ghrelin vs. leptin, ghrelin vs. adiponectin, and leptin vs. adiponectin. Conditional regularity or synchrony of point-by-point variations across two time series is quantified by this measure, which is distinct from cross-correlation analysis because cross-ApEn is independent of lag (41). The detailed technical description of cross-ApEn was provided in ref. 42.
ApEn and cross-ApEn data were presented as a normalized ratio defined by the mean ratio of absolute value to that of 1,000 randomly shuffled versions of the same time sequences (43).
Correlation Analysis. We conducted Pearson's correlations between leptin and BMI, leptin and percentage of body fat, adiponectin and BMI, and adiponectin and percentage of body fat.
Statistics. Results are presented as mean ± SE. We used Student's t test to make the two-group comparisons. P < 0.05 was considered statistically significant.
Results
Anthropometric Measures. The two groups were closely matched in age [24.2 ± 1.0 (lean) and 21.8 ± 1.6 (obese) years; difference not significant (NS)]. As expected, BMI and fat mass were significantly higher in the obese group (BMI, 35.0 ± 1.3 vs. 23.0 ± 0.3 kg/m2, P = 0.01; fat mass, 33.9 ± 2.4% vs. 18.8 ± 2.4%, P = 0.002).
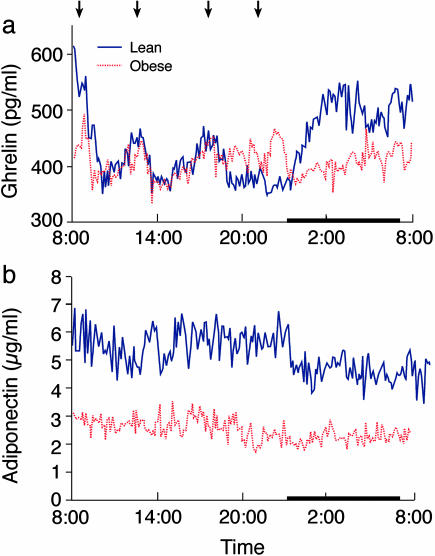
Plasma Ghrelin, Adiponectin, and Leptin Concentrations. The 24-h profiles and pulsatility parameters of circulating ghrelin, adiponectin, and leptin levels in the lean and obese groups are shown in Figs. 1 and 2 and in Table 1, respectively. Fig. 1a shows that the nocturnal rise in plasma ghrelin concentrations exceeded meal-associated increases in the plasma concentrations of this hormone in lean subjects. In the absence of obesity, the highest ghrelin levels occurred at early morning, not before meals. This substantial nocturnal rise could not be documented in the obese subjects we studied, even in the context of highly intensive sampling.
Fig. 1.
Twenty-four-hour profiles of rapidly sampled plasma ghrelin (a) and adiponectin (b) in lean (blue) and obese (red) male subjects. Arrows indicate standardized meals (breakfast, lunch, dinner, and evening snack), and the sleep period is demarcated by a black strip. Blood samples were collected in a general clinical research center after two nights of acclimatization in the research room. Lights were on during 0700–2300 hours and off during 2300–0700 hours, during which time subjects slept.
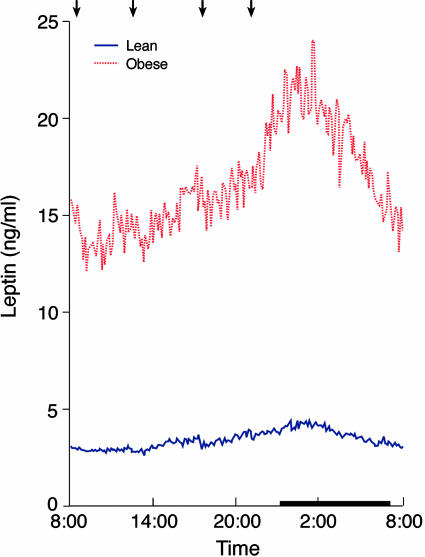
Fig. 2.
Twenty-four-hour profiles of rapidly sampled plasma leptin in lean (blue) and obese (red) male subjects. Arrows indicate standardized meals (breakfast, lunch, dinner, and evening snack), and the sleep period is demarcated by a black strip. Blood samples were collected in a general clinical research center after two nights of acclimatization in the research room. Lights were on during 0700–2300 hours and off during 2300–0700 hours, during which time subjects slept.
Table 1. Twenty-four-hour dynamics of ghrelin, adiponectin, and leptin in lean and obese men.
| Ghrelin
|
Adiponectin
|
Leptin
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Lean | Obese | P | Lean | Obese | P | Lean | Obese | P | |
| Mean hormone conc. | 437 ± 9 | 409 ± 39 | NS | 5.3 ± 0.3 | 2.5 ± 0.3 | <0.01 | 3.4 ± 0.6 | 16.7 ± 3.2 | <0.005 |
| Pulse freq. per 24 h | 21.8 ± 2.0 | 16.0 ± 1.3 | 0.05 | 28.8 ± 2.0 | 30.4 ± 2.3 | NS | 25.4 ± 1.7 | 26.4 ± 1.5 | NS |
| Interpeak interv., min | 65.4 ± 6.9 | 91.8 ± 11.2 | NS | 48.3 ± 3.6 | 46.3 ± 1.4 | NS | 56.0 ± 5.3 | 52.7 ± 3.4 | NS |
| Pulse duration, min | 49.7 ± 5.8 | 68.0 ± 10.9 | NS | 36.3 ± 3.1 | 34.5 ± 1.4 | NS | 40.8 ± 4.9 | 38.3 ± 2.7 | NS |
| Pulse height, conc. | 496 ± 10 | 461 ± 41 | NS | 6.7 ± 0.4 | 3.3 ± 0.3 | <0.001 | 3.8 ± 0.6 | 19.4 ± 3.5 | <0.05 |
| Pulse height, % incr. | 125 ± 3 | 123 ± 3 | NS | 149 ± 6 | 152 ± 5 | NS | 118 ± 1 | 124 ± 2 | <0.05 |
| Valley conc., ng/ml | 412 ± 11 | 384 ± 35 | NS | 4.8 ± 0.3 | 2.3 ± 0.2 | <0.001 | 3.3 ± 0.5 | 15.9 ± 3.0 | <0.05 |
| Relative increment, % | 8.7 ± 1.4 | 7.0 ± 0.7 | NS | 20.6 ± 2.8 | 21.3 ± 1.0 | NS | 7.5 ± 0.6 | 10.9 ± 1.2 | <0.05 |
| ApEn | 0.754 ± 0.076 | 0.752 ± 0.043 | NS | 0.937 ± 0.024 | 0.927 ± 0.039 | NS | 0.759 ± 0.041 | 0.807 ± 0.061 | NS |
conc., plasma hormone concentrations, ghrelin (pg/ml), adiponectin (μg/ml), leptin (ng/ml); freq., frequency; interv., interval; incr., increase. Data are mean ± SE. Each column represents the analysis of an endocrine time series containing 207 time points collected every 7 min for 24 h, starting at 0800 hours, in the course of a standardized isocaloric diet, after two nights of acclimatization. n = 5 per group.
We show the following significant correlations between 24-h mean adipocyte hormone concentrations and body weight and percentage of body fat: leptin–BMI, r = 0.82, P < 0.005; leptin–percentage of body fat, r = 0.93, P < 0.001; adiponectin–BMI, r = –0.91, P < 0.001; adiponectin–percentage of body fat, r = –0.78, P < 0.01.
Pulsatility Features. The circulating ghrelin concentrations in the lean subjects had significant pulsatility, with diurnal and ultradian rhythms, as assessed by frequent sampling (Table 1 and Fig. 1a). We show that rapidly sampled ghrelin concentrations exhibit 21.8 ± 2.0 pulses every 24 h in the lean men and 16.0 ± 1.3 pulses every 24 h in the obese men (P = 0.05). Circulating ghrelin pulse parameters were similar in the two groups (Table 1).
Adiponectin levels were significantly lower in the obese group, as expected (P < 0.01, Fig. 1b and Table 1). The obese group had significantly higher average pulse height and valley concentrations (P < 0.001 for both, Table 1), whereas the other pulsatility parameters, including pulse frequency, interpeak interval, pulse duration, pulse height as percentage increase, and relative increment, were all similar in the obese and lean groups (Table 1).
Circulating leptin concentrations were higher in the obese group throughout the 24-h period (24-h averages of 16.7 ± 3.2 in the obese and 3.4 ± 0.6 in the lean group, P < 0.005, Fig. 2 and Table 1). Leptin concentrations had similar pulse frequencies, interpeak intervals, and pulse durations in the lean and obese subjects. The obese subjects had significantly higher pulse height, pulse height as percentage increase, valley concentration, and relative increment (P < 0.05 for all comparisons, Table 1).
Ghrelin in Lean and Obese Subjects. In contrast to the healthy subjects, the obese subjects had no nocturnal rise of plasma ghrelin concentrations (Fig. 1a). The underlying pulsatility of ghrelin was similar in the obese and control subjects. When all ghrelin data points were compared between daytime and nighttime, the lean subjects had average plasma ghrelin concentrations during the period 0800–2000 hours (daytime) of 419 ± 5 pg/ml, which increased during the period 0000–0600 hours (nighttime) to 490 ± 5 pg/ml (P < 0.001). In contrast, the obese subjects had slightly lowered daytime average ghrelin concentrations of 407 ± 3pg/ml, with similar levels during nighttime (403 ± 3pg/ml, NS). Both the daytime and nighttime ghrelin levels were significantly lower in the obese subjects, compared with the lean subjects (P < 0.05 for daytime and P < 0.001 for nighttime).
Diurnal Variability of Ghrelin, Adiponectin, and Leptin Concentrations. Table 2 and Fig. 3 present the across-subjects estimates of diurnal variation for ghrelin, adiponectin, and leptin in the lean and obese groups. For both the lean and obese groups, we reported the parameter point-estimates and 95% confidence intervals by using the semiparametric mixed-effects model.
Table 2. Across-subject estimates of diurnal variation of ghrelin, adiponectin, and leptin in lean and obese men.
| Lean
|
Obese
|
|||
|---|---|---|---|---|
| Parameter | Estimate (95% CI) | P | Estimate (95% CI) | P |
| Ghrelin | ||||
| Baseline B | 436.9 (418.6 to 455.1) | 408.9 (332.8 to 485.0) | ||
| Coefficient α | -34.8 (-79.8 to 10.3) | <0.0001 | -4.4 (-47.0 to 38.2) | 0.6561 |
| Coefficient β | 50.8 (24.4 to 77.1) | -0.6 (-28.7 to 27.5) | ||
| Amplitude A | 61.5 (32.0 to 91.0) | NE | ||
| Peak time φ | 5:43 (0302 to 0822 hours) | NE | ||
| Adiponectin | ||||
| Baseline B | 5.31 (4.68 to 5.94) | 2.61 (2.15 to 3.06) | ||
| Coefficient α | 0.47 (-0.03 to 0.98) | 0.0633 | 0.31 (0.02 to 0.60) | 0.0914 |
| Coefficient β | -0.35 (-0.83 to 0.13) | 0.04 (-0.15 to 0.23) | ||
| Amplitude A | NE | NE | ||
| Peak time φ | NE | NE | ||
| Leptin | ||||
| Baseline B | 3.43 (2.35 to 4.51) | 16.75 (10.52 to 22.98) | ||
| Coefficient α | -0.49 (-0.80 to -0.18) | <0.0001 | -3.05 (-4.30 to -1.80) | <0.0001 |
| Coefficient β | -0.28 (-0.58 to 0.02) | -1.22 (-2.41 to -0.03) | ||
| Amplitude A | 0.57 (0.34 to 0.78) | 3.28 (1.84 to 4.73) | ||
| Peak time φ | 0:01 (2128 to 0233 hours) | 0:34 (2327 to 0138 hours) |
Columns report parameter point-estimates and 95% confidence intervals (CI) using a semiparametric mixed-effects model. Within a group, the diurnal variation across subjects is identified by rejecting diurnal coefficients α = β = 0 using an F test (P values reported above). For hormones without significant diurnal variation, A and φ are not estimable (NE).
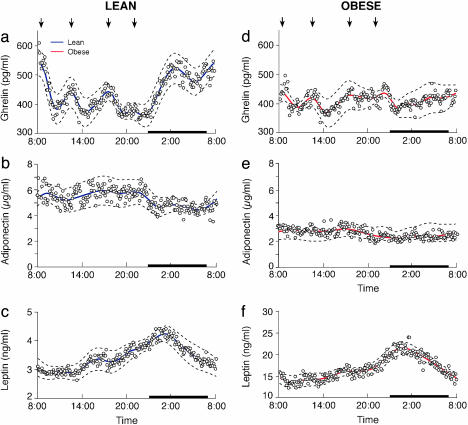
Fig. 3.
Estimated 24-h profiles of plasma ghrelin, adiponectin, and leptin in lean (a–c) and obese (d–f) subjects. For each group, mean hormone levels are plotted as circles. The semiparametric linear mixed-effects model, across-subject prediction is drawn as a solid line. Dashed lines trace out the prediction's approximate 95% confidence interval. Arrows indicate standardized meals (breakfast, lunch, dinner, and evening snack), and the sleep period is demarcated by a black strip.
We identified significant diurnal variation for leptin in both. Furthermore, there was no significant difference in peak times between the groups. In contrast, for the ghrelin results, only the lean subjects possessed significant diurnal variation after controlling for meal effects. There was no significant diurnal variation in the obese subjects. We did not find statistically significant diurnal variation in adiponectin in either the lean or the obese group.
Orderliness of Ghrelin, Adiponectin, and Leptin. ApEn was used to quantitate the regularity of the secretory patterns of ghrelin, adiponectin, and leptin in the lean and obese groups. The mean normalized ApEn values for ghrelin, adiponectin, and leptin were similar in both groups (lean vs. obese: ghrelin, 0.754 ± 0.076 vs. 0.752 ± 0.043, NS; adiponectin, 0.937 ± 0.024 vs. 0.927 ± 0.039, NS; leptin, 0.759 ± 0.041 vs. 0.807 ± 0.061, NS). There were no differences in ApEn for these three hormones, between the lean and obese groups. Interestingly, ghrelin and leptin secretion profiles had statistical structures that were significantly different from random, whereas the profile of pulsatile adiponectin secretion was not different from random.
Synchronicity of Ghrelin, Adiponectin, and Leptin. We assessed synchronicity of the pulses among the ghrelin, adiponectin, and leptin time series independently by cross-correlation analysis and cross-ApEn. Cross-correlations between plasma ghrelin and leptin, between ghrelin and adiponectin, and between plasma leptin and adiponectin concentrations were not significant at any lag across an interval of –200 through +200 min for the lean and obese groups. The mean normalized cross-ApEn values for ghrelin–leptin, ghrelin–adiponectin, and leptin–adiponectin were similar in the lean and obese groups and indicated temporally patterned synchronicity between plasma ghrelin and leptin, between ghrelin and adiponectin, and between plasma leptin and adiponectin concentrations for both the lean and obese groups (lean vs. obese: ghrelin–leptin, 0.771 ± 0.065 vs. 0.789 ± 0.047, NS; ghrelin–adiponectin, 0.868 ± 0.018 vs. 0.852 ± 0.019, NS; leptin–adiponectin, 0.879 ± 0.027 vs. 0.882 ± 0.024, NS).
Discussion
We report here that 24-h circulating ghrelin and adiponectin had pulsatile patterns in intensively sampled groups of young lean and young obese men. The main finding of this study is that ghrelin concentrations did not increase at night in the obese men as they did in healthy controls. The quantifiable levels of orderliness for the ghrelin concentrations were significantly different from random in both the lean and obese groups, as determined by ApEn.
The 24-h pulsatility parameters and the pattern of orderliness determined by ApEn were similar in the lean and obese subjects, whereas the nocturnal rise in ghrelin was obliterated in the obese subjects. Very frequent sampling was used to ensure that this newly observed feature of human obesity is not just reflective of insufficient sampling of a hormone that has a short half-life. This new feature of human obesity may help explain its pathophysiology and might point out new directions for treatment. Because we studied only young males, future work should further assess the complex relationships between hormones involved in food intake regulation in subjects of both sexes and across various age and ethnic groups.
The data demonstrate that although healthy men had a nocturnal rise in plasma ghrelin concentrations that exceeded the elevations seen at mealtimes, intensive sampling failed to document such a nocturnal increase in circulating ghrelin in sex- and age-matched obese subjects. It is noteworthy that breakfast, lunch, and dinner had a marked positive effect on plasma ghrelin levels but a smaller evening snack had a lesser effect in the lean subjects. We should also note that the obese subjects displayed blunted meal effects. It was reported previously in a group of subjects with an average BMI of 42.8 kg/m2 that a test meal failed to suppress ghrelin levels (44). Further studies would be required to test the hypothesis that meals of differing sizes or those taken at different times of day may have differential effects on appetite-regulating hormones.
Steady-state plasma ghrelin levels have been reported to be decreased in obesity (13, 45), and these modest decrements are thought to be compensatory, rather than causal, representing a physiological adaptation to the positive energy balance in human obesity. However, we show here that the major abnormality in human ghrelin concentrations was not at the steady-state level but in the organization of its dynamics. Our results show that, in obesity, there was maintenance of the diurnal architecture of leptin (albeit at higher concentrations) (Fig. 2); however, for ghrelin, such dynamics were altered (Fig. 1a). Consequently, in lean individuals, there was a concomitant rise of leptin and ghrelin at night; however, in the obese subjects, the marked nocturnal increase in leptin was not accompanied by a commensurate rise in ghrelin.
A previous report has shown that a low ghrelin level is independently associated with type 2 diabetes, insulin resistance, and elevated blood pressure (46). Therefore, this hormone might play a role in the etiology of type 2 diabetes and the regulation of blood pressure. Because both hypertension and diabetes are complications of obesity, it is possible that our findings of ghrelin dysregulation could contribute to explain these known complications of obesity. Moreover, we show that in healthy subjects, the highest levels of the orexigenic hormone ghrelin occurred in the early morning, when eating is precluded by sleep. In contrast, we show that in the obese subjects, ghrelin levels were higher when these individuals were awake and capable of eating, and that the plasma levels of this hormone did not rise at night during sleep.
The diurnal patterns of plasma ghrelin were examined in two previous studies, which contributed to advance the field but were not specifically designed to map the dynamics of ghrelin through very rapid sampling. Those studies did not show such marked increases in nocturnal ghrelin plasma levels in lean subjects as we show here (10, 14), but they had methodological features that preclude a direct comparison with our work. Whereas we focused on young men, lean vs. obese, the first of those articles reported on a group of subjects that was 90% female, with body weight ranging from normal to overweight (BMI, 22–30 kg/m2) and a very large age range (mean, 48 years; range, 29–64 years), in the context of a positive correlation between ghrelin levels and age (10). The other study was focused on cases of morbidly obese patients requiring a gastric bypass, with a normal-weight control group that was overweight (average BMI, 27.4 ± 0.9 kg/m2) (14). The study documented slightly lower plasma ghrelin concentrations at night in the morbidly obese subjects, and in the obese subjects studied before and after diet-induced weight loss (14). Those studies were specifically designed to assess the effects of meals (10) and of gastric bypass surgery (14). In contrast, our study was designed specifically to assess the pulsatility of ghrelin in young lean and young nonmorbidly obese men throughout the 24-h period, particularly at night, when the meal effect is not present. For this reason, we sampled five times more frequently throughout the 24-h period than in previous studies (10, 14) to obtain a detailed picture of the organization of plasma ghrelin concentrations.
Adiponectin is the most abundant circulating adipokine, accounting for 0.01% of total human plasma protein (19, 47). Single-point measurements of adiponectin showed that the concentrations of this adipokine were decreased in obesity (20, 47). We found that the mean 24-h concentrations of adiponectin were significantly lower in the obese subjects. The lower levels were associated with pulses of lower height and lower valley concentrations in the obese subjects, although the other pulsatility parameters were similar in both groups. Recently, Gavrila et al. (28) studied the dynamics of adiponectin concentrations in six healthy, young, normal-weight men and showed that this hormone showed ultradian pulsatility and diurnal variation. This study reported 10.0 ± 2.0 pulses/24 h for adiponectin by collecting blood samples every 15 min for 24 h (28). We show here that the pulse frequencies in the lean and obese men were 28.8 ± 2.0 and 30.4 ± 2.3/24 h, respectively. Because the pulse duration of adiponectin was short (36.3 ± 3.1 min for the lean men and 34.5 ± 1.4 for the obese men), we could determine that the adiponectin levels were highly pulsatile only by rapid sampling.
The diurnal and ultradian profiles of rapidly sampled 24-h leptin concentrations in this study were similar to those that we reported in ref. 29. The 24-h leptin concentrations were higher in the obese subjects, with higher pulse height and valley concentrations and higher relative increment.
We also assessed the pattern orderliness of the 24-h adiponectin and leptin concentrations by ApEn and found that, in contrast to the adipocyte product leptin, which was secreted in an orderly manner, adiponectin plasma concentrations did not appear to be different from random in the lean and obese subjects. We show here that adipocytes can secrete a hormone, such as leptin, in a highly organized fashion but that they secrete another hormone, adiponectin, in what appears to be a random fashion.
Recent evidence suggests that inverse changes in leptin and ghrelin levels are likely to be critical to the maintenance of energy homeostasis, although the nature of the relationship between ghrelin and leptin remains controversial (8, 48). It has been reported that ghrelin administration impairs the expression of adiponectin in adipocyte cell culture (49). Cross-sectional studies showed that single-point measurements of adiponectin are associated with central fat distribution (50, 51) but not with leptin (50). The mechanisms of the synchronicity of 24-h circulating ghrelin, adiponectin, and leptin that we report here, and the significance of these temporal relationships in the pathophysiology of human obesity, are yet to be elucidated.
In summary, by studying 6,210 data points of plasma ghrelin, adiponectin, and leptin concentrations, we report here that 24-h ghrelin showed similar and orderly patterns of ultradian release in lean and obese subjects, and we identify a previously undescribed biological feature of human obesity by rigorously documenting a blunted nocturnal rise of ghrelin in frequently sampled plasma concentrations.
Obesity is the outcome of the dysregulation of an elaborate central network of neuropeptidergic and monoaminergic circuits that provide an interface between genetic background and the environment. In addition to its direct effects as an orexigenic peptide, ghrelin appears to modulate other hormonal systems that participate in the regulation of food intake and body weight (52, 53). The blunting of the nocturnal rise in ghrelin concentrations might be important in the biology of obesity not only because of the direct effects of this hormone on energy homeostasis, but also because of indirect downstream effects that might emerge as a result of altered relationships among complex systems that regulate body-weight homeostasis. These effects might include possible alterations in the regulation of somatotrope cell function and growth hormone secretion by ghrelin (2). The hypothesis that the deletion of the nocturnal rise in plasma ghrelin is an element in the pathogenesis of obesity and not only the outcome of physiological adaptations to positive energy balance should therefore be tested.
Acknowledgments
We thank Mr. Robert Whitby, Ms. Li-Yin Liang, and Ms. Emily Bubbers for technical assistance. This work was supported by National Institutes of Health Grants K30HL04526, RR16996, HG002500, RR017611, DK063240, DK58851, RR017365, MH062777, and RR000865, and by awards from the Dana Foundation and Amgen Inc. (to J.L.) and the National Alliance for Research on Schizophrenia and Depression (to M.-L.W.).
Abbreviations: BMI, body mass index; NS, not significant; ApEn, approximate entropy.
References
- 1.Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H. & Kangawa, K. (1999) Nature 402, 656–660. [DOI] [PubMed] [Google Scholar]
- 2.Takaya, K., Ariyasu, H., Kanamoto, N., Iwakura, H., Yoshimoto, A., Harada, M., Mori, K., Komatsu, Y., Usui, T., Shimatsu, A., et al. (2000) J. Clin. Endocrinol. Metab. 85, 4908–4911. [DOI] [PubMed] [Google Scholar]
- 3.Peino, R., Baldelli, R., Rodriguez-Garcia, J., Rodriguez-Segade, S., Kojima, M., Kangawa, K., Arvat, E., Ghigo, E., Dieguez, C. & Casanueva, F. F. (2000) Eur. J. Endocrinol. 143, R11–R14. [DOI] [PubMed] [Google Scholar]
- 4.Date, Y., Kojima, M., Hosoda, H., Sawaguchi, A., Mondal, M. S., Suganuma, T., Matsukura, S., Kangawa, K. & Nakazato, M. (2000) Endocrinology 141, 4255–4261. [DOI] [PubMed] [Google Scholar]
- 5.Gnanapavan, S., Kola, B., Bustin, S. A., Morris, D. G., McGee, P., Fairclough, P., Bhattacharya, S., Carpenter, R., Grossman, A. B. & Korbonits, M. (2002) J. Clin. Endocrinol. Metab. 87, 2988–2991. [DOI] [PubMed] [Google Scholar]
- 6.Wren, A. M., Seal, L. J., Cohen, M. A., Brynes, A. E., Frost, G. S., Murphy, K. G., Dhillo, W. S., Ghatei, M. A. & Bloom, S. R. (2001) J. Clin. Endocrinol. Metab. 86, 5992–5995. [DOI] [PubMed] [Google Scholar]
- 7.Murray, C. D., Kamm, M. A., Bloom, S. R. & Emmanuel, A. V. (2003) Gastroenterology 125, 1492–1502. [DOI] [PubMed] [Google Scholar]
- 8.Zigman, J. M. & Elmquist, J. K. (2003) Endocrinology 144, 3749–3756. [DOI] [PubMed] [Google Scholar]
- 9.Cummings, D. E. & Shannon, M. H. (2003) Arch. Surg. (Chicago) 138, 389–396. [DOI] [PubMed] [Google Scholar]
- 10.Cummings, D. E., Purnell, J. Q., Frayo, R. S., Schmidova, K., Wisse, B. E. & Weigle, D. S. (2001) Diabetes 50, 1714–1719. [DOI] [PubMed] [Google Scholar]
- 11.Tschop, M., Wawarta, R., Riepl, R. L., Friedrich, S., Bidlingmaier, M., Landgraf, R. & Folwaczny, C. (2001) J. Endocrinol. Invest. 24, RC19–RC21. [DOI] [PubMed] [Google Scholar]
- 12.Tschop, M., Smiley, D. L. & Heiman, M. L. (2000) Nature 407, 908–913. [DOI] [PubMed] [Google Scholar]
- 13.Tschop, M., Weyer, C., Tataranni, P. A., Devanarayan, V., Ravussin, E. & Heiman, M. L. (2001) Diabetes 50, 707–709. [DOI] [PubMed] [Google Scholar]
- 14.Cummings, D. E., Weigle, D. S., Frayo, R. S., Breen, P. A., Ma, M. K., Dellinger, E. P. & Purnell, J. Q. (2002) N. Engl. J. Med. 346, 1623–1630. [DOI] [PubMed] [Google Scholar]
- 15.Asakawa, A., Inui, A., Kaga, T., Yuzuriha, H., Nagata, T., Fujimiya, M., Katsuura, G., Makino, S., Fujino, M. A. & Kasuga, M. (2001) Neuroendocrinology 74, 143–147. [DOI] [PubMed] [Google Scholar]
- 16.Anderwald, C., Brabant, G., Bernroider, E., Horn, R., Brehm, A., Waldhausl, W. & Roden, M. (2003) Diabetes 52, 1792–1798. [DOI] [PubMed] [Google Scholar]
- 17.Murdolo, G., Lucidi, P., Di Loreto, C., Parlanti, N., De Cicco, A., Fatone, C., Fanelli, C. G., Bolli, G. B., Santeusanio, F. & De Feo, P. (2003) Diabetes 52, 2923–2927. [DOI] [PubMed] [Google Scholar]
- 18.Barazzoni, R., Zanetti, M., Stebel, M., Biolo, G., Cattin, L. & Guarnieri, G. (2003) Gastroenterology 124, 1188–1192. [DOI] [PubMed] [Google Scholar]
- 19.Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. (1995) J. Biol. Chem. 270, 26746–26749. [DOI] [PubMed] [Google Scholar]
- 20.Weyer, C., Funahashi, T., Tanaka, S., Hotta, K., Matsuzawa, Y., Pratley, R. E. & Tataranni, P. A. (2001) J. Clin. Endocrinol. Metab. 86, 1930–1935. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay, R. S., Funahashi, T., Hanson, R. L., Matsuzawa, Y., Tanaka, S., Tataranni, P. A., Knowler, W. C. & Krakoff, J. (2002) Lancet 360, 57–58. [DOI] [PubMed] [Google Scholar]
- 22.Funahashi, T., Nakamura, T., Shimomura, I., Maeda, K., Kuriyama, H., Takahashi, M., Arita, Y., Kihara, S. & Matsuzawa, Y. (1999) Intern. Med. 38, 202–206. [DOI] [PubMed] [Google Scholar]
- 23.Berg, A. H., Combs, T. P. & Scherer, P. E. (2002) Trends Endocrinol. Metab. 13, 84–89. [DOI] [PubMed] [Google Scholar]
- 24.Berg, A. H., Combs, T. P., Du, X., Brownlee, M. & Scherer, P. E. (2001) Nat. Med. 7, 947–953. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi, T., Kamon, J., Waki, H., Terauchi, Y., Kubota, N., Hara, K., Mori, Y., Ide, T., Murakami, K., Tsuboyama-Kasaoka, N., et al. (2001) Nat. Med. 7, 941–946. [DOI] [PubMed] [Google Scholar]
- 26.Fruebis, J., Tsao, T. S., Javorschi, S., Ebbets-Reed, D., Erickson, M. R., Yen, F. T., Bihain, B. E. & Lodish, H. F. (2001) Proc. Natl. Acad. Sci. USA 98, 2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotta, K., Funahashi, T., Arita, Y., Takahashi, M., Matsuda, M., Okamoto, Y., Iwahashi, H., Kuriyama, H., Ouchi, N., Maeda, K., et al. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1595–1599. [DOI] [PubMed] [Google Scholar]
- 28.Gavrila, A., Peng, C. K., Chan, J. L., Mietus, J. E., Goldberger, A. L. & Mantzoros, C. S. (2003) J. Clin. Endocrinol. Metab. 88, 2838–2843. [DOI] [PubMed] [Google Scholar]
- 29.Licinio, J., Mantzoros, C., Negrao, A. B., Cizza, G., Wong, M. L., Bongiorno, P. B., Chrousos, G. P., Karp, B., Allen, C., Flier, J. S., et al. (1997) Nat. Med. 3, 575–579. [DOI] [PubMed] [Google Scholar]
- 30.Veldhuis, J. D. & Johnson, M. L. (1986) Am. J. Physiol. 250, E486–E493. [DOI] [PubMed] [Google Scholar]
- 31.Licinio, J., Negrao, A. B., Mantzoros, C., Kaklamani, V., Wong, M. L., Bongiorno, P. B., Negro, P. P., Mulla, A., Veldhuis, J. D., Cearnal, et al. (1998) J. Clin. Endocrinol. Metab. 83, 4140–4147. [DOI] [PubMed] [Google Scholar]
- 32.Wang, Y. (1998) J. R. Stat. Soc. B 60, 159–174 [Google Scholar]
- 33.Bingham, C., Arbogast, B., Guillaume, G. C., Lee, J. K. & Halberg, F. (1982) Chronobiologia 9, 397–439. [PubMed] [Google Scholar]
- 34.Ke, C. & Wang, Y. (2001) J. Am. Stat. Assoc. 96, 1272–1298. [Google Scholar]
- 35.McLean, R., Sanders, W. & Stroup, W. (1991) Am. Stat. 45, 54–64. [Google Scholar]
- 36.Pincus, S. M. & Keefe, D. L. (1992) Am. J. Physiol. 262, E741–E754. [DOI] [PubMed] [Google Scholar]
- 37.Pincus, S. M., Hartman, M. L., Roelfsema, F., Thorner, M. O. & Veldhuis, J. D. (1999) Am. J. Physiol. 277, E948–E957. [DOI] [PubMed] [Google Scholar]
- 38.Pincus, S. M. (1991) Proc. Natl. Acad. Sci. USA 88, 2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pincus, S. M., Gladstone, I. M. & Ehrenkranz, R. A. (1991) J. Clin. Monit. 7, 335–345. [DOI] [PubMed] [Google Scholar]
- 40.Pincus, S. M. & Huang, W. M. (1992) Commun. Stat. A 21, 3061–3077. [Google Scholar]
- 41.Pincus, S. M., Mulligan, T., Iranmanesh, A., Gheorghiu, S., Godschalk, M. & Veldhuis, J. D. (1996) Proc. Natl. Acad. Sci. USA 93, 14100–14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pincus, S. M. & Kalman, R. E. (1997) Proc. Natl. Acad. Sci. USA 94, 3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veldhuis, J. D. & Pincus, S. M. (1998) Eur. J. Endocrinol. 138, 358–362. [DOI] [PubMed] [Google Scholar]
- 44.English, P. J., Ghatei, M. A., Malik, I. A., Bloom, S. R. & Wilding, J. P. (2002) J. Clin. Endocrinol. Metab. 87, 2984–2987. [DOI] [PubMed] [Google Scholar]
- 45.Shiiya, T., Nakazato, M., Mizuta, M., Date, Y., Mondal, M. S., Tanaka, M., Nozoe, S., Hosoda, H., Kangawa, K. & Matsukura, S. (2002) J. Clin. Endocrinol. Metab. 87, 240–244. [DOI] [PubMed] [Google Scholar]
- 46.Poykko, S. M., Kellokoski, E., Horkko, S., Kauma, H., Kesaniemi, Y. A. & Ukkola, O. (2003) Diabetes 52, 2546–2553. [DOI] [PubMed] [Google Scholar]
- 47.Arita, Y., Kihara, S., Ouchi, N., Takahashi, M., Maeda, K., Miyagawa, J., Hotta, K., Shimomura, I., Nakamura, T., Miyaoka, et al. (1999) Biochem. Biophys. Res. Commun. 257, 79–83. [DOI] [PubMed] [Google Scholar]
- 48.Cummings, D. E. & Foster, K. E. (2003) Gastroenterology 124, 1532–1535. [DOI] [PubMed] [Google Scholar]
- 49.Ott, V., Fasshauer, M., Dalski, A., Meier, B., Perwitz, N., Klein, H. H., Tschop, M. & Klein, J. (2002) Horm. Metab. Res. 34, 640–645. [DOI] [PubMed] [Google Scholar]
- 50.Gavrila, A., Chan, J. L., Yiannakouris, N., Kontogianni, M., Miller, L. C., Orlova, C. & Mantzoros, C. S. (2003) J. Clin. Endocrinol. Metab. 88, 4823–4831. [DOI] [PubMed] [Google Scholar]
- 51.Staiger, H., Tschritter, O., Machann, J., Thamer, C., Fritsche, A., Maerker, E., Schick, F., Haring, H. U. & Stumvoll, M. (2003) Obes. Res. 11, 368–372. [DOI] [PubMed] [Google Scholar]
- 52.Weikel, J. C., Wichniak, A., Ising, M., Brunner, H., Friess, E., Held, K., Mathias, S., Schmid, D. A., Uhr, M. & Steiger, A. (2003) Am. J. Physiol. 284, E407–E415. [DOI] [PubMed] [Google Scholar]
- 53.Tassone, F., Broglio, F., Destefanis, S., Rovere, S., Benso, A., Gottero, C., Prodam, F., Rossetto, R., Gauna, C., Van Der Lely, et al. (2003) J. Clin. Endocrinol. Metab. 88, 5478–5483. [DOI] [PubMed] [Google Scholar]