Abstract
Background
Treatment-related toxicity and quality of life (QoL) considerations are important when counseling patients with localized prostate cancer.
Objective
To determine the incidence and longitudinal pattern of late genitourinary (GU) toxicity and QoL after high-dose intensity-modulated radiotherapy (IMRT).
Design, setting, and participants
A total of 268 patients with localized prostate cancer were treated between 06/2004 and 12/2008 at a tertiary referral center. Median follow-up was 5 (range, 3–7.7) years.
Intervention
Patients underwent IMRT to a total dose of 86.4 Gy, 50% of patients underwent neoadjuvant and concurrent androgen-deprivation therapy.
Outcome measurements and statistical analysis
Patients were evaluated with the prospectively obtained International Prostate Symptom Score (IPSS) questionnaire. GU toxicity was also scored using the Common Terminology Criteria for Adverse Events (CTCAE) v4.0; toxicity events were defined as increase over baseline. Differences in increases in IPSS sums and QoL index between baseline IPSS sum and QoL index groups were analyzed using the Kruskal-Wallis and Mann-Whitney tests. Univariate and multivariate Cox regression models were applied.
Results and limitations
The overall median IPSS sum increase during follow-up was 3, and was less pronounced among patients with severe compared with mild baseline symptoms (median increase 0 vs. 4; p < 0.0001). Overall, QoL index was unchanged after IMRT but appeared to improve in patients with dissatisfied baseline QoL compared with satisfied baseline QoL (p < 0.0001). Fifty-five (20%) and 2 (1%) patients developed grade 2 and 3 late GU toxicity; however, 28/57 (49%) of these resolved during follow-up. Even though the IPSS data was prospectively obtained, most patients were not treated within a prospective protocol.
Conclusions
Late GU toxicity after high-dose IMRT was mild and severe late GU toxicity was rare. Changes in IPSS sum and QoL index were dependent on the baseline GU function, which might be useful for future patient counseling.
Keywords: Urogenital abnormalities, radiotherapy, toxicity, prostatic neoplasms
1. Introduction
Radical prostatectomy as a monotherapy and high-dose external beam radiation therapy (RT) if indicated with concomitant androgen-deprivation therapy (ADT) are considered similarly effective for patients with localized prostate cancer [1]. Especially for early-stage patients, the decision to select a particular therapy is often based upon quality of life (QoL) considerations. Thus, treatment-related toxicity and QoL are important for patient counseling and decision making.
A significant proportion of patients with localized prostate cancer present with pre-RT genitourinary (GU) symptoms, predominantly voiding side effects of obstruction due to coexisting benign prostate hyperplasia [2]. These baseline symptoms should be incorporated into the definition of GU toxicity to more accurately identify patients with treatment-related toxicities. However, to the best of our knowledge, there are no such recommendations available for commonly used toxicity-grading systems as the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) or the Radiation Therapy Oncology Group (RTOG) system. One possibility is to characterize the presence of toxicity only when the symptom grade increases over baseline [3]. Impaired GU function at baseline is commonly described as a significant predictor of late GU toxicity after RT for prostate cancer [4–6]. This suggests that patients with adverse baseline symptoms are at higher risk for development of late GU symptoms after RT, but it may be an artifact, as baseline symptoms were not routinely accounted for in the definition of late GU toxicity events for these cohorts. Others have demonstrated that patients who had poor baseline GU function often improved after therapy, highlighting the critical need to account for baseline function [7].
High-dose RT (>74 Gy) showed improved biochemical recurrence-free survival compared with lower-dose RT for localized prostate cancer [8]; however, late GU toxicity also increased [9, 10]. As patient-reported toxicity is most reliable [11], this study incorporates data from patient-reported International Prostate Symptom Score (IPSS) questionnaires [12] from patients treated with 86.4 Gy IMRT to provide a detailed description of incidence and longitudinal pattern of GU toxicity.
2. Patients and methods
2.1 Patient selection
Between 08/1997 and 12/2008, 1002 consecutive patients with localized prostate cancer were treated with definitive IMRT to a prescribed dose of 86.4 Gy [13]. Of these, 268 patients were treated between 06/2004–12/2008, had available IPSS data at baseline with a minimum of 3 years follow-up, and available treatment-planning dosimetry. One patient who received salvage brachytherapy <3 years after IMRT was excluded and eight patients receiving salvage treatment ≥3 years post-IMRT were censored at salvage treatment. Research authorization was approved by the internal review board of the institution.
2.2 Treatment
All patients were treated using a 5- to 7-field IMRT plan with 15-MV photon beams using dose constraints as previously described [13]. Briefly, the clinical target volume consisted of the prostate, and seminal vesicles, with a 1-cm planning target volume (PTV) margin in all directions, except posteriorly (0.6 cm). When the small bowel constraints could not be met, a cone down was performed, excluding the upper part of the seminal vesicles from the PTV. Patient position was verified with weekly port films or daily using fiducial markers since 2007/2008. Patient positioning was prone prior to 2007 and changed to supine subsequently. Typically, patients were treated with an empty bladder, or full bladder when small bowel was in close proximal to the PTV. ADT was used for either volume reduction prior to therapy (in general patients with prostate size >75 cm3) or for the presence of high-risk features (Gleason Score 8–10 disease, prostate-specific antigen >20 ng/mL, or clinical cT3 disease). When administered, generally a 6-month course of ADT (3 months neoadjuvantly and 3 months concurrently) was used for low- and intermediate-risk patients, and a 6-month to 2-year ADT course for high-risk patients. All patients received 86.4 Gy in 48 fractions of 1.8 Gy, save for two patients who missed their last fraction and received 84.6 Gy.
2.3 Baseline symptoms and toxicity
The 7 IPSS questions and the QoL index were prospectively obtained at baseline and each subsequent visit. Patients were evaluated every 3 months for the first, every 6 months for the next 5 years, and yearly thereafter. For longitudinal analysis of IPSS sum and QoL index, 8 time periods were defined: baseline and 3–6, 6–12, 12–18, 18–24, 36–48, and 48–60 months. In cases of multiple values for a patient during one period, the maximal value was used. At baseline, during treatment and at each follow-up visit the GU morbidity/toxicity symptoms dysuria, incontinence, retention, frequency/urgency and hematuria were assessed using the CTCAE v4.0. Acute toxicity was defined as occurring during and until 3 months post-IMRT. Late toxicities were defined as occurring after 3 months; however, in the presence of acute toxicity, late toxicity was only assumed when occurring >6 months post-IMRT. To account for baseline symptoms, acute and late toxicities were defined as an increase over the baseline value. Amelioration of grade ≥2 late toxicity was defined as change into a lower grade or symptom disappearance.
2.4 Statistical analysis
The primary objective was to document the change in IPSS sum and QoL index; the secondary objective was late GU toxicity according to the CTCAE. For comparison acute GU toxicity rates according to the CTCAE were also described. Baseline IPSS sum values were grouped as mild (0–7), moderate (8–19) and severe (20–35), as recommended by the American Urological Association [12]. A clinically significant IPSS sum increase was defined as an increase of ≥5 during follow-up, as this change has been demonstrated as perceptible by patients [14]. We further calculated that an IPSS sum increase ≥5 increase correlated with an average QoL index drop of 2 points (data not shown). The baseline QoL index was dichotomized as 0–2 (satisfied) vs. 3–6 (dissatisfied). Follow-up duration was IPSS based and calculated from baseline to last available IPSS visit.
Differences in increases in IPSS sums and in IPSS sum groups between baseline IPSS sum groups were analyzed using the Kruskal-Wallis or Chi-squared test. Changes in QoL for different baseline QoL groups was compared using the Mann-Whitney test. Actuarial late GU toxicity rates and time to symptom resolution were estimated using the Kaplan-Meier method. Time to event was calculated from the IPSS baseline and the IMRT completion for the IPSS or CTCAE endpoints, respectively. Univariate and multivariate Cox regression models were used to separately correlate IPSS sum increase or late GU toxicity with the following dichotomized clinical variables: race (African American vs. others), diabetes, smoking (no vs. history vs. yes), risk group (low vs. intermediate vs. high), use of ADT, image-guided radiotherapy, patient positioning and performance of a cone down. Additionally, age, prostate volume, bladder volume, cross-sectional rectal area (rectal volume divided by rectal length), craniocaudal extent of the seminal vesicles, baseline IPSS, and the mean and maximal dose to the bladder wall were used as continuous variables. Two-sided p values <0.05 were considered statistically significant. The data was analyzed in SPSS (SPSS Inc., Chicago, IL, version 19.0).
3.0 Results
3.1 Patients
The median follow-up for the entire cohort was 5 (range, 3–7.7) years. Baseline demographics and clinical characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Total patients | n = 268 (%) |
|---|---|
| Median age, years (IQR) | 71.0 (11.0) |
| Race | |
| Caucasian | 227 (85) |
| African American/Black | 24 (9) |
| Other | 17 (6) |
| Comorbidity | |
| Diabetes | 49 (18) |
| Smoking | 28 (10) |
| Prostate volume, cm3 (IQR)# | 36.0 (25.0) |
| Bladder volume, cm3 (IQR) | 148.5 (169.2) |
| Cross-sectional rectal area, cm2(IQR) | 7.3 (3.2) |
| Extent of the seminal vesicles, cm (IQR) | 2.7 (0.6) |
| Risk group* | |
| Low | 58 (22) |
| Intermediate | 144 (53) |
| High | 66 (25) |
| Androgen deprivation therapy | |
| no | 134 (50) |
| yes | 134 (50) |
| Median duration, months (range) | 7 (1–35) |
| IGRT using fiducial markers | |
| no | 213 (79) |
| yes | 55 (21) |
| Cone down | |
| no | 214 (80) |
| yes+ | 54 (20) |
| Median FU, years (range) | 5 (3–7.7) |
| Patients available in FU periods | |
| Baseline | 268 (100) |
| 3–6 months | 208 (78) |
| 6–12 months | 176 (66) |
| 12–18 months | 184 (69) |
| 18–24 months | 122 (46) |
| 24–36 months | 220 (82) |
| 36–48 months | 236 (88) |
| 48–60 months | 166 (62) |
Abbreviations:
information lacking for 11 patients;
according to the National Comprehensive Cancer Network (NCCN);
cone downs were performed after a median dose of 54 (range, 30.6–72) Gy
IQR=interquartile range;
IGRT=image guided radiation therapy;
FU=follow-up
3.2 IPSS data
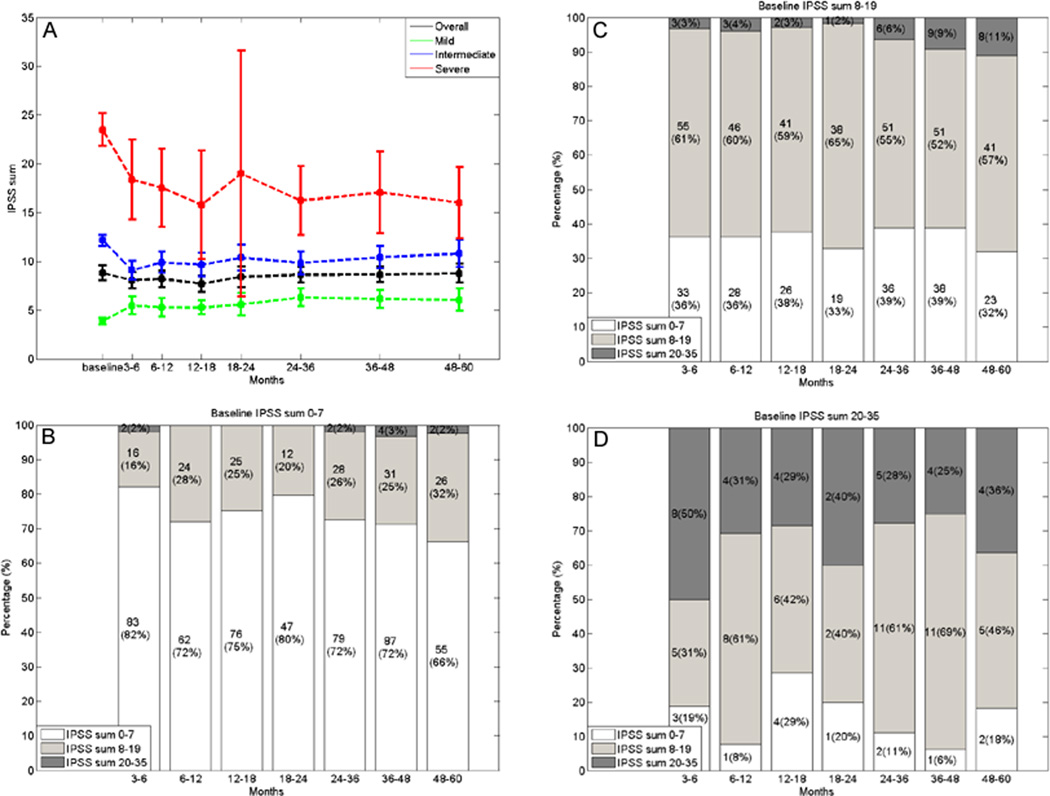
The median baseline IPSS sum was 7 (interquartile range [IQR], 8.75). 137 (51%), 111 (41%), and 20 (8%) patients had mild (0–7), moderate (8–19) and severe (20–35) baseline symptoms, respectively. Overall, there was no substantial quantitative increase in IPSS sum (Figure 1), with the median IPSS sum increase being 3 (IQR, 7). When stratified according to baseline group patients with severe baseline were less likely to have an increasing IPSS sum compared with patients with intermediate or mild baseline (median increase 0 vs. 2 vs. 4; p < 0.0001; Figure 1). Ninety-four out of 268 patients (35%) patients experienced an IPSS sum increase of ≥5 during follow-up and 63% remained event-free at 5 years. A lower baseline IPSS sum was the only variable associated with IPSS sum increases of ≥5 based on the multivariate analysis (p < 0.0001; Table 2).
Fig. 1.
Line chart showing the mean IPSS sum at baseline and during follow-up for all patients, stratified to their baseline function (mild, moderate, severe) with the 95% confidence intervals. (A) Bar chart showing the proportion of patients having an IPSS sum of the mild, moderate or severe category during follow-up, stratified to patients with mild (B), moderate (C), or severe (D) baseline symptoms. IPSS = International Prostate Symptom Score.
Table 2.
Univariate and multivariate associations with IPSS sum increase ≥5
| Variables | Associated level | IPSS sum increase ≥5 | |
|---|---|---|---|
| Hazard ratio (95% CI) | p-value | ||
| Univariate analysis | |||
| Race | African American | 1.276 (0.662–2.460) | 0.467 |
| Diabetes | Yes | 0.917 (0.535–1.570) | 0.752 |
| Smoking | Yes | 1.105 (0.894–1.366) | 0.357 |
| Risk group* | High | 1.064 (0.788–1.438) | 0.684 |
| ADT | Yes | 1.210 (0.806–1.816) | 0.358 |
| IGRT | Yes | 1.194 (0.718–1.984) | 0.494 |
| Positioning | Prone | 1.200 (0.723–1.993) | 0.481 |
| Cone down | Yes | 0.853 (0.510–1.427) | 0.544 |
| Age (years) | Continuous | 1.010 (0.982–1.039) | 0.495 |
| Prostate volume (cm3)# | Continuous | 1.003 (0.994–1.012) | 0.520 |
| Bladder volume (cm3) | Continuous | 1.000 (0.999–1.001) | 0.974 |
| Cross-sectional rectal area (cm2) | Continuous | 0.947 (0.871–1.029) | 0.199 |
| Craniocaudal extent of SV (cm) | Continuous | 1.142 (0.828–1.573) | 0.419 |
| Baseline IPSS sum | Continuous | 0.926 (0.891–0.963) | <0.0001 |
| Mean BW dose (Gy) | Continuous | 0.999 (0.981–1.017) | 0.914 |
| Maximal BW dose (Gy) | Continuous | 1.250 (1.018–1.534) | 0.033 |
| Multivariate analysis | |||
| Baseline IPSS sum | Continuous | 0.929 (0.894–0.966) | <0.0001 |
| Maximal BW dose (Gy) | Continuous | - | - |
Abbreviations:
according to the National Comprehensive Cancer Network (NCCN);
information lacking for 11 patients;
ADT=androgen deprivation therapy; IGRT=image guided radiation therapy; SV=seminal vesicles; BW=bladder wall; IPSS= International Prostate Symptom Score.
When the proportion of patients with mild, intermediate, or severe symptoms at different time periods during follow-up was analyzed, stratified according to the baseline group, we observed that over time 18%–34% of patients with mild baseline symptoms transferred into the intermediate or severe group (Figure 1). Of the patients with intermediate baseline symptoms, 32%–39% transferred into the mild symptom group and 2%–11% into the severe symptom group. Among patients with severe baseline symptoms 50%–75% transferred to the moderate or mild symptom group during follow-up. These differences were statistically significant for all evaluated time periods (p < 0.0001; Figure 1).
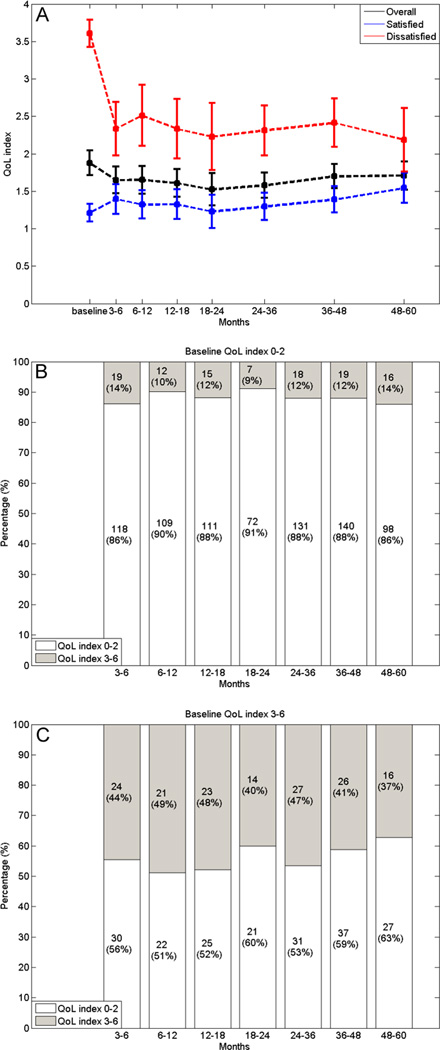
The median baseline QoL index was 2 (IQR, 2). Overall no significant worsening of QoL was evident during follow-up, with the median QoL index drop being 0 (IQR, 1). When stratified according to baseline QoL, patients with satisfied baseline had larger QoL index decreases compared with patients having dissatisfied baseline (median decrease, 1 vs. 0; p < 0.0001, Figure 2). Over time, 9%–14% of patients with satisfied baseline QoL transferred to dissatisfied while 51%–63% of patients with dissatisfied baseline QoL transferred to satisfied (Figure 2).
Fig. 2.
Line chart showing the Quality of Life (QoL) index at baseline and during follow-up for all patients and for those with pleased (0–2) or disturbed (3–6) baseline QoL with the 95% confidence intervals (A). Bar chart showing the proportion of patients having a pleased or disturbed QoL stratified according to pleased (B) or disturbed (C) baseline QoL.
3.3 CTCAE GU toxicity
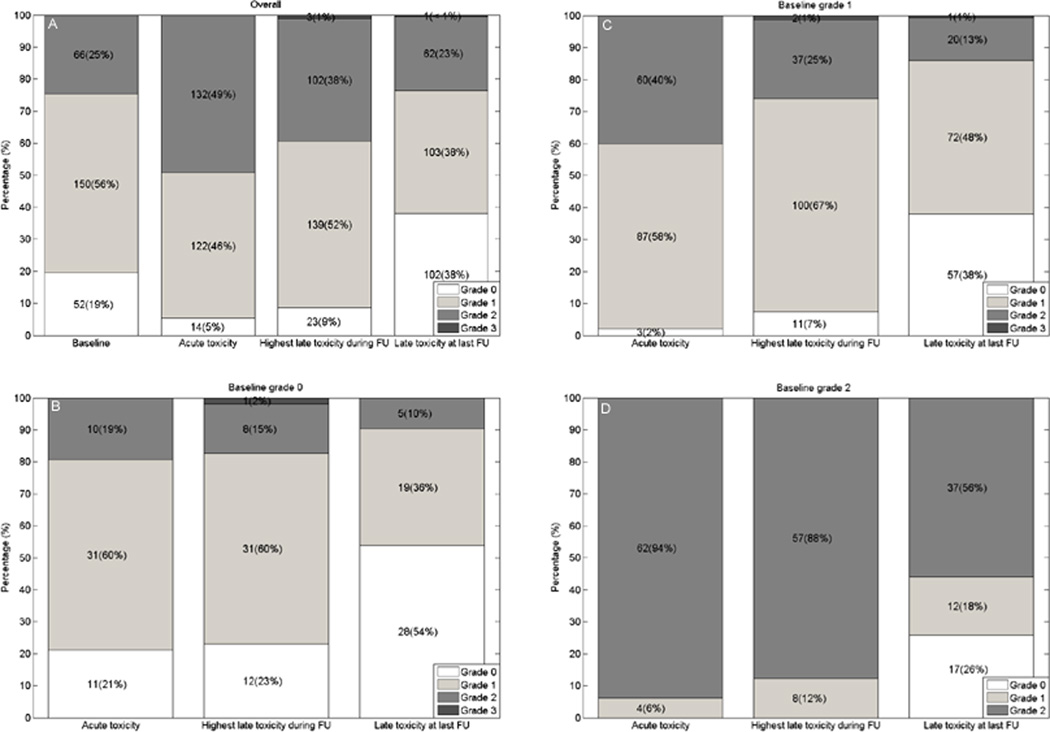
GU symptoms, GU toxicity, and medication used are summarized in Table 3. Sixty-six patients (25%) had grade 2 baseline GU symptoms; however, 44% of these patients experienced resolution of symptoms at the end of follow-up (Figure 3).
Table 3.
Baseline genitourinary symptoms and acute and late genitourinary symptoms and treatment related late toxicity.
| Baseline | Acute symptoms |
Acute toxicity |
Late symptoms |
Late toxicity |
Last late# symptoms |
Last late# toxicity |
||
|---|---|---|---|---|---|---|---|---|
| Toxicity | Grade | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Dysuria | 0 | 264 (99) | 167 (62) | 170 (63) | 247 (92) | 248 (93) | 268 (100) | 268 (100) |
| 1 | 4 (1) | 101 (38) | 98 (37) | 21 (8) | 20 (7) | 0 | 0 | |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Incontinence | 0 | 264 (99) | 262 (98) | 266 (99) | 212 (79) | 214 (80) | 245 (91) | 244 (91) |
| 1 | 4 (1) | 5 (2) | 1 (<1) | 49 (18) | 47 (17) | 19 (7) | 20 (8) | |
| 2 | 0 | 1 (<1) | 1 (<1) | 7 (3) | 7 (3) | 4 (2) | 4 (1) | |
| Retention | 0 | 102 (38) | 67 (25) | 188 (70) | 67 (25) | 186 (69) | 152 (57) | 228 (85) |
| 1 | 101 (38) | 68 (25) | 9 (4) | 101 (38) | 39 (15) | 59 (22) | 18 (7) | |
| 2 | 65 (24) | 133 (50) | 71 (26) | 98 (36) | 41 (15) | 56 (21) | 21(8) | |
| 3 | 0 | 0 | 0 | 2 (1) | 2 (1) | 1 (<1) | 1 (<1) | |
| Frequency/urgency | 0 | 80 (30) | 21 (8) | 202 (75) | 37 (14) | 198 (74) | 136 (51) | 234 (87) |
| 1 | 187 (70) | 242 (90) | 61 (23) | 209 (78) | 48 (18) | 124 (46) | 26 (10) | |
| 2 | 1 (<1) | 5 (2) | 5 (2) | 22 (8) | 22 (8) | 8 (3) | 8 (3) | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hematuria | 0 | 263 (98) | 263 (98) | 267 (100) | 230 (86) | 234 (87) | 257 (96) | 258 (96) |
| 1 | 5 (2) | 5 (2) | 1 (<1) | 38 (14) | 34 (13) | 11 (4) | 10 (4) | |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Highest GU* | 0 | 52 (19) | 14 (5) | 86 (32) | 23 (9) | 104 (39) | 102 (38) | 189 (71) |
| 1 | 150 (56) | 122 (46) | 108 (40) | 140 (52) | 107 (40) | 103 (38) | 50 (19) | |
| 2 | 66 (25) | 132 (49) | 74 (28) | 102 (38) | 55 (20) | 62 (23) | 28 (10) | |
| 3 | 0 | 0 | 0 | 2 (1) | 2 (1) | 1 (<1) | 1 (<1) | |
| GU medication used: | n (%) | n (%) | n (%) | n (%) | ||||
| Alpha blockers | 61 (23) | 132 (49) | 114 (42) | 56 (21) | ||||
| 5 Alpha reductase inhibitors | 13 (5) | 6 (2) | 10 (4) | 5 (2) | ||||
| Anticholinergics | 1 (<1) | 6 (2) | 23 (9) | 8 (3) | ||||
| NSAR | 0 | 60 (22) | 3 (1) | 1 (<1) | ||||
Abbreviations: GU=genitourinary; NSAR=non steroid anti-inflammatory drugs;
The highest toxicity in a patient was counted as a single event;
Incidence of late symptoms or late toxicity at last follow-up visit. There was no grade ≥ grade 4 GU toxicity observed.
Fig. 3.
Bar chart showing the distribution of baseline, acute, late, and at last follow-up visit Common Terminology Criteria for Adverse Events symptoms for all patients (A) and stratified according to baseline grade 0 symptoms (B), baseline grade 1 symptoms (C), and baseline grade 2 symptoms (D). FU = follow-up.
Seventy-four (28%) patients developed grade ≥2 acute GU toxicity, and 55 (20%) and 2 (1%) patients had grade 2 and 3 late GU toxicity, respectively. Late grade 3 toxicities were due to urinary retention, which developed in the absence of grade 2 baseline symptoms; one patient required an urethrotomy due to a bulbomembranous urethral stricture 17 months after IMRT; another required transurethral prostate resection, due to obstructive voiding 24 months after IMRT. There were no other grade 3 or grade 4 late toxicities.
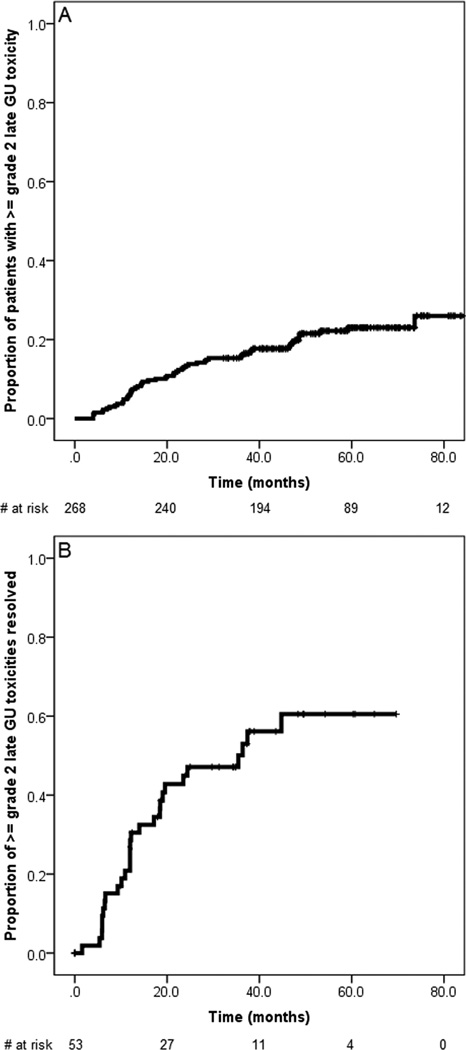
The 5-year incidence of grade ≥2 late GU toxicity was 23%. Of the 57 grade ≥2 late GU toxicity events, 28 (49%) resolved during follow-up, with the median time to resolution being 38 months (Figure 4). Of all tested variables smoking (p = 0.13) and use of ADT (p = 0.12) tended to be associated with late grade ≥2 GU toxicity based on the univariate analysis; however, statistical significance was not reached. At the last follow-up visit, ≥2 GU late toxicity was observed in 29 (11%) patients (Table 3).
Fig. 4.
Kaplan-Meier plot showing the grade ≥2 late genitourinary (GU) actuarial toxicity rate based on the Common Terminology Criteria for Adverse Events scoring system (A) and the probability of amelioration from these toxicity events (B).
4. Discussion
The observed changes in IPSS sum and QoL after high-dose IMRT indicated generally a very-well-tolerated treatment, as there was overall no substantial change in IPSS sum or QoL during follow-up. Likewise, the maximal CTCAE grade 2 and 3 late GU toxicity rates after consideration of baseline GU symptoms were 20% and 1% and declining to 10% and 1% at last follow-up. This was consistent with the observations of Fonteyne et al., who found that, 36 months after IMRT using ≤78 Gy, the observed grade 2 and 3 late GU toxicity rates were 19% and 3% after consideration of baseline symptoms, and 84% and 29% of these late GU toxicities ameliorated during follow-up [3].
Significantly, our results dispel a broadly accepted notion that prevalence of late GU toxicity increases even after 10 years post-RT [15]. In contrast, we found that a significant percentage of patients experienced resolution of their GU symptoms during follow-up. The actuarial late GU toxicity-free survival at 5 years was shown to be a rather poor measure of the prevalence of late GU toxicity, especially at last follow-up. Thus, the Kaplan-Meier estimate of incidence needs to be supplemented with analysis of prevalence of symptoms, because amelioration of symptoms was frequent and of clinical value. This disparity of measures has been recognized by others [16].
When changes in IPSS data were analyzed after stratification for baseline symptoms, the increase in IPSS sum and the worsening of QoL index was more pronounced in patients with favorable baseline functions, while patients with poor baseline functions experienced the most pronounced improvement after therapy. This was consistent with the findings of Chen et al. [7], who described patient-reported urinary function, based on Prostate Cancer Symptom Indices at 36 months after external beam RT and other local therapies. As in our cohort, increases in dysfunction were greatest among patients with normal or intermediate baseline function, while patients with poor baseline function experienced improvement after RT [7]. Our results also compare well with Malik et al. [17], who reported on 80 patients with impaired baseline IPSS sum ≥15 (median follow-up, 40 months) and 288 patients with baseline IPSS sum ≤14 (median follow-up 44 months) after a median dose of 75.6 Gy, respectively. The median baseline IPSS sum for patients with impaired IPSS sum at baseline was 18, declining to 13 at end of follow-up. Likewise, the mean QoL index dropped 0.7 points. For patients with a low IPSS sum at baseline, a minor but statistically significant rise of IPSS sum towards the end of follow-up was observed [17].
The results in this study disagree with previous work claiming a significant association between baseline GU symptoms and increased late GU toxicity [4, 5]. We find the opposite: patients with mild baseline symptoms were at risk of escalation of symptoms, while patients with worse baseline symptoms have the greatest chance for improved symptom profiles after IMRT. It seems plausible that patients with no significant baseline symptoms have more treatment-related dysfunction from a relative perspective because their superior baseline function increases the potential for loss of that function. The causes for the amelioration are probably multifactorial. The proportion of patients taking alpha blockers or anticholinergics increased from 23% or <1% at baseline to 49% or 2% during acute and 42% or 9% during late phase of follow-up (Table 2). Additional causes of GU symptom improvement can be the cytoreductive effect on the prostate gland due to neoadjuvant ADT as well as reduction of prostate cancer disease burden by both ADT and RT.
This work has several limitations. Even though IPSS was prospectively obtained, most of the patients were not treated within a prospective protocol. Thus, for example, the prescription of GU medication did not follow a predefined prospective policy. Medication was prescribed to patients with varying degrees of GU symptoms and it cannot be excluded that medication was preferably given to patients with higher baseline IPSS sum. Moreover, a control group with patients with benign prostate hyperplasia-related symptoms is lacking to separate the effect of IMRT from the effect of aging. We also recognize the irregular attendance of the recommended follow-up scheme as limitation. The strength of our study includes the relatively long follow-up duration and the detailed toxicity assessment involving both a patient- and a physician-reported scoring system.
Given the prevalence of pretreatment baseline GU dysfunction it appears mandatory to assess and use baseline GU symptoms for the definition of GU toxicity events e.g. to consider a true toxicity only when an increase over baseline was observed. This can be done both for patient and physician reported toxicity data and may warrant modification of commonly used current toxicity-grading systems.
5. Conclusions
Our findings may help to counsel patients with localized prostate cancer, who can be reassured that the risk of late GU toxicity and worsening of QoL after our protocol of high-dose IMRT was low. Moreover, the risk for development of late GU toxicity or worsening of QoL was dependent on baseline function such that patients with excellent baseline function were more prone to experience worsening, and patients with impaired baseline function are more likely to experience improvement after therapy.
TAKE HOME MESSAGE.
Late GU toxicity after high-dose IMRT is mild and patients with excellent GU baseline function are more likely to experience late GU toxicity. It is crucial to incorporate baseline GU function in the definition of GU toxicity events.
Acknowledgments
PG was supported financially by the Swiss Foundation for Medical-Biological Scholarships (SSMBS) as well as the Eugen & Elisabeth Schellenberg Foundation.
AJ was supported by NIH R01CA129182.
Footnotes
CONFLICTS OF INTEREST NOTIFICATION
The authors declare that there are no financial disclosures or conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 2.Platz EA, Joshu CE, Mondul AM, Peskoe SB, Willett WC, Giovannucci E. Incidence and progression of lower urinary tract symptoms in a large prospective cohort of United States men. J Urol. 2012;188(2):496–501. doi: 10.1016/j.juro.2012.03.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonteyne V, Villeirs G, Lumen N, De Meerleer G. Urinary toxicity after high dose intensity modulated radiotherapy as primary therapy for prostate cancer. Radiother Oncol. 2009;92:42–47. doi: 10.1016/j.radonc.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Peeters ST, Heemsbergen WD, van Putten WL, et al. Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys. 2005;61(4):1019–1034. doi: 10.1016/j.ijrobp.2004.07.715. [DOI] [PubMed] [Google Scholar]
- 5.Karlsdottir A, Muren LP, Wentzel-Larsen T, Dahl O. Late gastrointestinal morbidity after three-dimensional conformal radiation therapy for prostate cancer fades with time in contrast to genitourinary morbidity. Int J Radiat Oncol Biol Phys. 2008;70(5):1478–1486. doi: 10.1016/j.ijrobp.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 6.Budaus L, Bolla M, Bossi A, et al. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61(1):112–127. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Chen RC, Clark JA, Talcott JA. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J Clin Oncol. 2009;27(24):3916–3922. doi: 10.1200/JCO.2008.18.6486. [DOI] [PubMed] [Google Scholar]
- 8.Viani GA, da Silva LG, Stefano EJ. High-dose conformal radiotherapy reduces prostate cancer-specific mortality: results of a meta-analysis. Int J Radiat Oncol Biol Phys. 2012;83(5):e619–e625. doi: 10.1016/j.ijrobp.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 9.Beckendorf V, Guerif S, Le Prise E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80(4):1056–1063. doi: 10.1016/j.ijrobp.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 10.Zelefsky MJ, Fuks Z, Hunt M, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166(3):876–881. [PubMed] [Google Scholar]
- 11.Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol. 2006;7(11):903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- 12.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 64. [DOI] [PubMed] [Google Scholar]
- 13.Spratt DE, Pei X, Yamada J, Kollmeier MA, Cox B, Zelefsky MJ. Long-term Survival and Toxicity in Patients Treated With High-Dose Intensity Modulated Radiation Therapy for Localized Prostate Cancer. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154(5):1770–1774. doi: 10.1016/s0022-5347(01)66780-6. [DOI] [PubMed] [Google Scholar]
- 15.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(4):1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Peters LJ, Withers HR, Brown BW. Complicating issues in complication reporting. Int J Radiat Oncol Biol Phys. 1995;31(5):1349–1351. doi: 10.1016/0360-3016(95)00041-V. [DOI] [PubMed] [Google Scholar]
- 17.Malik R, Jani AB, Liauw SL. External beam radiotherapy for prostate cancer: urinary outcomes for men with high International Prostate Symptom Scores (IPSS) Int J Radiat Oncol Biol Phys. 2011;80(4):1080–1086. doi: 10.1016/j.ijrobp.2010.03.040. [DOI] [PubMed] [Google Scholar]